Abstract
Participants with mild cognitive impairment (MCI) have a higher likelihood of developing Alzheimer's disease (AD) compared to those without MCI, and functional magnetic resonance neuroimaging (fMRI) used with MCI participants may prove to be an important tool in identifying early biomarkers for AD. We tested the hypothesis that functional connectivity differences exist between older adults with and without MCI using resting-state fMRI. Data were collected on over 200 participants of the Rush Memory and Aging Project, a community-based, clinical-pathological cohort study of aging. From the cohort, 40 participants were identified as having MCI, and were compared to 40 demographically matched participants without cognitive impairment. MCI participants showed lesser functional connectivity between the posterior cingulate cortex and right and left orbital frontal, right middle frontal, left putamen, right caudate, left superior temporal, and right posterior cingulate regions; and greater connectivity with right inferior frontal, left fusiform, left rectal, and left precentral regions. Furthermore, in an alternate sample of 113, connectivity values in regions of difference correlated with episodic memory and processing speed. Results suggest functional connectivity values in regions of difference are associated with cognitive function and may reflect the presence of AD pathology and increased risk of developing clinical AD.
Keywords: Mild cognitive impairment (MCI), Resting-state fMRI, Functional connectivity, Posterior cingulate cortex, Memory, Basal ganglia, Striatum
Introduction
Alzheimer's disease (AD) currently affects an estimated 4.5 million persons in the United States (Hebert, Scherr, Bienias, Bennett, & Evans, 2003) and is predicted to affect more than 13.5 million persons by the year 2050 (Alzheimer's Association, 2009). In view of the heavy medical, social, and economic burdens of the disease, prevention is the best long-term strategy for addressing this significant public health problem. The identification of individuals at higher risk of developing a clinical diagnosis of AD is necessary for any comprehensive prevention program, and a variety of approaches have been used to identify predictive biomarkers (Sperling et al., 2011). In particular, functional neuroimaging biomarkers have significantly advanced our understanding of the progression to AD (Habeck et al., 2008), and advanced neuroimaging techniques will play an even greater role in the diagnosis of early AD according to recently revised criteria (McKhann et al., 2011). One approach which shows particular promise in the early detection of AD uses resting-state functional magnetic resonance imaging (fMRI) to assess functional connectivity in the brain by detecting gray matter regions that exhibit high temporal correlation of low frequency fluctuations. Because participants are asked to simply lie still during a conventional MR scan, resting-state fMRI can be used outside of specialized academic medical centers and thus has potential to become a widely used clinical diagnostic tool.
Resting-state fMRI has been increasingly used as a method for ascertaining functional connectivity of portions of the Default Mode Network (Buckner et al., 2005, 2009; Buckner and Vincent, 2007; Fleisher et al., 2009; Greicius, Srivastava, Reiss, & Menon, 2004; Hedden et al., 2009; Koch et al., 2010; Sperling et al., 2009; Wang et al., 2006), a network of brain regions including the posterior cingulate cortex, ventral anterior cingulate cortex, lateral parietal, temporal, and medial frontal regions that consistently show greater activity during periods of rest and less activity during periods of active cognitive engagement (Damoiseaux et al., 2006; Greicius, Krasnow, Reiss, & Menon, 2003; Greicius et al., 2008; Gusnard & Raichle, 2001; Harrison et al., 2008; Raiche et al., 2001). Beta amyloid deposition, which increases the risk of cognitive decline and dementia in aging (Sperling et al., 2011), appears to be greater in regions that comprise the Default Mode Network demonstrated by imaging using 11C-labeled Pittsburgh Compound B (Hedden et al., 2009; Sperling et al., 2009), and greater amyloid deposition in the Default Mode Network has been linked to worse performance in associative memory fMRI tasks in elderly controls and mild AD participants (Buckner et al., 2005).
Using resting-state fMRI to investigate functional connectivity in the Default Mode Network, several groups have shown less functional connectivity between the posterior cingulate and medial temporal regions in MCI and AD (Allen et al., 2007; Rombouts, Barkhof, Goekoop, Stam, & Sheltens, 2005; Sorg et al., 2007; Wang et al., 2006; Zhang et al., 2009; Zhou et al., 2008). However, some studies have found greater connectivity between the posterior cingulate cortex and frontal regions in persons with MCI (Bai et al., 2009) and others less connectivity (Gili et al., 2011), in addition to less connectivity between the posterior cingulate cortex and medial temporal lobe. The observation of increased functional connectivity between the posterior cingulate cortex and frontal regions suggests that as certain regions become functionally disconnected, other regions may become more functionally connected, perhaps as a compensatory response in older age. This is similar to what has been observed in some event-related fMRI studies (e.g., Cabeza, 2002).
Previous resting-state neuroimaging biomarker endeavors have focused on highly selected individuals with relatively low experimental group numbers. This focus may have contributed to the discrepant findings of prior studies. Furthermore, the use of highly selected individuals may limit generalization of this approach to the community where biomarker studies will be most widely used as a diagnostic tool. In the present study, we investigated differences in functional connectivity in persons with MCI compared to persons with no cognitive impairment participating in the Rush Memory and Aging Project, a community-based, clinical-pathological cohort study of aging and dementia (Bennett et al., 2005). We hypothesized that persons with mild cognitive impairment would show reductions in functional connectivity to the posterior cingulate cortex when compared to persons with no cognitive impairment. Furthermore, we hypothesized that functional connectivity values in regions of difference would correlate with measures of cognition.
Methods
Participants and Procedures
Participants were recruited from the Rush Memory and Aging Project, a community-based clinical-pathologic cohort study of aging and dementia (Bennett et al., 2005). Participants are free of clinically diagnosed dementia at baseline and are followed annually until death. They come from approximately 40 residential facilities across the greater Chicago metropolitan area, including subsidized senior housing facilities, retirement communities, retirement homes, local churches, and other community organizations. All participant procedures were approved by an Internal Review Board.
