Each aspect of sleep is a highly complex phenotype and little is currently known about their molecular bases. Nevertheless, genetic studies in model organisms have uncovered sleep regulatory mechanisms and distinct neurochemical processes that are conserved from Drosophila to rodents to humans.1 Although no dedicated “sleep genes” have been identified (if they exist), the adenosine neuromodulator/receptor system is believed to play an important role in sleep and sleep-wake regulation. Support for this hypothesis comes from the finding that the wake-promoting effects of caffeine, the major stimulant in the world, are mediated by antagonistic interaction with adenosine A1 and A2A receptors.2,3
Since people drink coffee, it is well-known that some individuals are sensitive to its stimulant effects whereas some others are not. The behavioral actions of caffeine in humans were first studied scientifically 100 years ago by Harry Levi Hollingworth.4 Hollingworth's studies set a new standard in psychopharmacological research because for the first time, they included double-blind and placebo-controlled experimental design. With respect to sleep disturbances, he concluded that “a few individuals show complete resistance to the effects of small doses of caffeine” (p. 100). Until recently, the biological reasons for these inter-individual differences remained unknown. Now we know they have a basis in genetics.
Because no consistent differences in caffeine pharmacokinetics were found between caffeine sensitive and insensitive subjects, Goldstein and colleagues proposed that endogenous diversity at the site of action of caffeine could influence its effects on sleep.5 Work in mice provided strong evidence that the stimulant promotes wakefulness primarily by blocking the A2A subtype of adenosine receptors.6 Thus, a moderate dose of caffeine (15 mg/kg) failed to disrupt sleep in mice with genetically abolished A2A receptor function. Conversely, the stimulant potently promoted wakefulness by 3-4 hours in wild-type animals, as well as in transgenic mice without functional A1 receptors. In accordance with these findings, a pharmacogenetic study in humans also suggested that common variation of the A2A receptor gene (ADORA2A) contributes to individual sensitivity to caffeine effects on sleep.7 More than 20,000 individuals were addressed with a brief questionnaire about self-rated caffeine sensitivity and sleep, and 4,329 people responded. Caffeine consumption was associated with subjectively reduced sleep quality in caffeine-sensitive respondents, but not in caffeine-insensitive respondents. Moreover, the distribution of individuals carrying C/C and T/T alleles of the c.1976T > C single nucleotide polymorphism (SNP) of ADORA2A (SNP-ID: rs5751876) differed between caffeine-sensitive and caffeine-insensitive individuals. Double-blind administration of the stimulant (2 × 200 mg) confirmed the classification of caffeine sensitivity based on questionnaire, whereas caffeine concentration in saliva did not differ. Intriguingly, the stimulant induced sleep EEG characteristics of insomnia in genotype-dependent manner. The results strongly suggested that genetic variation of ADORA2A is a determinant of individual sensitivity to subjective and objective effects of caffeine on sleep.
Independent replication is essential for establishing a credible genotype-phenotype association.8,9 In this issue of SLEEP, Byrne and colleagues10 report the independent confirmation of a role for ADORA2A in caffeine-related sleep disturbances. These authors conducted a genome-wide association study (GWAS) in a large number of twins and their families of the Australian Twin Registry (n = 2,402). More than 2 million common SNPs were examined. Caffeine-associated sleep disturbance was based on the participants' report of whether or not they have ever experienced caffeine-induced insomnia, statistically corrected by a “general insomnia factor score” derived from a questionnaire. Although no single SNP reached the stringent threshold of genome-wide significance (P < 7.2 × 10−8), a few genes showed evidence for meaningful association with caffeine-induced insomnia.
