Abstract
This paper describes a light-addressable electrolytic system used to perform an electrodeposition of calcium alginate hydrogels using a digital micromirror device (DMD). In this system, a patterned light illumination is projected onto a photoconductive substrate serving as a photo-anode to electrolytically produce protons, which can lead to a decreased pH gradient. The low pH generated at the anode can locally release calcium ions from insoluble calcium carbonate (CaCO3) to cause gelation of calcium alginate through sol-gel transition. By controlling the illumination pattern on the DMD, a light-addressable electrodeposition of calcium alginate hydrogels with different shapes and sizes, as well as multiplexed micropatterning was performed. The effects of the concentration of the alginate and CaCO3 solutions on the dimensional resolution of alginate hydrogel formation were experimentally examined. A 3 × 3 array of cell-encapsulated alginate hydrogels was also successfully demonstrated through light-addressable electrodeposition. Our proposed method provides a programmable method for the spatiotemporally controllable assembly of cell populations into cellular microarrays and could have a wide range of biological applications in cell-based biosensing, toxicology, and drug discovery.
INTRODUCTION
Recently, cellular microarrays have been proven to be a powerful experimental tool for biological studies and drug discovery1 because of their ability to provide more information from smaller sample volumes while reducing both reagent consumption and the number of cells required for a wide range of assays. Thus, a variety of novel methods have been developed to assemble and cultivate cells at specific addresses for cellular microarrays.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 Among these methods, an acidic polysaccharide of sodium alginate, which can form a calcium alginate gel in the presence of calcium ions (Ca2+), is widely used to entrap and immobilize prokaryotes and eukaryotic cells, such as alginate beads,6, 7, 8 fibers/tubes,9, 10 and a three-dimensional (3D) cellular microarray.11, 12 Electroaddressing is an attractive technique for depositing calcium alginate gel13, 14, 15 or pH-responsive chitosan16, 17 at specific addresses and in specific shapes on the preformed metal thin-film electrode surfaces that can generate protons or hydroxide ions by electrolysis on the anodes or cathodes of the electrodes. This technique is used for the assembly of cells within polysaccharide hydrogels at specific addresses with a controllable pattern. Payne et al.13 reported the electroaddressing of calcium alginate hydrogels, with the ability to entrap viable cell populations within the electrodeposited films. However, gel patterns involving locations, shapes and dimensions are completely subject to pre-defined configurations of microelectrodes. Changing the pattern requires the re-design and re-fabrication of the photo-masks, microelectrodes and sometimes the chip structures themselves. This inflexibility of the microelectrodes has hampered the feasibility in biological applications of achieving dynamical and multiplexed micropatterning of cell-encapsulated alginate hydrogels with different cell types on the same device. Moreover, electrodeposition was typically performed by immersing glass slides with pre-defined electrodes into a centimeter-scale electrolytic container, which resulted in a low current density (3 A m−2) during electrolysis. The low current density leads to a longer time (5 min) required for the formation of the calcium alginate hydrogels.
Here, we propose an alternative approach to produce cell-encapsulated alginate hydrogels by utilizing a light-addressable electrolytic system to perform an electrodeposition of calcium alginate hydrogels in microchambers/microchannels using a digital micromirror device (DMD). The DMD provides spatio-temporal illumination pattern switching, which is used to achieve flexible electrode patterning for the dynamical and multiplexed electrodeposition of calcium alginate hydrogels. Changing the patterns desired do not require to the re-design and re-fabrication of the photo-masks. Moreover, the dynamical photo-mask eliminates the need to align two or more different photo-masks to achieve the precise control of the illumination patterns for multiplexed electrodeposition. By controlling the illumination pattern on the DMD, a light-addressable electrodeposition of calcium alginate hydrogels with different shapes and sizes, as well as multiplexed micropatterning, were performed. The effects of the concentration of the alginate and CaCO3 solutions on the dimensional resolution of alginate hydrogel formation were experimentally examined. Finally, a 3 × 3 array of cell-encapsulated alginate hydrogels was successfully created without destroying viability through light-addressable electrodeposition.
