Summary
Septins are conserved GTP-binding proteins that associate with cellular membranes and the actin and microtubule cytoskeletons. They polymerize to form filamentous structures that act as diffusion barriers between different membrane domains and as molecular scaffolds for membrane- and cytoskeleton-binding proteins. In yeast, septins are central to the spatio-temporal coordination of membrane polarity and cell division, but the roles of their mammalian counterparts have remained poorly understood. However, recent findings have shed light on the dynamics and regulation of mammalian septin assembly and our understanding of septin functions in cytoskeleton and membrane organization. The mammalian septins appear to form a novel network of hetero-polymers that are multi-functional, inter-changeable and respond dynamically to signals that coordinate events at the interface between cytoskeleton and membrane biology. Hence, studies of these molecules might provide new insights not only into how cells coordinate their functions, but also into the pathogenesis of cancer and other diseases in which septins are abnormally expressed.
Keywords: Septins, Cytoskeleton, Membranes, Vesicles, Mitosis, Intracellular traffic
In the beginning: septins in budding yeast
In Saccharomyces cerevisiae, septins, a conserved family of GTP-binding proteins, are central to the spatio-temporal coordination of membrane polarity and cell division. Originally identified as the targets of a group of mutations that cause cell cycle arrest and aberrant bud growth, septins are recruited to the incipient site of bud growth, where they polymerize to form a network of highly ordered filaments (septin rings, also referred to as the septin collar in budded cells) (Byers and Goetsch, 1976; Hartwell, 1971; Longtine et al., 1996). Septin assembly is temporally linked to G1-specific cell cycle signals and is regulated by the Ras-like Cdc42 signaling module (Cid et al., 2001; Gladfelter et al., 2002; Longtine and Bi, 2003). At the mother-bud neck, the septin collar persists throughout the yeast cell cycle, acting as a spatial landmark for the proper localization of many proteins required for polarized membrane growth, spindle positioning, cytokinesis, cell wall synthesis, and the selection of a new site of bud growth after cell division (Faty et al., 2002; Gladfelter et al., 2001; Kusch et al., 2002).
In addition, septins ensure that progression through the cell cycle is coupled to bud growth and chromosome inheritance. The membrane-bound septin collar serves as a scaffold for protein kinases that cause the gradual degradation of Swe1p, an inhibitor of cyclin-dependent kinase 1 (Cdk1), which in turn promotes entry into mitosis (Sakchaisri et al., 2004). Mitotic exit is also linked to the assembly of the septin collar. Here, septins function as a diffusion barrier restricting the mitotic exit activator Lte1p to the growing bud (Castillon et al., 2003). This ensures exit from mitosis only when the mitotic spindle has positioned one of its poles in the bud, which is crucial for the inheritance of an equal number of chromosomes by the two daughter cells.
The molecular details of septin functions in higher eukaryotes are poorly understood. Septins are essential for the development of adult flies and worms (Adam et al., 2000; Finger et al., 2003; Neufeld and Rubin, 1994; Nguyen et al., 2000; White et al., 1982). In mice, deletion of septin genes results in physiological abnormalities [unregulated platelet secretion in Sept5-null mice (Dent et al., 2002)] and morphogenetic defects [deformed and immotile spermatozoa in Sept4-null mice (Ihara et al., 2005; Kissel et al., 2005)], and in some instances embryonic development is fatally impaired (Sept9-null mice; E. M. Fuechtbauer, Aarhus University, Aarhus, Denmark, personal communication). In humans, growing evidence has linked abnormalities in septin expression to cancer and a variety of other diseases (reviewed by Hall and Russell, 2004).
Thus, similar to their yeast homologs, mammalian septins play key roles in promoting tissue biogenesis and maintenance. Indeed, new reports link mammalian septins to signaling pathways and mechanisms that regulate membrane and cytoskeleton organization and function. Here, we review these findings and discuss how septins might coordinate intracellular organization in response to cell signaling. The assembly, regulation and function of yeast septins are extensively reviewed elsewhere (Longtine and Bi, 2003; Versele and Thorner, 2005); here, we refer to recent studies in yeast as a framework for considering the functions of mammalian septins.
Assembly and regulation of septin polymers
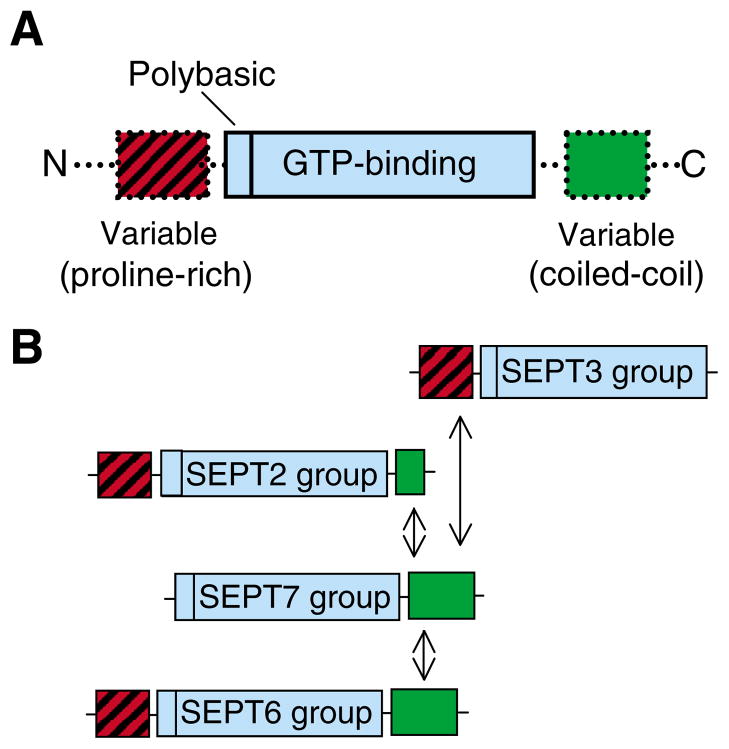
