Abstract
Neuroimmunology was once referred to in terms of its pathological connotation only and was generally understood as covering the deleterious involvement of the immune system in various diseases and disorders of the central nervous system (CNS). However, our conception of the function of the immune system in the structure, function, and plasticity of the CNS has undergone a sea change after relevant discoveries over the past two decades, and continues to be challenged by more recent studies of neurodevelopment and cognition. This review summarizes the recent advances in understanding of immune-system participation in the development and functioning of the CNS under physiological conditions. Considering as an example Rett syndrome a devastating neurodevelopmental disease, we offer a hypothesis that might help to explain the part played by immune cells in its etiology, and hence suggests that the immune system might be a feasible therapeutic target for alleviation of some of the symptoms of this and other autism spectrum disorders.
Keywords: Rett syndrome, neuroimmunology, T cells, immune system, neurodevelopment, protective immunity
Function of the immune system in central nervous system injury
The immune system and the central nervous system (CNS) are characterized by more similarities than differences. Both systems learn from experience. Both communicate discrete pieces of information over very short distances through synapses and over very long distances through intricate chemical signaling networks. Although both systems are critical to an organism’s survival, they are commonly regarded as separate entities in terms of their ability to communicate with and influence one another. This systemic segregation, which underlies the concept of the brain’s ‘immune privilege,’ was long believed to be almost absolute. It was only within the last couple of decades that this particularly resilient scientific dogma has been seriously challenged.
Initial steps were taken toward a more nuanced way of thinking about the immune system in terms of its function in relation to such ‘privileged’ parts of the whole organism in pioneering work showing that a well-controlled amplification of the autoimmune response could be correlated with improved neuronal survival in acute CNS injury.1,2 Those studies were followed by others from diverse groups, all implicating immune factors—often autoreactive T cells—as the key to effective repair and maintenance of the CNS.1,3–10 Further studies showed the pivotal function of the innate immune system, implicating such cells as CNS microglia11,12 and peripheral myeloid-derived cells13 in CNS regeneration and neuroprotection.1,3,7,8 Recent data point to even more complex heterogeneity in innate immune activation and function than was originally thought.14,15 Thus, for example, recognition of innate immune-cell phenotypes as pro-inflammatory (M1) or anti-inflammatory (M2)15–17 is attracting increasing interest as the basis for a possible downstream mechanism linking the action of T lymphocytes to healthy CNS function.
Function of the immune system in higher brain functions
It is now generally accepted that the immune system can be benign, even protective,18–20 in the context of a well-controlled response to CNS pathology. Acute injury or inflammation, however, is an entirely different entity from the physiological assembly and maintenance of the complex neural circuits underlying higher brain functions. Thus, it was tantalizing to discover that mice with severe combined immunodeficiency showed marked impairment in behavioral tasks (such as tests of spatial learning and memory in the Morris Water Maze) and, as a corollary, that injection of adult T-cell-deficient mice with wild-type splenocytes improved their spatial learning and memory performance to a level comparable with that of normal mice.21 Transfusion of T-cell-depleted splenocytes had no such effect on cognitive function, indicating that the observed improvement was dependent on the presence of T cells.22 A clue to the nature of the specific T cells needed for such learning and memory was provided by experiments with TMBP and TOVA transgenic mice (respectively engineered to express T cells specific for an epitope of either the myelin basic protein (MBP) autoantigen or the irrelevant antigen ovalbumin (OVA)). When their performance in the Morris Water Maze was compared with that of the wild-type control, the TMBP mice performed no worse and even slightly better than the wild type, suggesting that a single population of T cells reactive to a CNS autoantigen was sufficient to support spatial learning and memory functions. The performance of the TOVA mice, whose T cells lack the capacity to recognize and react with CNS antigen, reflected significant learning impairment. Taken together, the results showed that T cells specific to CNS antigen were key supporters of spatial learning and memory.
Although the function of the healthy brain is clearly affected by the immune system,21,23 no T cells or peripheral myeloid-derived cells are found in the CNS parenchyma under normal physiological conditions. The brain is surrounded, however, by the meninges, which provide a tripartite membranous covering, under which flows cerebrospinal fluid produced by the choroid plexus epithelium.24,25 The cerebrospinal fluid drains into cervical lymph nodes,26 thereby allowing peripheral T cells to respond to CNS antigens. Thus, whereas in the healthy brain, access of immune cells to the CNS parenchyma is restricted, this is not the case for their access to the ventricular choroid plexus and the meninges. These latter tissues are indeed densely populated by myeloid-derived cells such as macrophages and dendritic cells,27,28 and by substantial numbers of T cells.29,30 Therefore, it is reasonable to suggest that neuroimmune interactions affecting learning and memory might originate in the meninges and choroid plexus/ventricular areas rather than in the parenchyma.
Indeed, recent work in our laboratory has shown that immune changes within the meningeal milieu surrounding the CNS have a significant impact on CNS function (Derecki et al., unpublished observations). We showed that T-cell deficiency, either chronic (in severe combined immunodeficiency mice) or acute (pharmaceutically induced), skews meningeal myeloid immunity toward a pro-inflammatory (M1) phenotype. We believe that this skewed pro-inflammatory meningeal response could contribute to the learning and memory impairment seen in the abovementioned experiments on acute and chronic T-cell deficiency. We further show that successful performance of the Morris Water Maze task is accompanied by the accumulation of interleukin (IL)-4 producing (TH2) cells in the meninges, pointing to a critical function for IL-4 in learning and memory (Derecki et al., unpublished observations).
Function of the immune system in CNS development
The amazingly intricate network assembled in the course of wiring the mammalian CNS testifies to the ability of single genes to dictate multiple phenotypic outcomes. Indeed, many genes that were once thought to encode proteins relevant only to the immune system—including cytokines, chemokines, major histocompatibility complex (MHC), and complement factors31–34—are now known to have major functions in the CNS at all stages of development.
