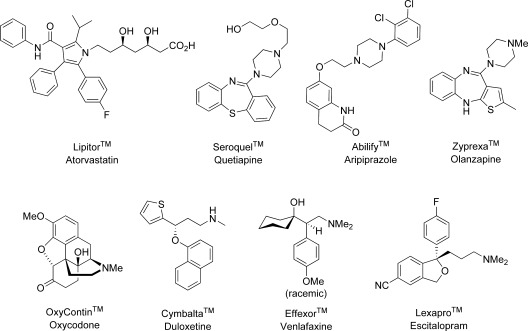
Figures were recently released for the top 200 prescription drugs of 2009 (1,2). As for the past several years, Pfizer’s Lipitor was the number one product, with total sales of >$7.5 billion; however, Lipitor will face patent expiration and generic challengers within the next 2 years. As a class, antipsychotics were the highest grossing, with combined sales of $14.6 billion and three drugs in the top 20 (all atypical antipsychotics: AstraZeneca’s Seroquel, Bristol-Myers-Squibb/Otsuka’s Abilify, and Eli Lilly’s Zyprexa) account for two-thirds of the total sales of the class (1,2). The efficacy of Seroquel is due to inhibition of a variety of neurotransmitters and biogenic amines (D1, D2, 5HT1A, 5HT2A, α1, α2), but unlike clozapine, it possesses higher affinity for serotonin (5-HT2A) receptors than dopamine (D2) receptors and no affinity for muscarinic receptors (3). Abilify is unique in that instead of antagonizing the D2 receptor, it acts as a partial agonist. Abilify is also a partial agonist at the 5-HT1A receptor and antagonizes 5-HT2A4. Zyprexa, a close cousin of clozapine, distinguishes itself (much like Seroquel) by possessing higher affinity for serotonin (5-HT2A) receptors than dopamine (D2) receptors, along with lower affinity at histamine, muscarinic, and adrenergic receptors (5). These three class-leading drugs highlight the value of polypharmacology for the treatment of diseases like schizophrenia, with complex multidimensional symptom clusters.
OxyContin is Purdue Pharma’s brand for a time-release version of the opioid analgesic derived from thebaine, oxycodone (6). In 2009, it was the only pain medication in the top 20, with total sales in excess of $2.9 billion (1,2). The last three CNS drugs in the top 20 for 2009 are antidepressants of the neurotransmitter reuptake class. Eli Lilly’s Cymbalta, a serotonin−norepinephrine reuptake inhibitor (SNRI), leads this class with >$2.8 billion in US sales with approvals for the treatment of major depressive disorder and general anxiety disorder (1,2,7). Like many SNRIs, Cymbalta also inhibits dopamine reuptake, but to a lesser extent. Wyeth’s (now Pfizer’s) Effexor is close behind with US sales of ∼$2.7 billion in 2009. Effexor, another SNRI, is sold as a racemic mixture and is also approved for major depressive disorder and general anxiety disorder (1,2,8). Finally, Lundbeck-Forest’s Lexapro is a selective serotonin reuptake inhibitor (SSRI) with a comparable ∼$2.7 billion in sales. Interestingly, Lexapro is the pure (S)-enantiomer of the older racemic Lundbeck drug citalopram that was approved for the same indications (1,2,9).
After the antipsychotic class of drugs at no. 1 ($ 14.9 billion) come lipid regulators ($14.3 billion, with Lipitor sales accounting for >50%), followed by proton pump inhibitors (∼$12 billion), and finally antidepressants ($9.9 billion). Overall in the US, drug sales totaled >$300 billion (a 5.1% increase over 2008) with over 3.9 billion prescriptions written (1,2). The trend of generic drugs claiming more market share continued in 2009, with sales of $74 billion, accounting for ∼75% of all prescriptions written. This trend can only increase as most major pharmaceutical companies have billion dollar “blockbuster” drugs facing patent expiration in 2011 and 2012. As expected, biologics continue to do well, with several in the top 20 in 2009 (1,2).