The biological functions of septins stem from their intrinsic ability to assemble into polymers that compartmentalize cell membranes (diffusion barriers) and form scaffolds for interacting proteins at specific intracellular locations (Fig. 1). Humans have thirteen septin genes (SEPT1-SEPT13), whose RNAs contain multiple translation initiation sites and are alternatively spliced to yield an even greater number of septin proteins, some of which are tissue specific (Hall et al., 2005). Analyses of their amino acid sequence similarity indicate that mammalian septins contain highly conserved polybasic and GTP-binding domains and allow them to be classified into four groups (SEPT2, SEPT3, SEPT6 and SEPT7; Fig. 2A,B) (Kartmann and Roth, 2001; Kinoshita, 2003). [Note that, with the discovery of SEPT13, SEPT7 has been classified under SEPT2 (Hall et al., 2005).] Whether mammalian septins function as monomers is unclear, but some monomeric septins are unstable in vivo and form insoluble aggregates in vitro (Kinoshita et al., 2002; Sheffield et al., 2003). Recombinant septins form stable heterodimers in vitro and the basic unit of a septin polymer appears to be a heterotrimeric complex in which three different homodimers associate in equimolar ratios to form a hexamer of 2:2:2 stoichiometry (Joberty et al., 2001; Kinoshita et al., 2002; Sheffield et al., 2003). Although little is known about the exact composition of septin complexes in vivo, SEPT7 is thought to be a unique and perhaps irreplaceable subunit that binds proteins of the SEPT2 (SEPT1, SEPT4, SEPT5) and SEPT6 (SEPT8, SEPT10, SEPT11) groups, which differ in the length of their C-terminal coiled-coil regions, these being conserved domains that mediate most septin interactions (Kinoshita, 2003; Versele and Thorner, 2005). SEPT9 isoforms of the SEPT3 group, which lack a C-terminal coiled-coil domain, might associate with SEPT7 heterodimers through their variable N-terminal sequences, as demonstrated for SEPT9_v3α (also known as SEPT9b) in the assembly of a SEPT7–SEPT11–SEPT9_v3α complex (Nagata et al., 2004).
Fig. 1.
Mammalian septins localize to the plasma membrane and with the actin and microtubule cytoskeletons. (A,B) SEPT2 localizes to the cell cortex, the microtubule spindle apparatus and the midbody of mammalian cells (MDCKs) in mitosis. (C) SEPT2 (red) localizes to membranes (green) labeled with gpiYFP (glycosylphosphatidylinositol linked to yellow fluorescence protein) in interphase MDCK cells. The outlined area shows a region of cell-cell contact in which SEPT2 elements are closely apposed to the plasma membrane. (D) SEPT2 elements (green) colocalize with the microtubule (blue; arrow heads) and actin (red; arrows) cytoskeletons in interphase MDCK cells. All images represent ~1 μm optical sections obtained by confocal microscopy. Bars, ~10 μm.
Fig. 2.
(A) Septin structure. All mammalian septins consist of a polybasic region and a GTP-binding domain of the P-loop superfamily of GTPases. Their N- and C-terminal regions vary in length and amino acid composition, and contain proline-rich (red) and α-helical coiled-coil (green) domains. (B) Assembly and classification of mammalian septins based on amino acid similarity (Kartmann and Roth, 2001; Kinoshita, 2003; Hall et al., 2005). Septins are classified into four groups: SEPT3 (SEPT3, SEPT9 and SEPT12), SEPT2 (SEPT2, SEPT1, SEPT4 and SEPT5), SEPT7 (SEPT7 and SEPT13) and SEPT6 (SEPT6, SEPT8, SEPT10 and SEPT11). Note that proteins of the SEPT7 group were recently classified under the SEPT2 group (Hall et al., 2005). Proteins of the SEPT3 group lack C-terminal coiled-coil domains, and the SEPT2 group contains a shorter coiled-coil region than SEPT6. Septins bind one another through their coiled-coil domains to form hetero-oligomers and hetero-polymers (Sheffield et al., 2003). SEPT9 isoforms (SEPT3 group) associate with other septins through their N-terminal domain (Nagata et al., 2004).
Real-time dynamics of septin assembly into oligomeric complexes and highly ordered polymers have been probed only recently. Septin rings in unbudded (G1) yeast cells dynamically exchange their subunits with an unassembled pool of proteins (Caviston et al., 2003; Dobbelaere et al., 2003). In later stages (G2/M) of the yeast cell cycle, when septins serve as diffusion barriers for the compartmentalization of the cell membrane, septin rings become more stable and little exchange of septin proteins is observed (Caviston et al., 2003; Dobbelaere et al., 2003). This change is genetically linked to protein kinases and phosphatases, which indicates that the phosphorylation state of septins might regulate their polymerization into highly ordered structures (Dobbelaere et al., 2003). In mammalian cells, SEPT2-containing filaments and rings are also highly dynamic structures that exhibit rapid exchange of cytoplasmic SEPT2 molecules in and out of their respective polymers (Schmidt and Nichols, 2004b) (E.T.S. and W.J.N., unpublished observations). Hence, septin polymers appear to be in a dynamic equilibrium with unbound oligomers and, importantly, this dynamic exchange is sensitive to cell-cycle-specific transitions.
In budding yeast, septin polymer dynamics and assembly/disassembly are regulated by post-translational modifications and effectors of Cdc42 (reviewed by Longtine and Bi, 2003; Versele and Thorner, 2005). Septins contain a highly conserved GTP-binding domain that is distantly related to that of the Ras/EF-Tu family of small GTPases, but their intrinsic GTPase activity is not thought to play a regulatory role in the assembly of septin hetero-oligomers beyond the initial folding and dimerization of septin monomers (Mitchison and Field, 2002; Sheffield et al., 2003). Mammalian septin polymers have little or no GTPase activity in vitro and the same is observed for their yeast counterparts in vivo (Kinoshita et al., 2002; Sheffield et al., 2003; Vrabioiu et al., 2004). Although GTPase activity is dispensable for the assembly of the yeast septin collar, GTP binding is required for the establishment of highly ordered polymers (Versele and Thorner, 2004). In fact, regulation of septin assembly/disassembly depends on protein partners that might induce conformational changes by direct binding, GTP loading or covalent addition/removal of phosphate and small ubiquitin-like modifier (SUMO) residues (see below).