Signaling by cytokines is crucial for neural-tube development and neural/glial specification. Expression of bone morphogenetic proteins—members of the transforming growth-factor β superfamily—potently suppresses neural induction unless these proteins are themselves actively repressed.35 This action is facilitated by the factors noggin and chordin,36 and allows Notch-dependent differentiation of neuroepithelial cells into radial glial cells. In subsequent differentiation steps, IL-6 and neuropoietic cytokines, such as leukemia inhibitory factor, ciliary neurotrophic factor, and cardiotrophin 1, turn on or off downstream genes responsible for determining the fate of neuroepithelial cells as radial glial cells, neurons, or glia by signaling through glycoprotein 130 and the transcription-factor STAT3.37 STAT3 signaling is itself regulated by the protein-tyrosine phosphatase SHP2. Interestingly, both STAT3 and SHP2 are important in both the developing and adult immune systems.38 Numerous other cytokines with clearly defined functions in the immune system, including IL-1β, IL-2, -3, -4, -5, -7, -9, and -11, are also expressed in the developing CNS.39 Although the spatial and temporal expression for some of these cytokines are well defined,40,41 insight into their precise developmental functions will require further elucidation.
Immune-derived chemokines in the periphery act through gradients to attract cells bearing cognate receptors. In the CNS, chemokines have been shown to perform in a similar way, thereby regulating neural-cell migration. Several chemokine receptors are expressed in stereotypic patterns in the CNS,31 but for most of them, specific functions remain obscure. This may be due to the promiscuity of most chemokine ligands and receptors, as well as the fact that individual neurons usually express multiple chemokine receptors.42 A notable exception is the CXCL12/CXCR4 pairing, which shows strong mutual selectivity,43 and consequently, much is known about their function in the CNS. Mutant mice lacking either partner of this pair show significant defects in cerebellar44 and hippocampal45 development. The reported finding that meninges are a primary source of CXCL1246 is particularly interesting in view of recent results in our laboratory showing the importance of meningeal immunity for CNS function. One theory postulates that meningeal CXCL12, secreted adjacent to brain parenchyma, maintains cerebellar granule cells in proximity to mitogenic sonic hedgehog before their migration.47 In the mouse hippocampus, meningeal CXCL12 attracts nascent neurons into place in the dentate gyrus.48 CXCL12 and CXCR4 also participate in axonal guidance, interacting with canonical guidance molecules, including semaphorins 3A and C, Robo, and Slit-2.49,50 Consequently, knockout mice show abnormalities in axonal projection.51
Recent work has uncovered unexpected functions in the CNS for MHC-I and complement-factor C1q. MHC-I, a membrane-bound structure found in nucleated cells, is active in the presentation of cytosolic polypeptide (self) fragments to T lymphocytes; cells presenting foreign or unrecognizable polypep-tides are eliminated by the immune system.32 The complement system comprises a family of some 25 small proteins that bind to extracellular immune complexes or plasma-membrane components and mediate the removal of unwanted cells and debris.52 In screening for changes in activity-dependent gene expression in the developing lateral geniculate nucleus, Shatz and coworkers noted that fluctuations in neuronal MHC-I occur synchronously with the synaptic refinement needed to establish visual circuitry in the CNS.34 In line with that observation, mutant mice deficient in MHC-I were found to show significant aberrations in retinogeniculate axonal refinement.53 Accordingly, it was postulated that the function of MHC-I in synaptic elimination may be analogous to its function in the periphery as a mediator of the removal of cells presenting ‘non-self’ antigen.33 The complement-factors C1q and C3 were subsequently identified by Barres and coworkers32 in a similar function in the same pathway. Most recently, it was suggested that the two immune proteins may actually partner one another in achieving synaptic refinement in the lateral geniculate nucleus.54
Immune factors occupy a prominent place in the developing CNS, in which they regulate cellular differentiation and migration, network formation, and—as described above—even synaptic refinement. Thus, immune dysfunction during pre- or post-natal CNS development is likely to have serious ramifications in terms of emergent cognitive functions. Immune mediators might have central or contributory functions in both the etiology and the amelioration of pathologies that manifest themselves during this time—namely, pervasive developmental disorders, such as Rett syndrome (RTT), for example, and other autism spectrum disorders (ASD).
Rett syndrome
RTT is a pervasive developmental disorder that is grouped with the ASD. RTT affects 1 in 8500 females;55 in rare cases, males with X-chromosome aneuploidy or somatic mosaicism have presented with the classical RTT phenotype.56 Patients with RTT have normal psychomotor and head circumference development up to the age of about 5 months, followed by deceleration of head growth accompanied by impaired language development, psychomotor retardation, stereotyped motor deficits and behaviors, and loss of social engagement before the age of 4 years.57 Morphologically, RTT patients exhibit small brains with no evidence of atrophy, a phenotype consistent with a lack of proper development rather than ongoing degeneration. Neurons in RTT patients display decreased dendritic arborization, fewer dendritic spines, and increased packing density.58 Magnetic resonance spectroscopy shows a progressively increasing glia-to-neuron ratio in the white matter, suggesting progressive axonal damage and inflammatory astrocytosis indicative of mild white-matter pathology. In addition, there are indications of increased glutamine–glutamate cycling at the synapse, pointing to increased excitatory neurotrans-mission in younger RTT patients.59
In approximately 80% of cases, RTT is linked to a mutation in the MECP2 gene on the X chromosome. MECP2 encodes a methyl CpG-binding protein that binds to methylated DNA and acts as either a transcriptional activator or a repressor, depending on genetic context.60 Only one mutated X chromo-some is necessary for the disease to manifest, so differences in X-chromosome inactivation among female patients partially explains the variability seen in the disease phenotype.61
Immune system and RTT
