Abstract
In this paper, we present a microfluidic platform for the continuous generation of stable, monodisperse lipid vesicles 20–110 μm in diameter. Our approach utilizes a microfluidic flow-focusing droplet generation design to control the vesicle size by altering the system’s fluid flow rates to generate vesicles with narrow size distribution. Double emulsions are first produced in consecutive flow-focusing channel geometries and lipid membranes are then formed through a controlled solvent extraction process. Since no strong solvents are used in the process, our method allows for the safe encapsulation and manipulation of an assortment of biological entities, including cells, proteins, and nucleic acids. The vesicles generated by this method are stable and have a shelf life of at least 3 months. Here, we demonstrate the cell-free in vitro synthesis of proteins within lipid vesicles as an initial step towards the development of an artificial cell.
INTRODUCTION
Living systems share a common unit structure known as a cell, a small but complex bioreactor enclosed by phospholipid membranes. The confinement of crucial biochemical reactions within these compartments is essential to life, and for such, phospholipid vesicles have been the preferred model for these bioreactors. Liposomes (lipid vesicles) have served as artificial cells for the controlled study of biological phenomena1, 2 or in vitro production of biomolecules such as DNA and protein.3, 4 Water-in-oil emulsions have been used to conduct various biochemical reactions such as green fluorescent protein (GFP) expression,5, 6 directed evolution of proteins,7, 8 and polymerase chain reaction (PCR),9 however the components that form the oil-water (O/W) interface of these systems are not composed of lipid bilayers, thus are very different from that of a cell membrane. As the cell membrane plays several crucial roles such as in determining transport of material in and out of a cell, and in the integration of membrane proteins for signal transduction, the lack of a lipid membrane of these oil-in-water emulsions limits the extent of bio-mimicry that can be achieved. For example, the transport of hydrophilic material in and out of the aqueous core is difficult in water-in-oil emulsions. Protein expression in water-in-oil-in-water (W/O/W) double emulsions have been achieved recently, however the thick oil layer limits diffusion of hydrophilic molecules across the interfaces and does not allow for the incorporation of membrane proteins.10 The liposome shares a likeness in composition and structure to a cell in its lipid membrane; thus it can better serve as a cell model and form a template for the insertion of a membrane protein of interest and facilitate the study of the protein’s behavior in a controlled environment.11 Liposomes have also become an ideal candidate for applications ranging from targeted therapeutic delivery to in vitro synthesis of biological molecules due to their cell membrane-like structure, ability to adopt both hydrophilic and hydrophobic molecules, and ease of functionalization. These features of liposomes have also been used in transfection procedures to insert DNA into host cells and as a mechanism of therapeutic delivery.12, 13
However, current methods of liposome production result in liposomes of polydisperse size populations, limited shelf life, and varying degrees of multilamellarity. Encapsulation efficiency is particularly poor in conventional liposome formation methods. For instance, with the lipid film hydration method, encapsulation is the result of arbitrary swelling of a lipid membrane around the cargo of interest. Encapsulation efficiency can be increased by increasing the cargo concentration, yet this also results in a greater amount of material lost in the external suspension. Both micron- and nanometer-sized liposomes have been produced in microfluidic devices, however these methods suffer from either polydisperse size populations or poor encapsulation efficiency.14, 15 Other methods have been presented which implement an ink-jet powered technique that produces monodisperse lipid vesicles, but the vesicles generated are hundreds of micrometers in diameter.16 Another method uses a lipid bubble blowing process but is limited in its ability to vary the size of the vesicles generated.17 A double emulsion method was used to form lipid vesicles in glass microcapillary tubes, however this method employs strong organic solvents which can harm cells or other biological materials.18 Unlike droplet microfluidics in microfabricated channels, this method does not easily allow upstream mixing of multiple reagents prior to droplet generation or the controlled fusion and splitting of droplets.
In this paper, we implement droplet-based microfluidics which involves the generation and manipulation of discrete droplets inside microfluidic flow-focusing devices as our strategy for forming monodisperse lipid vesicles.19 To form the phospholipid membrane, a solvent extraction method similar to ones used to synthesize polymer particles was utilized.20, 21 In addition, we show a selective hydrophilic surface treatment that is used to aid in the formation of double emulsions. The flow-focusing configuration allows controlled encapsulation of a variety of materials. Such a device has several advantages over conventional and other microfluidic synthesis methods, enabling size control of the lipid vesicles with high efficiency encapsulation of a wide range of biological entities. First, the sizes of the vesicles can be easily controlled through the flow rates of the fluids and the geometry of the microfluidic channels. And because of the nature of laminar flow within the flow-focusing nozzle, the size and structure of the vesicles are well-defined and consistent. The process by which the lipid vesicles are generated ensures that the entire solution is formed into these vesicles, resulting in high encapsulation efficiency and little wasted material. Additionally, the lipid vesicles are generated without the use of toxic solvents lending itself suitable for pharmaceutical use or biological studies. The lipid vesicles generated in this system also have a long stability period of >3 months compared to <1 week reported in the literature.22, 23 Finally, microfluidic configurations allow for the controlled encapsulation or fusion of multiple reagents at once inside the vesicles for multiplexed and programmable assays.