Macara and coworkers have demonstrated direct binding of mammalian septin complexes (SEPT2-SEPT6-SEPT7) to a downstream effector of Cdc42 called Borg3, which contains a Cdc42/Rac-interaction binding (CRIB) motif (Joberty et al., 2001). Overexpression of Borg3 disrupts assembly of septins into fibrillar structures, and the effect is ameliorated by expression of a constitutively active mutant of Cdc42, which inhibits the Borg3-septin interaction (Joberty et al., 2001). New studies also link Rho signaling to the regulation of septin assembly. Expression of constitutively active Rho disrupts the filamentous organization of SEPT9_v3α (SEPT9b) and Ito et al. have identified Rhotekin as the downstream effector that controls its assembly into filaments that colocalize with actin stress fibers (Ito et al., 2005). Moreover, Rhotekin accompanies SEPT9_v3α during its assembly and disassembly on actin stress fibers in response to activation of G-protein-coupled receptors and Rho signaling (Ito et al., 2005). In contrast to Rhotekin, which does not bind directly to SEPT9_v3α, a septin-associated RhoGEF (SA-RhoGEF) has now been shown to be a direct SEPT9_v3α-binding partner whose overexpression leads to disruption of SEPT9_v3α polymers (Nagata and Inagaki, 2005).
How effectors of Cdc42 and Rho regulate mammalian septin polymerization is currently unknown. In budding yeast, phosphorylation and sumoylation of septins as a direct consequence of binding to upstream effectors might cause conformational changes that alter the dynamics of septin polymerization. Phosphorylation of the yeast septin Cdc10p is observed upon its binding to Cla4p, an ortholog of mammalian p21-activated protein kinases (PAKs) that contains a CRIB domain and is a downstream effector of Cdc42 (Versele and Thorner, 2004). Similarly, two-hybrid interactions have been found between Drosophila septins and the ubiquitin-activating (E1) and -conjugating (E2) enzymes Dmuba2 and Dmubc9, which catalyze the conjugation of the SUMO1-like protein Smt3 (Shih et al., 2002). Notably, yeast septins are abundantly sumoylated in G2/M-arrested cells, and the ubiquitin-protein ligase (E3) Siz1p is required for septin sumoylation both in vivo and in vitro (Johnson and Blobel, 1999; Johnson and Gupta, 2001). To date, sumoylation of mammalian septins has not been reported. Recent studies have shown that mammalian septins (e.g. SEPT2 and SEPT3) contain multiple phosphorylation sites and are phosphorylated by Ser/Thr kinases such as protein kinase C (PKC) and cGMP-dependent protein kinase I (PKG-I) in post-mitotic neurons (She et al., 2004; Xue et al., 2004a; Xue et al., 2004b; Xue et al., 2000) and by Aurora-B kinase in mitotic cells (e.g. SEPT1) (Qi et al., 2005). Thus, sumoylation and phosphorylation of septins might act as conformational switches that alter their polymerization and thereby functions.
Septins in cytoskeleton organization and function
In marked contrast to yeast septins, which polymerize mainly at cortical sites of the mother-bud neck, mammalian septins localize not only to the plasma membrane but also throughout the cytoplasm with the microtubule and actin cytoskeletons (Fig. 1). Recent studies suggest that mammalian septins function in cytoskeleton organization by acting as scaffolds for cytoskeleton-binding proteins. Hence, mammalian septins appear to have conserved the scaffolding function of their yeast counterparts while adapting it to a range of molecular mechanisms distal to the plasma membrane.
Two reports have independently implicated mammalian septins in microtubule-dependent functions by showing colocalization and copurification of α-tubulin with isoforms of SEPT9 in interphase and mitotic cells (Nagata et al., 2003; Surka et al., 2002). Recent work has extended these studies to SEPT6 and SEPT2; the latter forms a novel network of fibrillar structures at the mitotic midplane of epithelial cells (Spiliotis et al., 2005). Unexpectedly, SEPT2 is required for proper localization of centromere-associated protein E (CENP-E), a mitotic checkpoint and microtubule motor protein required for stable kinetochore attachment to the depolymerizing ends of spindle microtubules. In SEPT2-depleted cells, chromosomes not only fail to align properly but do not segregate, owing to a lack of spindle elongation during anaphase (Spiliotis et al., 2005). Because CENP-E does not account entirely for the range of microtubule-based defects seen after SEPT2 depletion, septin polymers might also act as scaffolds for other microtubule motors and/or microtubule-associated proteins. Recombinant SEPT2-SEPT6-SEPT7 binds directly to microtubule-associated protein 4 (MAP4). This inhibits binding to and bundling of microtubules by MAP4 in vitro (Kremer et al., 2005). Furthermore, septin depletion results in marked stabilization of microtubules and mitotic defects in vivo (Kremer et al., 2005). Note that MAP4 and CENP-E are phosphorylated by kinases of the MARK/Par-1 and ERK families, respectively (Drewes et al., 1998; Zecevic et al., 1998). Hence, similar to yeast septins, which scaffold kinases for the regulation of Swe1p, mammalian septins might act as scaffolds for kinases that regulate binding of MAPs and motors to the microtubule cytoskeleton in interphase and mitotic cells.
In various cell types, actin stress fibers colocalize with fibrillar septin polymers and both networks are structurally interdependent; actin depolymerization results in loss of septin fibers, and septin depletion in turn leads to loss of actin bundles (Kinoshita et al., 2002). The functional interdependence of septins and actin has been difficult to dissect. Kinoshita et al. found that reconstitution of filamentous septin assembly requires F-actin bundles and anillin, an actin-bundling protein that links septins to actin (Kinoshita et al., 2002). Because anillin is mainly sequestered to the nucleus during interphase, Rhotekin and/or SA-RhoGEF could be involved in septin assembly on actin bundles (Ito et al., 2005), but the exact mechanism is unknown. Conversely, binding of SEPT9_v3α to SA-RhoGEF, an activator of Rho, inhibits Rho activation, which implicates septins in feedback regulation of actin assembly (Nagata and Inagaki, 2005). Indeed, excessive actin protrusions are observed upon overexpression of SEPT9_v4, a SEPT9 isoform that exhibits altered expression in breast and ovarian cancer (Chacko et al., 2005).
