Abstract
Prenatal exposure both to maternal psychiatric illness and psychiatric medication has been linked with adverse child outcomes that affect physiological, emotional and psychiatric development. Studies suggest that epigenetic mechanisms, such as DNA methylation, may facilitate these effects. In this report, we explore the association between maternal psychiatric illness and treatment during pregnancy and neonatal DNA methylation patterns in a prospectively-characterized clinical cohort of 201 dyads. Associations between the percent of umbilical cord blood DNA methylated at 27,578 CpG sites and maternal psychiatric diagnosis, symptoms and antidepressant use were evaluated by fitting a separate linear mixed effects model for each CpG site. There were no significant changes in neonatal DNA methylation attributable to maternal psychiatric diagnosis or depressive symptoms during pregnancy. Exposure to an antidepressant medication was associated with differential methylation of CpG sites in TNFRSF21 and CHRNA2 (false discovery rate < 0.05), but the average difference in methylation for both CpG sites was less than 3% between each group. The results were not specific to type of antidepressant or duration of the exposure. This study suggests that there are no large effects of maternal psychiatric illness, depressive symptoms or prenatal exposure to antidepressants on neonatal DNA methylation. Delineation of the influence of maternal psychiatric illness and pharmacological exposures on the developing fetuses has critical implications for clinical care during pregnancy.
Keywords: antidepressants, depressive symptoms, DNA Methylation, HumanMethylation27 BeadChip, Infinium, prenatal exposures
Introduction
Major depressive disorder is nearly twice as prevalent in women as it is in men and affects up to 12.8% of women during pregnancy.1 Accompanying depressive symptoms do not abate during pregnancy, and depressed women are more likely to deliver prematurely or to deliver growth-restricted neonates.2,3 The children of depressed mothers score lower on the Neonatal Behavioral Assessment Scale (NBAS)4,5 and show reduced mental, motor and emotional development as infants.6,7 Over time, the children of depressed mothers are at higher risk of developing depression,8 violent behavior9 and anxiety.10
Nearly 8% of women in the United States are treated with an antidepressant during pregnancy.11 Women who discontinue antidepressant treatment proximate to conception are more likely to experience a depressive relapse during gestation12 so treatment decisions during pregnancy must weigh the risks of maternal depression during pregnancy against those of fetal antidepressant exposure.13 Several antidepressants and their metabolites cross the placenta and are detectable in umbilical cord blood at birth.14 Typically, umbilical cord blood antidepressant concentrations are lower than those in corresponding maternal blood.15 Reports describing the effects of in utero antidepressant exposure on the developing fetus have been mixed. Some studies suggest that antidepressants increase the risk of adverse child outcomes such as cardiac defects,16 attention deficit/hyperactivity disorder17 and motor development issues.18,19 Other studies suggest that there is little to no significant risk linked to antidepressant exposure, or that comparable risk could result from untreated depression.20,21
Prenatal exposure to psychotropic medications, maternal psychiatric illness, or the combination of the two could influence offspring outcomes through changes in DNA methylation at CpG dinucleotides. Human and animal studies report links between prenatal or early life stress and offspring DNA methylation.22 In one such study, umbilical cord blood DNA methylation of NR3C1 was associated with cortisol response to a stress challenge at three months in the offspring of depressed mothers taking an SSRI.23 This study also observed that offspring DNA methylation patterns were associated with maternal mood during pregnancy, but not with SSRI exposure. Similarly, neonates born to women depressed during the second trimester have significant methylation differences in the serotonin transporter (SLC6A4), though differential methylation of this gene did not associate with SSRI exposure or maternal depression in the third trimester.24
It is important to delineate the potential effects of psychiatric illness and symptoms from treatment on the developing fetus. Previous efforts in this regard have been limited to selected candidate genes. In this study, we examined the methylation patterns of > 27,000 CpG sites across the genome in umbilical cord blood-derived DNA from the offspring of women undergoing treatment for a mood disorder during the perinatal period.
Results
Maternal diagnosis and symptoms
To assess the potential contribution of maternal psychiatric illness, current (n = 118 vs. 83) and lifetime (n = 146 vs. 55) maternal diagnosis of major depressive disorder (MDD) was evaluated as well as the presence or absence of major depressive episodes during pregnancy (n = 86 and 115, respectively). Similarly, current (n = 50 vs. 151) and lifetime (n = 55 vs. 146) maternal diagnosis of bipolar disorder was examined. No association with neonatal DNA methylation was observed at any of 27,578 CpG sites, based on a false discovery rate (FDR) cutoff of 0.05. Similarly, the severity of maternal depressive symptoms (HRSD17; n = 178; 16.3 ± 5.9 and BDI; n = 179; 17.3 ± 10.8) and the occurrence of clinically significant depressive symptoms at any point during pregnancy (HRSD17 > 15; 109 vs. 69 or BDI > 10; 125 vs. 54) did not associate with neonatal DNA methylation at any CpG site.
