Abstract
Ouabain, an potent inhibitor of the Na+/K+-ATPase pump, selectively destroys spiral ganglion neurons (SGNs) in gerbils and mice whereas in guinea pigs it preferentially damages cochlear hair cells. To elucidate the effects of ouabain on the rat inner ear, a species widely used in research, 5 µl of 1 mM or 10 mM ouabain was applied to the round window membrane. Distortion product otoacoustic emissions (DPOAE) and auditory brainstem responses (ABR) were used identify functional deficits in hair cells and neurons respectively and histological techniques were used to characterize cochlear pathologies. High-frequency ABR thresholds were elevated after treatment with 1 mM ouabain whereas DPOAEs remained normal. In contrast, 10 mM ouabain increased ABR thresholds and reduced DPOAE amplitudes. Consistent with the physiological changes, 1 mM ouabain only damaged the SGNs and auditory nerve fibers in the basal turn of the cochlea whereas 10 mM ouabain destroyed both SGNs and cochlear hair cells; damage was greatest near the base and decreased toward the apex. The nuclei of degenerating SGNs and hair cells were condensed and fragmented and many cells were TUNEL-positive, morphological features of apoptotic cell death. Thus, ouabain-induced cochlear degeneration in rats is apoptotic and concentration dependent; low concentrations preferentially damage SGNs in the base of the cochlea, producing an animal model of partial auditory neuropathy, whereas high concentrations damage both hair cells and SGNs with damage decreasing from the base towards the apex.
Keywords: ouabain, hair cell, spiral ganglion neuron, degeneration, apoptosis, TUNEL
Introduction
The sodium, potassium-ATPase (Na+/K+-ATPase) pump is a membrane protein that is responsible for establishing the sodium-potassium electrochemical gradient across the plasma membrane and plays a major role in maintaining ion homeostasis throughout the body, particularly in epithelial cells such as those found in the inner ear (Rajasekaran et al., 2003, Rajasekaran and Rajasekaran, 2003). The large cation gradients that exist between the endolymph and perilymph in the inner ear are maintained by several energy consuming ion pumps. The Na+/K+-ATPase pump is highly expressed in several regions of the inner ear including type II fibrocytes of the spiral ligament, spiral limbus, hair cells and spiral ganglion neurons (SGNs) as well as the marginal cells of the stria vascularis where it helps to maintain the endolymphatic potential (EP) (Hamada and Kimura, 1999, Ichimiya et al., 1994, Kuijpers and Bonting, 1969, McLean et al., 2009, Nakazawa et al., 1995, ten Cate et al., 1994).
Ouabain is a cardiac glycoside which selectively inhibits Na+/K+-ATPase activity. Ouabain has been used for many years to investigate the role of Na+/K+-ATPase in maintaining ion homeostasis in the inner ear and to determine how Na+/K+-ATPase inhibition alters various cochlear potentials (Lang et al., 2005, Schmiedt et al., 2002, Wang et al., 2006). There has been renewed interest in using ouabain as a research tool because it selectively destroy type I SGNs in the mouse and gerbil inner ear thereby creating an animal model of auditory neuropathy (Lang et al., 2011, Schmiedt et al., 2002). Some of the physiological characteristics of selective, ouabain-induced SGN loss in animals that mimic auditory neuropathy in humans are retention of nearly normal otoacoustic emission and reduction or complete loss of the auditory brainstem response (ABR) beginning with wave I generated by the auditory nerve (Lang et al., 2008, Santarelli et al., 2008, Schmiedt et al., 2002, Starr et al., 1996). In addition, gerbils with selective loss of SGNs are now being used to study the ability of transplanted stem cells to differentiate into new neurons that repopulate Rosenthal’s canal and establish synaptic contact with the remaining hair cells in the organ of Corti (Corrales et al., 2006, Lang et al., 2011, Lang et al., 2008, Lang et al., 2005, Matsuoka et al., 2007, Schmiedt et al., 2002). While ouabain can selectively destroy type I SGNs in gerbils and mice, its destructive potential in other laboratory species commonly used in auditory research have not been explored except in guinea pigs. That damage patterns seen in guinea pig are dramatically different from those seen in gerbils and mice; ouabain administered to guinea pig round window preferentially damages cochlear hair cells and limbal fibrocytes (Hamada and Kimura, 1999). The factors that contribute to different patterns of cochlear damage in guinea pigs, gerbils and mice are unknown, but could be related to mode of drug delivery, concentration or duration of drug delivery or anatomical difference in these species. The rat is widely used in auditory research and studies of neural regeneration and repair; however, the effects of ouabain on its inner ear have not been investigated. The objective of the present study was to identify the histopathological and functional changes in the rat after applying ouabain to the round window membrane. In addition, distortion product otoacoustic emissions (DPOAEs) and auditory brainstem responses (ABR) were measured before and after ouabain treatment to assess the functional status of the outer hair cells (OHCs) and neural output of the peripheral auditory system.