Cytoplasmic pools of particular septin isoforms (e.g. SEPT2; see Fig. 1D) often colocalize with actin filaments and microtubules, and Drosophila septins purified by F-actin affinity column chromatography rebind microtubules in vitro (Sisson et al., 2000). The involvement of septins in both microtubule and actin cytoskeleton organization and function raises the question of whether they participate in the cross-talk between these two cytoskeleton systems. In light of recent evidence showing that polarization of migrating cells requires actin flow and microtubule capture at the leading edge (Gomes et al., 2005), both of which are mediated by two distinct branches of the Cdc42 signaling pathway (Cau and Hall, 2005; Gomes et al., 2005), septins might coordinate the two cytoskeletons through their interaction with effectors of Cdc42/Rho signaling pathways. The finding that expression of GTP-binding mutants of SEPT9_v4 results in loss of directional cell migration and cytoskeletal polarization in wound-healing assays is consistent with this possibility (Chacko et al., 2005).
Septins in membrane organization and vesicle targeting
Yeast and mammalian septins associate with biological membranes through a highly conserved polybasic region at the N-terminus of the GTP-binding domain (Fig. 2A). Through this region, recombinant yeast septins associate preferentially with phosphatidylinositol (4)-phosphate [PtdIns(4)P] and phosphatidylinositol (5)-phosphate [PtdIns(5)P], whereas recombinant SEPT4 specifically binds phosphatidylinositol (4,5)-bisphosphate [PtdIns(4,5)P2] and phosphatidylinositol (3,4,5)-trisphosphate [PtdIns(3,4,5)P3] (Casamayor and Snyder, 2003; Zhang et al., 1999). In yeast cells that exhibit defective PtdIns(4)P synthesis, septins fail to assemble properly at the mother-bud neck (Casamayor and Snyder, 2003). In mammalian cells, sequestration of PtdIns(4,5)P2 and reduction of its overall level at the membrane leads to the loss of SEPT4 filaments (Zhang et al., 1999), and lipid-based signaling events and PtdIns(4,5)P2-mediated regulation of the actin cytoskeleton might indirectly influence septin polymerization. Alternatively, septins might bind membranes through adaptor proteins such as anillin, which contains an inositol-binding pleckstrin-homology (PH) domain. [Note that, in Drosophila, anillin mutations disrupt septin localization, resulting in the collapse of newly formed plasma membranes during cellularization (Field et al., 2005).]
Association of septins with phospholipids is crucial to the formation and maintenance of membrane domains. Barral and colleagues demonstrated that septins compartmentalize yeast cell membranes (e.g. the plasma membrane and endoplasmic reticulum membrane) during interphase and mitosis (Barral et al., 2000; Dobbelaere and Barral, 2004; Luedeke et al., 2005). In interphase cells, during isotropic bud growth, disruption of the septin collar results in passive diffusion of bud-restricted membrane proteins into the mother cell (Barral et al., 2000). In the bud cortex, actin patches, presumed to be sites of endocytosis, become unstable, which indicates that there are also changes in membrane organization (Barral et al., 2000). In wild-type cells, the septin collar splits into two rings, demarcating a distinct membrane compartment at the plane of cytokinesis during cell division. Therein, septins act as diffusion barriers for the concentration of cortical factors that mediate actomyosin contraction, and membrane growth and abscission at the mother-bud neck (Dobbelaere and Barral, 2004).
Lateral diffusion measurements suggest that the plasma membrane might be similarly compartmentalized during mammalian cytokinesis. In contrast to proteins bound to the outer-leaflet membrane, which freely diffuse in the cortical continuum of a dividing cell, transmembrane and inner-leaflet-bound proteins do not diffuse across the plane of cytokinesis (Schmidt and Nichols, 2004a). On the cytoplasmic side of the cleavage furrow, septins and/or other proteins might therefore corral the plasma membrane, blocking diffusional exchange of membrane proteins. Remarkably, transmembrane and inner-leaflet-bound proteins are also excluded from the centre of the midbody, where septins are particularly enriched (Schmidt and Nichols, 2004a). These observations raise the possibility either that mammalian septins are bona fide components of the plasma membrane skeleton that contribute to the formation of distinct membrane domains, or that their function is coupled to other aspects of membrane organization such as vesicle targeting and fusion.
These two possibilities might not be mutually exclusive. For example, in spermatozoa from male Sept4-knockout mice, both cortical organization and intraflagellar transport are disrupted (Ihara et al., 2005; Kissel et al., 2005). Here, the distribution of kinesin motor proteins is significantly altered, which is reminiscent of the role of septins in the proper localization of MAP4 and CENP-E described above. Interestingly, the presence of MAP4 on microtubules could affect organelle transport independently of its role in microtubule stabilization (Bulinski et al., 1997). Moreover, the presence of SEPT4 on both synaptic vesicles and presynaptic plasma membranes suggests that the function of septins in membrane organization might be reinforced by their involvement in vesicle transport and fusion (Caltagarone et al., 1998).
Among the few interactions reported for mammalian septins is their physical association with components of the machinery for vesicle docking and fusion. Septins copurify and co-immunoprecipitate with the mammalian exocyst complex and soluble N-ethylmaleimide-sensitive fusion (NSF) protein attachment protein (SNARE) receptors, which mediate vesicle docking and fusion, respectively (Beites et al., 1999; Hsu et al., 1998; Vega and Hsu, 2003). Recent evidence suggests that SNARE complexes associate with SEPT5, which competes for binding with the NSF protein and its attachment receptor (SNAP); the latter are known to mediate dissociation of SNARE complexes following membrane fusion (Beites et al., 2005). Hence, septins might regulate the availability of SNAREs for membrane fusion. This would be consistent with data showing inhibition of exocytosis upon overexpression of SEPT5 in insulin-secreting cells and upregulated release of serotonin in platelets from Sept5-knockout mice (Beites et al., 1999; Dent et al., 2002). It is unclear how this specialized SEPT5 role can be reconciled with the ability of septins to bind the exocyst complex, delineate distinct membrane domains and form large cytoplasmic polymers. However, localization of septins to the midbody, a putative region of focused exocytic activity during abscission (Joo et al., 2005), adds weight to the idea that septins perform all of these functions to coordinate membrane targeting and fusion during abscission. This degree of multi-functionality may indeed be achievable within a dynamic network of septin hetero-oligomers and hetero-polymers.