Medication exposure
Exposure to an antidepressant (n = 151 vs. 50) was associated with differential methylation of two CpG sites (FDR < 0.05). Methylation of a CpG site, cg22464186, in a CpG island in exon 1 of tumor necrosis factor receptor subfamily 21 (TNFRSF21) was decreased by an average of 1.9% in the umbilical cord blood DNA of neonates exposed to antidepressants (t = -4.85; p = 2.8 × 10−6; Figure 1a). Methylation of a CpG site, cg02953306, in exon 1 of cholinergic receptor, nicotinic, α 2 (CHRNA2) was increased by an average of 3% (t = 4.83; p = 3.1 × 10−6; Figure 1b). These results were not specific to either class 1 (i.e., SSRIs, SNRIs, and TCAs; n = 132) or class 2 (bupropion; n = 40) antidepressants. Likewise methylation was not influenced by the number of weeks of exposure (24.1 ± 17.4).
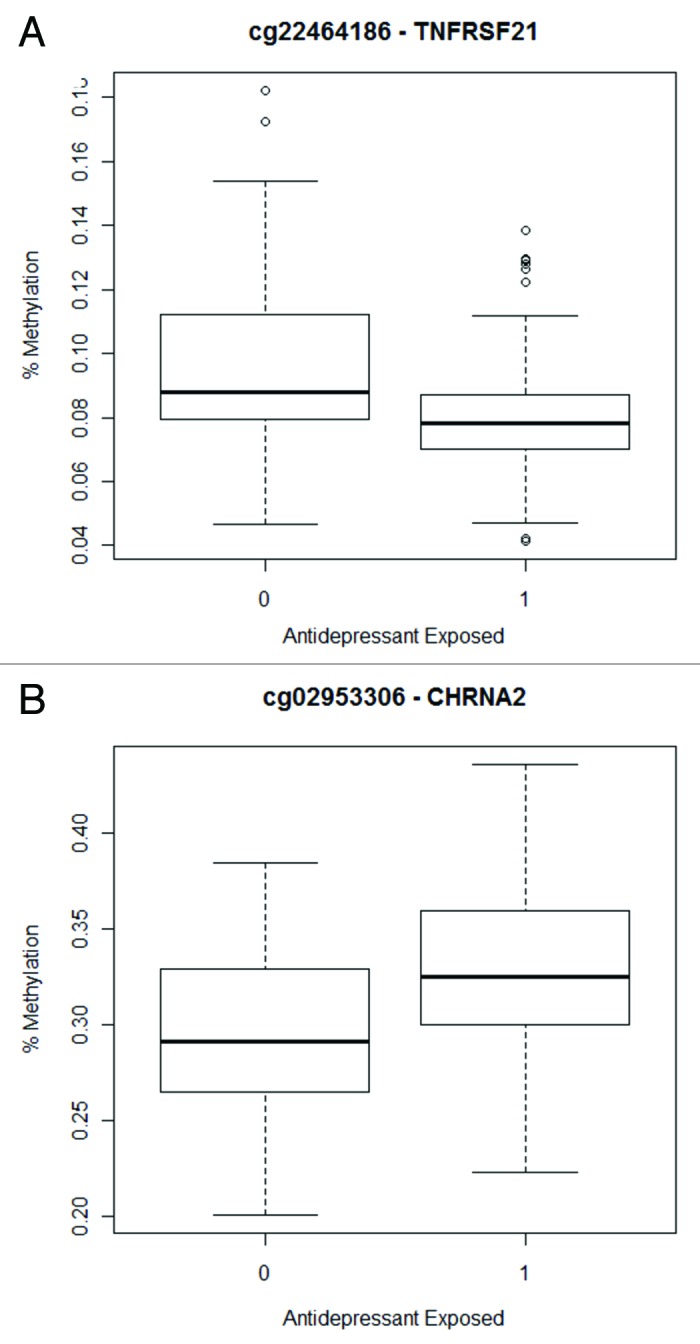
Figure 1. Box plots indicate methylation values for neonates that were not exposed to antidepressants (0) compared with those that were exposed (1) for the CpG sites associated with antidepressant exposure (Class 1 and 2 combined): (A) cg22464186 (TNFRSF21) and (B) cg02953306 (CHRNA2).