The Rush Memory and Aging Project has a rolling admission that started in 1997. Brain imaging was initiated in 2008. At the time of analyses, 1299 participants had enrolled and completed their baseline evaluation, 443 died, and 77 refused further participation before scan data collection began. Of the remaining 779, a total of 260 had MRI contraindications or were unable to sign informed consent leaving 519 eligible for scanning. Of these, 155 (29.9%) refused, 214 were scanned, and the remaining 150 were still being scheduled for scanning. From the 214 that were scanned, 14 were dropped due to excessive motion, 7 were dropped due to scanning data acquisition problems, leaving 193 participants. Of these, 40 were found to have MCI (as described below), and 40 demographically matched participants without cognitive impairment (also described as “no cognitive impairment” or “NCI”) were selected at random by a statistician for initial analyses to determine peak voxels of difference. The remaining 113 non-cognitively impaired participants were used as an “alternate sample” to test the association between observed regions of difference (coordinates defined by between-group differences) and measures of cognition. The alternate sample was used to avoid any issues of multicollinearity, circularity, or selection bias since the MCI and non-cognitive impaired groups were distinguished by performance on cognitive measures.
Diagnostic classification was performed by a clinician with expertise in the evaluation of older persons after review of clinical data. A battery of 21 cognitive performance tests was administered by trained technicians in an approximately hour-long session during baseline and annual follow-up sessions. Measures of cognitive function assessed a broad range of dissociable cognitive abilities that are consistent with functioning of different anatomic substrates commonly affected by aging and AD (Bennett et al., 2006; Wilson, Barnes, & Bennett, 2003). Episodic memory measures included Word List Memory, Word List Recall and Word List Recognition from the procedures established by the CERAD; immediate and delayed recall of Logical Memory Story A and the East Boston Story. Semantic memory measures included Verbal Fluency, Boston Naming, subsets of items from Complex Ideational Material, and the National Adult Reading Test. Working memory measures included the Digit Span subtests (forward and backward) of the Wechsler Memory Scale-Revised and Digit Ordering. Measures of perceptual speed included the oral version of the Symbol Digit Modalities Test and Number Comparison. Measures of visuospatial ability included Judgment of Line Orientation and Standard Progressive Matrices. Raw scores on each test were converted to standard z-scores using the mean and standard deviation from the baseline evaluation. A person's standard scores across 19 tests were averaged to yield a single overall cognitive composite score (Fleischman, Wilson, Bienias, & Bennett, 2005). A composite score for five cognitive domains (episodic memory, semantic memory, working memory, perceptual speed, visuospatial ability) was created by averaging the z-scores of all measures within a domain, as previously described (Fleischman et al., 2005). A composite score has the advantage of increasing power by reducing random variability and floor and ceiling effects. An experienced neuro-psychologist with expertise in aging and AD and blinded to participant age, sex, and race reviewed all results of cognitive measures and rendered a judgment as to cognitive impairment. Next, an experienced clinician with expertise in the diagnosis of AD reviewed all available participant information (cognitive data, medical history, neurological exam, brain scan) and rendered a judgment as to dementia in accordance with NINCDS/ADRDA criteria. Finally, any participant with cognitive impairment but no dementia was deemed to have MCI (Bennett et al., 2002; Boyle, Wilson, Aggarwal, Tang, & Bennett, 2005). This diagnostic characterization of MCI by the Rush Alzheimer's Disease Center closely resembles the condition of “Cognitive Impaired Not Demented” or otherwise known as CIND (Graham et al., 1997).
Image Acquisition and Processing
MRI scans were conducted on a 1.5 Tesla clinical scanner (General Electric, Waukesha, WI), equipped with a standard quadrature head coil, located within the community of the sample. High data quality was ensured through daily quality assurance tests. High-resolution T1-weighted anatomical images were collected with a 3D magnetization-prepared rapid acquisition gradient-echo (MPRAGE) sequence with the following parameters: repetition time (TR) = 6.3 ms; echo time (TE) = 2.8 ms; preparation time = 1000 ms; flip angle = 8°; 160 sagittal slices; 1 mm slice thickness; field of view (FOV)=24cm × 24cm; acquisition matrix 224 × 192, reconstructed to a 256 × 256 image matrix; scan time = 10 min and 56 s. Two copies of the T1-weighted data were acquired on each subject. Resting-state MRI data was acquired using a two-dimensional (2D) spiral in/out echo-planar imaging (EPI) sequence with the following parameters: TR=2000 ms; TE=33 ms; flip angle=85°; 26 oblique axial slices; 5 mm slice thickness; acquisition/reconstruction matrix 64×64; FOV=24cm×24cm; 240 time-points/volumes; scan time = 8 min. Participants were not given instructions regarding the opening and closing of eyes.
The skull was removed from each structural MRI dataset using FreeSurfer's Hybrid Watershed Algorithm (Segonne et al., 2004). Structural scans were also manually edited when necessary to remove residual non-brain material. Brain segmentation into gray matter, white matter and CSF was also performed using FreeSurfer (http://surfer.nmr.mgh.harvard.edu/). Whole brain volume was also derived, and thus proportions of each compartment were calculated.
The first five image volumes of resting-state data were discarded at the scanner to avoid using data collected before reaching signal equilibrium. Images were reconstructed on Linux machines from the acquired k-space data (Glover & Thomason, 2004). Using the Statistical Parametric Mapping software (Friston et al., 1995; http://www.fil.ion.ucl.ac.uk/spm/) version 8 (SPM8), all volumes were corrected for motion, co-registered to the high-resolution T1-weighted data, and spatially normalized to the Montreal Neurological Institute (MNI) template. The normalized image volumes were spatially smoothed with a 4-mm full-width half-maximum (FWHM) Gaussian kernel. Next, a band-pass filter of 0.01 to 0.08 Hz was applied to the data in temporal frequency space to minimize low-frequency signal drift and high frequency variations due to cardiac and respiratory effects. To remove any residual effects of motion and other non-neuronal factors, 6 head motion parameters, as well as parameters for the white matter signal, global mean signal, and cerebrospinal fluid signal were used as nuisance variables (Buckner et al., 2009) in functional connectivity analysis using the Resting-State fMRI Data Analysis Toolkit (REST: http://restfmri.net/forum/REST).