MATERIALS AND METHODS
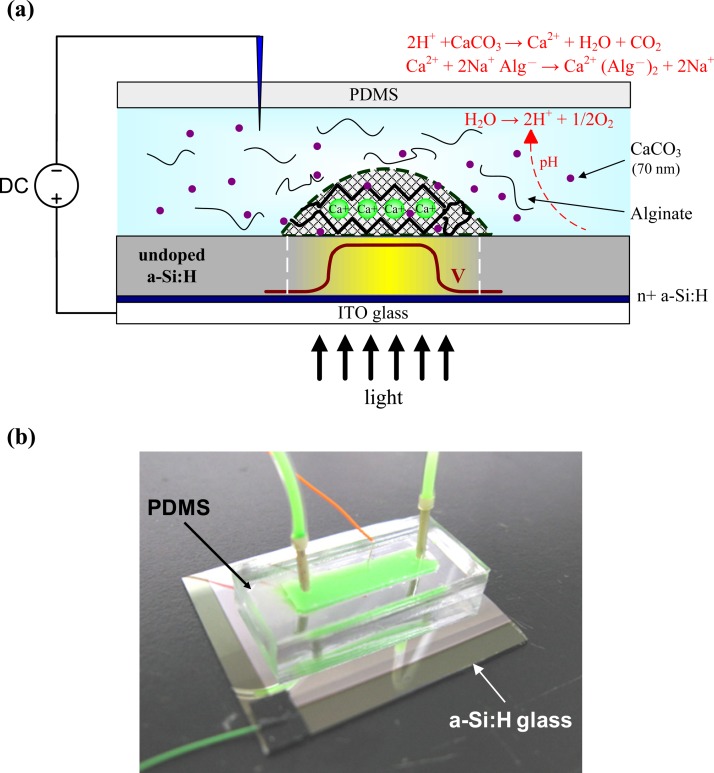
Figure 1 shows a light-addressable electrolytic system used to perform an electrodeposition of calcium alginate hydrogels using a DMD. A photoconductive substrate, which consists of 0.2 μm of heavily doped hydrogenated amorphous silicon (n+ a-Si:H) and 1 μm of undoped a-Si:H on a 700-μm indium tin oxide (ITO) glass substrate, serves as a light-addressable electrode. A microchamber either 0.5 or 1 mm in height was fabricated of polydimethylsiloxane (PDMS) and then bonded to the photoconductive substrate. The deposition solution, which contains soluble sodium alginate (80–120 mPa·S, Sigma-Aldrich) plus insoluble calcium carbonate (CaCO3) nanoparticles (70 nm, Specialty Minerals, United Kingdom), was introduced into the microchamber [Fig. 1a]. We used CaCO3 nanoparticles (70 nm in diameter) as the calcium complex to drastically increase the sedimentation time to over 10 min and to ensure a homogeneous dispersion within the microchamber, which is important for the production of calcium alginate hydrogels with high shape fidelity and small sizes of less than 100 μm.6 An anodic voltage was applied to the photoconductive substrate via the ITO layer, and metallic or platinum wire was inserted into the microchamber to serve as the cathode. Figure 1b shows the image of the PDMS microchamber bonded to the photoconductive substrate with a metallic wire inserted into the microchamber as the cathode (green dye was introduced into the microchamber for visibility purposes). When a DC voltage is applied concurrently, light illumination on a photoconductive substrate generates a conducting point that acts as a photo-anode to electrolytically produce protons, which can lead to a decreased pH gradient. The low pH generated at the anode can locally release calcium ions (Ca2+) from insoluble CaCO3 nanoparticles Eq. 1 and cause gelation of the calcium alginate through sol-gel transition Eq. 2.
| (1) |
| (2) |
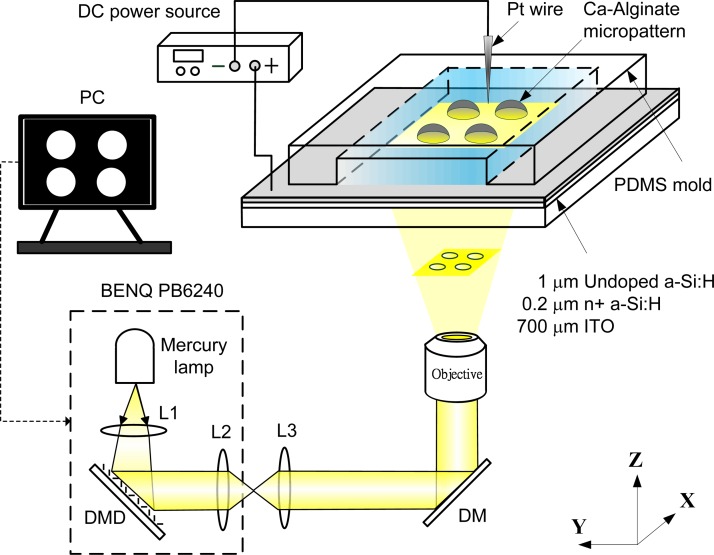
Figure 2 shows an experimental setup for a light-addressable electrolytic system for performing electrodeposition of calcium alginate hydrogels. This setup uses a modified commercial DMD projector (BENQ PB6240) that is equipped with a mercury lamp unit as the light source. We modified the commercial DMD projector by simply removing the original lamp and the color filter wheel.18 The original projection lens was replaced with a commercial projection lens (ENL20853/100, JML) with a suitable focal length and adjustable apertures. The structured light patterns of the DMD were controlled by a computer. The continuous light illuminated the DMD uniformly through the built-in condensing lens (L1) within the DMD projector and then spatially projected it onto the photoconductive substrate through a projection lens (L2), a focus lens (L3), a 50/50 dichroic mirror (DM), and an objective lens with 10X magnification. An anodic voltage was applied to the photoconductive substrate via a DC power source (50 W, HP/Agilent 6613C), and metallic wire served as the cathode. Focal light illumination locally increases the conductivity of the photoconductive substrate and thus creates a virtual photo-electrode with flexible addressability and patternability, which is a substitute candidate for conventional metal microelectrodes. We can control the illumination pattern on the DMD, which enables the performance of an electrodeposition of calcium alginate hydrogels with different shapes and sizes and multiplexed micropatterning.