Exposure to neurotropic medications taken concurrently with antidepressants was independently evaluated, but there was no association between any CpG site and duration of exposure to hypnotics (n = 31 vs. 170), antiemetics (n = 41 vs. 160) or benzodiazepines (n = 39 vs. 162). In contrast, methylation of a CpG site, cg23034818, in BTB (POZ) domain containing 6 (BTBD6) was associated with exposure to an atypical antipsychotic (n = 32 vs. 169), but this association did not remain significant upon removal of a single influential outlier.
Discussion
Using a genome-wide approach, we observed no association between neonatal umbilical cord blood DNA methylation and maternal psychiatric diagnosis or clinically significant depressive symptoms. Lack of association can occur due to insufficient power; however, our analysis had > 80% power to detect group differences explaining > 14.5% of variation in methylation at a single CpG site even after applying the conservative Bonferroni correction. Although it is possible that we did not detect more subtle effects due to limited power, our study was well-powered to detect larger effects. This suggests that the previously reported association between maternal psychiatric illness and adverse offspring outcomes may be due to genetic variants that remain undiscovered or to environmental or behavioral factors that do not correspond with DNA methylation changes present at birth.
Our sample was restricted to women with a lifetime history of a mood disorder diagnosis, which may have resulted in a more restricted range of depressive symptoms experienced during pregnancy. Nevertheless, this study failed to support the hypothesis that clinically significant maternal mood disturbance in pregnancy is associated with DNA methylation changes in cord blood. This finding was somewhat unexpected given the results of previous candidate gene studies. Despite having 90–99% power to identify a nominal association with CpG sites in NR3C1 or SLC64 as strong as those previously reported (semi-partial R2 from 0.05-.11),23,24 we observed no association (p < 0.05) between methylation of these genes and maternal psychiatric illness or treatment. This disparity may reflect the limitations of candidate gene studies or it may reflect the fact that we did not interrogate the exact CpG sites that were previously studied.
Prenatal exposure to an antidepressant was associated with differential methylation of CpG sites in TNFRSF21 and CHRNA2. TNFRSF21, also known as death receptor 6 (DR6), is expressed in both developing neurons25,26 and developing lymphocytes.27,28 Because of its role in apoptosis, it is involved in refinement of neuronal connections during development.25,26,29 Alterations in TNFRSF21 methylation or gene expression influence complex traits ranging from learning and memory and emotional responses to stressful events to cancer.30,31 CHRNA2 is a broadly-expressed subunit of nicotinic acetylcholine receptors.32 In addition to its role in nicotine dependence and neurocognitive functioning,33,34 CHRNA2 is located in a region of chromosome 8p that is suggested to contribute to psychiatric and neurodegenerative disorders.35 Interestingly, nominal differential methylation of CHRNA2 was recently reported in monozygotic twins discordant for psychosis.36
Though the results of this analysis are statistically significant, the magnitude of methylation differences between those with and without antidepressant exposure is less than 3% for both CpG sites (Fig. 1), which is unlikely to be biologically significant. They would not satisfy a more conservative Bonferroni criterion (p ≤ 1.81 x 10−6) nor would they remain associated under a FDR strategy that adjusted for the number of phenotypes tested. Thus, these results are most likely false positives.
A potential limitation to this study that DNA extracted from whole umbilical cord blood may not reflect changes in relevant tissues within the neonatal central nervous system. However, both stress-responsive substrates such as cortisol and the medications examined in this study cross the placental barrier and are detectable in umbilical cord blood.15,37,38 The study is strengthened by the rich, prospective characterization of the course of psychiatric illness and treatment as well as other environmental exposures throughout pregnancy.
Delineation of the influence of maternal psychiatric illness and treatment on developing fetuses is vital for informing clinical care decisions of pregnant women. The results of this study suggest that there are no large effects of maternal psychiatric illness, depressive symptoms or prenatal antidepressant exposure on neonatal DNA methylation though we cannot rule out subtle effects. The potential role of maternal psychiatric illness and treatment on long-term offspring behavior and neurocognitive development warrants further attention.
Methods
Subject enrollment
Subjects were recruited from the Specialized Center of Research for Sex and Gender Effects (SCOR) or the Translational Research Center for Behavioral Sciences (TRCBS) at the Emory Women’s Mental Health Program (WMHP), a tertiary referral center for the treatment of perinatal psychiatric illness. Mothers were evaluated prospectively at 4–6 week intervals with serial measures of psychiatric symptoms and pharmacologic exposures throughout pregnancy.