MATERIALS AND METHOD
Lipid reagents and solutions
The aqueous solution which comprised the inner phase consists of a 1:1 mixture of Pluronic F68 (Sigma Aldrich) and ultrapure water obtained from a millipore system. The lipid solution is composed of 56 μM DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine, Avanti Polar Lipids) dissolved in oleic acid (Fisher Scientific). The fluorescent probe DiI-C18 (1,1′-dilinoleyl—3,3,3′,3′-tetramethylindocarbocyanine perchlorate, molecular probes) was added at 0.1 wt. % for fluorescence microscopy imaging. The external aqueous solution consists of a mixture of 2.85% v/v Pluronic F68 (10% solution, Sigma-Aldrich), 14% v/v glycerol (EMD Biosciences), 14% v/v ethanol (Sigma Aldrich), and ultrapure water.
A cell-free protein expression kit (RTS 500, Roche Applied Science) was used for the GFP expression experiments. Solutions were prepared according to the manufacturer’s instructions and then aliquoted into 15 reaction sets with the final GFP plasmid concentration of 0.5 μg/μl. The reagents were stored at −20°C and thawed and kept on ice until ready to be used.
Photolithography and fabrication
Microfluidic devices were fabricated in poly(dimethyl) siloxane (PDMS) using conventional soft lithography techniques.24 First, three inch silicon wafers were spin-coated with a 50 μm layer of SU8-50 (MicroChem) photoresist, baked to improve the adhesion of the SU-8 to the silicon wafer and then patterned with multiple copies of the device by exposure to UV light through a high resolution 20 000 dpi photomask containing the channel design. After post-exposure baking, the wafer is then submerged in SU8 developer to form the channel pattern. The remaining cross-linked SU-8 resist forms a positive mold for the silicone polymer.
PDMS (Sylgard 184, Dow Corning) was mixed at a 10:1 pre-polymer base to curing agent ratio and poured over the patterned wafer. The polymer mix was cured at 65 °C for at least 4 h. After curing, the PDMS is peeled off the mold, cut into individual devices, and connection holes were bored into the device using flat end dispensing needles (Integrated Dispensing Solutions Inc.). The devices were then cleaned before bonding via air plasma treatment to a cleaned glass slide (Corning). The plasma activates the surfaces of the PDMS and glass and allows for irreversible bonding between the two materials.
Surface treatment
After bonding and sealing of the channels, a hydrophilic surface treatment was applied to the external droplet generation junction to render the channel surface hydrophilic.25 As PDMS is inherently hydrophobic and the continuous phase used for the inner droplet generation is water, the hydrophilic surface treatment ensures complete wetting of the walls by the aqueous solution. Briefly, the channels were filled with a 1 wt. % PVA (polyvinyl alcohol, average MW 30 000-70 000, 87%-90% hydrolyzed, Sigma Aldrich) solution after bonding and incubated at room temperature. The excess solution is then removed by vacuum and the device is baked in a 120 °C oven to fully dry the device and improve bonding strength of the coating. The device is then ready for use and can be stored at room temperature as the hydrophilic coating lasts for months.
Experimental set-up
The lipid solution is prepared by dispensing powdered DOPC lipid into oleic acid and sealing in a glass vial. The solution is then dissolved with the aid of a 37 °C sonication bath. The solutions are contained in 1 ml Luer Norm-Ject syringes (Henke Sass Wolf) and the flow rate is adjusted by a custom-made LabVIEW control for syringe pumps (Pico Plus, Harvard Apparatus). Clear polypropylene tubing (Tygon, Cole-Palmer) is used to connect the syringes to inlets of the device and the outlet to the collection vial.
Analysis and imaging
We captured still images and movies of the lipid vesicles using an inverted Nikon microscope and high-speed camera (Fastcam PCI-10K, PhotronLTd.). We used an image analysis program (ImageJ, NIH) for vesicle size measurement.
To visualize the lipid vesicles, the membranes were tagged with a lipophilic probe, DiI and imaged with brightfield, fluorescence, and confocal microscopy. Imaging of the stained lipid membranes were performed on an inverted fluorescence microscope (Nikon) and illuminated by a mercury lamp. Excitation of the DiI probe was observed at 570 nm using a TRITC filter, and the vesicles were imaged at 10× magnification with a high resolution CCD camera (ORCA-D2, Hamamatsu).