Previous research suggests that the functional connectivity map generated when using the posterior cingulate as a seed region of interest may provide the best match with the hypothesized Default Mode Network (Greicius et al., 2003; Buckner et al., 2009). Therefore, a spherical seed ROI with a radius of 4 mm was prescribed in the posterior cingulate cortex, with MNI coordinates of x = 0, y = −53, z = 26 in accordance with previous work (Hedden et al., 2009). A mean signal time course for the seed was calculated and used as a reference. Cross-correlation analysis was then conducted between the reference signal time course and the time series of each other voxel in the brain. The voxels showing significant functional connectivity to the posterior cingulate seed ROI were identified as those voxels whose cross-correlation differed significantly (alpha = 0.001) from 0, based on whole-brain Fisher's z-transformation of the correlations at the individual level. Seed-based functional connectivity analysis was conducted with the Data Processing Assistant for Resting-State fMRI (DPARSF; http://restfmri.net/forum/DPARSF) and SPM8.
Statistical Analyses
Statistical analyses proceeded in several steps. We first examined between-group differences in demographic variables (age, education, sex, Mini-Mental State Examination [MMSE], race), cognitive performance data (episodic memory, semantic memory, working memory, processing speed, perceptual organization, global cognition), brain volumetry (total gray matter volume and posterior cingulate cortex volume), and clinical diagnosis (MCI versus no cognitive impairment) using between-group analyses (age, education, MMSE, total gray matter volume, posterior cingulate volume), or Chi-square tests (sex, race). We then verified the functional connectivity of the posterior cingulate cortex to other regions associated with the Default Mode Network by determining within-group whole brain z-transformed functional clusters of significance, for both clinical diagnoses (MCI and no cognitive impairment), after controlling for the effects of total gray matter volume. To control for multiple comparisons, within-group whole brain functional imaging results were controlled by using a false-discovery rate (FDR) of p <.01. After verifying the functional connectivity of the posterior cingulate cortex to other regions associated with the Default Mode Network, we conducted voxel-wise, between-group comparisons of z-transformed functional connectivity values (MCI versus no cognitive impairment) while adjusting for the effects of total gray matter volume. The chance of spurious findings was controlled by using a voxel height threshold of p<.001 and a cluster size threshold of five voxels. Finally, in each subject of an alternate sample, we extracted functional connectivity z-scores at the same coordinates of the maximum intensity voxels of clusters of difference from our between-group comparisons and conducted exploratory partial correlations of these connectivity z-scores with measures of cognitive performance. A non-cognitively impaired alternate sample was used for the partial correlation analyses to avoid any issues of multicollinearity, circularity, or selection bias since MCI and non-cognitively impaired participants were grouped based on cognitive performance. Partial correlations were adjusted for age, education, and gender as these factors have been known to correlate with cognitive performance. Significance was determined at p <.05.
Results
Demographic, Cognitive, and Brain Volumetry Differences Between MCI and Those Without Cognitive Impairment
Demographic, cognitive, and brain volumetry characteristics are shown in Table 1. Most participants were white. As expected, persons with MCI had lower MMSE scores, but did not significantly differ in terms of age, sex, or education. Furthermore, persons with MCI had lower scores on the global cognition measure and all five cognitive domain scores. Total gray matter volume and posteror cingulate cortex volume did not differ significantly between groups.
Table 1.
Statistics for demographic, cognitive, and brain volumetry variables for the non-cognitive impaired participants (NCI, N = 40) and mild cognitively impaired participants (MCI, N = 40)
| NCI n = 40 |
MCI n = 40 |
Whole sample n = 80 |
t or χ2 | p value | |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean (SD) | 86.28 (4.39) | 86.26 (4.49) | 86.27 (4.41) | .025 | .98 |
| Range | 74–94 | 75–94 | 74–94 | ||
| Education (years) | |||||
| Mean (SD) | 15.98 (2.86) | 15.00 (3.22) | 15.49 (3.07) | 1.43 | .16 |
| Range | 12–23 | 8–25 | 8–25 | ||
| MMSE (total score)*** | |||||
| Mean (SD) | 28.68 (1.23) | 27.10 (1.96) | 27.59 (1.81) | 4.31 | <.001 |
| Range | 26–30 | 23–30 | 23–30 | ||
| Sex | |||||
| % Female | 62.5 (n = 25) | 82.5 (n = 33) | 72.5 (n = 58) | 3.07 | .08 |
| Race | |||||
| % White | 100(n = 40) | 100(n = 40) | 100(n = 80) | - | - |
| Global Cognition (z-score)*** | 0.49 (0.41) | −0.18 (0.50) | 6.59 | <.001 | |
| Episodic Memory (z-score)*** | 0.68 (0.52) | −0.23 (0.85) | 5.74 | <.001 | |
| Semantic Memory (z-score)*** | 0.56 (0.63) | −0.07 (0.75) | 4.02 | <.001 | |
| Working Memory (z-score)** | 0.31 (0.73) | −0.16 (0.83) | 2.69 | .009 | |
| Processing Speed (z-score)** | 0.25 (0.63) | −0.16 (0.73) | 2.70 | .008 | |
| Perceptual Organization (z-score)*** | 0.45 (0.53) | −0.33 (0.91) | 4.64 | <.001 | |
| Total Gray Matter (mm3) | 268.10 (26.85) | 270.34 (21.03) | 20.42 | .68 | |
| Posterior Cingulate Cortex (mm3) | 2.98 (0.60) | 3.04 (0.57) | 20.49 | .62 |
Note. Data are summarized as Mean (standard deviation = SD) or as number (%). Age and education are presented in years; MMSE is total score; global cognition, episodic memory, semantic memory, working memory, processing speed, and perceptual organization are presented as z-scores; and total gray matter and posterior cingulate cortex volumes are presented as mm3. Asterisks indicate significant differences between diagnostic groups.
p<.01
p<.001.