The maternal inclusion criteria for this study included: 1) > 17 y of age; 2) written and verbal fluency in English; 3) a live singleton delivery; 4) availability of DNA from umbilical cord blood collected at delivery; and 5) maternal lifetime diagnosis of a mood disorder. Exclusion criteria included: 1) unstable non-psychiatric medical illnesses requiring pharmacological treatment during pregnancy (e.g., asthma, autoimmune disorders); 2) abnormal thyroid stimulating hormone (TSH); or 3) use of lithium, stimulants, or migraine medications. A total of 201 mothers (average age 33.77 ± 4.93) and their neonates were included in the study (Table 1).
Table 1. Demographic and clinical characteristics of the subjects.
| Demographics | N (%) Total n = 201 |
|---|---|
| Child Gender Male Female |
100 (49.8) 101 (50.2) |
| Child Race Caucasian African-American |
183 (91) 18 (9) |
| Medication Exposure during Pregnancy | |
|---|---|
| No Antidepressant |
50 (24.9) |
| Any Antidepressant Class 1 (SRIs, SNRIs, TCAs) Class 2 (Bupropion) |
151 (75.1) 132(65.7) 40 (19.9) |
| Atypical Antipsychotics |
32 (15.9) |
| Hypnotics |
31 (15.4) |
| Benzodiazepines |
39 (19.4) |
| Antiemetics | 41 (20.4) |
| Maternal Diagnosis and Symptoms | |
|---|---|
| Lifetime history of MDD Current MDD diagnosis |
146 (72.6) 118 (58.7) |
| Lifetime history of BPD Current BPD diagnosis |
55 (27.4) 50 (24.9) |
| Other Current Diagnoses |
33 (16.4) |
| Maximum Prenatal HRSD17 < 15 ≥ 15 |
178 69 (38.8) 109 (61.2) |
| Maximum Prenatal BDI < 10 ≥ 10 |
179 54 (30.2) 125 (69.8) |
| Prenatal Major Depressive Episode | 86 (42.8) |
Data was not available for all measures including 18 missing data points for class 2 antidepressants and benzodiazepine, 20 for atypical antipsychotics and antiemetics, 16 for hypnotics, 23 for HRSD17, and 22 for BDI. The following abbreviations were used: major depressive disorder (MDD), all bipolar disorders (BPD), bipolar disorder 1 (BP1), bipolar disorder 2 (BP2) and other bipolar disorder (BPO). Other current diagnoses included schizophrenia (1), substance abuse (2), anxiety (29), and binge eating (1). Lifetime BPD includes BP1 (78.2%), BP2 (12.7%) and BPO (9.1%), and current BPD has similar proportions.
A Structured Clinical Interview for Diagnosis (SCID) was used to assess lifetime diagnosis according to DSM-IV criteria, and the SCID Mood Module was used to assess major depressive episodes at each visit during pregnancy (average 5 visits per subject).39 No subject met criteria for a manic episode during pregnancy according to the SCID Mood Module. Depressive symptoms were assessed using the 17-item Hamilton Rating Scale for Depression (HRSD17)40 and the Beck Depressive Inventory (BDI).41 Symptom scores at each visit were used to calculate the area under the curve (AUC) for symptoms across pregnancy and were normalized to 40 weeks to account for differences in timing of delivery.
Antidepressants were categorized by mechanism of action. Class 1, serotonin reuptake inhibitors (SRIs), included selective serotonin reuptake inhibitors (SSRIs), dual serotonin–norepinephrine reuptake inhibitors (SNRIs), and tricyclic antidepressants (TCAs), that act primarily through serotonergic pathways, while class 2 consisted of bupropion, which does not act primarily through serotonergic pathways.37,42,43 The number of weeks of medication exposure was used to calculate the AUC for the pregnancy and normalized to 40 weeks.
All mothers provided written informed consent prior to study enrollment and the Institutional Review Board of Emory approved all procedures. This study was conducted in accordance with the Helsinki Declaration of 1975.