RESULTS AND DISCUSSION
Selective hydrophilic patterning
Channel surface wettability plays an important role in determining the type of emulsions that can be generated within the device. Given a hydrophobic surface, co-flowing streams of water and oil will generate water emulsions due to the preferential wetting of oil to the channel surface. With a hydrophilic channel, this situation is reversed and water favors the surface of the channel wall, resulting in the formation of oil droplets. To form double emulsions, it is ideal to pattern different channels with hydrophilic or hydrophobic surface wettability. Since PDMS is naturally hydrophobic, a hydrophilic surface coating can be applied for the formation of oil-in-water emulsions. Previous methods for generating double emulsions relied on separate channel layers for the formation of the inner and outer droplets or selective UV surface treatment using photomasks which can be complicated to carry out as the processes require careful alignment.26, 27 Recently, a selective hydrophilic surface treatment for PDMS was demonstrated with a sol-gel coating, however the treatment requires the use of several chemical entities and thermal or UV-initiated reactions.28 A much simpler approach using the selective coating of PVA has been developed. PVA treatment has been shown to irreversibly adsorb to a number of materials including silicon capillary channels and some hydrophobic polymer surfaces.25 Wu et al.29 showed that repeated cycles of adsorption/drying and heat immobilization of PVA onto PDMS results in a stable hydrophilic treatment that we have found to last for several months.29
We use an effective and simple hydrophilic treatment to selectively coat the external channel of our platform, such that the region forming water-in-oil droplets is hydrophobic and the region forming oil-in-water droplets is hydrophilic. This process of selective hydrophilic treatment for double emulsion formation was first described by Teh and Lee.30 Unlike other hydrophilic surface treatments, this process does not require mask alignment or hazardous chemicals, and it takes only 10 min to complete. Hydrophobic channels are required for the generation of the water-in-oil droplets, whereas the hydrophilic surface is needed for generation of the double emulsion. The process for selective hydrophilic treatment is shown in Figure 1. First, immediately after plasma bonding, a 100 μl drop of 1 wt. % PVA solution is applied at the external phase inlet, while continuously applying negative pressure at the device outlet. This is achieved by connecting the device reservoir to the house vacuum with tubing. This negative pressure pulls PVA through the device such that only the external phase channels are coated. The vacuum prevents the PVA solution from coating the single emulsion generating channels, forcing air through the lipid and inner aqueous phase inlets, and enabling surface treatment to be applied to only the external channels. The PVA solution is exposed to the channels for at least 5 min at room temperature to provide sufficient time for the PVA monomers to assemble onto the channel surface. The channels are then vacuumed until excess PVA solution is removed. Then, the device is placed in a 120 °C oven for at least 5 min to heat immobilize the PVA monomers onto the surface. This surface treatment allows the device to remain hydrophilic for several months. It is important that during the treatment process, to remove as much excess PVA as possible prior to the baking step, since the remaining PVA will harden and can result in the clogging of channels. One should take care to visually inspect treated channels before the baking step to ensure sufficient removal of excess PVA.
Figure 1.
Schematic of the selective PVA treatment process. Vacuum is applied constantly to the outlet of the device as a 100 μl drop of 1 wt. % PVA is placed at the inlet of the device and drawn through the external channel by vacuum. Then, the device is incubated at 120 °C for 5 min. The device remains hydrophilic for several months.
Device design
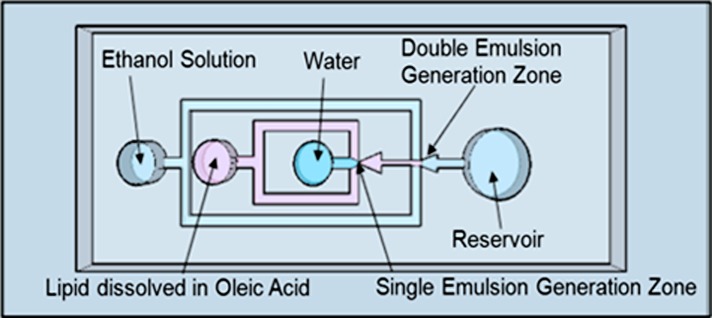
The device is designed to generate water-oil-water double emulsions, which is implemented with the use of two consecutive flow-focusing emulsification junctions. Lipid vesicles are formed when oil is removed from the middle layer of the double emulsion by a solvent extraction process.31 A schematic of the device is shown in Figure 2. The device has three inlets–one each for the internal phase, the lipid phase, and the external phase–and one outlet reservoir for collection of the vesicles. The internal phase is an aqueous solution, which is sheared into monodispersed droplets by the lipid solution that is composed of a 6.5 mM DOPC/oleic acid mixture. This concentration was chosen such that there would be a sufficient number of phospholipids to form a lipid bilayer around a ∼50 μm diameter vesicle. For a DOPC phospholipid with a headgroup area of approximately 70 Å and vesicle surface area of 7850 μm2, at least 3.73 × 10−14 moles of phospholipids are needed to compose the lipid bilayer. We calculate the volume of oleic acid in a double emulsion to be 17pL by assuming a 5 μm thickness surrounding a 50 μm vesicle. With a 6.5 mM lipid concentration, this results in the lipid layer containing 3.03 × 10−13 moles of phospholipid, which is eight times the amount of phospholipid needed to form a lipid bilayer around the vesicle. The volume of lipid mixture comprising the middle layer of the double emulsion could also be increased or decreased by changing the ratio of the external and middle fluid flow rates.
Figure 2.
Schematic of double emulsion device. The platform consists of PDMS-molded channels, air plasma bonded to a PDMS-coated glass slide. There are two flow-focusing junctions placed in sequence to generate water-in-oil-in-water double emulsions.
These single emulsions are formed at the first flow-focusing junction, labeled “single emulsion generation zone.” This stream of droplets is then sheared at the second junction, labeled “double emulsion generation zone,” into double emulsions by the external aqueous solution which is composed of a water-ethanol mixture.
As fluid flow in microfluidic systems is dominated by viscous forces, characterized by low Reynolds number, and thus laminar flow, we implement the flow-focusing nozzle geometry to enable the breakup of the fluidic streams in a microscale environment. Due to the expansion geometry following the nozzle orifice, a velocity gradient is formed such that the maximum velocity occurs at a single point at the narrowest region of the nozzle orifice.32 This ensures that droplet break-off occurs at this point due to the shear gradient. The channels are 50 μm in height and the first and second shear-focusing orifices are 15 μm and 45 μm in width, respectively. The channel geometry and solution flow rates can be altered to provide precise control over the vesicle diameter in a range of sizes. The second junction orifice diameter is larger to avoid shear-induced breakage of the internal aqueous phase.