Resting-State Functional Connectivity Differences Between MCI and Those Without Cognitive Impairment
Seeding the posterior cingulate cortex yielded a network of functionally related regions consistent with the Default Mode Network in analyses controlling for total gray matter volume among cognitively intact and MCI participants in within-group analyses (Figure 1 and Table 2). These regions included large clusters of significance centering in widespread bilateral posterior cingulate, medial and lateral parietal, temporal, occipital, basal ganglia, and thamalic regions (t = 51.6306), widespread bilateral medial frontal and anterior cingulate regions (t = 9.0885), and smaller clusters of significance in left (t = 7.2618; t = 4.2848) and right (t = 6.7549) middle temporal, left (t = 4.6772) and right (t = 6.0595; t = 4.5446) cerebellum, left superior temporal (t = 4.9723), left (t = 3.5388) and right (t = 4.5782) orbital frontal, right insula (t = 4.2836), left (t = 4.2180) and right (t =3.9550) thalamus, left brainstem (t =4.1424), right cuneus (t = 4.0907), right caudate (t = 3.9912), and left (t = 3.6707) and right (t = 3.8836; t = 3.7365) inferior frontal regions for those without cognitive impairment. For participants with MCI, large clusters of significance were observed centering in widespread bilateral posterior cingulate, medial and lateral parietal, temporal, occipital, basal ganglia, and thalamic regions (t = 53.8787), widespread bilateral medial, frontal, and anterior cingulate regions (t = 10.1883), and smaller clusters of significance in the left (t = 7.9563) and right (t =7.4053; t = 3.7045; t = 3.9644; t = 3.7045) middle temporal, right (t = 6.5141; t = 4.5733; t = 4.3973) and left (t = 6.4655; t = 5.1676; t = 4.2190) cerebellum, right parahippocampal (t = 4.6878), right superior temporal (t = 4.5437), right thalamus (t = 4.3221), left brainstem (t = 4.1668), left caudate (t = 4.1087), and right anterior cingulate (t = 3.8263) regions.
Fig. 1.
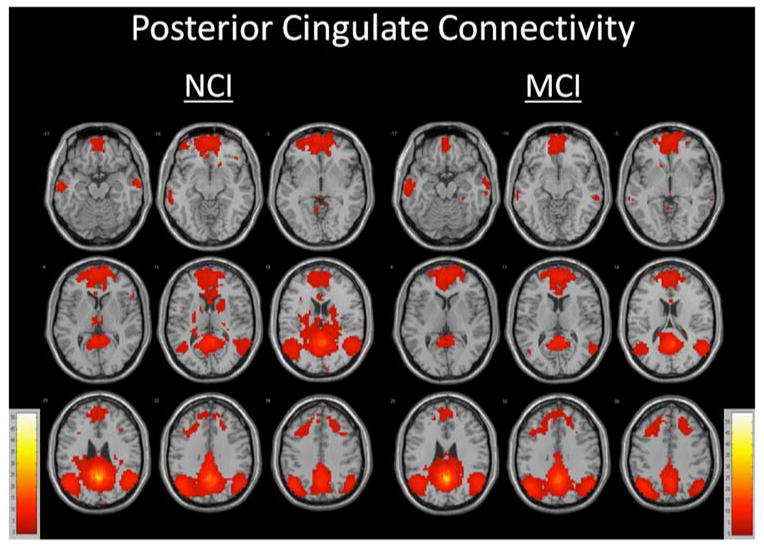
Functionally connected clusters indicated by a seed region of interest (ROI) prescribed in the posterior cingulate cortex for participants with no cognitive impairment (NCI, N = 40) and participants with mild cognitive impairment (MCI, N = 40). Seed ROI MNI coordinates: x = 0, y = −53, z = 26; radius=4mm, p<.001, cluster size >5 voxels, false-discovery rate (FDR) corrected at p < .01.
Table 2.