The neurological abnormalities in RTT are accompanied by fundamental immunological changes both in the CNS and in the periphery. MECP2, apart from its central function in the etiology of RTT, has been shown to have a contemporaneous function in the development and regulation of the immune system;62–64 notably, it was recently named as a candidate susceptibility gene for the autoimmune disorder systemic lupus erythymatosus.65 Nevertheless, research into immune-system abnormalities in RTT has been limited. In the few such studies conducted to date, however, it has become increasingly clear that mutations in MECP2 not only affect the CNS directly, but also profoundly alter the expression of genes influencing the functional capabilities, activation states, cytokine profiles, and access to the CNS of T lymphocytes.66–70
Gene expression profiles of T cells harboring the MECP2 mutation reflect the complexity inherent in predicting downstream effects of a transcriptional mediator. As an example, T cell clones from RTT patients show decreased mRNA transcripts for T-box 21.66 T-box 21 encodes T-bet, a master transcription factor that controls lineage commitment of CD4+ T-helper cells thereby promoting TH1 differentiation in part by allowing access to the interferon-γ promoter.67 Taken alone, T-bet downregulation would indicate a marked TH2 skew in RTT patients. However, studies have also disclosed increased transcript levels of L-RAP (leukocyte-derived arginine aminopeptidase), an interferon-γ-induced gene,68 as well as of CD6. The latter is transcriptionally regulated by RUNX1/3,71 which inhibits TH2 commitment.72 Thus, the inflammatory skewing effects of MECP2 require further clarification. Notably, CD6 is also a co-stimulatory molecule that participates in T-cell activation and differentiation,71 and its ligand, ALCAM (activated leukocyte cell adhesion molecule), has a function in leukocyte migration across the blood–brain barrier.66,73 Thus, T cells in RTT patients would probably have relatively readier access to the CNS. This would emphasize the critical importance of their cytokine profile, as the data support a destructive function for supranormal levels of pro-inflammatory cytokines in the CNS.74
Although there is general agreement that the total numbers of circulating lymphocytes in RTT patients are normal,69,70 IL-2 receptor (CD25)-positive (canonically activated) T lymphocytes could not be detected in serum from RTT patients,69 and HLA-DR+ T lymphocytes were found to be increased.75 Other groups have reported a decrease in CD8+ T cells and CD57+ natural killer cells and increased serum levels of soluble IL-2 receptor.70 Abnormally large numbers of HLA-DR+ T cells could indicate that a component of RTT is immune dependent, and possibly autoimmune in nature.76
Possible immune etiology and therapeutic intervention for RTT
Whereas initial studies strongly supported an exclusively neuronal function for MECP2 in RTT pathology, it has since been shown that other cells, specifically glia, have a major function in the disease.77,78 Genetic manipulations in mouse models were used to show that specific neuron-restricted expression of wild-type MECP2 is insufficient for rescue of the phenotype;77,79 its pan-neural expression is needed.80 Later studies disclosed that MECP2 is also expressed in glia, and that astrocytes expressing a mutant form of the protein can confer a diseased ‘stunted’ phenotype in wild-type neurons in vitro; the reciprocal experiment showed that wild-type astrocytes could rescue stunted neurons and promote normal neurite development.78 As both astrocytes and microglia provide support for neuronal development, and because these two cell types represent a connection between the adaptive immune system and the CNS parenchyma, it seems reasonable to suggest that glial pathology is indicative of larger immune problems implicated in RTT.
Increased numbers of HLA-DR+ T cells together with a striking decrease in IL-2 receptor expression could point to the presence of an autoimmune component of RTT dysfunction and other ASD;76 alternatively, it could be indicative of an anergic T-cell phenotype, which might similarly implicate a potential function for T lymphocytes in RTT. Preliminary data by our group, aimed at characterizing the meningeal immunity of MECP2 mutant mice, are largely in agreement with the published data indicating severe deficits in activation of T lymphocytes in human beings; we observed a similar lack of IL2R expression in the meninges after training in spatial learning and memory assays (Derecki et al., unpublished observations).
Furthermore, dysregulation of the expression of brain-derived neurotrophic factor (BDNF) is a mechanism by which mutations of the MECP2 gene might lead to the neuropathological findings and developmental problems of RTT.81 During experience-dependent neuronal activation, calcium influx and Ca2+ /calmodulin-dependent protein kinase II mediate selective phosphorylation of MECP2 in the brain. This phosphorylation represses the ability of MECP2 to bind to the Bdnf promoter, thus blocking disinhibition of Bdnf transcription during times of neuronal activity and affecting dendritic arborization and spine formation.81 This is particularly interesting in view of the fact that BDNF production is not limited to neurons. In fact, glial cells produce substantial amounts, as do immune cells such as T and B lymphocytes, and monocytes.82
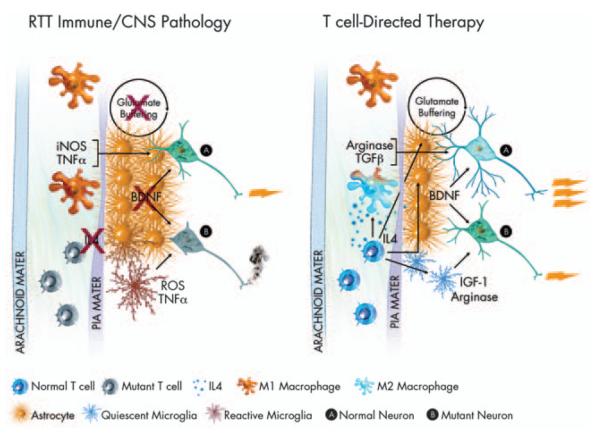
Taking the above observations into consideration, possible immune therapies for RTT might be directed toward modification of glial phenotypes by manipulation of meningeal immunity (Figure 1). The cells most immediately affected by the meningeal cytokines are likely to be astrocytes, which—because of their interaction with the pia mater (the inner membrane of the meninges)—are the neural cells closest to the meninges.24,83 Astrocytes have numerous functions in the brain, including growth-factor production, extra-synaptic glutamate buffering, and water metabolism.84 They also support brain plasticity and synaptogenesis in both the normal and the diseased CNS.85,86 Astrocytes express receptors for most of the known cytokines and have been shown to acquire a neurotoxic phenotype on stimulation with pro-inflammatory cytokines such as IL-1β and tumor necrosis factor-α.87,88 Activation of astrocytes by T cells or anti-inflammatory cytokines, such as IL-4,89,90 results in their better glutamate buffering under normoxic conditions, and restores glutamate buffering under oxidative stress conditions.89,90 Moreover, microglia, which heavily populate the parenchyma (and comprise approximately 25% of the glia limitans, which lies immediately adjacent to the pia mater), respond robustly to cytokine stimulation and accordingly can acquire both protective and destructive phenotypes.91,92 Microglia activated by IL-4 produce insulin-like growth-factor 1,93 a factor that supports cognitive function and neural growth, whereas microglia activated by pro-inflammatory cytokines express large amounts of inducible nitric oxide synthase and other neurodestructive molecules91,94 that could indeed impair learning processes.