Lipid vesicle formation
The double emulsions serve as a template for the formation of lipid vesicles. As shown in Figure 3, monodisperse single and double emulsions are formed in the flow-focusing nozzles. At the first junction orifice, monodisperse water droplet emulsions are formed in a phospholipid mixture. Due to their amphiphilic nature, the phospholipids arrange themselves at the oil/water interface such that the hydrophilic heads point inwards and hydrophobic tails point into the oleic acid, forming a lipid monolayer around the water droplet. This water-in-oil emulsion stream is then sheared into double emulsions at the second junction orifice by an aqueous solution consisting of a mixture of ethanol, glycerol, and pluronic surfactant in water.
Figure 3.
Schematic and microscope images of single and double emulsion formation. First, water droplets are sheared by the continuous phase consisting of DOPC/oleic acid which enter the junction from the top and bottom channels. Then, this stream is sheared by a stream composed of an ethanol solution which arrives at the second junction from the top and bottom channels. Images were captured with the Photron Fastcam.
Glycerol is a viscous water soluble liquid that is commonly used in pharmaceutical formulations. Glycerol was included in the outer aqueous phase to increase the viscosity of the continuous phase to improve shearing of the viscous oil by water. Glycerol has also been shown to interact with lipid bilayers to decrease membrane fluidity and increase stability.33 Pluronic F68 is a diblock co-polymer that is used to prevent cell membranes from hydrodynamic damage and has been used to re-stabilize cell membranes following electroporation.34 Pluronic F-68 is also used in large scale mammalian cell culture and was found that at low concentrations, can enhance cell growth.35 Pluronic F68 is used in the external solution formulation to serve as a surfactant to prevent coalescence of the double emulsions and to improve stability of the vesicles. Ethanol serves to extract the oleic acid from the middle layer. Since oleic acid is highly soluble in ethanol, it dissolves into the ethanol solution over time leaving behind a concentrated ring of phospholipids around the water droplet.31 The spontaneous formation of a closed lipid bilayer is energetically favored due to the hydrophilic interactions between the polar head groups, van der Waal’s forces between the hydrocarbon tails, and hydrophilic interactions with the aqueous medium.36 This results in the self-assembling of the phospholipids at the oil/water interfaces such that the phospholipid tails point inward and the head groups outward into the external aqueous solution mirroring the inner surface of the ring, resulting in the formation of a lipid bilayer. The ethanol in the external solution is evaporated away and since oleic acid is less dense than water or the vesicles, it floats to the top of the vesicle solution where it can be easily removed.
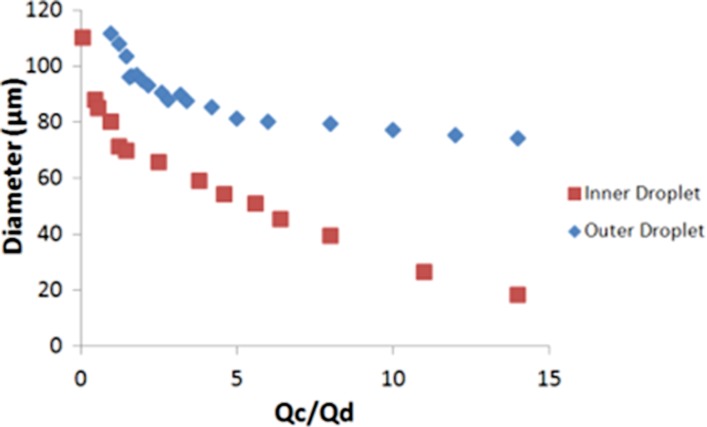
The size of the inner and outer droplet can be tuned by changing the ratio of the flow rates of the discrete and continuous phases, denoted by Qd and Qc respectively as shown in Figure 4. Qd represents the phase which is sheared, and Qc is the phase that causes the shearing. In terms of the inner droplet, the internal aqueous phase is Qd, whereas Qc is the DOPC/oleic acid mixture. In the case of the second emulsification step, the DOPC/oleic acid mixture is Qd, and Qc is the external ethanol solution. The inner droplet diameter increases when the inner aqueous flowrate increases or if the lipid flowrate decreases. In other words, the inner droplet diameter will increase with decreasing ratio of lipid to inner aqueous flowrates and vice versa. Likewise, the outer droplet diameter decreases as the ratio of the flowrates of the external solution to DOPC/oleic acid solution increases. In general, this effect is more apparent for lower internal aqueous flowrates. The inner aqueous droplet serves as the core of the lipid vesicle, and as the lipid membrane thickness is negligible, compared to the overall diameter of the vesicle, the diameter of the inner droplet determines the diameter of the resultant lipid vesicle. For the inner droplet, the size increases from 18 μm to 110 μm when Qc/Qd is decreased from 14 to 0.1. The outer droplet diameter ranges from 74 μm at a ratio of 14 to approximately 110 μm when the ratio is decreased to 1. Control over the diameter of the outer droplet, and thus the volume and thickness of the middle phase, ensures that there is an adequate number of phospholipids present to form the lipid membrane. This ability to control both the inner and outer droplet diameter is useful in determining the size of vesicles that can be generated from the device.
Figure 4.
Graph showing correlation between ratio of fluid flowrates of the continuous to discrete phase with diameter of the inner and outer droplet.