Septin polymers coordinate intracellular organization and functions
Septins are clearly essential for the spatio-temporal coordination of many crucial events in cell division. Yeast septins demarcate membrane domains for the spatial coordination of cytokinesis and this function is integrated with the molecular machinery that confers temporal control of cell division. Similarly, mammalian septins, in addition to their putative role in membrane organization during cytokinesis, form scaffolds for microtubule motor and mitotic checkpoint proteins, which couple microtubule-chromosome interactions with entry into anaphase.
The role of septins in cell division provides a paradigm for how these proteins might coordinate intracellular membrane traffic and the cytoskeleton during interphase in mammalian cells. As noted above, septins associate with both actin and microtubule cytoskeletons, and regulate the localization of cytoskeleton-binding and motor proteins, and bind target membranes and intracellular vesicles. Hence, septins might spatially influence the directionality of vesicle movement and/or catalyze membrane fission and fusion. This might be significant in polarized epithelia and neurons, in which vesicles must be targeted to specific domains. In this context, abnormalities in septin expression are common in neurological diseases and carcinomas (Hall and Russell, 2004), in which loss of cell polarity, increased cell motility and defects in cell division are central to tumor progression and metastasis.
If septins indeed coordinate many aspects of intracellular organization, how do they achieve such a multi-faceted task? Mammalian septins represent a cytoplasmic network of polymers that dynamically rearranges under the guidance of signaling modules (e.g. Cdc42 and Rho) to support various intracellular functions (Fig. 3). In recent years, it has become evident that, in contrast to polymeric GTP-binding proteins (e.g. tubulin) and monomeric GTPases (e.g. Rabs), septins are novel GTPases that combine both structural and regulatory properties. Thereby, septins form a unique network of polymers characterized by three principles: (1) multi-functionality – septin scaffolds and diffusion barriers serve a variety of roles that regulate membrane and cytoskeleton functions; (2) inter-changeability – different septin proteins (>13) bind one another in a combinatorial fashion to form hetero-oligomers, which in turn assemble into polymeric structures of various size and composition; and (3) dynamic responsiveness – assembly of septin hetero-oligomers is dynamic and highly responsive to signaling modules.
Fig. 3.
Septins at the interface between cell signaling, and membrane and cytoskeleton biology. Mammalian septins assemble into hetero-polymers under the influence of the Rho and Cdc42 signaling modules. Phosphorylation of mammalian septins by protein kinases in neurons suggests that post-translational modifications (e.g. phosphorylation and sumoylation) might further modulate the dynamics of their assembly. Hence, septins can dynamically rearrange under the guidance of signaling pathways to support various intracellular functions, including organization and function of the actin and microtubule (MT) cytoskeletons, formation and maintenance of plasma membrane domains, and vesicle transport and fusion.
In the future: septins in mammalian cells and human disease
Clearly, more work is required if we are to understand the functions of all mammalian septins and their concerted operation as a network of polymers that coordinate many intracellular processes. This will not be trivial because different septin isoforms are expressed in different cell types (e.g. neurons, epithelia, immune cells) and some septins might be present in both the cytoplasm and the nucleus (Hall et al., 2005). Although mammalian septins seem largely to have conserved the functions of their yeast counterparts, we have made several assumptions. For example, there is still no formal evidence for rapid changes in mammalian septin dynamics in response to signaling modules. Furthermore, the conformational transitions and morphogenetic properties of mammalian septins are largely unknown. Dissecting septin properties and functions remains a challenging task. Identification of septin-binding partners has been slow, owing to insolubility issues and perturbed affinities; biochemical approaches either disrupt or fail to recapitulate affinities between native septin complexes and their binding partners. Interpretation of results from overexpression, gene ablation and silencing approaches has been hindered by pleiotropic phenotypes as a result of the diverse functions of septins, which are often hard to interpret. Imaging septin interactions in situ and probing their dynamics of assembly in real time using fluorescence-based approaches [e.g. fluorescence resonance energy transfer (FRET) and fluorescence recovery after photobleaching (FRAP)] could be helpful to identify new septin interactions and at the same time aiding our understanding of their morphogenetic properties. The development of chemical inhibitors that specifically interfere with septin assembly might overcome some of these limitations. For example, forchlorfenuron, a synthetic plant cytokinin, was recently found to disrupt yeast septin organization in a specific and reversible manner; however, this drug has yet to be tested in mammalian cells (Iwase et al., 2004). In future studies, the challenge is to understand how septins coordinate intracellular organization through an intricate network of hetero-oligomers and hetero-polymers. With the rise of computational biology and in silico modeling, we can begin to model the septin network and test some complex hypotheses in virtual reality. Finally, understanding how septins are linked to the pathogenesis of cancer and other diseases in which they are abnormally expressed remains the final frontier.
Acknowledgments
We apologize to our colleagues for having to omit, owing to space limitations, many references that detail the studies reported in this commentary. We thank E.-M. Fuechtbauer, I. G. Macara, P. A. Hall and J. Thorner for sharing unpublished observations and manuscripts, and all our colleagues at the 1st International Septin Workshop for stimulating discussions. Work in the Nelson laboratory is supported by National Institutes of Health grant GM35527 to W.J.N. E.T.S. was supported by a postdoctoral fellowship from the Jane Coffin Childs Memorial Fund for Medical Research.