Methods
Subjects
Eighteen female SASCO Sprague Dawley rats 4–8 months of age and weighing between 250 and 350 g were used for this experiment. The right ear of each animal in group I was treated with 1 mM ouabain (n=8); the right ear of those in group II were treated with 10 mM ouabain (n=10). The left ear of the rats in group I and group II were used as normal controls. This research was approved by the University of Buffalo Institutional Animal Care and Use Committee and experiments were carried out in accordance with NIH guidelines.
Ouabain treatment
Animals were anesthetized with isoflurane (4% for induction, 1.5% for maintenance, 0.4 L/min O2 flow rate). Body temperature was maintained at 37 °C using a heating pad. Using sterile techniques, ouabain was applied to the round window membrane by making a post-auricular incision to expose the bulla. A hole of approximately 1.5 mm diameter was made using a sharp scalpel to thin the bone. The blade was gently rotated to drill open the bulla and expose the round window niche. Afterwards, 5 µl of ouabain dissolved in normal saline at a concentration of either 1 mM or 10 mM was applied to the round window membrane of the right ear using a 10 µl syringe equipped with 31 gauge needle. After 30 minutes, the ouabain solution on the round window membrane was carefully wicked away with a small piece of absorbent filter paper. Afterwards, the hole on the surface of the bulla was closed with dental cement and the overlying incision was sutured close. Two animals in group II were sacrificed 2 days post-ouabain treatment for histological studies to test for the presence of apoptotic markers in SGNs and cochlear hair cells. The remaining animals from both groups were allowed to recover for 7 days.
ABR
ABRs were measured before and seven days following ouabain treatment using procedures described in detail in earlier publications (Chen et al., 2010). Briefly, rat were anesthetized with isoflurane (4% for induction, 1.5% for maintenance, 0.4 L/min O2 flow rate) and placed on a heating pad to maintain body temperature at 37 °C in a sound attenuating booth. Subdermal needle electrodes (Grass Technologies, West Warwick, RI) were placed at the vertex (non-inverting), the ipsilateral mastoid (inverting), and contralateral mastoid (ground). Test stimuli were 4, 8, 12, 16, 20 and 32 kHz tone bursts (5 ms duration, 1 ms rise/fall time, cosine-squared gating, alternating starting phase) generated using TDT hardware (RA16, RP2) and software (Sig-Gen). Stimuli were attenuated with a programmable attenuator (TDT PA-4) and presented through a high-frequency transducer placed at a 90 degree azimuth 10 cm from the test ear. The non-test ear was occluded with an earplug during testing. The presentation level was attenuated in 5 dB steps from 100 down to −10dB (SPL). Stimuli were calibrated with a sound level meter system (Larson Davis Inc., model 825,) equipped with a half-inch condenser microphone (Larson Davis, model 2540). ABR responses were filtered (100–3000 Hz, 60 Hz notch filter), amplified (5020X) and averaged for 100–600 stimulus presentations using TDT hardware and software. Replications were obtained at stimulus levels near threshold. The lowest stimulus level that elicited a repeatable was considered as the threshold.