It is important to note that although the size of the vesicles produced can be decreased by lowering the flowrate ratio of inner-to-middle phase, the range of sizes of vesicles tunable in this manner is limited by the channel geometry. For instance, submicron vesicles could be generated with a smaller droplet generation junction orifice diameter.
Solvent extraction process/vesicle characterization
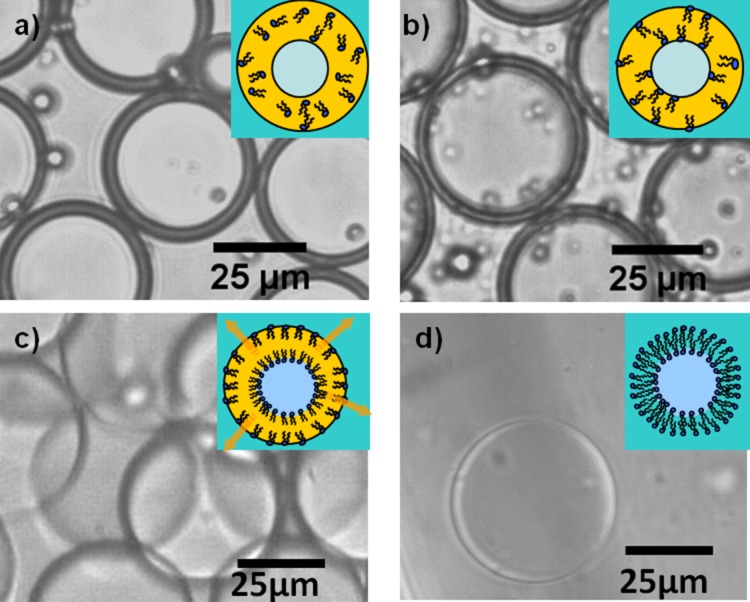
Monodispersed water-oil-water double emulsions were successfully generated using this device. The double emulsions are incubated at room temperature in the external ethanol solution and observed over 24 h. As shown in Figure 5, oleic acid is extracted by ethanol, resulting in a thinning middle phase layer. Over time, the middle layer becomes thinner as ethanol dissolves away oleic acid until exposure to aqueous environments on both sides of the middle layer forces the phospholipids to reorient into a bilayer, forming stable liposomes.
Figure 5.
Time-lapse images of solvent removal process at 0,5, 10, and 15 h. (a)-(d) Oleic acid is extracted out by ethanol, as is indicated by the reduction in the thickness of the middle layer of the double emulsion. As the solvent is removed, the phospholipids are forced to self-assemble along the water-oil interface to form a lipid membrane.
The presence of the lipid membrane can be indirectly verified with the use of a membrane probe. To visualize the vesicle membrane, we dissolve the lipophilic probe, DiI, along with the phospholipids into oleic acid at 0.1 wt. %. DiI is a fluorescent tracer which is commonly used for tagging and studying cell morphology. It is soluble and weakly fluorescent in water, but when incorporated into a membrane, it is highly fluorescent and photostable and will display a rhodamine-like fluorescence.37 As shown in Figure 6a, the vesicle membranes are thin and fluoresce brightly, suggesting the presence of a lipid membrane. While it may be possible the vesicles are not unilamellar, such that there may be residual oleic acid or excess phospholipid that may be in the lipid shell, this does not significantly impact applications involving the encapsulation or expression of proteins. The incorporation of unsaturated fatty acid into the membrane would increase membrane fluidity which is stabilized with the pluronic surfactant. Previous work from this lab has shown that ion exchange can occur between the external aqueous environment and the vesicle interior which would have been limited if there were a thick oil layer separating the vesicle interior from the outside.31 These results suggest that the vesicles are likely to be unilamellar, however further investigation will need to be done.
Figure 6.
(a) Fluorescent image of DiI stained lipid vesicles. DiI fluoresces brightly when inserted into a lipid membrane. (b) Size distribution plot showing that vesicle diameter can be controlled by changing the fluid flowrates. The ratio of the external aqueous to oil to internal aqueous phase are listed in the legend.
Vesicle size control
We show the generation of vesicles ranging from 20–120 μm in diameter. As shown in Figure 6b, the size population is monodisperse with less than 2% variation. As mentioned in Sec. 3D, the lipid vesicle diameter can be tuned by changing the relative fluid flow rates. As shown in the graph, as the ratio of the flowrate of the inner aqueous phase increases, larger lipid vesicles are generated. Smaller vesicles can be formed by decreasing the inner aqueous flow rate. The ability to control the size of lipid vesicles provides an additional parameter that can be varied to elucidate cell function from cell structure. As cellular processes occur over a variety of length scales and are dictated by local spatial confinements, the ability to change the size of the liposomes offers the potential to investigate the effect of various geometrical constraints on these processes.
The droplets were generated at a throughput rate of up to 200 droplets/s. The generation rate is also dependent on the fluid flowrates. An increase in the continuous phase flowrate will result in a higher generation rate. While the generation rate is slow compared to conventional means, a number of methods for scaling up droplet generation have been presented.38, 39, 40 Parallel processing of multiple flow-focusing platforms is one such example of a method we could implement with our generation system.
We also observe that up to 75% of the lipid vesicles remain stable in solution for up to 3 months, which is an important characteristic for use in applications involving long-term incubation periods.