References
- Adam JC, Pringle JR, Peifer M. Evidence for functional differentiation among Drosophila septins in cytokinesis and cellularization. Mol Biol Cell. 2000;11:3123–3135. doi: 10.1091/mbc.11.9.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral Y, Mermall V, Mooseker MS, Snyder M. Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol Cell. 2000;5:841–851. doi: 10.1016/s1097-2765(00)80324-x. [DOI] [PubMed] [Google Scholar]
- Beites CL, Xie H, Bowser R, Trimble WS. The septin CDCrel-1 binds syntaxin and inhibits exocytosis. Nat Neurosci. 1999;2:434–439. doi: 10.1038/8100. [DOI] [PubMed] [Google Scholar]
- Beites CL, Campbell KA, Trimble WS. The septin Sept5/CDCrel-1 competes with alpha-SNAP for binding to the SNARE complex. Biochem J. 2005;385:347–353. doi: 10.1042/BJ20041090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulinski JC, McGraw TE, Gruber D, Nguyen HL, Sheetz MP. Overexpression of MAP4 inhibits organelle motility and trafficking in vivo. J Cell Sci. 1997;110:3055–3064. doi: 10.1242/jcs.110.24.3055. [DOI] [PubMed] [Google Scholar]
- Byers B, Goetsch L. A highly ordered ring of membrane-associated filaments in budding yeast. J Cell Biol. 1976;69:717–721. doi: 10.1083/jcb.69.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caltagarone J, Rhodes J, Honer WG, Bowser R. Localization of a novel septin protein, hCDCrel-1, in neurons of human brain. NeuroReport. 1998;9:2907–2912. doi: 10.1097/00001756-199808240-00042. [DOI] [PubMed] [Google Scholar]
- Casamayor A, Snyder M. Molecular dissection of a yeast septin: distinct domains are required for septin interaction, localization, and function. Mol Cell Biol. 2003;23:2762–2777. doi: 10.1128/MCB.23.8.2762-2777.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillon GA, Adames NR, Rosello CH, Seidel HS, Longtine MS, Cooper JA, Heil-Chapdelaine RA. Septins have a dual role in controlling mitotic exit in budding yeast. Curr Biol. 2003;13:654–658. doi: 10.1016/s0960-9822(03)00247-1. [DOI] [PubMed] [Google Scholar]
- Cau J, Hall A. Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J Cell Sci. 2005;118:2579–2587. doi: 10.1242/jcs.02385. [DOI] [PubMed] [Google Scholar]
- Caviston JP, Longtine M, Pringle JR, Bi E. The role of Cdc42p GTPase-activating proteins in assembly of the septin ring in yeast. Mol Biol Cell. 2003;14:4051–4066. doi: 10.1091/mbc.E03-04-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko AD, Hyland PL, McDade SS, Hamilton PW, Russell SH, Hall PA. SEPT9_v4 expression induces morphological change, increased motility and disturbed polarity. J Pathol. 2005;206:458–465. doi: 10.1002/path.1794. [DOI] [PubMed] [Google Scholar]
- Cid VJ, Adamikova L, Sanchez M, Molina M, Nombela C. Cell cycle control of septin ring dynamics in the budding yeast. Microbiology. 2001;147:1437–1450. doi: 10.1099/00221287-147-6-1437. [DOI] [PubMed] [Google Scholar]
- Dent J, Kato K, Peng XR, Martinez C, Cattaneo M, Poujol C, Nurden P, Nurden A, Trimble WS, Ware J. A prototypic platelet septin and its participation in secretion. Proc Natl Acad Sci USA. 2002;99:3064–3069. doi: 10.1073/pnas.052715199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelaere J, Barral Y. Spatial coordination of cytokinetic events by compartmentalization of the cell cortex. Science. 2004;305:393–396. doi: 10.1126/science.1099892. [DOI] [PubMed] [Google Scholar]
- Dobbelaere J, Gentry MS, Hallberg RL, Barral Y. Phosphorylation-dependent regulation of septin dynamics during the cell cycle. Dev Cell. 2003;4:345–357. doi: 10.1016/s1534-5807(03)00061-3. [DOI] [PubMed] [Google Scholar]
- Drewes G, Ebneth A, Mandelkow EM. MAPs, MARKs and microtubule dynamics. Trends Biochem Sci. 1998;23:307–311. doi: 10.1016/s0968-0004(98)01245-6. [DOI] [PubMed] [Google Scholar]
- Faty M, Fink M, Barral Y. Septins: a ring to part mother and daughter. Curr Genet. 2002;41:123–131. doi: 10.1007/s00294-002-0304-0. [DOI] [PubMed] [Google Scholar]
- Field CM, Coughlin M, Doberstein S, Marty T, Sullivan W. Characterization of anillin mutants reveals essential roles in septin localization and plasma membrane integrity. Development. 2005;132:2849–2860. doi: 10.1242/dev.01843. [DOI] [PubMed] [Google Scholar]
- Finger FP, Kopish KR, White JG. A role for septins in cellular and axonal migration in C. elegans. Dev Biol. 2003;261:220–234. doi: 10.1016/s0012-1606(03)00296-3. [DOI] [PubMed] [Google Scholar]
- Gladfelter AS, Pringle JR, Lew DJ. The septin cortex at the yeast mother-bud neck. Curr Opin Microbiol. 2001;4:681–689. doi: 10.1016/s1369-5274(01)00269-7. [DOI] [PubMed] [Google Scholar]
- Gladfelter AS, Bose I, Zyla TR, Bardes ES, Lew DJ. Septin ring assembly involves cycles of GTP loading and hydrolysis by Cdc42p. J Cell Biol. 2002;156:315–326. doi: 10.1083/jcb.200109062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes ER, Jani S, Gundersen GG. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell. 2005;121:451–463. doi: 10.1016/j.cell.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Hall PA, Russell SE. The pathobiology of the septin gene family. J Pathol. 2004;204:489–505. doi: 10.1002/path.1654. [DOI] [PubMed] [Google Scholar]
- Hall PA, Jung K, Hillan KJ, Russell SE. Expression profiling the human septin gene family. J Pathol. 2005;206:269–278. doi: 10.1002/path.1789. [DOI] [PubMed] [Google Scholar]