Figure 1.
(a) Schematic diagram of a light-addressable electrolytic system for performing the electrodeposition of calcium alginate hydrogels using a DMD. (b) An image of the PDMS microchamber bonded to the photoconductive substrate with a metallic wire inserted into the microchamber as the cathode (green dye was introduced into the microchamber for visibility purposes).
Figure 2.
Schematic diagram of an experimental setup for a light-addressable electrolytic system for performing the electrodeposition of calcium alginate hydrogels. A modified commercial DMD projector (BENQ PB6240) that is equipped with a mercury lamp unit as the light source. L1: condensing lens, L2: projection lens, L3: focus lens, and DM: dichroic mirror.
EXPERIMENTAL RESULTS AND DISCUSSION
Light-addressable electrodeposition of alginate hydrogels
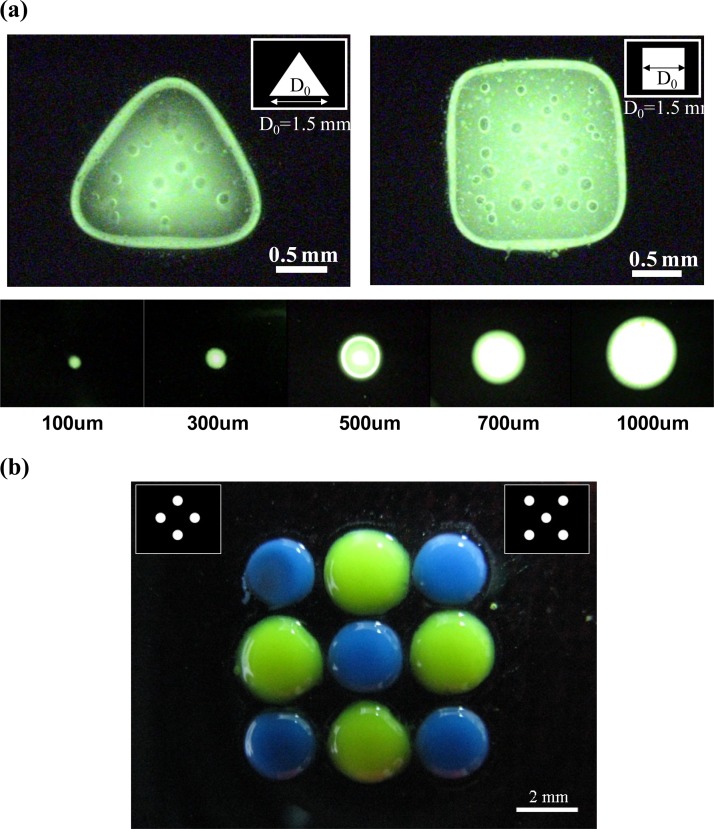
Flexible addressability and patternability of photo-electrodes would allow the generation of desired alginate hydrogel patterns at a specific address, providing a great advantage over the electroaddressing of calcium alginate hydrogels using conventional metal microelectrodes.13 To demonstrate the feasibility of the light-addressable electrolytic system for performing the electrodeposition of calcium alginate hydrogels, calcium alginate hydrogels of different shapes and sizes and with multiplexed micropatterning were micropatterned, as shown in Fig. 3. In these experiments, the insoluble CaCO3 powder (70-nm particles; 0.5 wt. %) was blended into a sodium alginate (1.0 wt. %) solution and sonicated for 10 min. FITC fluorescent dye (Sigma-Aldrich) was also blended into the deposition solution, so that it was easy to observe. Then, the deposition solution was introduced into the microchamber. A DC voltage was applied between the photoconductive substrate and metallic wire for 15 s to achieve a current density of 180 A m−2 (typical voltage was about 10 V). A high current density with a short reaction time was used to prevent destruction of the photoconductive layer. The illumination patterns on the DMD can be either triangular or square shapes with a characteristic length of D0 = 1.5 mm or circular shapes with different diameters ranging from 100 to 1000 μm, as shown in Fig. 3a. The shaped light pattern was then projected onto the photoconductive substrate to electrolytically produce protons for the electrodeposition of calcium alginate hydrogels. After 15 s, we turned off the light pattern and DC power supply and immediately introduced deionized (DI) water into the microchamber to flush away the deposition solution. The calcium alginate hydrogels deposited on the photoconductive layer were imaged using a fluorescence microscope (Olympus IX-71) connected to a digital camera (WAT-221s, Watec) to examine the shape fidelity between the alginate hydrogels produced by the process and the illuminated DMD. As shown in Fig. 3a, the produced calcium alginate hydrogels showed a high shape fidelity to the illumination patterns. These calcium alginate hydrogels contained several trapped bubbles due to the generation of CO2 during electrolysis, as indicated in Eq. 1. To demonstrate the dimension limitation of the produced alginate hydrogels for our proposed method, we projected an illumination pattern of circular shapes with different diameters ranging from 100 to 1000 μm that was passed