Cell-free protein synthesis
An artificial cell holds the advantage of having well-defined components that will allow scientists to study biological activities otherwise impossible. Micron-sized aqueous compartments that are capable of performing biological reactions are the first step to creating an artificial cell. The biological cell has many important functions, however its two fundamental roles include: (1) its lipid membrane serves as a selective barrier to protect the cell from external contaminants and to control the passage of nutrients, waste, and signaling molecules; and (2) it contains the components necessary to carry out gene transcription and protein translation. As the liposome is similar in structure and composition to that of a cell, it is ideal for replicating the basic functions of a cell.
We demonstrate the ability of these lipid vesicles to serve as a template for an artificial cell and to show its potential function as a microbioreactor. We modified the original double emulsion device design to include two inner aqueous inlets, creating a platform that simultaneously and continuously generates lipid vesicles and carries out cell-free protein expression. As a proof of concept, we chose to synthesize GFP, as the proper transcription and translation of GFP as well as correct folding of the protein structure can be easily detected by the presence of its fluorescence.
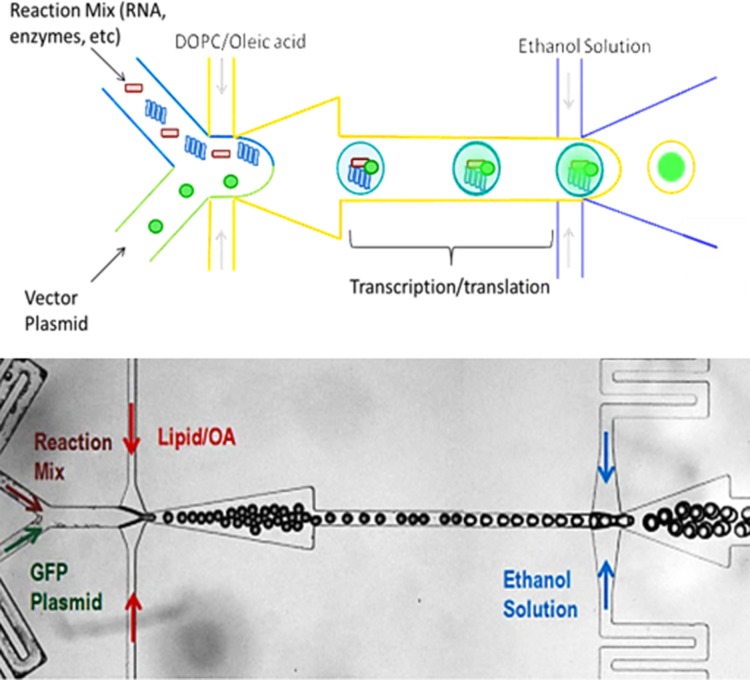
A schematic of the encapsulation of the protein synthesis reagents into double emulsions is shown in Figure 7. The reaction mix and plasmid solution were prepared according to the RTS500 manufacturer’s instructions. This device was designed with two inlets to ensure that the reaction mix containing the necessary reagents for transcription and translation is not exposed to the GFP vector prior to encapsulation within the droplet. Although it is not critical for the expression of GFP, the ability to control the interaction between two reagents would be critical in assays such as interrogating protein-protein interactions or time-sensitive reactions.
Figure 7.
(a) Schematic of protein synthesis in the microfluidic device. The transcription/translation reagents and vector of interest are encapsulated and sheared into droplets by the DOPC/oleic acid mixture. Then, the droplets are sheared into double emulsions and allowed to incubate to complete vesicle and protein formation. (b) Microscope image of single and double emulsion formed in the device.
At the first junction, the protein expression reagents are introduced at 0.5 μl/min and sheared by a 5 wt. % mixture of DOPC/oleic acid into droplets (1 μl/min). The droplets generated were 25 μm in diameter and highly monodispersed. At the second junction, these droplets are sheared into double emulsions by an ethanol solution (1 μl/min). The emulsions are incubated on chip at room temperature and the fluorescence level inside the vesicles was monitored over several hours.
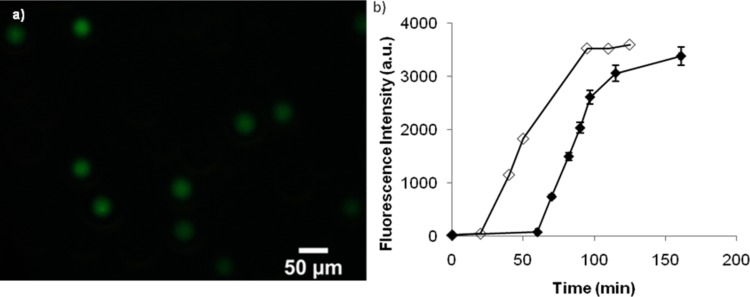
As shown in Figure 8, we observe the expression of GFP in monodispersed lipid vesicles. DiI was not added to the lipid mixture in these experiments to prevent overlap in the fluorescence intensity measurements. Fluorescent images of the vesicles were taken every 15 min during the 3 h incubation period. As seen in the graph, GFP was continuously expressed during the 3 h period. The fluorescence level was not detectable until after approximately 60 min. This time delay is believed to be attributed to the limit of fluorescence detection and the low incubation temperature of 23 °C. The initial delay period allowed for formation of the lipid membrane, multiple-step enzymatic reactions involved in transcription and translation, and folding of the amino acid chain into a three-dimensional protein. Incorrectly folded GFP molecules do not have a strong fluorescent signal, and it can take minutes to hours for GFP to re-fold correctly and reach a detectable signal.41 The fluorescence signal begins to plateau after approximately 100 min, which indicates that protein expression has completed. The protein expression in the vesicles was also compared to expression in bulk. The same volume of transcription/translation reagents and GFP vector was mixed in a small vial and allowed to incubate at room temperature. The delay period for bulk expression is about 15 min which is the typical delay time due to gradual reagent consumption, also shown by other research groups.5 It was observed that the time to reach maximum expression and total amount of GFP expressed for both the bulk and droplet method were similar, demonstrating no significant loss of material through the droplet microfluidic device. These results indicate that we were successful in the expression of GFP in lipid vesicles and that these vesicles have the potential to serve as artificial cells.