Sample collection and DNA methylation analysis
Sample collection and DNA methylation assays have been previously described in detail.44 Briefly, umbilical cord blood was collected at birth, stored on ice, and processed within 2 h of delivery. DNA extraction and processing of the HumanMethylation27 BeadChip45,46 (Illumina, San Diego, CA) was performed according to manufacture instructions at the Emory Biomarker Service Center. A single female genomic DNA sample was run on each BeadChip as a technical control. Three samples with probe detection call rates < 90% or with an average intensity value of either < 50% of the experiment-wide sample mean or < 2000 arbitrary units (AU) were excluded from the analysis. We normalized the signal data to adjust for technical variability between samples by utilizing the information from 16 negative control probes that are included on the Illumina BeadChip and are designed to detect a true methylated and unmethylated signal at zero. The signals from methylated (M) and unmethylated (U) bead types were then used to determine a β value, calculated as β = M/(U+M), or the proportion of DNA methylated at a particular CpG site.
Statistical analysis
All analyses used a logit-transformed β value equal to log(β/(1-β)). The associations between this function of β, the proportion of DNA methylated, and each maternal diagnosis, symptom, or exposure were evaluated by fitting a separate linear mixed effects model for each CpG site. For each CpG site log(β/(1-β)) was then modeled as a function of the measures of offspring exposure to maternal medication(s), psychiatric diagnosis, or symptoms.
Dichotomous variables examined included: prenatal exposure to either any antidepressant or to specific antidepressant categories (Class 1 or class 2); exposure to atypical antipsychotics, benzodiazepines, antiemetics, and hypnotics (each analyzed independently); lifetime and current principal diagnosis of major depressive disorder or bipolar disorder. Continuous variables examined included: measures of depressive symptoms (HRSD17 and BDI) and the number of weeks of exposure to these antidepressant variables, adjusted for a standard 40 week pregnancy, both across the entire pregnancy and separately by trimester. Additionally, depressive symptoms were dichotomized based on the maximum maternal depression scale score at any point during pregnancy such that clinically-significant symptoms were present if a subject scored > 15 on the HRSD17 or > 10 on the BDI.
For each analysis, neonatal gender, race, and gestational age were included as covariates because they have been shown to exert independent effects on methylation patterns in this and other studies.44,47-50 Random effects for chips were included in the model to allow for chip-to-chip differences in measurement of the proportion of DNA methylated. To account for the 27,578 tests performed for each diagnosis or exposure, the false discovery rate (FDR) was controlled at 0.05 using the method of Storey et al.51 For CpG sites in two genes previously reported to be differentially methylated with maternal mood, we also considered nominal p-values (α = 0.05).
Power calculations
Power was calculated using Quanto (http://hydra.usc.edu/gxe/), to assess whether a lack of significant differences is likely due to insufficient sample size vs. an absence of differential methylation. For analyses of 201 individuals, there was > 80% power to detect between-group methylation differences with an R2 > 0.145 after correction for 27,578 tests.
Acknowledgments
The authors gratefully acknowledge the women who participated in this study and the community obstetrical practices in the Atlanta area. This work is supported by RC1 MH088609 (PIs: AKS and PB), Specialized Center for Research P50 MH 68036 (PI: ZNS), and the Translational Research Center in Behavioral Sciences (TRCBS) P50 MH077928 (PI: ZNS). Salary support for AKS was provided by MH085806. This work was also supported, in part, by the Emory Biomarker Service Center.
Lifetime Disclosures
The following lifetime disclosures were reported. AKS has received research support from NIH, AFSP and Schering Plough Pharmaceuticals. PB has received research support from NIH and NARSAD. BTK has received research support from NIH, NARSAD, Wyeth, BMS, Cyberonics, Eli Lilly, Forest, Janssen and Novartis. A family member is a GSK employee and holds GSK stock options. DJN has received research support from Eli Lilly, GSK, Janssen, NIH, NARSAD and Wyeth, has served on speakers or advisory boards for Astra-Zeneca, Eli Lilly, GSK, Pfizer and Wyeth and has received honoraria from Astra-Zeneca, Eli Lilly, GSK, Pfizer, and Wyeth. ZNS has received research support from NIH, GSK, Pfizer and Wyeth, has served on speakers or advisory boards for Pfizer, Eli Lilly, Wyeth, BMS, and GSK, and has received honoraria from Eli Lilly, GSK, Pfizer, and Wyeth.