- Hartwell LH. Genetic control of the cell division cycle in yeast. IV Genes controlling bud emergence and cytokinesis. Exp Cell Res. 1971;69:265–276. doi: 10.1016/0014-4827(71)90223-0. [DOI] [PubMed] [Google Scholar]
- Hsu SC, Hazuka CD, Roth R, Foletti DL, Heuser J, Scheller RH. Subunit composition, protein interactions, and structures of the mammalian brain sec6/8 complex and septin filaments. Neuron. 1998;20:1111–1122. doi: 10.1016/s0896-6273(00)80493-6. [DOI] [PubMed] [Google Scholar]
- Ihara M, Tomimoto H, Kitayama H, Morioka Y, Akiguchi I, Shibasaki H, Noda M, Kinoshita M. Association of the cytoskeletal GTP-binding protein Sept4/H5 with cytoplasmic inclusions found in Parkinson’s disease and other synucleinopathies. J Biol Chem. 2003;278:24095–24102. doi: 10.1074/jbc.M301352200. [DOI] [PubMed] [Google Scholar]
- Ihara M, Kinoshita A, Yamada S, Tanaka H, Tanigaki A, Kitano A, Goto M, Okubo K, Nishiyama H, Ogawa O, et al. Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev Cell. 2005;8:343–352. doi: 10.1016/j.devcel.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Ito H, Iwamoto I, Morishita R, Nozawa Y, Narumiya S, Asano T, Nagata KI. Possible role of Rho/Rhotekin signaling in mammalian septin organization. Oncogene. 2005;24:7064–7072. doi: 10.1038/sj.onc.1208862. [DOI] [PubMed] [Google Scholar]
- Iwase M, Okada S, Oguchi T, Toh-e A. Forchlorfenuron, a phenylurea cytokinin, disturbs septin organization in Saccharomyces cerevisiae. Genes Genet Syst. 2004;79:199–206. doi: 10.1266/ggs.79.199. [DOI] [PubMed] [Google Scholar]
- Kissel H, Georgescu M-M, Larisch S, Manova K, Hunnicutt GR, Steller H. The Sept4 septin locus is required for sperm terminal differentiation in mice. Dev Cell. 2005;8:353–364. doi: 10.1016/j.devcel.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Joberty G, Perlungher RR, Sheffield PJ, Kinoshita M, Noda M, Haystead T, Macara IG. Borg proteins control septin organization and are negatively regulated by Cdc42. Nat Cell Biol. 2001;3:861–866. doi: 10.1038/ncb1001-861. [DOI] [PubMed] [Google Scholar]
- Johnson ES, Blobel G. Cell cycle-regulated attachment of the ubiquitin-related protein SUMO to the yeast septins. J Cell Biol. 1999;147:981–994. doi: 10.1083/jcb.147.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ES, Gupta AA. An E3-like factor that promotes SUMO conjugation to the yeast septins. Cell. 2001;106:735–744. doi: 10.1016/s0092-8674(01)00491-3. [DOI] [PubMed] [Google Scholar]
- Joo E, Tsang CW, Trimble WS. Septins: traffic control at the cytokinesis intersection. Traffic. 2005;6:626–634. doi: 10.1111/j.1600-0854.2005.00305.x. [DOI] [PubMed] [Google Scholar]
- Kartmann B, Roth D. Novel roles for mammalian septins: from vesicle trafficking to oncogenesis. J Cell Sci. 2001;114:839–844. doi: 10.1242/jcs.114.5.839. [DOI] [PubMed] [Google Scholar]
- Kinoshita M. Assembly of mammalian septins. J Biochem (Tokyo) 2003;134:491–496. doi: 10.1093/jb/mvg182. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Field CM, Coughlin ML, Straight AF, Mitchison TJ. Self- and actin-templated assembly of mammalian septins. Dev Cell. 2002;3:791–802. doi: 10.1016/s1534-5807(02)00366-0. [DOI] [PubMed] [Google Scholar]
- Kremer BE, Haystead T, Macara IG. Mammalian septins regulate microtubule stability through Interaction with the microtubule-binding protein MAP4. Mol Biol Cell. 2005;16:4648–4659. doi: 10.1091/mbc.E05-03-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusch J, Meyer A, Snyder MP, Barral Y. Microtubule capture by the cleavage apparatus is required for proper spindle positioning in yeast. Genes Dev. 2002;16:1627–1639. doi: 10.1101/gad.222602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, Bi E. Regulation of septin organization and function in yeast. Trends Cell Biol. 2003;13:403–409. doi: 10.1016/s0962-8924(03)00151-x. [DOI] [PubMed] [Google Scholar]
- Longtine MS, DeMarini DJ, Valencik ML, Al-Awar OS, Fares H, De Virgilio C, Pringle JR. The septins: roles in cytokinesis and other processes. Curr Opin Cell Biol. 1996;8:106–119. doi: 10.1016/s0955-0674(96)80054-8. [DOI] [PubMed] [Google Scholar]
- Luedeke C, Frei SB, Sbalzarini I, Schwarz H, Spang A, Barral Y. Septin-dependent compartmentalization of the endoplasmic reticulum during yeast polarized growth. J Cell Biol. 2005;169:897–908. doi: 10.1083/jcb.200412143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison TJ, Field CM. Cytoskeleton: what does GTP do for septins? Curr Biol. 2002;12:R788–R790. doi: 10.1016/s0960-9822(02)01295-2. [DOI] [PubMed] [Google Scholar]
- Nagata K, Inagaki M. Cytoskeletal modification of Rho guanine nucleotide exchange factor activity: identification of a Rho guanine nucleotide exchange factor as a binding partner for Sept9b, a mammalian septin. Oncogene. 2005;24:65–76. doi: 10.1038/sj.onc.1208101. [DOI] [PubMed] [Google Scholar]
- Nagata K, Kawajiri A, Matsui S, Takagishi M, Shiromizu T, Saitoh N, Izawa I, Kiyono T, Itoh TJ, Hotani H, et al. Filament formation of MSF-A, a mammalian septin, in human mammary epithelial cells depends on interactions with microtubules. J Biol Chem. 2003;278:18538–18543. doi: 10.1074/jbc.M205246200. [DOI] [PubMed] [Google Scholar]
- Nagata K, Asano T, Nozawa Y, Inagaki M. Biochemical and cell biological analyses of a mammalian septin complex, Sept7/9b/11. J Biol Chem. 2004;279:55895–55904. doi: 10.1074/jbc.M406153200. [DOI] [PubMed] [Google Scholar]