through an objective lens with 10X magnification. As shown in Fig. 3a, we can successfully produce 100-μm-sized alginate hydrogels on the photoconductive substrate after flushing with DI water. However, the alginate hydrogels smaller than 100 μm were flushed away from the photoconductive substrate due to poor adhesion between the alginate hydrogels and the photoconductive substrate. This problem might be overcome by treating the surface of the photoconductive substrate with poly-L-lysine (PLL). The positively charged PLL can promote the attachment of the negatively charged polysaccharide constituent of alginate upon gelation.11 We also demonstrated the capability for multiplexed micropatterning of calcium alginate hydrogels by performing light-addressable electrodepositions sequentially, as shown in Fig. 3b. Two different deposition solution colors were electrodeposited using two different illumination light patterns. We first performed an electrodeposition of alginate hydrogels with a green color using an illumination pattern with four circles, and then we repeated the electrodeposition to form alginate hydrogels with a blue color adjacent to the former using an illumination pattern with five circles.
Figure 3.
(a) Images of calcium alginate hydrogels with triangular and square shapes with characteristic lengths of D0 = 1.5 mm and circular shapes with diameters ranging from 100 to 1000 μm. (b) An image of multiplexed micropatterning of calcium alginate hydrogels created by performing a light-addressable electrodeposition using two different illumination light patterns in sequence.
Dissolution and re-formation of alginate hydrogels using light-addressable electrodeposition
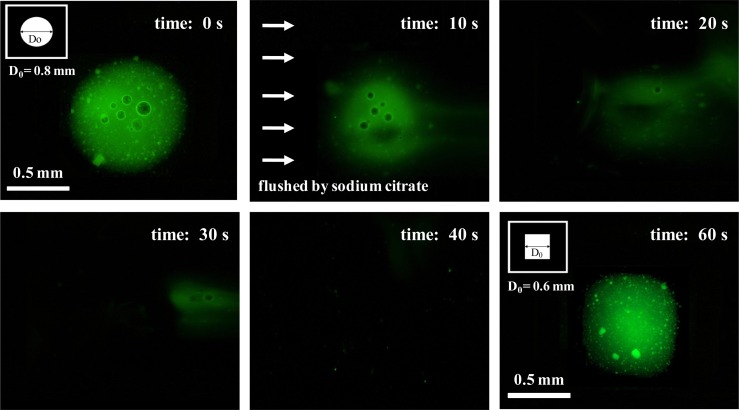
The ability to dissolve the hydrogels is very useful, as we can release the encapsulated materials after observation/screening. This will allow post-analysis (for instance, DNA analysis of cells) to be carried out if desired. It is well known that the formation of calcium alginate hydrogel by the reaction of Ca2+ with sodium alginate is reversible.13 When a suitable calcium sequestrant is added, the Ca2+ is removed and the hydrogel structure collapses. This reversibility also allows us to rinse and reset the device, resulting in a reusable platform. To demonstrate this ability, we first performed a light-addressable electrodeposition to form alginate hydrogels with a circular shape, then dissolved the alginate hydrogels using sodium citrate, and then re-formed alginate hydrogels with a square shape, as shown in Fig. 4. We first performed an electrodeposition of alginate hydrogels with a circular shape of D0 = 0.8 mm in diameter at a time equal to 0 s. When a solution of sodium citrate (500 mm) was introduced into the device at a flow rate of 3 ml/min, the formed alginate hydrogel was rapidly dissolved by a time of 10 s and gradually removed after approximately 40 s. After completing the removal of the alginate hydrogel, we then performed a secondary electrodeposition of alginate hydrogels with a square shape of D0 = 0.6 mm in width at a time equal to 60 s. The results demonstrate the ability to reset the device for reusable platforms and to achieve flexible photo-electrode patterns for dynamical and multiplexed micropatterning of alginate hydrogels by the spatio-temporal switching of illumination patterns through the DMD.