Footnotes
Previously published online: www.landesbioscience.com/journals/epigenetics/article/19551
References
- 1.Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol. 2004;103:698–709. doi: 10.1097/01.AOG.0000116689.75396.5f. [DOI] [PubMed] [Google Scholar]
- 2.Beydoun H, Saftlas AF. Physical and mental health outcomes of prenatal maternal stress in human and animal studies: a review of recent evidence. Paediatr Perinat Epidemiol. 2008;22:438–66. doi: 10.1111/j.1365-3016.2008.00951.x. [DOI] [PubMed] [Google Scholar]
- 3.Wadhwa PD, Culhane JF, Rauh V, Barve SS, Hogan V, Sandman CA, et al. Stress, infection and preterm birth: a biobehavioural perspective. Paediatr Perinat Epidemiol. 2001;15(Suppl 2):17–29. doi: 10.1046/j.1365-3016.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- 4.Field T, Diego M, Hernandez-Reif M, Schanberg S, Kuhn C, Yando R, et al. Pregnancy anxiety and comorbid depression and anger: effects on the fetus and neonate. Depress Anxiety. 2003;17:140–51. doi: 10.1002/da.10071. [DOI] [PubMed] [Google Scholar]
- 5.Brazelton TB. Assessment of the infant at risk. Clin Obstet Gynecol. 1973;16:361–75. doi: 10.1097/00003081-197303000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Jones NA, Field T, Fox NA, Davalos M, Lundy B, Hart S. Newborns of depressed mothers are physiologically less developed. Infant Behav Dev. 1998;21:537–41. doi: 10.1016/S0163-6383(98)90027-3. [DOI] [Google Scholar]
- 7.Zuckerman B, Bauchner H, Parker S, Cabral H. Maternal depressive symptoms during pregnancy, and newborn irritability. J Dev Behav Pediatr. 1990;11:190–4. doi: 10.1097/00004703-199008000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Silberg JL, Maes H, Eaves LJ. Genetic and environmental influences on the transmission of parental depression to children’s depression and conduct disturbance: an extended Children of Twins study. J Child Psychol Psychiatry. 2010;51:734–44. doi: 10.1111/j.1469-7610.2010.02205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hay DF, Pawlby S, Waters CS, Perra O, Sharp D. Mothers’ antenatal depression and their children’s antisocial outcomes. Child Dev. 2010;81:149–65. doi: 10.1111/j.1467-8624.2009.01386.x. [DOI] [PubMed] [Google Scholar]
- 10.Gerardin P, Wendland J, Bodeau N, Galin A, Bialobos S, Tordjman S, et al. Depression during pregnancy: is the developmental impact earlier in boys? A prospective case-control study. J Clin Psychiatry. 2011;72:378–87. doi: 10.4088/JCP.09m05724blu. [DOI] [PubMed] [Google Scholar]
- 11.Andrade SE, Raebel MA, Brown J, Lane K, Livingston J, Boudreau D, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198:194–, e1-5. doi: 10.1016/j.ajog.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 12.Cohen LS, Nonacs RM, Bailey JW, Viguera AC, Reminick AM, Altshuler LL, et al. Relapse of depression during pregnancy following antidepressant discontinuation: a preliminary prospective study. Arch Womens Ment Health. 2004;7:217–21. doi: 10.1007/s00737-004-0059-3. [DOI] [PubMed] [Google Scholar]
- 13.Petersen I, Gilbert RE, Evans SJ, Man SL, Nazareth I. Pregnancy as a major determinant for discontinuation of antidepressants: an analysis of data from The Health Improvement Network. J Clin Psychiatry. 2011;72:979–85. doi: 10.4088/JCP.10m06090blu. [DOI] [PubMed] [Google Scholar]
- 14.Hostetter A, Ritchie JC, Stowe ZN. Amniotic fluid and umbilical cord blood concentrations of antidepressants in three women. Biol Psychiatry. 2000;48:1032–4. doi: 10.1016/S0006-3223(00)00958-6. [DOI] [PubMed] [Google Scholar]
- 15.Hendrick V, Stowe ZN, Altshuler LL, Hwang S, Lee E, Haynes D. Placental passage of antidepressant medications. Am J Psychiatry. 2003;160:993–6. doi: 10.1176/appi.ajp.160.5.993. [DOI] [PubMed] [Google Scholar]
- 16.Cole JA, Modell JG, Haight BR, Cosmatos IS, Stoler JM, Walker AM. Bupropion in pregnancy and the prevalence of congenital malformations. Pharmacoepidemiol Drug Saf. 2007;16:474–84. doi: 10.1002/pds.1296. [DOI] [PubMed] [Google Scholar]