Functionally connected clusters as indicated by a seed region of interest (ROI) prescribed in the posterior cingulate cortex and covarying for the effects of total gray matter volume for non-cognitively impaired participants (NCI) and mild cognitive impaired participants (MCI)
| Group | Region | Cluster Size (# voxels) | Maximum Intensity Voxel coordinates | t-value | ||
|---|---|---|---|---|---|---|
| NCI | ||||||
| Widespread bilateral posterior cingulate, medial and lateral parietal, temporal, occipital, basal ganglia, thalamus | 5393 | 0 | −54 | 27 | 51.6306 | |
| Widespread bilateral medial frontal, anterior cingulate | 2573 | −3 | 63 | 0 | 9.0885 | |
| L middle temporal | 160 | −63 | −15 | −18 | 7.2618 | |
| R middle temporal | 95 | 63 | −9 | −21 | 6.7549 | |
| R cerebellum | 90 | 6 | −54 | −42 | 6.0595 | |
| L superior temporal | 5 | −39 | 21 | −30 | 4.9723 | |
| L cerebellum | 74 | −30 | −78 | −39 | 4.6772 | |
| R orbital frontal | 6 | 45 | 30 | −12 | 4.5782 | |
| R cerebellum | 61 | 33 | −75 | −39 | 4.5446 | |
| L middle temporal | 5 | −48 | 6 | −36 | 4.2848 | |
| R insula | 5 | 36 | −12 | 21 | 4.2836 | |
| L thalamus | 26 | −9 | −21 | 6 | 4.2180 | |
| L brainstem | 8 | 0 | −33 | −6 | 4.1424 | |
| R cuneus | 9 | 9 | −93 | 21 | 4.0907 | |
| R caudate | 8 | 18 | 21 | −6 | 3.9912 | |
| R thalamus | 12 | 6 | −18 | 3 | 3.9550 | |
| R inferior frontal | 11 | 57 | 24 | 3 | 3.8836 | |
| R inferior frontal | 5 | 36 | 24 | 24 | 3.7365 | |
| L inferior frontal | 10 | −51 | 24 | 6 | 3.6707 | |
| L orbital frontal | 6 | −48 | 33 | −12 | 3.5388 | |
| MCI | ||||||
| Widespread bilateral posterior cingulate, medial and lateral parietal, temporal, occipital, basal ganglia, thalamus | 4131 | 0 | −54 | 27 | 53.8787 | |
| Widespread bilateral medial frontal, anterior cingulate | 2426 | 3 | 54 | 12 | 10.1883 | |
| L middle temporal | 216 | −66 | −12 | −18 | 7.9563 | |
| R middle temporal | 142 | 63 | −3 | −24 | 7.4053 | |
| R cerebellum | 41 | 9 | −54 | −45 | 6.5141 | |
| L cerebellum | 29 | −9 | −57 | −45 | 6.4655 | |
| L cerebellum | 48 | −30 | −81 | −33 | 5.1676 | |
| R parahippocampal | 5 | 27 | −33 | −18 | 4.6878 | |
| R cerebellum | 36 | 42 | −69 | −39 | 4.5733 | |
| R superior temporal | 17 | 45 | 21 | −33 | 4.5437 | |
| R cerebellum | 74 | 30 | −84 | −30 | 4.3973 | |
| R thalamus | 5 | 9 | −12 | 9 | 4.3221 | |
| L cerebellum | 15 | −42 | −63 | −48 | 4.2190 | |
| L brainstem | 9 | −3 | −30 | −6 | 4.1668 | |
| L caudate | 8 | −15 | 18 | −3 | 4.1087 | |
| R middle temporal | 20 | 63 | −33 | −9 | 3.9644 | |
| R anterior cingulate | 9 | 3 | 18 | 18 | 3.8263 | |
| R middle temporal | 9 | 63 | −24 | −18 | 3.7045 | |
Seed ROI MNI coordinates: x = 0, y = −53, z = 26; radius = 4mm; p < 0.001, cluster size >5 voxels, false-discovery rate (FDR) corrected at p < .01.
Multiple regions of functional connectivity differences were observed between MCI and NCI subjects in between-group analyses (Figure 2 and Table 3). Persons with MCI were characterized by less functional connectivity in left (t = 4.2153) and right (t = 3.9043) orbital frontal, right middle frontal (t = 3.8609), left putamen (t = 4.9792), right caudate (t = 4.1524; t = 4.0735), left superior temporal (t = 3.9342), and right posterior cingulate (t = 4.7002) regions. By contrast, persons with MCI had greater functional connectivity in the left fusiform (t = 4.6011), left rectal (t = 3.9158), right inferior frontal (t = 3.8467), and left precentral (t = 4.1251) regions.
Fig. 2.
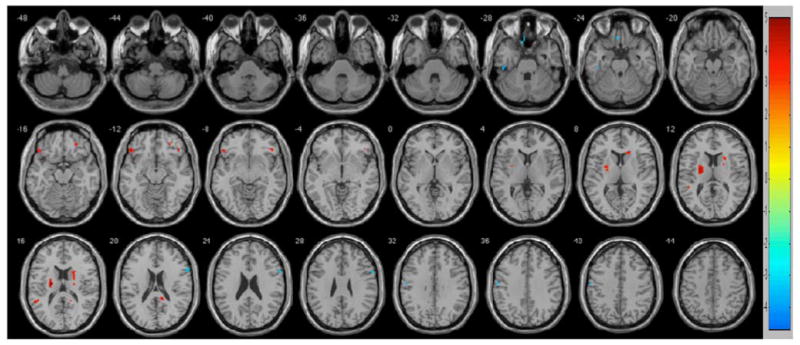
Regions of contrast between participants with no cognitive impairment (NCI) and mild cognitive impairment (MCI). Regions of greater connectivity for the NCIs are in reds. Regions of greater connectivity for the MCIs are in blues. To control for multiple comparisons and spurious findings, we used the following consistent with our false discovery rate corrected within group analyses: p < .001, cluster size >5 voxels.
Table 3.
Results of voxel-wise, between-group contrasts of z-transformed functional connectivity values (MCI versus NCI) while accounting for the effects of total gray matter volume
| Location of Maximum Intensity Voxel |
# Voxels | MNI Coordinates | t-value | ||
|---|---|---|---|---|---|
| NCI>MCI | |||||
| L orbital frontal | 13 | −48 | 30 | −12 | 4.2153 |
| R orbital frontal | 8 | 45 | 30 | −12 | 3.9043 |
| R middle frontal | 5 | 24 | 42 | −15 | 3.8609 |
| L putamen | 52 | −24 | −6 | 12 | 4.9792 |
| R caudate 1 | 7 | 18 | 27 | 6 | 4.1524 |
| R caudate 2 | 22 | 21 | −12 | 18 | 4.0735 |
| L superior temporal | 10 | −54 | −48 | 15 | 3.9342 |
| R posterior cingulate | 13 | 12 | −42 | 18 | 4.7002 |
| MCI>NCI | |||||
| L fusiform | 5 | −45 | −27 | −27 | 4.6011 |
| L rectal | 7 | −6 | 30 | −27 | 3.9158 |
| R inferior frontal | 12 | 60 | 12 | 24 | 3.8467 |
| L precentral | 6 | −57 | −9 | 36 | 4.1251 |
To control for multiple comparisons and spurious findings, we used the following consistent with our within-group false discovery rate threshold: p<.001, cluster size >5 voxels.