Figure 4.
Images of the sequence in which the light-addressable electrodeposition is performed to form alginate hydrogels with a circular shape, the alginate hydrogels are dissolved using sodium citrate, and then the alginate hydrogels are reformed with a square shape.
Characteristics of light-addressable electrodeposition of alginate hydrogels
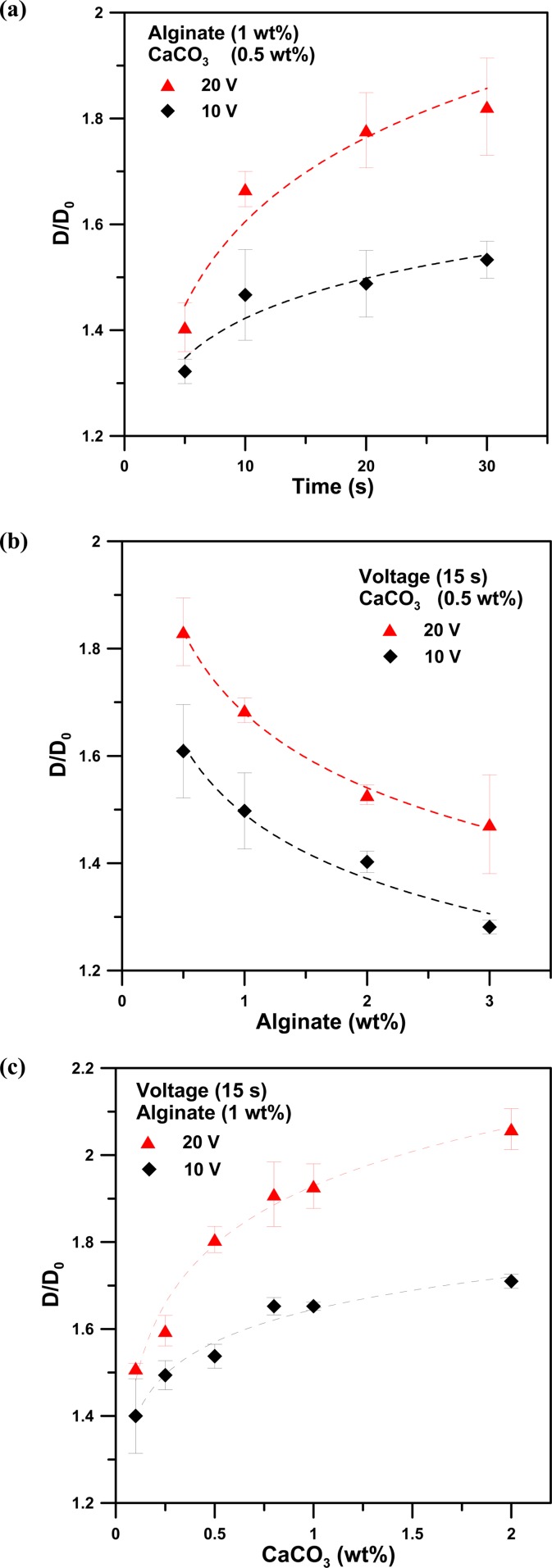
To characterize the light-addressable electrodeposition of alginate hydrogels, we examined the effect of the concentration of the alginate and CaCO3 solutions on the dimensional resolution of calcium alginate formation, as shown in Fig. 5. The ratio of D/D0 is defined as the dimensional resolution, where Do and D denotes an illumination pattern of circular shapes with D0 = 600 μm in diameter and the corresponding diameter (D) of the calcium alginate hydrogel produced by the illumination pattern, respectively. Figure 5a shows the effect of the illumination time of the light pattern on the dimensional resolution (D/D0) for the deposition solution (1% alginate; 0.5% CaCO3) with applied voltages of 10 and 20 V. The data indicate the average value of the five experiments, and the error bar shows the standard deviation at the same operating conditions. The ratio of D/D0 increases with increasing illumination time for applied voltages of both 10 and 20 V, whereas for a fixed illumination time the D/Do for an applied voltage of 20 V is larger than that for an applied voltage of 10 V. These results indicate that an increase in the illumination time or in the applied voltage for electrolysis results in an increase in the calcium ions released from the insoluble CaCO3 nanoparticles. The calcium ions diffuse and then react with the alginate solution to produce alginate hydrogels of larger sizes (D). These results demonstrate that alginate hydrogels of varying sizes can be controllably electrodeposited by changing either the illumination time or the applied voltages.