Figure 1.
Hypothesis—immune function in RTT. RTT is most often caused by a mutation in the MECP2 gene. As MECP2 is X-linked, because of X-inactivation, some cells in afflicted females express the mutant form of the gene, whereas others do not. In the above illustration, mutant T cells are shown in an inactive or anergic state. Inactive T cells produce low levels of cytokines, in particular IL4. Loss of T-cell modulation of meningeal immunity has been shown to lead to an M1 meningeal myeloid skew. M1 myeloid cells produce high levels of pro-inflammatory neurodestructive cytokines (iNOS, TNF-α)asin turn do reactive microglia. Reactive astrocytes buffer glutamate at reduced levels, and produce substantially diminished levels of BDNF. All these factors combined lead to an environment in CNS impoverished in critical neuronal growth factors, leading to massive dysfunction in mutant neurons (B), and diminished function in normal neurons (A). Amelioration of T-cell function could return homeostasis to the meningeal immune milieu. Normal levels of IL4 would push meningeal myeloid cells to an M2 fate, encouraging production of neuroprotective factors such as TGFβ and arginase. T-cell-derived interferon-γ (not shown) and IL4 would also support improved glutamate buffering and improved BDNF production by astrocytes as well as production of arginase and IGF-1 by microglia. An improved meningeal and glial environment would lead to strong support for normal neurons (A) and mutant neurons (B) alike, thus improved neural function overall and amelioration of RTT. Several immune-related genes (e.g. MHC) have been connected to ASD in linkage analysis studies, thus some ideas expressed in this paradigm can be generalized.
Synaptic stripping, a neuronal phenotype seen in RTT, was recently shown to be mediated by microglia. One interesting suggestion was that synaptic stripping may actually be a neuroprotective microglial response to activation of the immune response in the CNS;14,95 thus, the pathology seen in RTT might be the result of a dysregulated immune response rather than simply neuronal in origin. Magnetic resonance spectroscopy shows a progressively increasing glia-to-neuron ratio in the white matter of RTT patients, suggesting ongoing axonal damage and astrocytosis. In addition, there are indications of increased glutamine–glutamate cycling at the synapse, leading to an excess of extracellular glutamate96 in RTT, which would also be consistent with microglial and astroglial dysfunction. It may be that RTT is a disease not of neurons, but rather of glia, a proposal that is supported by recent evidence.77,78,80 If this is indeed the case, then manipulations at the level of the immune system aimed at modifying microglial phenotype, rather than the neuronally directed therapies considered up to now, might actually offer a better chance of disease amelioration, and additionally obviate a need for direct access to and genetic manipulation of neuronal targets.
Another candidate therapy for amelioration of RTT pathology is the oral administration of copolymer (Cop)-1. Cop-1 is a synthetic polypeptide (4.7–11.0 kDa) comprising four amino acids, l-alanine, l-lysine, l-glutamic acid, and l-tyrosine, in a defined molar ratio. It was originally synthesized in an attempt to mimic the activity of MBP in the induction of experimental autoimmune encephalomyelitis in laboratory animals.97 Serendipitously, it was found to be non-encephalitogenic and actually suppressive of MBP-induced encephalomyelitis.98 Presently, Cop-1 is the most frequently prescribed drug for the treatment of multiple sclerosis. Recent results suggest that Cop-1 induces an M2 phenotype in myeloid-derived cells.99 Strikingly, M2 monocytes in adoptive transfer models can provide robust protection against encephalomyelitis.100 Presumably, therefore, by suitably shaping and regulating both microglial and T-cell phenotypes in the context of neuroinflammation, it might be possible to control CNS inflammation in a location-specific and context-specific manner.
In addition to manifesting anti-inflammatory properties in the CNS, Cop-1 also exhibits neuroprotective activity in acute and chronic neurodegenerative pathologies,101,102 for example in models of Parkinson’s and Alzheimer’s diseases, by driving the uptake of cytotoxic compounds. Recent results in an optic nerve crush injury model showed that Cop-1, when delivered by dendritic cells loaded with the drug ex vivo, exerts a direct neuroprotective effect on mechanically injured neurons.103 Although prevented by its size and charge from traversing the blood–brain barrier on its own, Cop-1 is readily internalized by the dendritic cells and is capable of enhancing their ability to cross the blood–brain barrier and to be released on the parenchymal side. It thus provides a possible route for the entry of Cop-1 into the CNS parenchyma.104
Concluding remarks
Recent discoveries, some of which are outlined in this review, have altered traditional views of the function of immune cells in the CNS and changed our perception of neuroimmunology as a term that covers pathological conditions into one that refers to physiological interactions between the two systems. There is general agreement by now that a well controlled and properly functioning immune system is a prerequisite for normal functioning of the brain. It has yet to be established, however, whether the function of immune cells is restricted to maintenance of CNS homeostasis, or whether the immune system is directly involved in brain function. Thus, for example, in the case of cognitive function, it is not yet known whether the immune system participates actively in learning and memory processes or simply helps the brain to cope with stress and thus allows the learning process to be more efficient. Nevertheless, as clearly shown by findings summarized in this review, we can no longer ignore immune malfunction as a potentially contributory or even causative factor in the etiology of neurodevelopmental, cognitive, and psychiatric diseases. We put forward here the hypothesis that immune malfunction is responsible, at least in part, for several manifestations of RTT. Were the immune system of RTT patients intact (that is bearing the wild-type MECP2 allele), the disease progression might be slowed down and several of the symptoms ameliorated. On the basis of this hypothesis, studies should be aimed at deciphering the function of the immune system in progression of the disease in animal models of RTT, and on the consequent development of immune-based therapies.
Here, we singled out one disease from a wide spectrum of neurodevelopmental syndromes commonly called ASD. A central characteristic of ASD patients seems to be autoimmune dysfunction, as is suggested by several lines of evidence on the basis of data indicative of abnormal T-lymphocyte activation, the presence of CNS autoantibodies, and signs of innate immune-system activation in the CNS. As overall lymphocyte numbers are normal in these patients, the CD4+ population (shown to specifically support cognitive function21,23,105) is diminished. Given the critical functions played in CNS development, organization, and function by cytokines, chemokines, macrophages, and T cells, immune malfunction might well have a function in ASD etiology and hence serve as a future therapeutic target.