Relationship Between Regions of Functional Connectivity Differences and Cognitive Performance
When exploring the relationship between cognition and connectivity values in the regions with functional connectivity differences, the level of connectivity correlated with measures of episodic memory and processing speed in three of the 12 regions in partial correlations that adjusted for age, education, and gender in an alternate group (N = 113; Table 4) of non-cognitively impaired older adults (Table 5). Specifically, episodic memory domain z-scores correlated inversely with the right caudate and directly with the left superior temporal region at the p < .05 level. Processing speed domain z-scores correlated directly with the right inferior frontal region at the p < .05 level.
Table 4.
Statistics for demographic and cognitive variables for the non-cognitive impaired alternate sample of participants (N = 113)
| Alternate sample n = 113 |
|
|---|---|
| Age (years) | |
| Mean (SD) | 79.67 (7.09) |
| Range | 60–93 |
| Education (years) | |
| Mean (SD) | 15.34 (3.15) |
| Range | 8–28 |
| MMSE (total score) | |
| Mean (SD) | 28.89 (1.18) |
| Range | 25–30 |
| Sex | |
| % Female | 77.6 (n = 90) |
| Race | |
| % White | 94.8 (n = 110) |
| Global Cognition (z-score) | 0.44 (0.43) |
| Episodic Memory (z-score) | 0.56 (0.51) |
| Semantic Memory (z-score) | 0.45 (0.51) |
| Working Memory (z-score) | 0.31 (0.65) |
| Processing Speed (z-score) | 0.36 (0.69) |
| Perceptual Organization (z-score) | 0.39 (0.62) |
Data are summarized as mean (standard deviation 5 SD) or as number (%). Age and education are presented in years; MMSE is total score; global cognition, episodic memory, semantic memory, working memory, processing speed, and perceptual organization are presented as z-scores.
Table 5.
Partial correlations between cognitive abilities and levels of connectivity in regions of interest after controlling for age, education, and sex in the alternate sample (N = 113) of non-cognitively impaired older adults.
| Alternate sample partial correlations | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L Orbital Frontal | R Orbital Frontal | R Middle Frontal | L Putamen | R Caudate 1 | R Caudate 2 | L Superior Temporal | R Posterior Cingulate | L Fusiform | L Rectal | R Inferior Frontal | L Precentral | |
| Episodic Memory | 0.044 | 0.068 | 0.076 | 0.141 | –0.220* | 0.052 | 0.202* | 0.077 | –0.032 | 0.057 | 0.026 | –0.025 |
| Semantic Memory | –0.200 | 0.000 | 0.084 | 0.101 | 0.031 | 0.091 | –0.001 | –0.031 | 0.125 | 0.092 | –0.017 | 0.013 |
| Working Memory | –0.027 | 0.009 | –0.039 | –0.017 | –0.082 | –0.023 | 0.009 | –0.123 | 0.056 | 0.032 | 0.101 | –0.024 |
| Processing Speed | 0.013 | 0.058 | 0.016 | 0.040 | 0.036 | –0.037 | 0.081 | –0.056 | –0.056 | 0.108 | 0.206* | 0.162 |
| Perceptual Organization | 0.105 | –0.012 | –0.078 | –0.102 | –0.029 | –0.044 | 0.065 | 0.029 | –0.138 | 0.165 | 0.088 | –0.005 |
| Global Cognition | –0.007 | 0.051 | 0.032 | 0.077 | –0.111 | 0.017 | 0.135 | –0.020 | –0.015 | 0.116 | 0.123 | 0.040 |
Two-tailed Pearson r values presented. Note. Coordinates are presented in MNI space.
p < .05.
Discussion
This study examined functional connectivity in the Default Mode Network in a well-characterized sample of older persons with and without mild cognitive impairment. A different pattern of functional connectivity between the posterior cingulate and specific brain regions was observed between the two groups after adjusting for potential confounding variables. Specifically, there was less functional connectivity between the posterior cingulate cortex and the left and right orbital frontal, right middle frontal, left putamen, right caudate, left superior temporal, and right posterior cingulate regions in persons with MCI. There was also greater functional connectivity between the posterior cingulate cortex and right inferior frontal, left fusiform, left rectal, and left precentral regions in persons with MCI. Furthermore, functional connectivity values in the right caudate, left superior temporal, and right inferior frontal regions correlated with measures of cognition after adjusting for the effects of age, education, and gender.
An association between the Default Mode Network, amyloid burden, and episodic memory has been previously demonstrated (Buckner et al., 2005). In addition, recent evidence (Wang et al., 2010) suggests that functional connectivity of the posteriomedial cortices and the temporal lobe may be associated with memory performance on cognitive tasks. Our results are consistent with these findings and extend them to other brain regions and another domain of cognition beyond episodic memory.
A central finding of the present study is that reduced functional connectivity in the striatum was observed in MCI. This is particularly noteworthy in that striatal structures are not typically considered part of the Default Mode Network. Although the striatum have previously received relatively little attention as key brain structures in cognitive aging and the development and progression of Alzheimer's disease, it is known that amyloid deposition occurs in the striatum in AD (Braak & Braak, 1990; Klunk et al., 2004), reduced putamen volume is associated with global cognitive performance in AD (De Jong et al., 2008), and recent work using 11C-labeled Pittsburgh Compound B (Koivunen et al., 2011) and novel anatomical connectivity mapping approaches (Bozzali et al., 2011) have implicated striatal structures as particularly sensitive to AD progression. Generally speaking, striatal caudate and putamen structures have robust connections with anterior and posterior cortices, and functional connectivity changes in these structures may be a reasonable indicator for significant intrinsic network alterations secondary to AD pathological changes. The striatum has projections to the prefrontal cortex, the primary and supplementary motor area, the primary somatosensory area, the premotor area, the temporal lobes, the occipital lobes, the cerebellum, and the thalamus, making the striatum a set of structures that may be sensitive to functional changes in multiple brain regions. It is difficult, however, to determine if functional connectivity differences in the striatum are due to primary changes in the function of the striatum or secondary changes in the regions associated with the striatum through their projections. More work is needed to clarify the role of the functional connectivity of the striatum in cognitive aging and AD.