Figure 5.
(a) The effect of the illumination time of the light pattern on the dimensional resolution (D/D0) for a deposition solution (1% alginate; 0.5% CaCO3) with applied voltages of 10 and 20 V. The effect of (b) the concentrations of alginate solutions with 0.5% CaCO3 and (c) the concentrations of CaCO3 nanoparticles dispersed within 1 wt. % alginate solutions on the dimensional resolution (D/D0) for a deposition solution with applied voltages of 10 and 20 V, for an illumination time of 15 s. (D0 and D denote the illumination pattern of circular shapes with D0 = 600 μm in diameter and the corresponding diameter (D) of the calcium alginate hydrogels produced by the illumination pattern).
Figure 5b shows the effect of the concentration of an alginate solution with 0.5% CaCO3 on the dimensional resolution (D/D0) for a deposition solution with an applied voltage of 10 or 20 V and an illumination time of 15 s. The ratio of D/Do decreases as the concentration of the alginate solution increases for applied voltages of both 10 and 20 V. We attribute this trend to the viscosity effect; the increase in the alginate concentration leads to an increase in the viscosity of the deposition solution, which hinders the diffusion of the produced Ca2+ ions within the illumination area during formation of alginate hydrogels. As the alginate concentration increases, the value of D/Do approaches 1.0, indicating high shape fidelity between the illumination pattern and the produced alginate hydrogels. However, for a concentration of the alginate solution higher than 4 wt. %, the deposition solution is too viscous for the CaCO3 nanoparticles to be homogeneously dispersed, which can significantly influence the formation of alginate hydrogels. Figure 5c shows the effect of the concentration of CaCO3 nanoparticles dispersed within a 1 wt. % alginate solution on the dimensional resolution (D/D0) for a deposition solution with applied voltages of 10 and 20 V and an illumination time of 15 s. The results show that the D/Do increases as the concentration of CaCO3 nanoparticles increases for applied voltages of both 10 and 20 V. Increasing the concentration of CaCO3 nanoparticles can result in more Ca2+ ions being released from the insoluble CaCO3 nanoparticles during electrolysis. The calcium ions diffuse and then react with the alginate solution to produce larger-sized alginate hydrogels. However, as the concentration of CaCO3 nanoparticles increases past 1 wt. %, the D/D0 gradually increases to a constant value, such as D/D0 = 1.6 and 2.0 for applied voltages of 10 and 20 V, respectively. This might be due to the fact that the low pH generated at the photo-anode, which was operated at a fixed applied voltage and illumination time, can release only a certain amount of Ca2+ ions from the insoluble calcium carbonate (CaCO3). Increasing the concentration of CaCO3 nanoparticles over a critical value cannot further increase the number of Ca2+ ions released from the insoluble CaCO3 nanoparticles to cause gelation of the alginate hydrogel.
Light-addressable electrodeposition of cell-encapsulated alginate hydrogels
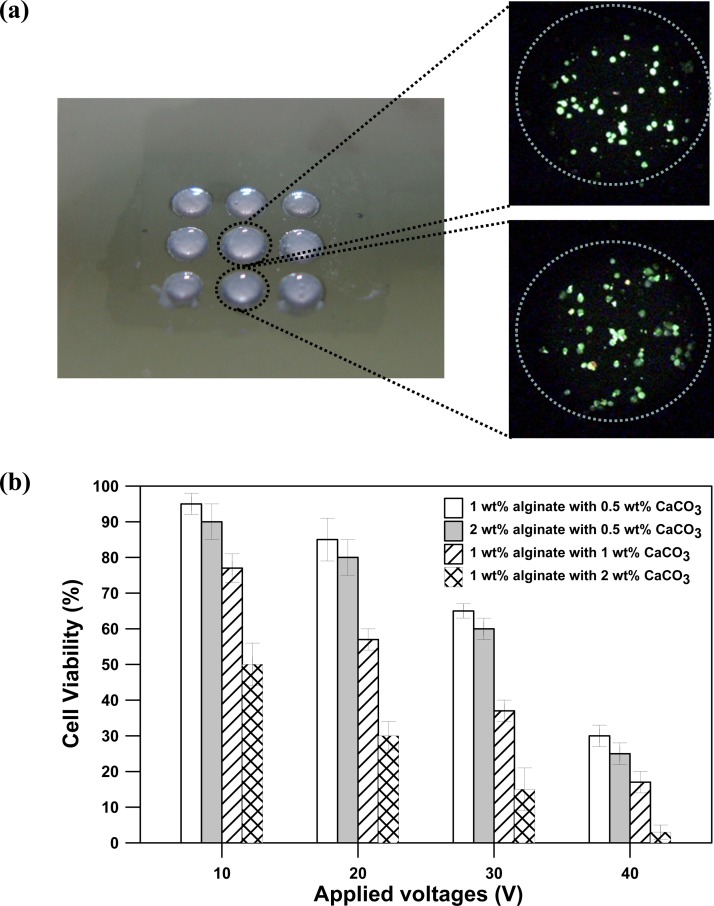
We demonstrated the potential for light-addressable electrodeposition of cell-encapsulated alginate hydrogels for 3D cellular microarrays as shown in Fig. 6. Baby hamster kidney-21 fibroblast cells (BHK-21) were homogeneously suspended in deposition solutions of either 1or 2 wt. % sodium alginate solution with CaCO3 concentrations of 0.5, 1 and 2 wt. %. The concentrations of 1% and 2% for the alginate solution were chosen for this experiment because they are optimal concentrations with suitably low viscosities for facilitating the homogeneous dispersion of BHK-21 cells and CaCO3 nanoparticles within the deposition solutions. Figure 6a shows a 3 × 3 array of cell-encapsulated alginate hydrogels produced through light-addressable electrodeposition. Cell viability was determined using a live/dead assay (Invitrogen, CA) containing calcein AM (live cells, green) and ethidium homodimer (dead cells, red). Cell-encapsulated alginate hydrogels were incubated for 1 h after electrodeposition, after which they were stained by incubation with the live/dead assay agents for 10 min to allow the stain to diffuse into the hydrogels. The stain was removed by washing the hydrogels with culture media before the hydrogels were imaged. The average cell viability percentage was calculated by counting the number of pixels in the green (living cells) channel from three different experiments.