DPOAE
Rats were anesthetized with isoflurane as noted above, placed on a heating pad in a sound attenuating chamber and body temperature maintained at 37 °C. DPOAEs at 2f1-f2 were recorded using the Smart Distortion Product Otoacoustic Emission System (Intelligent Hearing System, Miami, FL, USA) as described in our earlier publications (Chen et al., 2010, Jamesdaniel et al., 2008). The earpiece containing a microphone (Etymotic10B+) and two sound delivery tubes was inserted into the ear canal. Two IHS-3738 high frequency transducers (Intelligent Hearing System, Miami, FL, USA) delivered the primary tones, f1 and f2, to the ear canal via flexible tubes connected to the earpiece. The f2/f1 ratio was set at 1.2. DPOAE amplitudes at f2 of 4, 8, 12, 16, 20 and 32 kHz were collected as a function of f2 stimulation intensity (DP I/O function). The intensity of f1 was varied from 25 to 70 dB (SPL) in 5-dB steps and the intensity of f2 was 10 dB lower than that of f1. At 4, 8, 12 and 16 kHz, the output of the microphone was sampled at 40 kHz over a period of 204 ms; the spectrum of each sweep was computed and averaged over 32 non-rejected sweeps. Sweeps with an average noise level exceeding the initial noise floor by 10 dB were rejected. The noise floor was measured in a 24 Hz band surrounding 2f1-f2. At 20 and 32 kHz, the output of the microphone was sampled at 127 kHz over a period of 64 ms; the spectrum of each sweep was computed and averaged over 32 non-rejected sweeps. The noise floor was measured in a 46.7 Hz band surrounding 2f1-f2.
Cochlear anatomy
Seven days after ouabain treatment, animals were given a lethal injection of sodium pentobarbital and then perfused intracardially with normal saline followed by 10% formalin in phosphate buffer. Each temporal bone was removed, immersed in fixative at 4 °C for 24 h, decalcified with 10% EDTA at 4 °C for 4 days and then rinsed with 0.1 M phosphate buffered saline (PBS). The cochleas of half of the animals in group I (n=4) and group II (n=4) were prepared as cochlear surface preparations as described previously (Ding et al., 2001). After fixation and decalcification, the cochleae were stained with Harris’ hematoxylin solution, microdissected out as flat surface preparations, mounted in glycerin on glass slides and cover slipped. The cochlear surface preparation was examined with a compound microscope (Zeiss Standard, 400X) and the numbers of missing inner hair cells (IHCs) and outer hair cells (OHCs) were determined over 0.24-mm intervals along the entire basilar membrane. Hair cells were counted as present if the cell body and the cuticular plate were intact. Data were entered into a computer to obtain a cochleogram showing percent IHC and OHC loss as a function of percent total distance from the apex; cochleograms were based on laboratory norms of hair cell density for young normal rats (Ding et al., 2001). Percent distance from the apex was related to frequency using a frequency-place map. Mean cochleograms were computed for each treatment group as described previously (Ding et al., 2001).
Spiral Ganglion Neurons and Auditory Nerve Fibers
The temporal bones from half of the animals in group I (n=4) and group II (n=4) were used to evaluate the SGN in Rosenthal’s canal and the auditory nerve fibers in the habenula perforata as described previously (Ding et al., 1999b, Wang et al., 2003). Briefly, samples were immersed in 2% osmium tetroxide for 2 h, then dehydrated through a serially graded ethanol solutions ending at 100% and embedded in Epon 812. After polymerization, 4 µm thick serial sections were cut parallel to the cochlear modiolus using an ultramicrotome (Reichert Supernova) and glass knives. Sections were collected on glass slides, stained with toluidine blue and examined under a light microscope (Zeiss Axioskop). Specimens were photographed with a digital camera (SPOT Insight, Diagnostic Instruments Inc.) and processed with imaging software (SPOT Software, version 4.6; Adobe Photoshop 5.5). For each cochlea, representative photomicrographs of Rosenthal’s canal were obtained from the hook region, upper basal turn, and second turn of the cochlea. SGNs were counted from the hook region, upper basal turn and the second turn of the cochlea. As described previously, counts from each cochlear location were obtained from 5 slides per animal (every 5th section through the modiolus) and the mean value computed for each cochlea location (McFadden et al., 1999). The numbers of nerve fibers in a 100 µm2 grid centered over each opening of the habenula perforata were counted (i.e., nerve fibers per 100 µm2); counts were obtained from 5 habenular openings of each slide in the hook region, upper basal turn and second turn of the cochlea. Counts were obtained from 5 slides per animal (every 5th section) and the mean value computed for each cochlea location.