Despite a turbulent year in terms of mergers, acquisitions, and product lawsuits, US pharmaceutical sales continue to increase. Notably, 7 of the top 20 drugs in 2009 were for CNS indications and two of the highest grossing classes were antipsychotics (no. 1) and antidepressants (no. 4). Looking ahead, patent expirations and generic competition will challenge many of the leading CNS drugs. What can be done? Clearly, it is time to invest heavily in the development of therapeutics focused on novel targets and mechanisms for the treatment of CNS and other pathologies. If successful, these next generation drugs will capture a significant share of their respective markets, address serious unmet medical needs and improve the lives of patients as well as companies bottom lines.
Figure 1.
Structures of the top prescription drugs of 2009 in the US. As always, Lipitor was the highest grossing single medication (no. 1, ∼$7.5 billion). The top 20 of 2009 consisted of seven CNS drugs: the atypical antipsychotic Serquel (no. 5, ∼$4.1 billion), the atypical antipsychotic Abilify (no. 6, ∼$3.9 billion), the atypical antipsychotic Zyprexa (no. 20, ∼$2.7 billion), the opioid analgesic OxyContin (no. 15, ∼$2.9 billion), the SNRI antidepressant Cymbalta (no. 16, ∼$2.8 billion), the SNRI antidepressant Effexor (no. 17, ∼$2.7 million), and SSRI antidepressant Lexapro (sold as a racemate, no. 18, ∼$2.7 billion).
References
- Bartholow M. (2010) Top prescription drugs of 2009. Pharmacy Times, May 2010 (www.pharmacytimes.com/issue/pharmacy/2010/May2010/RxFocusTopDrugs-0510). [Google Scholar]
- IMS Health (April 1, 2010). www.imshealth.com
- Casey D. E. (1996) Seroquel (quetiapine): preclinical and clinical findings of a new antipsychotic. Exp. Opin. Invest. Drugs 5(8), 939–957for more information see: www.astrazenca-us.com. [Google Scholar]
- Shapiro D. A.; Renock S.; Arrington E.; Chiodo L. A.; Liu L.-X.; Sibley D. R.; Roth B. L.; Mailman R. (2003) Neuropsychopharmacology 28(8), 1400–1411for more information see: www.bms.com. [DOI] [PubMed] [Google Scholar]
- Tamminga C. A.; Kane J. M. (1997) Olanzapine (Zyprexa): Characteristics of a new antipsychotic. Exp. Opin. Invest. Drugs 6(11), 1743–1752for more information see: www.lilly.com. [DOI] [PubMed] [Google Scholar]
- Sunshine A.; Olson N. Z.; Colon A.; Rivera J.; Kaiko R. F.; Fitzmartin R. D.; Reder R. F.; Goldenheim P. D. (1996) Analgesic efficacy of controlled-release oxycodone in postoperative pain. J. Clin. Pharm. 36(7), 595–603for more information see: www.purduepharma.com. [DOI] [PubMed] [Google Scholar]
- Wong D. T.; Robertson D. W.; Bymaster F. P.; Krushinski J. H.; Reid L. R. (1998) LY227942, an inhibitor of serotonin and norepinephrine uptake: Biochemical pharmacology of a potential antidepressant drug. Life Sci. 43(24), 2049–2057for more information see: www.lilly.com. [DOI] [PubMed] [Google Scholar]
- Yardley J. P.; Husbands G. E. M.; Stack G.; Butch J.; Bicksler J.; Moyer J. A.; Muth E. A.; Andree T.; Fletcher H. III; James M. N. G.; Sielecki A. R. (1990) 2-Phenyl-2-(1-hydroxycycloalkyl)ethylamine derivatives: synthesis and antidepressant activity. J. Med. Chem. 33(10), 2899–905for more information see: www.pfizer.com. [DOI] [PubMed] [Google Scholar]
- Chen F.; Larsen M. B.; Sanchez C.; Wiborg O. (2005) The S-enantiomer of R,S-citalopram increases inhibitor binding to the human serotonin transporter by an allosteric mechanism. Comparison with other serotonin transporter inhibitors. Eur. Neuropsychopharmacol. 15(2), 193–198for more information see: www.frx.com. [DOI] [PubMed] [Google Scholar]