Our hypothesis, unlike theories suggesting that a leading factor for ASD development and progression is immune-system overactivation, proposes that abnormal neurodevelopment and function in ASD are an outcome of the lack or malfunction of the relevant T cells, probably autoimmune in nature. Once the function of the immune system in the etiology of these diseases is better understood, bone marrow transplantation and vaccines aimed at boosting immune function might be included in future therapies.
Acknowledgments
We thank Amber Cardani for her critical reading and discussion of the manuscript and Shirley Smith for editing the manuscript. This work was supported in part by NICHD (R21HD056293) and NINDS (R01NS061973) awards to JK, by Training in Neuro-biology and Behavioral Development (T32HD007323) award to NCD, and by Medical Scientist Training Program (T32GM007267) award to EP.
Footnotes
Conflict of interest The authors declare no conflict of interest.
References
- 1.Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen IR, Schwartz M. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- 2.Cohen IR, Schwartz M. Autoimmune maintenance and neuro-protection of the central nervous system. J Neuroimmunol. 1999;100:111–114. doi: 10.1016/s0165-5728(99)00190-3. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz M, Moalem G, Leibowitz-Amit R, Cohen IR. Innate and adaptive immune responses can be beneficial for CNS repair. Trends Neurosci. 1999;22:295–299. doi: 10.1016/s0166-2236(99)01405-8. [DOI] [PubMed] [Google Scholar]
- 4.Serpe CJ, Tetzlaff JE, Coers S, Sanders VM, Jones KJ. Functional recovery after facial nerve crush is delayed in severe combined immunodeficient mice. Brain Behav Immun. 2002;16:808–812. doi: 10.1016/s0889-1591(02)00017-x. [DOI] [PubMed] [Google Scholar]
- 5.Serpe CJ, Coers S, Sanders VM, Jones KJ. CD4+ T, but not CD8+ or B, lymphocytes mediate facial motoneuron survival after facial nerve transection. Brain Behav Immun. 2003;17:393–402. doi: 10.1016/s0889-1591(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 6.Byram SC, Carson MJ, DeBoy CA, Serpe CJ, Sanders VM, Jones KJ. CD4-positive T cell-mediated neuroprotection requires dual compartment antigen presentation. J Neurosci. 2004;24:4333–4339. doi: 10.1523/JNEUROSCI.5276-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hofstetter HH, Sewell DL, Liu F, Sandor M, Forsthuber T, Lehmann PV, et al. Autoreactive T cells promote post-traumatic healing in the central nervous system. J Neuroimmunol. 2003;134:25–34. doi: 10.1016/s0165-5728(02)00358-2. [DOI] [PubMed] [Google Scholar]
- 8.Kipnis J, Schwartz M. Dual action of glatiramer acetate (Cop-1) in the treatment of CNS autoimmune and neurodegenerative disorders. Trends Mol Med. 2002;8:319–323. doi: 10.1016/s1471-4914(02)02373-0. [DOI] [PubMed] [Google Scholar]
- 9.Karman J, Chu HH, Co DO, Seroogy CM, Sandor M, Fabry Z. Dendritic cells amplify T cell-mediated immune responses in the central nervous system. J Immunol. 2006;177:7750–7760. doi: 10.4049/jimmunol.177.11.7750. [DOI] [PubMed] [Google Scholar]
- 10.Frenkel D, Huang Z, Maron R, Koldzic DN, Moskowitz MA, Weiner HL. Neuroprotection by IL-10-producing MOG CD4+ T cells following ischemic stroke. J Neurol Sci. 2005;233:125–132. doi: 10.1016/j.jns.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Butovsky O, Landa G, Kunis G, Ziv Y, Avidan H, Greenberg N, et al. Induction and blockage of oligodendrogenesis by differently activated microglia in an animal model of multiple sclerosis. J Clin Invest. 2006;116:905–915. doi: 10.1172/JCI26836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Persson M, Brantefjord M, Hansson E, Ronnback L. Lipopolysaccharide increases microglial GLT-1 expression and glutamate uptake capacity in vitro by a mechanism dependent on TNF-alpha. Glia. 2005;51:111–120. doi: 10.1002/glia.20191. [DOI] [PubMed] [Google Scholar]
- 13.Shechter R, London A, Varol C, Raposo C, Cusimano M, Yovel G, et al. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6:e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 15.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Michelucci A, Heurtaux T, Grandbarbe L, Morga E, Heuschling P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: effects of oligomeric and fibrillar amyloid-beta. J Neuroimmunol. 2009;210:3–12. doi: 10.1016/j.jneuroim.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 18.Kipnis J, Mizrahi T, Hauben E, Shaked I, Shevach E, Schwartz M. Neuroprotective autoimmunity: naturally occurring CD4+ CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system. Proc Natl Acad Sci USA. 2002;99:15620–15625. doi: 10.1073/pnas.232565399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz M, Kipnis J. Protective autoimmunity and neuroprotection in inflammatory and noninflammatory neurodegenerative diseases. J Neurol Sci. 2005;233:163–166. doi: 10.1016/j.jns.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Yoles E, Hauben E, Palgi O, Agranov E, Gothilf A, Cohen A, et al. Protective autoimmunity is a physiological response to CNS trauma. J Neurosci. 2001;21:3740–3748. doi: 10.1523/JNEUROSCI.21-11-03740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci USA. 2004;101:8180–8185. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brynskikh A, Warren T, Zhu J, Kipnis J. Adaptive immunity affects learning behavior in mice. Brain Behav Immun. 2008;22:861–869. doi: 10.1016/j.bbi.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 24.Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 25.Cserr HF, Knopf PM. Cervical lymphatics, the blood-brain barrier and the immunoreactivity of the brain: a new view. Immunol Today. 1992;13:507–512. doi: 10.1016/0167-5699(92)90027-5. [DOI] [PubMed] [Google Scholar]