- 17.Figueroa R. Use of antidepressants during pregnancy and risk of attention-deficit/hyperactivity disorder in the offspring. J Dev Behav Pediatr. 2010;31:641–8. doi: 10.1097/DBP.0b013e3181e5ac93. [DOI] [PubMed] [Google Scholar]
- 18.Galbally M, Lewis AJ, Buist A. Developmental outcomes of children exposed to antidepressants in pregnancy. Aust N Z J Psychiatry. 2011;45:393–9. doi: 10.3109/00048674.2010.549995. [DOI] [PubMed] [Google Scholar]
- 19.Casper RC, Fleisher BE, Lee-Ancajas JC, Gilles A, Gaylor E, DeBattista A, et al. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr. 2003;142:402–8. doi: 10.1067/mpd.2003.139. [DOI] [PubMed] [Google Scholar]
- 20.Lorenzo L, Byers B, Einarson A. Antidepressant use in pregnancy. Expert Opin Drug Saf. 2011;10:883–9. doi: 10.1517/14740338.2011.583917. [DOI] [PubMed] [Google Scholar]
- 21.Henry AL, Beach AJ, Stowe ZN, Newport DJ. The fetus and maternal depression: implications for antenatal treatment guidelines. Clin Obstet Gynecol. 2004;47:535–46. doi: 10.1097/01.grf.0000135341.48747.f9. [DOI] [PubMed] [Google Scholar]
- 22.Zhang TY, Meaney MJ. Epigenetics and the environmental regulation of the genome and its function. Annu Rev Psychol. 2010;61:439–66, C1-3. doi: 10.1146/annurev.psych.60.110707.163625. [DOI] [PubMed] [Google Scholar]
- 23.Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 24.Devlin AM, Brain U, Austin J, Oberlander TF. Prenatal exposure to maternal depressed mood and the MTHFR C677T variant affect SLC6A4 methylation in infants at birth. PLoS One. 2010;5:e12201. doi: 10.1371/journal.pone.0012201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan G, Bauer JH, Haridas V, Wang S, Liu D, Yu G, et al. Identification and functional characterization of DR6, a novel death domain-containing TNF receptor. FEBS Lett. 1998;431:351–6. doi: 10.1016/S0014-5793(98)00791-1. [DOI] [PubMed] [Google Scholar]
- 26.Nikolaev A, McLaughlin T, O’Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–9. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Zhao H, Yan M, Wang H, Erickson S, Grewal IS, Dixit VM. Impaired c-Jun amino terminal kinase activity and T cell differentiation in death receptor 6-deficient mice. J Exp Med. 2001;194:1441–8. doi: 10.1084/jem.194.10.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt CS, Liu J, Zhang T, Song HY, Sandusky G, Mintze K, et al. Enhanced B cell expansion, survival, and humoral responses by targeting death receptor 6. J Exp Med. 2003;197:51–62. doi: 10.1084/jem.20020617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholson DW. Neuroscience: Good and bad cell death. Nature. 2009;457:970–1. doi: 10.1038/457970a. [DOI] [PubMed] [Google Scholar]
- 30.Chambers RA, Potenza MN, Hoffman RE, Miranker W. Simulated apoptosis/neurogenesis regulates learning and memory capabilities of adaptive neural networks. Neuropsychopharmacology. 2004;29:747–58. doi: 10.1038/sj.npp.1300358. [DOI] [PubMed] [Google Scholar]
- 31.Carvalho JR, Filipe L, Costa VL, Ribeiro FR, Martins AT, Teixeira MR, et al. Detailed analysis of expression and promoter methylation status of apoptosis-related genes in prostate cancer. Apoptosis. 2010;15:956–65. doi: 10.1007/s10495-010-0508-6. [DOI] [PubMed] [Google Scholar]
- 32.Bonati MT, Combi R, Asselta R, Duga S, Malcovati M, Oldani A, et al. Exclusion of linkage of nine neuronal nicotinic acetylcholine receptor subunit genes expressed in brain in autosomal dominant nocturnal frontal lobe epilepsy in four unrelated families. J Neurol. 2002;249:967–74. doi: 10.1007/s00415-002-0763-8. [DOI] [PubMed] [Google Scholar]
- 33.Philibert RA, Todorov A, Andersen A, Hollenbeck N, Gunter T, Heath A, et al. Examination of the nicotine dependence (NICSNP) consortium findings in the Iowa adoption studies population. Nicotine Tob Res. 2009;11:286–92. doi: 10.1093/ntr/ntn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rigbi A, Kanyas K, Yakir A, Greenbaum L, Pollak Y, Ben-Asher E, et al. Why do young women smoke? V. Role of direct and interactive effects of nicotinic cholinergic receptor gene variation on neurocognitive function. Genes Brain Behav. 2008;7:164–72. doi: 10.1111/j.1601-183X.2007.00329.x. [DOI] [PubMed] [Google Scholar]