- Neufeld TP, Rubin GM. The Drosophila peanut gene is required for cytokinesis and encodes a protein similar to yeast putative bud neck filament proteins. Cell. 1994;77:371–379. doi: 10.1016/0092-8674(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Nguyen TQ, Sawa H, Okano H, White JG. The C. elegans septin genes, unc-59 and unc-61, are required for normal postembryonic cytokineses and morphogenesis but have no essential function in embryogenesis. J Cell Sci. 2000;113:3825–3837. doi: 10.1242/jcs.113.21.3825. [DOI] [PubMed] [Google Scholar]
- Qi M, Yu W, Liu S, Jia H, Tang L, Shen M, Yan X, Saiyin H, Lang Q, Wan B, et al. Septin1, a new interaction partner for human serine/threonine kinase aurora-B. Biochem Biophys Res Commun. 2005;336:994–1000. doi: 10.1016/j.bbrc.2005.06.212. [DOI] [PubMed] [Google Scholar]
- Sakchaisri K, Asano S, Yu LR, Shulewitz MJ, Park CJ, Park JE, Cho YW, Veenstra TD, Thorner J, Lee KS. Coupling morphogenesis to mitotic entry. Proc Natl Acad Sci USA. 2004;101:4124–4129. doi: 10.1073/pnas.0400641101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K, Nichols BJ. A barrier to lateral diffusion in the cleavage furrow of dividing mammalian cells. Curr Biol. 2004a;14:1002–1006. doi: 10.1016/j.cub.2004.05.044. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Nichols BJ. Functional interdependence between septin and actin cytoskeleton. BMC Cell Biol. 2004b;5:43. doi: 10.1186/1471-2121-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She YM, Huang YW, Zhang L, Trimble WS. Septin 2 phosphorylation: theoretical and mass spectrometric evidence for the existence of a single phosphorylation site in vivo. Rapid Commun Mass Spectrom. 2004;18:1123–1130. doi: 10.1002/rcm.1453. [DOI] [PubMed] [Google Scholar]
- Sheffield PJ, Oliver CJ, Kremer BE, Sheng S, Shao Z, Macara IG. Borg/septin interactions and the assembly of mammalian septin heterodimers, trimers, and filaments. J Biol Chem. 2003;278:3483–3488. doi: 10.1074/jbc.M209701200. [DOI] [PubMed] [Google Scholar]
- Shih HP, Hales KG, Pringle JR, Peifer M. Identification of septin-interacting proteins and characterization of the Smt3/SUMO-conjugation system in Drosophila. J Cell Sci. 2002;115:1259–1271. doi: 10.1242/jcs.115.6.1259. [DOI] [PubMed] [Google Scholar]
- Sisson JC, Field C, Ventura R, Royou A, Sullivan W. Lava lamp, a novel peripheral golgi protein, is required for Drosophila melanogaster cellularization. J Cell Biol. 2000;151:905–918. doi: 10.1083/jcb.151.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiliotis ET, Kinoshita M, Nelson WJ. A mitotic septin scaffold required for mammalian chromosome congression and segregation. Science. 2005;307:1781–1785. doi: 10.1126/science.1106823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surka MC, Tsang CW, Trimble WS. The mammalian septin MSF localizes with microtubules and is required for completion of cytokinesis. Mol Biol Cell. 2002;13:3532–3545. doi: 10.1091/mbc.E02-01-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega IE, Hsu SC. The septin protein Nedd5 associates with both the exocyst complex and microtubules and disruption of its GTPase activity promotes aberrant neurite sprouting in PC12 cells. NeuroReport. 2003;14:31–37. doi: 10.1097/00001756-200301200-00006. [DOI] [PubMed] [Google Scholar]
- Versele M, Thorner J. Septin collar formation in budding yeast requires GTP binding and direct phosphorylation by the PAK, Cla4. J Cell Biol. 2004;164:701–715. doi: 10.1083/jcb.200312070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versele M, Thorner J. Some assembly required: yeast septins provide the instruction manual. Trends Cell Biol. 2005;15:414–424. doi: 10.1016/j.tcb.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrabioiu AM, Gerber SA, Gygi SP, Field CM, Mitchison TJ. The majority of the Saccharomyces cerevisiae septin complexes do not exchange guanine nucleotides. J Biol Chem. 2004;279:3111–3118. doi: 10.1074/jbc.M310941200. [DOI] [PubMed] [Google Scholar]
- White JG, Horvitz HR, Sulston JE. Neurone differentiation in cell lineage mutants of Caenorhabditis elegans. Nature. 1982;297:584–587. doi: 10.1038/297584a0. [DOI] [PubMed] [Google Scholar]
- Xue J, Wang X, Malladi CS, Kinoshita M, Milburn PJ, Lengyel I, Rostas JA, Robinson PJ. Phosphorylation of a new brain-specific septin, G-septin, by cGMP-dependent protein kinase. J Biol Chem. 2000;275:10047–10056. doi: 10.1074/jbc.275.14.10047. [DOI] [PubMed] [Google Scholar]
- Xue J, Milburn PJ, Hanna BT, Graham ME, Rostas JA, Robinson PJ. Phosphorylation of septin 3 on Ser-91 by cGMP-dependent protein kinase-I in nerve terminals. Biochem J. 2004a;381:753–760. doi: 10.1042/BJ20040455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Tsang CW, Gai WP, Malladi CS, Trimble WS, Rostas JA, Robinson PJ. Septin 3 (G-septin) is a developmentally regulated phosphoprotein enriched in presynaptic nerve terminals. J Neurochem. 2004b;91:579–590. doi: 10.1111/j.1471-4159.2004.02755.x. [DOI] [PubMed] [Google Scholar]
- Zecevic M, Catling AD, Eblen ST, Renzi L, Hittle JC, Yen TJ, Gorbsky GJ, Weber MJ. Active MAP kinase in mitosis: localization at kinetochores and association with the motor protein CENP-E. J Cell Biol. 1998;142:1547–1558. doi: 10.1083/jcb.142.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Kong C, Xie H, McPherson PS, Grinstein S, Trimble WS. Phosphatidylinositol polyphosphate binding to the mammalian septin H5 is modulated by GTP. Curr Biol. 1999;9:1458–1467. doi: 10.1016/s0960-9822(00)80115-3. [DOI] [PubMed] [Google Scholar]