- 26.Widner H, Moller G, Johansson BB. Immune response in deep cervical lymph nodes and spleen in the mouse after antigen deposition in different intracerebral sites. Scand J Immunol. 1988;28:563–571. doi: 10.1111/j.1365-3083.1988.tb01488.x. [DOI] [PubMed] [Google Scholar]
- 27.McMenamin PG, Wealthall RJ, Deverall M, Cooper SJ, Griffin B. Macrophages and dendritic cells in the rat meninges and choroid plexus: three-dimensional localisation by environmental scanning electron microscopy and confocal microscopy. Cell Tissue Res. 2003;313:259–269. doi: 10.1007/s00441-003-0779-0. [DOI] [PubMed] [Google Scholar]
- 28.McMenamin PG. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol. 1999;405:553–562. [PubMed] [Google Scholar]
- 29.Kivisakk P, Mahad DJ, Callahan MK, Trebst C, Tucky B, Wei T, et al. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci USA. 2003;100:8389–8394. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qing Z, Sewell D, Sandor M, Fabry Z. Antigen-specific T cell trafficking into the central nervous system. J Neuroimmunol. 2000;105:169–178. doi: 10.1016/s0165-5728(99)00265-9. [DOI] [PubMed] [Google Scholar]
- 31.Tran PB, Miller RJ. Chemokine receptors: signposts to brain development and disease. Nat Rev Neurosci. 2003;4:444–455. doi: 10.1038/nrn1116. [DOI] [PubMed] [Google Scholar]
- 32.Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 33.Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Corriveau RA, Huh GS, Shatz CJ. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- 35.Hawley SH, Wunnenberg-Stapleton K, Hashimoto C, Laurent MN, Watabe T, Blumberg BW, et al. Disruption of BMP signals in embryonic Xenopus ectoderm leads to direct neural induction. Genes Dev. 1995;9:2923–2935. doi: 10.1101/gad.9.23.2923. [DOI] [PubMed] [Google Scholar]
- 36.Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, May SR, et al. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–661. doi: 10.1038/35001072. [DOI] [PubMed] [Google Scholar]
- 37.Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Ohtani T, Ishihara K, Atsumi T, Nishida K, Kaneko Y, Miyata T, et al. Dissection of signaling cascades through gp130 in vivo: reciprocal roles for STAT3- and SHP2-mediated signals in immune responses. Immunity. 2000;12:95–105. doi: 10.1016/s1074-7613(00)80162-4. [DOI] [PubMed] [Google Scholar]
- 39.Mehler MF, Kessler JA. Hematolymphopoietic and inflammatory cytokines in neural development. Trends Neurosci. 1997;20:357–365. doi: 10.1016/s0166-2236(96)01045-4. [DOI] [PubMed] [Google Scholar]
- 40.Lovett-Racke AE, Smith ME, Arredondo LR, Bittner PS, Ratts RB, Shive CL, et al. Developmentally regulated gene expression of Th2 cytokines in the brain. Brain Res. 2000;870:27–35. doi: 10.1016/s0006-8993(00)02398-2. [DOI] [PubMed] [Google Scholar]
- 41.Dziegielewska KM, Moller JE, Potter AM, Ek J, Lane MA, Saunders NR. Acute-phase cytokines IL-1beta and TNF-alpha in brain development. Cell Tissue Res. 2000;299:335–345. doi: 10.1007/s004419900157. [DOI] [PubMed] [Google Scholar]
- 42.Gillard SE, Lu M, Mastracci RM, Miller RJ. Expression of functional chemokine receptors by rat cerebellar neurons. J Neuroimmunol. 2002;124:16–28. doi: 10.1016/s0165-5728(02)00005-x. [DOI] [PubMed] [Google Scholar]
- 43.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 44.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, et al. The chemokine SDF1 regulates migration of dentate granule cells. Development. 2002;129:4249–4260. doi: 10.1242/dev.129.18.4249. [DOI] [PubMed] [Google Scholar]
- 46.Borrell V, Marin O. Meninges control tangential migration of hem-derived Cajal-Retzius cells via CXCL12/CXCR4 signaling. Nat Neurosci. 2006;9:1284–1293. doi: 10.1038/nn1764. [DOI] [PubMed] [Google Scholar]
- 47.Zhu Y, Yu T, Zhang XC, Nagasawa T, Wu JY, Rao Y. Role of the chemokine SDF-1 as the meningeal attractant for embryonic cerebellar neurons. Nat Neurosci. 2002;5:719–720. doi: 10.1038/nn881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reiss K, Mentlein R, Sievers J, Hartmann D. Stromal cell-derived factor 1 is secreted by meningeal cells and acts as chemotactic factor on neuronal stem cells of the cerebellar external granular layer. Neuroscience. 2002;115:295–305. doi: 10.1016/s0306-4522(02)00307-x. [DOI] [PubMed] [Google Scholar]
- 49.Chalasani SH, Sabol A, Xu H, Gyda MA, Rasband K, Granato M, et al. Stromal cell-derived factor-1 antagonizes slit/robo signaling in vivo. J Neurosci. 2007;27:973–980. doi: 10.1523/JNEUROSCI.4132-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chalasani SH, Sabelko KA, Sunshine MJ, Littman DR, Raper JA. A chemokine, SDF-1, reduces the effectiveness of multiple axonal repellents and is required for normal axon pathfinding. J Neurosci. 2003;23:1360–1371. doi: 10.1523/JNEUROSCI.23-04-01360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lieberam I, Agalliu D, Nagasawa T, Ericson J, Jessell TM. A Cxcl12-CXCR4 chemokine signaling pathway defines the initial trajectory of mammalian motor axons. Neuron. 2005;47:667–679. doi: 10.1016/j.neuron.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 52.Pettigrew HD, Teuber SS, Gershwin ME. Clinical significance of complement deficiencies. Ann N Y Acad Sci. 2009;1173:108–123. doi: 10.1111/j.1749-6632.2009.04633.x. [DOI] [PubMed] [Google Scholar]