No attempt was made to distinguish between type I and type II SGNs or between efferent and afferent nerve fibers.
Apoptosis and TUNEL
Two animals in group II were sacrificed 2 d after ouabain-treatment. The sensory epithelium from each cochlea was removed, stained with hematoxylin to label cells nuclei and prepared as a surface preparation as described above and in an earlier publication (Ding et al., 2001). Surface preparations were used to identify cells with condensed or fragmented nuclei, morphological features of apoptotic cell death. Samples were examined with a microscope (Zeiss Axioskop), photographed with a digital camera (SPOT Insight, Diagnostic Instruments Inc.) and processed with imaging software (SPOT Software, version 4.6; Adobe Photoshop 5.5).
The remaining modiolar portion of each cochlea was decalcified in freshly prepared 10% EDTA solution (ethylenediamine tetraacetic acid, Sigma, Lot 34H0285) for four days, rinsed in PBS, embedded in OCT media (Tissue Tek, Fischer Scientific), and cut parallel to the modiolar axis at a thickness of 30 µm using a cryostat as described previously (Ding et al., 1999a). Sections were prepared for TUNEL (terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling) labeling using the APO-BrdU TUNEL Assay Kit (A-23210, Molecular Probes, Inc, Eugene, OR) according to the manufacturer’s protocol and as described in detail in our previous publication (Qi et al., 2008). Briefly, specimens were transferred to ice-cold 70% ethanol overnight, and then rinsed in washing buffer at room temperature and incubated with 50 µl of DNA-labeling solution overnight at room temperature. Specimens were washed twice in rinse buffer and then incubated in 50 µl of freshly prepared antibody labeling solution for 1 h at room temperature. Afterwards, the samples were rinsed in 0.1 M PBS and immersed overnight at 4 °C in a solution containing a monoclonal antibody against neuronal class III β-tubulin (Covance, MMS-435P). The antibody was diluted 1:100 in blocking solution (Triton X-100, 1%; goat serum 3%; 0.1 M PBS, 96% v/v). Specimens were rinsed three times with 0.1 M PBS and incubated for 1 h with a secondary antibody labeled with Alexa Fluor 555 (goat anti-mouse IgG, Invitrogen, A21426) dissolved in 0.1 M PBS (1:300). The specimens were rinsed with 0.1 M PBS and mounted in glycerin on glass slides and coverslipped (Ding et al., 2002). Specimens were examined under a confocal microscope (Zeiss LSM-510 meta, step size 0.5 µm per slice) using appropriate filters for Alexa Fluor 555 labeled product in nerve fibers and SGNs (excitation 555 nm, emission 565 nm) and green fluorescence of Alexa Fluor 488 dye labeled TUNEL positive cells with DNA fragmentation (excitation 495 nm, emission 519 nm). Confocal images were stored on disk and processed using Zeiss LSM Image Examiner (Carl Zeiss MicroImaging GmbH) and Adobe Photoshop (version 5.5) as described previously (Ding et al., 2011, Qi et al., 2008).