Figure 8.
(a) Fluorescence microscopy image of GFP in monodisperse lipid vesicles following in vitro expression. (b) Graph of the fluorescence intensity of GFP within the vesicles during the course of the incubation period (filled) and a bulk solution (open). We observe the continuous expression of GFP for up to 3 h.
CONCLUSIONS
We have presented a microfluidic platform that is capable of synthesizing long-lasting, monodispersed lipid vesicles using a biocompatible solvent extraction process. Precise control over a wide range of liposome sizes through manipulation of flow rate ratios was demonstrated. As the vesicles were aimed to serve as the basis of artificial cells; cell-free expression of green fluorescent protein was shown inside the microfluidically produced lipid vesicles. We believe this platform shows great promise as a tool to enable further development of artificial cell technology by providing a continuous process that enables the production of well-controlled vesicles with efficient encapsulation of biologically active reagents. To further demonstrate the platform’s capabilities, we plan to functionalize the membrane through the expression of membrane proteins inside the vesicles, which will facilitate in the study of transport across the lipid membrane.
ACKNOWLEDGMENTS
This work was partially funded by the Micro/nano Fundamentals Focus (MF3) Center under the DARPA N/MEMS Science and Technology Fundamentals Program, Grant No. HR0001-06-1-0500, and the President’s Dissertation Fellowship. We thank Robert Lin for his help in editing of the manuscript, and Lisen Wang, Tim Tseng, and Roger Shih for their help in the cleanroom.
References
- Pohorille A. and Deamer D., Trends Biotechnol. 20, 123 (2002). 10.1016/S0167-7799(02)01909-1 [DOI] [PubMed] [Google Scholar]
- Luisi P., Ferri F., and Stano P., Naturwiss. 93, 1 (2006). 10.1007/s00114-005-0056-z [DOI] [PubMed] [Google Scholar]
- Walde P. and Ichikawa S., Biomol. Eng. 18, 143 (2001). 10.1016/S1389-0344(01)00088-0 [DOI] [PubMed] [Google Scholar]
- Noireaux V., Bar-Ziv R., Godefroy J., Salman H., and Libchaber A., Phys. Biol. 2, P1 (2005). 10.1088/1478-3975/2/3/P01 [DOI] [PubMed] [Google Scholar]
- Dittrich P. S., Jahnz M., and Schwille P., ChemBioChem, 6, 811 (2005). 10.1002/cbic.200400321 [DOI] [PubMed] [Google Scholar]
- Osaki T., Yoshizawa S., Kawano R., Sasaki H., and Takeuchi S., Anal. Chem. 83, 3186 (2011). 10.1021/ac2000048 [DOI] [PubMed] [Google Scholar]
- Griffiths A. D. and Tawfik D. S., EMBO J. 22, 24 (2003). 10.1093/emboj/cdg014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller O. J., Bernath K., Agresti J. J., Amitai G., Kelly B. T., Mastrobattista E., Taly V., Magdassi S., Tawfik D. S., and Griffiths A. D., Nat. Methods 3, 561 (2006). 10.1038/nmeth897 [DOI] [PubMed] [Google Scholar]
- Williams R., Peisajovich S. G., Miller O. J., Magdassi S., Tawfik D. S., and Griffiths A. D., Nat. Methods 3, 545 (2006). 10.1038/nmeth896 [DOI] [PubMed] [Google Scholar]
- Wu N., Oakeschott J., Easton C., Peat T., Surjadi R., and Zhu Y., J. Micromech. Microeng. 21, 054032 (2011). 10.1088/0960-1317/21/5/054032 [DOI] [Google Scholar]
- Christensen S. M. and Stamou D., Soft Matter 3, 828 (2007). 10.1039/b702849k [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Trends Biotechnol. 13, 527 (1995). 10.1016/S0167-7799(00)89017-4 [DOI] [PubMed] [Google Scholar]
- Felgner P. L. and Ringold G. M., Nature 337, 387 (1989). 10.1038/337387a0 [DOI] [PubMed] [Google Scholar]
- Kuribayashi K., Tresset G., Coquet P., Fujita H., and Takeuchi S., Measurement Sci. Technol. 17, 3121 (2006). 10.1088/0957-0233/17/12/S01 [DOI] [Google Scholar]
- Jahn A., Vreeland W. N., DeVoe D. L., Locascio L. E., and Gaitan M., Langmuir 23, 6289 (2007). 10.1021/la070051a [DOI] [PubMed] [Google Scholar]