- 53.Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McConnell MJ, Huang YH, Datwani A, Shatz CJ. H2-K(b) and H2-D(b) regulate cerebellar long-term depression and limit motor learning. Proc Natl Acad Sci USA. 2009;106:6784–6789. doi: 10.1073/pnas.0902018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laurvick CL, Msall ME, Silburn S, Bower C, de Klerk N, Leonard H. Physical and mental health of mothers caring for a child with Rett syndrome. Pediatrics. 2006;118:e1152–e1164. doi: 10.1542/peds.2006-0439. [DOI] [PubMed] [Google Scholar]
- 56.Moog U, Smeets EE, van Roozendaal KE, Schoenmakers S, Herbergs J, Schoonbrood-Lenssen AM, et al. Neurodevelopmental disorders in males related to the gene causing Rett syndrome in females (MECP2) Eur J Paediatr Neurol. 2003;7:5–12. doi: 10.1016/s1090-3798(02)00134-4. [DOI] [PubMed] [Google Scholar]
- 57.Shetty AK, Chatters R, Tilton AH, Lacassie Y. Syndrome of microcephaly, mental retardation, and tracheoesophageal fistula associated with features of Rett syndrome. J Child Neurol. 2000;15:61–63. doi: 10.1177/088307380001500114. [DOI] [PubMed] [Google Scholar]
- 58.Armstrong DD. Neuropathology of Rett syndrome. J Child Neurol. 2005;20:747–753. doi: 10.1177/08830738050200090901. [DOI] [PubMed] [Google Scholar]
- 59.Horska A, Farage L, Bibat G, Nagae LM, Kaufmann WE, Barker PB, et al. Brain metabolism in Rett syndrome: age, clinical, and genotype correlations. Ann Neurol. 2009;65:90–97. doi: 10.1002/ana.21562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 61.Bao X, Jiang S, Song F, Pan H, Li M, Wu X. X chromosome inactivation in Rett syndrome and its correlations with MECP2 mutations and phenotype. J Child Neurol. 2008;23:22–25. doi: 10.1177/0883073807307077. [DOI] [PubMed] [Google Scholar]
- 62.Tong Y, Aune T, Boothby M. T-bet antagonizes mSin3a recruitment and transactivates a fully methylated IFN-gamma promoter via a conserved T-box half-site. Proc Natl Acad Sci USA. 2005;102:2034–2039. doi: 10.1073/pnas.0409510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lal G, Zhang N, van der Touw W, Ding Y, Ju W, Bottinger EP, et al. Epigenetic regulation of Foxp3 expression in regulatory T cells by DNA methylation. J Immunol. 2009;182:259–273. doi: 10.4049/jimmunol.182.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Balmer D, Arredondo J, Samaco RC, LaSalle JM. MECP2 mutations in Rett syndrome adversely affect lymphocyte growth, but do not affect imprinted gene expression in blood or brain. Hum Genet. 2002;110:545–552. doi: 10.1007/s00439-002-0724-4. [DOI] [PubMed] [Google Scholar]
- 65.Webb R, Wren JD, Jeffries M, Kelly JA, Kaufman KM, Tang Y, et al. Variants within MECP2, a key transcription regulator, are associated with increased susceptibility to lupus and differential gene expression in patients with systemic lupus erythematosus. Arthritis Rheum. 2009;60:1076–1084. doi: 10.1002/art.24360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Delgado IJ, Kim DS, Thatcher KN, LaSalle JM, Van den Veyver IB. Expression profiling of clonal lymphocyte cell cultures from Rett syndrome patients. BMC Med Genet. 2006;7:61. doi: 10.1186/1471-2350-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 68.Tanioka T, Hattori A, Mizutani S, Tsujimoto M. Regulation of the human leukocyte-derived arginine aminopeptidase/endoplasmic reticulum-aminopeptidase 2 gene by interferon-gamma. FEBS J. 2005;272:916–928. doi: 10.1111/j.1742-4658.2004.04521.x. [DOI] [PubMed] [Google Scholar]
- 69.Plioplys AV, Greaves A, Kazemi K, Silverman E. Lymphocyte function in autism and Rett syndrome. Neuropsychobiology. 1994;29:12–16. doi: 10.1159/000119056. [DOI] [PubMed] [Google Scholar]
- 70.Fiumara A, Sciotto A, Barone R, D’Asero G, Munda S, Parano E, et al. Peripheral lymphocyte subsets and other immune aspects in Rett syndrome. Pediatr Neurol. 1999;21:619–621. doi: 10.1016/s0887-8994(99)00053-3. [DOI] [PubMed] [Google Scholar]
- 71.Arman M, Aguilera-Montilla N, Mas V, Puig-Kroger A, Pignatelli M, Guigo R, et al. The human CD6 gene is transcriptionally regulated by RUNX and Ets transcription factors in T cells. Mol Immunol. 2009;46:2226–2235. doi: 10.1016/j.molimm.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 72.Komine O, Hayashi K, Natsume W, Watanabe T, Seki Y, Seki N, et al. The Runx1 transcription factor inhibits the differentiation of naive CD4+ T cells into the Th2 lineage by repressing GATA3 expression. J Exp Med. 2003;198:51–61. doi: 10.1084/jem.20021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cayrol R, Wosik K, Berard JL, Dodelet-Devillers A, Ifergan I, Kebir H, et al. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat Immunol. 2008;9:137–145. doi: 10.1038/ni1551. [DOI] [PubMed] [Google Scholar]
- 74.Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener. 2009;4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Holling TM, van der Stoep N, Quinten E, van den Elsen PJ. Activated human T cells accomplish MHC class II expression through T cell-specific occupation of class II transactivator promoter III. J Immunol. 2002;168:763–770. doi: 10.4049/jimmunol.168.2.763. [DOI] [PubMed] [Google Scholar]
- 76.Latham KA, Whittington KB, Zhou R, Qian Z, Rosloniec EF. Ex vivo characterization of the autoimmune T cell response in the HLA-DR1 mouse model of collagen-induced arthritis reveals long-term activation of type II collagen-specific cells and their presence in arthritic joints. J Immunol. 2005;174:3978–3985. doi: 10.4049/jimmunol.174.7.3978. [DOI] [PubMed] [Google Scholar]