Greater functional connectivity of the right inferior frontal region with the posterior cingulate cortex was observed among MCI participants, and functional connectivity values were directly correlated with measures of processing speed in the alternate non-cognitively impaired group. Strengthening of the functional connectivity of the posterior cingulate cortex and right frontal regions is consistent with the findings of another resting-state functional connectivity study in MCI (Bai et al., 2009) and with the hypothesis that right frontal regions may serve a compensatory response among those at risk for AD (Han et al., 2007). Longitudinal studies are needed to determine if functional connectivity changes between the posterior cingulate cortex and the right inferior frontal region are associated with development of MCI and subsequent AD.
In this study, posterior cingulate cortex functional connectivity differences correlated with scores in the cognitive domains of episodic memory and processing speed. To date, the relation between functional connectivity of the posterior cingulate cortex and performance in cognitive domains has been somewhat unclear. While functional connectivity of the posterior cingulate cortex and associated structures has been associated with other cognitive abilities such as processing speed and attention in studies with relatively low participant numbers (e.g., Bai et al., 2009; Sorg et al., 2007), our findings indicate that functional connectivity of these structures may have particular significance for episodic memory and processing speed in the context of MCI and may reflect the presence of widespread AD pathology and an impending diagnosis of clinical AD. Furthermore, it was noted that functional connectivity increases in the striatum and decreases in the superior temporal region correlated with episodic memory performance. This functional connectivity direction difference highlights another contribution of the present study, that cognitive changes may be associated with co-occurring increases and decreases in functional connectivity of specific brain regions.
Limitations of this study include the restriction of the seed region to the posterior cingulate cortex. However, the posterior cingulate cortex has been established as a reliable seed region of interest for establishing the functional connectivity of the Default Mode Network (Greicius et al., 2003; Bai et al., 2009), and the reliability of other seed regions is less known. Even so, it is possible that seeding regions other than the posterior cingulate cortex may reveal a different pattern of functional connectivity between persons with and without MCI. Another limitation is that the analysis of the association between cognitive performance and connectivity levels in regions with significant group differences in functional connectivity was not controlled for multiple comparisons. Controlling for multiple comparisons yielded no significant correlations. These results are thus exploratory but nonetheless we believe they have important clinical implications. An additional limitation is the lack of inclusion of another set of MCI participants in our alternate sample for our cognitive measure partial correlation analyses. Our alternate sample is not reflective of the combined sample's characteristics since it is a non-cognitively impaired sample; however, we viewed this as a conservative approach to protect against the threat of multicollinearity and circularity of findings since our MCI and non-cognitive impaired groups were already distinguished based on cognitive measures. The lack of racial diversity of our sample yields another significant limitation as the present results may not be generalizable to members of other racial backgrounds. A final limitation is the study design is cross-sectional. However, the participants are followed annually and we will have the opportunity to examine the association between functional connectivity patterns and transition to MCI and AD in future analyses.
Strengths of this study include the use of a large community-based sample, examination of the relation between multiple cognitive domains and resting-state functional connectivity values, and control of factors known to influence cognition and resting-state functional connectivity values: age, education, sex, and total gray matter volume. Future research is needed to establish the time course relationship between changes in functional connectivity and cognitive performance; it is not known whether changes in functional connectivity precede a change in cognition or vice versa. Regardless of directionality, resting-state functional imaging has strong potential to become a widely used community-based neuroimaging biomarker for identifying persons at risk of developing AD, particularly when used in combination with other established biomarkers for the disease.
Acknowledgments
Supported by National Institute on Aging Grant R01AG17917, the Illinois Department of Public Health, and The Marsha K. Dowd Philanthropic Fund. We gratefully acknowledge the assistance of Dr. Randy Buckner, Dr. Gary Glover, and Dr. Jeffrey Rosengarten with this project. We thank Niranjini Rajendran, MS, and Woojeong Bang, MS, for image post-processing and statistical analyses. We also thank the Rush Memory and Aging Project staff and participants. There were no actual or potential conflicts of interest for any of the authors.