Figure 6.
(a) An image of a 3 × 3 array of cell-encapsulated alginate hydrogels produced through light-addressable electrodeposition. (b) The average cell viability percentage of cell-encapsulated alginate hydrogels at either 1 or 2 wt. % sodium alginate with 0.5–2.0 wt. % CaCO3 concentrations and applied voltages of 1–40 V for 15 s.
Figure 6b shows the average percentage of viable cells for cell-encapsulated alginate hydrogels from either 1 or 2 wt. % sodium alginate with 0.5–2.0 wt. % CaCO3 concentrations and voltages of 1–40 V that were applied for 15 s. Cell viability for either 1or 2 wt. % sodium alginate with 0.5 wt. % CaCO3 was found to be high (>90%) at 10 V for 15 s, but significantly decreased for both concentrations with increasing the applied voltage from 20 to 40 V. We attribute this phenomenon to two contributing factors: (a) low pH generation and (b) increase in the Ca2+ ions. Increasing the applied voltage can prompt the electrolysis to produce more protons, which can lead to a decreased pH. The low pH can decrease cell viability. The low pH also induces more Ca2+ ions to be released from the insoluble CaCO3 nanoparticles. The increase of Ca2+ ions results in cytotoxicity, which decreases cell viability. Note that for 1 or 2 wt. % sodium alginate mixed with 0.5 wt. % CaCO3 there is no apparent change in the cell viabilities for applied voltages from 20 to 40 V. However, for 1 wt. % sodium alginate at a fixed applied voltage, the cell viabilities decreased as the CaCO3 concentration increased from 0.5 to 2 wt. %. For example, the cell viabilities for 1 wt. % sodium alginate with 0.5, 1, and 2 wt. % CaCO3 at 10 V are 95%, 77%, and 52%, respectively. Increasing the concentration of CaCO3 nanoparticles can result in the release of more Ca2+ ions from the insoluble CaCO3 nanoparticles during electrolysis, which results in cytotoxicity and decreases cell viability.
CONCLUSION
In this study, we proposed a novel electrolytic system for performing an electrodeposition of calcium alginate hydrogels at desired locations on a photoconductive electrode substrate. Light illumination spatially patterned by the DMD produces a virtual photo-anode (i.e., a pH decrease) that triggers a localized release of Ca2+ from insoluble CaCO3 nanoparticles to cause gelation of the calcium alginate. Flexible addressability, or patternability of the photo-electrodes, would allow the generation of desired alginate hydrogel patterns at a specific address, providing a great advantage over the electroaddressing of calcium alginate hydrogels using conventional metal microelectrodes. We demonstrate the ability to perform micropatterning of calcium alginate hydrogels with different shapes and sizes and with multiplexed micropatterning. A 3 × 3 array of cell-encapsulated alginate hydrogels was also successfully created without destroying viability through light-addressable electrodeposition. We also examine the effects of varying the concentration of the alginate and CaCO3 solutions on the dimensional resolution of alginate hydrogel formation and the cell viability of cell-encapsulated alginate hydrogels after light-addressable electrodeposition. Our proposed electrolytic system does not need metal microelectrodes and provides both a reagentless method and the flexible electroaddressing of cell-encapsulated alginate hydrogels into cellular microarrays inside a microfluidic chamber or channel. We anticipate that this simple, rapid, and flexible method for light-addressable electrodeposition of cell populations into cellular microarrays could have a wide range of applications in drug discovery, toxicology, stem cell research, and potentially therapy.