Notably, the previously suggested association between genetic variation of ADORA2A and disturbed sleep after caffeine was successfully replicated. This finding is remarkable in the genetics of complex traits because only a small minority of candidate genes has typically been confirmed by GWAS.9 Although the original SNP (rs5751876) was not typed in the present sample, it forms a perfect linkage-disequilibrium with several SNPs of ADORA2A that significantly affect caffeine-induced sleep disturbance.10 Apart from ADORA2A, some other “suggestive hits” may be targeted for future replication. Among the most interesting of them is MTNR1B, which codes for high-affinity melatonin MT2 receptors. Genetic variants of MTNR1B modulate fasting blood glucose concentrations11 and may contribute to the intriguing yet poorly understood relationships among caffeine consumption, short duration and poor quality of sleep, and the risk to develop type-2 diabetes.12
Rétey et al.7 combined self-reports and polysomnography after double-blind caffeine administration, to document individual differences in the effects of caffeine on sleep. By contrast, the present work was restricted to self-classification of caffeine sensitivity. The successful replication of the previously reported association with this less accurate and less reliable (i.e., subjective) phenotype indicates that questionnaires are useful as an initial step in large-scale epidemiological studies, which are followed-up by physiological studies aimed to provide insights into the molecular bases of sleep-wake regulation (e.g., pharmacogenetics). For example, such an approach may lead to a better understanding of individual vulnerability to sleep deprivation, which is intensively investigated in sleep research. Interestingly, caffeine sensitive and insensitive individuals appear to be differently affected by sleep loss.13 Together with the present results, this observation suggests that genetic variants of ADORA2A may alter the accumulation of homeostatically regulated sleep propensity during prolonged wakefulness. Convergent findings in mice14 and humans15 are consistent with this notion. They indicate that the sleep deprivation-induced rebound of EEG delta activity in NREM sleep, the most reliable marker of sleep homeostasis, depends on the functional state of A2A receptors (Figure 1).
Figure 1.
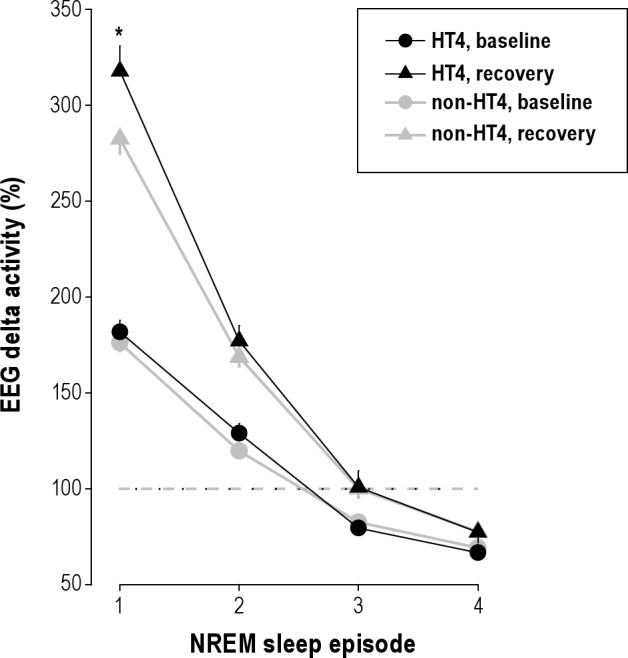
Genetic variation of ADORA2A modulates the rebound of EEG delta oscillations (0.75-4.5 Hz) after sleep loss. Delta activity in baseline (circles) and recovery (triangles) nights across consecutive NREM sleep episodes was expressed as a percentage of the all-night value in baseline (NREM sleep stages 1-4, horizontal dashed line). Data represent means ± SEM in carriers of HT4 (black symbols, n = 14) and non-HT4 haplotype (gray symbols, n = 31) alleles of ADORA2A. Forty hours prolonged wakefulness induced a larger relative rebound in delta activity in HT4 haplotype than in non-HT4 haplotype (“haplotype” × “night” × “NREM sleep episode”: F6,125 = 68.95, P < 0.0001). *P < 0.02 (HT4 vs. non-HT4, unpaired 2-tailed t-test). Figure corresponds to supplementary Figure S3 in15 (re-plotted with permission).