- 77.Maezawa I, Swanberg S, Harvey D, LaSalle JM, Jin LW. Rett syndrome astrocytes are abnormal and spread MeCP2 deficiency through gap junctions. J Neurosci. 2009;29:5051–5061. doi: 10.1523/JNEUROSCI.0324-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ballas N, Lioy DT, Grunseich C, Mandel G. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci. 2009;12:311–317. doi: 10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alvarez-Saavedra M, Saez MA, Kang D, Zoghbi HY, Young JI. Cell-specific expression of wild-type MeCP2 in mouse models of Rett syndrome yields insight about pathogenesis. Hum Mol Genet. 2007;16:2315–2325. doi: 10.1093/hmg/ddm185. [DOI] [PubMed] [Google Scholar]
- 80.Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- 82.Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Engelhardt B, Ransohoff RM. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 2005;26:485–495. doi: 10.1016/j.it.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 84.Allen NJ, Barres BA. Neuroscience: Glia—more than just brain glue. Nature. 2009;457:675–677. doi: 10.1038/457675a. [DOI] [PubMed] [Google Scholar]
- 85.Ullian EM, Christopherson KS, Barres BA. Role for glia in synaptogenesis. Glia. 2004;47:209–216. doi: 10.1002/glia.20082. [DOI] [PubMed] [Google Scholar]
- 86.Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 87.Chung IY, Benveniste EN. Tumor necrosis factor-alpha production by astrocytes. Induction by lipopolysaccharide, IFN-gamma, and IL-1 beta. J Immunol. 1990;144:2999–3007. [PubMed] [Google Scholar]
- 88.Chao CC, Hu S, Sheng WS, Bu D, Bukrinsky MI, Peterson PK. Cytokine-stimulated astrocytes damage human neurons via a nitric oxide mechanism. Glia. 1996;16:276–284. doi: 10.1002/(SICI)1098-1136(199603)16:3<276::AID-GLIA10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 89.Garg SK, Banerjee R, Kipnis J. Neuroprotective immunity: T cell-derived glutamate endows astrocytes with a neuroprotective phenotype. J Immunol. 2008;180:3866–3873. doi: 10.4049/jimmunol.180.6.3866. [DOI] [PubMed] [Google Scholar]
- 90.Garg SK, Kipnis J, Banerjee R. IFN-gamma and IL-4 differentially shape metabolic responses and neuroprotective phenotype of astrocytes*. J Neurochem. 2009;108:1155–1166. doi: 10.1111/j.1471-4159.2009.05872.x. [DOI] [PubMed] [Google Scholar]
- 91.Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, et al. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/ progenitor cells. Mol Cell Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 92.Schwartz M, Butovsky O, Bruck W, Hanisch UK. Microglial phenotype: is the commitment reversible? Trends Neurosci. 2006;29:68–74. doi: 10.1016/j.tins.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 93.Zhao W, Xie W, Xiao Q, Beers DR, Appel SH. Protective effects of an anti-inflammatory cytokine, interleukin-4, on motoneuron toxicity induced by activated microglia. J Neurochem. 2006;99:1176–1187. doi: 10.1111/j.1471-4159.2006.04172.x. [DOI] [PubMed] [Google Scholar]
- 94.Beers DR, Henkel JS, Zhao W, Wang J, Appel SH. CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc Natl Acad Sci USA. 2008;105:15558–15563. doi: 10.1073/pnas.0807419105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baracskay KL, Kidd GJ, Miller RH, Trapp BD. NG2-positive cells generate A2B5-positive oligodendrocyte precursor cells. Glia. 2007;55:1001–1010. doi: 10.1002/glia.20519. [DOI] [PubMed] [Google Scholar]
- 96.Pan JW, Lane JB, Hetherington H, Percy AK. Rett syndrome: 1H spectroscopic imaging at 4.1 Tesla. J Child Neurol. 1999;14:524–528. doi: 10.1177/088307389901400808. [DOI] [PubMed] [Google Scholar]
- 97.Teitelbaum D, Arnon R, Sela M. Copolymer 1: from basic research to clinical application. Cell Mol Life Sci. 1997;53:24–28. doi: 10.1007/PL00000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Teitelbaum D, Fridkis-Hareli M, Arnon R, Sela M. Copolymer 1 inhibits chronic relapsing experimental allergic encephalomyelitis induced by proteolipid protein (PLP) peptides in mice and interferes with PLP-specific T cell responses. J Neuroimmunol. 1996;64:209–217. doi: 10.1016/0165-5728(95)00180-8. [DOI] [PubMed] [Google Scholar]
- 99.Weber MS, Hohlfeld R, Zamvil SS. Mechanism of action of glatiramer acetate in treatment of multiple sclerosis. Neurotherapeutics. 2007;4:647–653. doi: 10.1016/j.nurt.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weber MS, Prod’homme T, Youssef S, Dunn SE, Rundle CD, Lee L, et al. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med. 2007;13:935–943. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- 101.Frenkel D, Maron R, Burt DS, Weiner HL. Nasal vaccination with a proteosome-based adjuvant and glatiramer acetate clears beta-amyloid in a mouse model of Alzheimer disease. J Clin Invest. 2005;115:2423–2433. doi: 10.1172/JCI23241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Benner EJ, Mosley RL, Destache CJ, Lewis TB, Jackson-Lewis V, Gorantla S, et al. Therapeutic immunization protects dopaminergic neurons in a mouse model of Parkinson’s disease. Proc Natl Acad Sci USA. 2004;101:9435–9440. doi: 10.1073/pnas.0400569101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kipnis J, Yoles E, Porat Z, Cohen A, Mor F, Sela M, et al. T cell immunity to copolymer 1 confers neuroprotection on the damaged optic nerve: possible therapy for optic neuropathies. Proc Natl Acad Sci USA. 2000;97:7446–7451. doi: 10.1073/pnas.97.13.7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu J, Johnson TV, Lin J, Ramirez SH, Bronich TK, Caplan S, et al. T cell independent mechanism for copolymer-1-induced neuro-protection. Eur J Immunol. 2007;37:3143–3154. doi: 10.1002/eji.200737398. [DOI] [PubMed] [Google Scholar]
- 105.Wolf SA, Steiner B, Akpinarli A, Kammertoens T, Nassenstein C, Braun A, et al. CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neuro-genesis. J Immunol. 2009;182:3979–3984. doi: 10.4049/jimmunol.0801218. [DOI] [PubMed] [Google Scholar]