ACKNOWLEDGMENTS
This work was partially supported by the National Science Council, Taiwan, through Grant No. NSC 99-2627-B-019-003.
References
- Fernandes T. G., Diogo M. M., Clark D. S., Dordick J. S., and Cabral J. M. S., Trends Biotechnol. 27, 342 (2009). 10.1016/j.tibtech.2009.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y. C., Chuang Y. S., Chen Y. Y., Shu A. C., Hsu H. Y., Chang H. Y., and Yew T. R., Biosens. Bioelectron. 23, 1856 (2008). 10.1016/j.bios.2008.03.005 [DOI] [PubMed] [Google Scholar]
- Nie Z. H. and Kumacheva E., Nature Mater. 7, 277 (2008). 10.1038/nmat2109 [DOI] [PubMed] [Google Scholar]
- Xu T., Petridou S., Lee E. H., Roth E. A., Vyavahare N. R., Hickman J. J., and Boland T., Biotechnol. Bioeng. 85, 29 (2004). 10.1002/bit.v85:1 [DOI] [PubMed] [Google Scholar]
- Flickinger M. C., Schottel J. L., Bond D. R., Aksan A., and Scriven L. E., Biotechnol. Prog. 23, 2 (2007). 10.1021/bp060347r [DOI] [PubMed] [Google Scholar]
- Tan W. H. and Takeuchi S., Adv. Mater. 19, 2696 (2007). 10.1002/adma.v19:18 [DOI] [Google Scholar]
- Workman V. L., Dunnett S. B., Kille P., and Palmer D. D., Macromol. Rapid Commun. 29, 165 (2008). 10.1002/marc.v29:2 [DOI] [Google Scholar]
- Capretto L., Mazzitelli S., Balestra C., Tosi A., and Nastruzzi C., Lab Chip 8, 617 (2008). 10.1039/b714876c [DOI] [PubMed] [Google Scholar]
- Lee K. H., Shin S. J., Park Y., and Lee S. H., Small 5, 1264 (2009). 10.1002/smll.200801667 [DOI] [PubMed] [Google Scholar]
- Shin S. J., Park J. Y., Lee J. Y., Park H., Park Y. D., Lee K. B., Whang C. M., and Lee S. H., Langmuir 23, 9104 (2007). 10.1021/la700818q [DOI] [PubMed] [Google Scholar]
- Lee M. Y., Kumar R. A., Sukumaran S. M., Hogg M. G., Clark D. S., and Dordick J. S., Proc. Natl. Acad. Sci. U.S.A. 105, 59 (2008). 10.1073/pnas.0708756105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes T. G., Kwon S. J., Bale. S. S.., Lee M. Y., Diogo M. M., Clark D. S., Cabral J. M.S., and Dordic J. S., Biotechnol. Bioeng. 106, 106 (2010). 10.1002/bit.22661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X. W., Tsao C. Y., X. Y., Liu Y., Dykstra P., Rubloff G. W., Ghodssi R., Bentley W. E., and Payne G. F., Adv. Funct. Mater. 19, 2074 (2009). 10.1002/adfm.200900026 [DOI] [Google Scholar]
- Liu Y., Kim E., Ulijn R. V., Bentley W. E., and Payne G. F., Adv. Funct. Mater. 21, 1575 (2011). 10.1002/adfm.201002020 [DOI] [Google Scholar]
- Liu Y., Kim E., Ghodssi R., Rubloff G. W., Culver J. N., Bentley W. E., and Payne G. F., Biofabrication 2, 022002 (2010). 10.1088/1758-5082/2/2/022002 [DOI] [PubMed] [Google Scholar]
- Hyunmin Y., Wu L. Q., Bentley W. E., Ghodssi R., Rubloff G. W., Culver J. N., and Payne G. F., Biomacromolecules 6, 2881 (2005). 10.1021/bm050410l [DOI] [PubMed] [Google Scholar]
- Koev S. T., Dykstra P. H., Luo X., Rubloff G. W., Bentley W. E., Payne G. F., and Ghodssi R., Lab Chip 10, 3026 (2010). 10.1039/c0lc00047g [DOI] [PubMed] [Google Scholar]
- Huang S. H., Tsai C. H., Wu C. C., and Wu C. J., Sensors and Actuators, A 165, 139 (2011). 10.1016/j.sna.2010.10.011 [DOI] [Google Scholar]