Results
ABR threshold shift
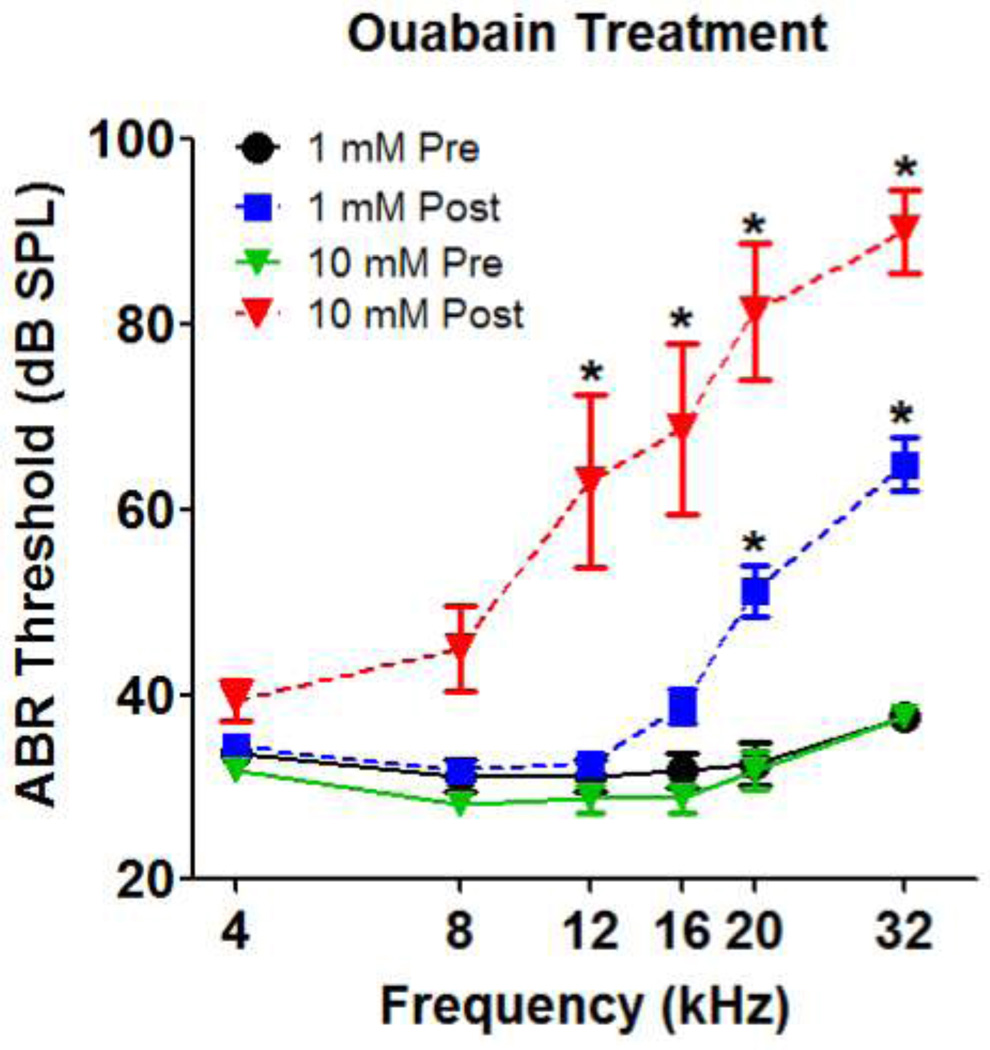
Mean ABR thresholds before ouabain treatment ranged from 20–45 dB SPL across frequency with the lowest threshold at 16 kHz (Fig. 1). Seven days after ouabain treatment, significant elevations of ABR thresholds were detected in the ears treated with 1 mM or 10 mM on the round window. After treatment with 1 mM ouabain, ABR thresholds were elevated at the high frequency. A repeated measures, two-way analysis of variance showed a significant effect of ouabain treatment (p<0.0001), frequency (p<0.0001) and interaction of treatment and frequency (p<0.0001). A Bonferroni post-hoc analysis showed that ABR thresholds were elevated significantly at 20 and 32 kHz after ouabain treatment (p<0.001). ABR threshold shifts were larger and extended over a broader range of frequencies in rats treated with 10 mM ouabain (Fig. 1). A repeated measures, two-way analysis of variance showed a significant effect of 10 mM ouabain treatment (p<0.0001), frequency (p<0.0001) and interaction of treatment and frequency (p<0.0002). ABR thresholds were elevated significantly from 12 to 32 kHz after 10 mM ouabain treatment (Bonferroni post-hoc analysis, p<0.001).