References
- Allen G, Barnard H, McColl R, Hester AL, Fields JA, Weiner MF, et al. Cullum M. Reduced hippocampal functional connectivity in Alzheimer's disease. Archives of Neurology. 2007;64(10):1482–1487. doi: 10.1001/archneur.64.10.1482. [DOI] [PubMed] [Google Scholar]
- Alzheimer's Association. 2009 Alzheimer's disease facts and figures. Alzheimer's and Dementia. 2009;5(3):234–270. doi: 10.1016/j.jalz.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Bai F, Watson DR, Hui Y, Shi Y, Yuan Y, Zhang Z. Abnormal resting-state functional connectivity of posterior cingulate cortex in amnestic type mild cognitive impairment. Brain Research. 2009;1302:167–174. doi: 10.1016/j.brainres.2009.09.028. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, et al. Wilson RS. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community-based cohort compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27:169–176. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: Study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25(4):163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, et al. Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: Risk of Alzheimer disease and rate of cognitive decline. Neurology. 2005;67:441–445. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- Bozzali M, Parker GJ, Serra L, Embleton K, Gili T, Perri R, et al. Cercignani M. Anatomical connectivity mapping: A new tool to assess brain disconnection in Alzheimer's disease. Neuroimage. 2011;54:2045–2051. doi: 10.1016/j.neuroimage.2010.08.069. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Alzheimer's disease: Striatal amyloid deposits and neurofibrillary changes. Journal of Neuropathology and Experimental Neurology. 1990;49:215–224. [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, et al. Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer's disease. The Journal of Neuroscience. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Synder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, et al. Mintun MA. Molecular, structural, and functional characterization of Alzheimer's disease: Evidence for a relationship between default activity, amyloid, and memory. The Journal of Neuroscience. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Vincent JL. Unrest at rest: Default activity and spontaneous network correlations. Neuroimage. 2007;37:1091–1096. doi: 10.1016/j.neuroimage.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychology and Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong LW, van der Hiele K, Veer IM, Houwig JJ, Westendorp RG, Bollen EL, et al. van der Grond J. Srongly reduced volumes of putamen and thalamus in Alzheimer's disease: An MRI study. Brain. 2008;131:3277–3285. doi: 10.1093/brain/awn278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher AS, Sherzai A, Talyor C, Langbaum JB, Chen K, Buxton RB. Resting- state BOLD networks versus task-associated functional MRI for distinguishing Alzheimer's disease risk groups. Neuroimage. 2009;47(4):1678–1690. doi: 10.1016/j.neuroimage.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman DA, Wilson RS, Bienias JL, Bennett DA. Parkinsonian signs and cognitive function in old age. Journal of the International Neuropsychological Society. 2005;11:591–597. doi: 10.1017/S1355617705050708. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Passingham RE, Hutt JG, Heather JD, Sawle GV, Frackowiak RSJ. Spatial registration and normalization of images. Human Brain Mapping. 1995;3(3):165–189. [Google Scholar]
- Gili T, Cercignani M, Serra L, Perri R, Giove F, Maraviglia B, et al. Bozzali M. Regional brain atrophy and functional disconnection across Alzheimer's disease evolution. Journal of Neurology, Neurosurgery, and Psychiatry. 2011;82(1):58–66. doi: 10.1136/jnnp.2009.199935. [DOI] [PubMed] [Google Scholar]
- Glover GH, Thomason ME. Improved combination of spiral-in/out images for BOLD fMRI. Magnetic Resonance in Medicine. 2004;51:863–868. doi: 10.1002/mrm.20016. [DOI] [PubMed] [Google Scholar]
- Graham JE, Rockwood K, Beattie BL, Eastwood R, Gauthier S, Tuokko H, McDowell I. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;49:1793–1796. doi: 10.1016/S0140-6736(97)01007-6. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Kiviniemi V, Tervonen O, Vainionpaa V, Alahuhta S, Reiss AL, Menon V. Persistent default mode network connectivity during light sedation. Human Brain Mapping. 2008;29:839–847. doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: Evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Habeck C, Fostern NL, Pernecsky R, Kurz A, Alexopoulos P, Koeppe RA, et al. Stern Y. Multivariate and univariate neuroimaging biomarkers of Alzheimer's disease. Neuroimage. 2008;40(4):1503–1515. doi: 10.1016/j.neuroimage.2008.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SD, Houston WS, Jak AJ, Eyler LT, Nagel BJ, Fleisher AS, et al. Bondi MW. Verbal paired-associate learning by APOE genotype in nondemented adults: fMRI evidence of a right hemispheric compensatory response. Neuro-biology of Aging. 2007;28(2):238–247. doi: 10.1016/j.neurobiolaging.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Lopez-Sola M, Hernandez-Ribas R, Deus J, Ortiz H, et al. Cardoner N. Consistency and functional specialization in the default mode brain network. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(28):9781–9786. doi: 10.1073/pnas.0711791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KR, Becker JA, Mehta A, Sperling RA, Johnson KA, Buckner RA. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. The Journal of Neuroscience. 2009;29(40):12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the U.S. population: Prevalence estimates using the 2000 census. Archives of Neurology. 2003;60(8):1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Långström B. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Annals of Neurology. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Koch W, Teipel S, Buerger K, Bokde AL, Hampel H, Coates U, et al. Meindl T. Effects of aging on default mode network activity in resting state fMRI: Does the method of analysis matter? Neuroimage. 2010;51(1):280–287. doi: 10.1016/j.neuroimage.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Koivunen J, Scheinin N, Virta JR, Aalto S, Vahlberg T, Nagren K, et al. Rinne JO. Amyloid PET imaging in patients with mild cognitive impairment: A 2-year follow-up study. Neurology. 2011;76(12):1085–1090. doi: 10.1212/WNL.0b013e318212015e. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. Phelps CH. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging and the Alzheimer's Association workgroup. Alzheimer's and Dementia. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiche ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Sheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: An fMRI study. Human Brain Mapping. 2005;26:231–239. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, et al. Wohlschlager AM. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Phelps CH. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging and the Alzheimer's Association workgroup. Alzheimer's & Dementia. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, LaViolette PS, O'Keefe K, O'Brien J, Rentz D, Pihlajamaki M, et al. Johnson KA. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Laviolette P, O'Keefe K, Putcha D, Bakkour A, Van Dijk KR, et al. Sperling RA. Intrinsic connectivity between the hippocampus and posteromedical cortex predicts memory performance in cognitively intact older individuals. Neuroimaging. 2010;51(2):910–917. doi: 10.1016/j.neuroimage.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L, et al. Li K. Changes in hippocampal connectivity in the early stages of Alzheimer's disease: Evidence from resting state fMRI. Neuroimage. 2006;31:496–504. doi: 10.1016/j.neuroimage.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Bennett DA. Assessment of lifetime participation in cognitively stimulating activities. Journal of Clinical and Experimental Neuropsycholology. 2003;25:634–642. doi: 10.1076/jcen.25.5.634.14572. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Wang SJ, Xing J, Liu B, Ma ZL, Yang M, et al. Teng GJ. Detection of PCC functional connectivity characteristics in resting-state fMRI in mild Alzheimer's disease. Behavioural Brain Research. 2009;197:103–108. doi: 10.1016/j.bbr.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Dougherty JH, Hubner KF, Bai B, Cannon RL, Hutson RK. Abnormal connectivity in the posterior cingulated and hippocampus in early Alzheimer's disease and mild cognitive impairment. Alzheimer's and Dementia. 2008;4:265–270. doi: 10.1016/j.jalz.2008.04.006. [DOI] [PubMed] [Google Scholar]