- Stachowiak J. C., Richmond D. L., Li T. H., Liu A. P., Parekh S. H., and Fletcher D. A., Proc. Natl. Acad. Sci. USA 105, 4697 (2008). 10.1073/pnas.0710875105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota S., Yoshizawa S., and Takeuchi S., Angew. Chem., Int. Ed. 48, 6533 (2009). 10.1002/anie.200902182 [DOI] [PubMed] [Google Scholar]
- Shum H. C., Lee D., Yoon I., Kodger T., and Weitz D. A., Langmuir 24, 7651 (2008). 10.1021/la801833a [DOI] [PubMed] [Google Scholar]
- Teh S. Y., Lin R., Hung L. H., and Lee A. P., Lab Chip 8, 198 (2008). 10.1039/b715524g [DOI] [PubMed] [Google Scholar]
- Hung L.-H., Teh S.-Y., Jester J., and Lee A. P., Lab Chip 10, 1820 (2010). 10.1039/c002866e [DOI] [PubMed] [Google Scholar]
- Freitas S., Merkle H. P., and Gander B., J. Controlled Release 102, 313 (2005). 10.1016/j.jconrel.2004.10.015 [DOI] [PubMed] [Google Scholar]
- Xia S. and Xu S., Food Res. Int. 38, 289 (2004). 10.1016/j.foodres.2004.04.010 [DOI] [Google Scholar]
- Yang T., Cui F.-D., Choi M.-K., Cho J.-W., Chung S.-J., Shim C.-K., and Kim D.-D., Int. J. Pharmaceutics 338, 317 (2007). 10.1016/j.ijpharm.2007.02.011 [DOI] [PubMed] [Google Scholar]
- Whitesides G. M., Ostuni E., Takayama S., Jiang X. Y., and Ingber D. E., Annu. Rev. Biomed. Eng. 3, 335 (2001). 10.1146/annurev.bioeng.3.1.335 [DOI] [PubMed] [Google Scholar]
- Kozlov M., Quarmyne M., Chen W., and McCarthy T. J., Macromolecules 36, 6054 (2003). 10.1021/ma021681g [DOI] [Google Scholar]
- Okushima S., Nisisako T., Torii T., and Higuchi T., Langmuir 20, 9905, (2004). 10.1021/la0480336 [DOI] [PubMed] [Google Scholar]
- Hu S. W., Ren X. Q., Bachman M., Sims C. E., Li G. P., and Allbritton N. L., Anal. Chem. 76, 1865 (2004). 10.1021/ac049937z [DOI] [PubMed] [Google Scholar]
- Abate A. R., Thiele J., Weinhart M., and Weitz D. A., Lab Chip 10, 1774 (2010). 10.1039/c004124f [DOI] [PubMed] [Google Scholar]
- Wu D. P., Luo Y., Zhou X. M., Dai Z. P., and Lin B. C., Electrophoresis 26, 211 (2005). 10.1002/elps.v26:1 [DOI] [PubMed] [Google Scholar]
- Teh S. Y. and Lee A. P., in Proceedings of The Thirteenth International Conference on Miniaturized Systems for Chemistry and Life Sciences (uTAS 2009) (Jeju, Korea, 2009), pp. 1353–1355.
- Tan Y. C., Hettiarachchi K., Siu M., Pan Y. P., and Lee A. P., J. Am. Chem. Soc. 128, 5656 (2006). 10.1021/ja056641h [DOI] [PubMed] [Google Scholar]
- Tan Y. C., Cristini V., and Lee A. P., Sens. Actuators B 114, 350 (2006). 10.1016/j.snb.2005.06.008 [DOI] [Google Scholar]
- Witold K S., Chem. Phys. Lipids 34, 363 (1984). 10.1016/0009-3084(84)90010-0 [DOI] [Google Scholar]
- Lee R. C., River L. P., Pan F. S., Ji L., and Wollmann R. L., Proc. Natl Acad. Sci. U.S.A. 89, 4524 (1992). 10.1073/pnas.89.10.4524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellung-Larsen P., Assaad F., Pankratova S., Saietz B. L., and Skovgaard L. T., J. Biotechnol. 76, 185 (2000). 10.1016/S0168-1656(99)00188-1 [DOI] [PubMed] [Google Scholar]
- Frezard F., Braz. J. Med. Biol. Res. 32, 181 (1999). 10.1590/S0100-879X1999000200006 [DOI] [PubMed] [Google Scholar]
- Johnson I. and T M.. Spence Z., Molecular Probes Handbook, A guide to Fluorescent Probes and Labeling Technologies (Life Technologies, Eugene, OR, 2010). [Google Scholar]
- Nisisako T. and Torii T., Lab Chip 8, 287 (2008). 10.1039/b713141k [DOI] [PubMed] [Google Scholar]
- Hettiarachchi K., Talu E., Longo M., Dayton P., and Lee A., in The Eleventh International Conference on Miniaturized Systems for Chemistry and Life Sciences (uTAS 2007) (Paris, France, 2007), pp. 664–666.
- Nisisako T., Torii T., Takahashi T., and Takizawa Y., Adv. Mater. 18, 1152 (2006). 10.1002/adma.v18:9 [DOI] [Google Scholar]
- Andrews B. T., Gosavi S., Finke J. M., Onuchic J. N., and Jennings P. A., Proc. Natl. Acad. Sci. 105, 12283 (2008). 10.1073/pnas.0804039105 [DOI] [PMC free article] [PubMed] [Google Scholar]