One century after Hollingworth, pharmacogenetic studies of caffeine not only revealed insights into a distinct molecular contribution to individual caffeine sensitivity, but also indicate that A2A receptors are part of a biological pathway that regulates sleep in mammals. These findings could have important implications for the pathophysiology and the rational treatment of insomnia, as well as for recommendations for the critical use of caffeine, which is consumed on a daily basis by up to 90% of adults in western societies.
DISCLOSURE STATEMENT
The author's research is supported by the Swiss National Science Foundation; there are no competing interests to declare.
CITATION
Landolt HP. “No thanks, coffee keeps me awake”: individual caffeine sensitivity depends on ADORA2A genotype. SLEEP 2012;35(7):899-900.
REFERENCES
- 1.Andretic R, Franken P, Tafti M. Genetics of Sleep. Annu Rev Genet. 2008;42:361–88. doi: 10.1146/annurev.genet.42.110807.091541. [DOI] [PubMed] [Google Scholar]
- 2.Fredholm BB. Adenosine, adenosine receptor and the action of caffeine. Pharmacol Toxicol. 1995;76:93–101. doi: 10.1111/j.1600-0773.1995.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 3.Landolt HP. Sleep homeostasis: A role for adenosine in humans? Biochem Pharmacol. 2008;75:2070–9. doi: 10.1016/j.bcp.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 4.Hollingworth HL. The influence of caffein alkaloid on the quality and amount of sleep. Am J Psychol. 1912;23:89–100. [Google Scholar]
- 5.Goldstein A, Warren R, Kaizer S. Psychotropic effects of caffeine in man. I. Individual differences in sensitivity to caffeine-induced wakefulness. J Pharmacol Exp Ther. 1965;149:156–9. [PubMed] [Google Scholar]
- 6.Huang ZL, Qu WM, Eguchi N, et al. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci. 2005;8:858–9. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- 7.Rétey JV, Adam M, Khatami R, et al. Genetic variation in the adenosine A2A receptor gene (ADORA2A) contributes to individual sensitivity to caffeine effects on sleep. Clin Pharmacol Ther. 2007;81:692–8. doi: 10.1038/sj.clpt.6100102. [DOI] [PubMed] [Google Scholar]
- 8.Chanock SJ, Manolio T, Boehnke M, et al. Replicating genotype-phenotype associations. Nature. 2007;447:655–60. doi: 10.1038/447655a. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy MI, Abecasis GR, Cardon LR, et al. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat Rev Genet. 2008;9:356–69. doi: 10.1038/nrg2344. [DOI] [PubMed] [Google Scholar]
- 10.Byrne EM, Johnson J, McRae AF, et al. A genome-wide association study of caffeine-related sleep disturbance: conformation of a role for a common variant in the adenosine receptor. Sleep. 2012;35:967–975. doi: 10.5665/sleep.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prokopenko I, Langenberg C, Florez JC, et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009;41:77–81. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferré S, Bach Jensen M, Kempf K, et al. What do you see as the main priorities, opportunities, and challenges in caffeine research in the next five years? J Caffeine Res. 2011;1:5–12. [Google Scholar]
- 13.Rétey JV, Adam M, Gottselig JM, et al. Adenosinergic mechanisms contribute to individual differences in sleep-deprivation induced changes in neurobehavioral function and brain rhythmic activity. J Neurosci. 2006;26:10472–9. doi: 10.1523/JNEUROSCI.1538-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayaishi O, Urade Y, Eguchi N, Huang Z-L. Genes for prostaglandin D synthase and receptor as well as adenosine A2A receptor are involved in the homeostatic regulation of NREM sleep. Arch Ital Biol. 2004;142:533–9. [PubMed] [Google Scholar]
- 15.Bodenmann S, Hohoff C, Freitag C, et al. Polymorphisms of ADORA2A modulate psychomotor vigilance and the effects of caffeine on neurobehavioral performance and sleep EEG after sleep deprivation. Br J Pharmacol. 2012;165:1904–13. doi: 10.1111/j.1476-5381.2011.01689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