- 35.Tabarés-Seisdedos R, Rubenstein JL. Chromosome 8p as a potential hub for developmental neuropsychiatric disorders: implications for schizophrenia, autism and cancer. Mol Psychiatry. 2009;14:563–89. doi: 10.1038/mp.2009.2. [DOI] [PubMed] [Google Scholar]
- 36.Dempster EL, Pidsley R, Schalkwyk LC, Owens S, Georgiades A, Kane F, et al. Disease-associated epigenetic changes in monozygotic twins discordant for schizophrenia and bipolar disorder. Hum Mol Genet. 2011;20:4786–96. doi: 10.1093/hmg/ddr416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Capello CF, Bourke CH, Ritchie JC, Stowe ZN, Newport DJ, Nemeroff A, et al. Serotonin transporter occupancy in rats exposed to serotonin reuptake inhibitors in utero or via breast milk. J Pharmacol Exp Ther. 2011;339:275–85. doi: 10.1124/jpet.111.183855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith AK, Jeffrey Newport D, Ashe MP, Brennan PA, Laprairie JL, Calamaras M, et al. Predictors of Neonatal Hypothalamic-Pituitary-Adrenal Axis Activity at Delivery. Clin Endocrinol (Oxf) 2011 doi: 10.1111/j.1365-2265.2011.03998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.First MB, Spitzer RL. M. G, W. WJB. Structured Clinical Interview for DSM-IV-TR Axis I disorders, Research Version, Patient Edition (SCID-I/P). New York: New York State Psychiatric Institute, 2002. [Google Scholar]
- 40.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 42.Terry P, Katz JL. Dopaminergic mediation of the discriminative stimulus effects of bupropion in rats. Psychopharmacology (Berl) 1997;134:201–12. doi: 10.1007/s002130050443. [DOI] [PubMed] [Google Scholar]
- 43.Learned-Coughlin SM, Bergström M, Savitcheva I, Ascher J, Schmith VD, Långstrom B. In vivo activity of bupropion at the human dopamine transporter as measured by positron emission tomography. Biol Psychiatry. 2003;54:800–5. doi: 10.1016/S0006-3223(02)01834-6. [DOI] [PubMed] [Google Scholar]
- 44.Schroeder JW, Conneely KN, Cubells JC, Kilaru V, Newport DJ, Knight BT, et al. Neonatal DNA methylation patterns associate with gestational age. Epigenetics. 2011;6:1498–504. doi: 10.4161/epi.6.12.18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bibikova M, Le J, Barnes B, Saedinia-Melnyk S, Zhou L, Shen R, et al. Genome-wide DNA methylation profiling using Infinium(®) assay. Epigenomics. 2009;1:177–200. doi: 10.2217/epi.09.14. [DOI] [PubMed] [Google Scholar]
- 46.Bock C, Tomazou EM, Brinkman AB, Müller F, Simmer F, Gu H, et al. Quantitative comparison of genome-wide DNA methylation mapping technologies. Nat Biotechnol. 2010;28:1106–14. doi: 10.1038/nbt.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adkins RM, Krushkal J, Tylavsky FA, Thomas F. Racial differences in gene-specific DNA methylation levels are present at birth. Birth Defects Res A Clin Mol Teratol. 2011;91:728–36. doi: 10.1002/bdra.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu J, Hutchison K, Perrone-Bizzozero N, Morgan M, Sui J, Calhoun V. Identification of genetic and epigenetic marks involved in population structure. PLoS One. 2010;5:e13209. doi: 10.1371/journal.pone.0013209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christensen BC, Houseman EA, Marsit CJ, Zheng S, Wrensch MR, Wiemels JL, et al. Aging and environmental exposures alter tissue-specific DNA methylation dependent upon CpG island context. PLoS Genet. 2009;5:e1000602. doi: 10.1371/journal.pgen.1000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bjornsson HT, Sigurdsson MI, Fallin MD, Irizarry RA, Aspelund T, Cui H, et al. Intra-individual change over time in DNA methylation with familial clustering. JAMA. 2008;299:2877–83. doi: 10.1001/jama.299.24.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Storey J. A direct approach to false discovery rates. J R Stat Soc, B. 2002;64:479–98. doi: 10.1111/1467-9868.00346. [DOI] [Google Scholar]