Figure 1.
Mean (+/− SEM, n=8/group) ABR thresholds before and after treatment with 1 mM or 10 mM ouabain. * denotes frequencies at which a significant threshold shift occurred post-treatment.
DPOAE amplitude reduction
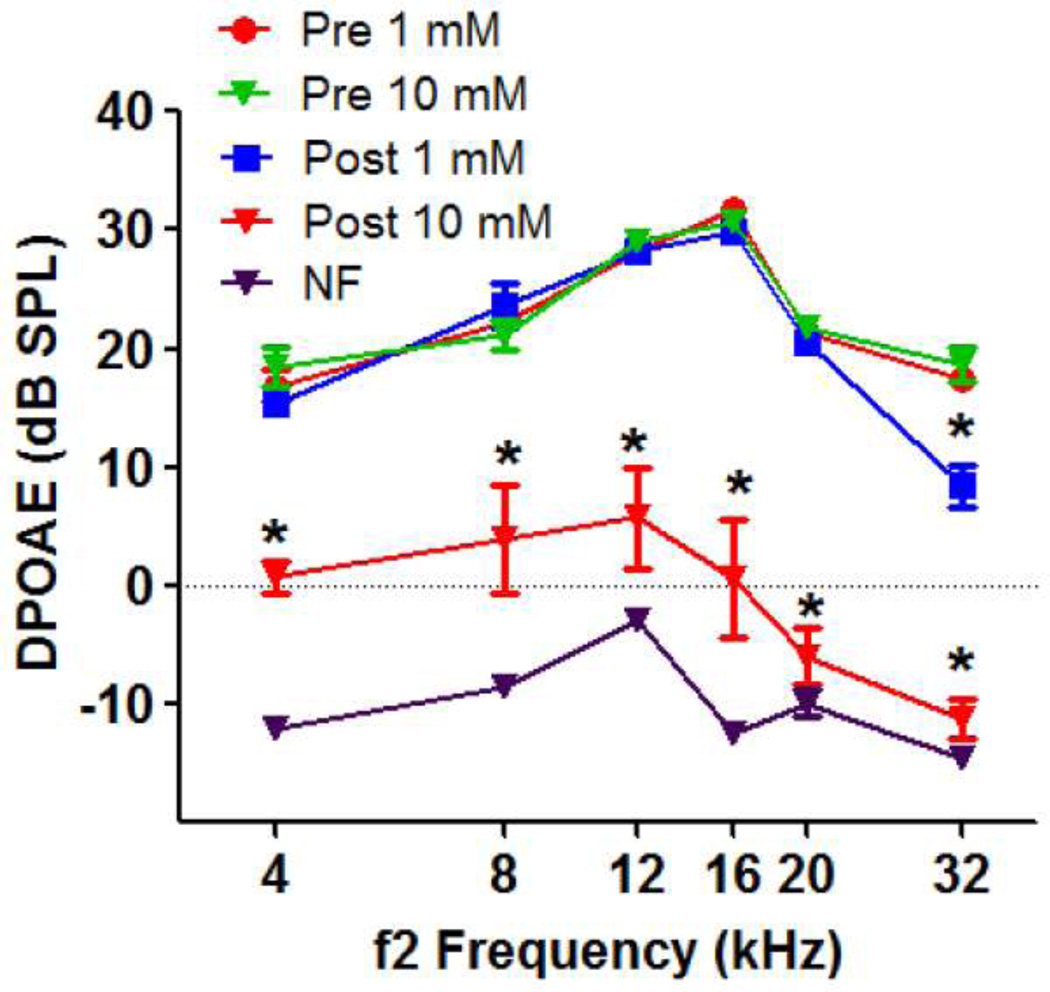
DPOAEs were measured before and after ouabain treatment. Mean DPOAEs amplitude to 65/55 dB SPL stimuli in group I and group II at f2 frequencies of 4, 8, 12, 16, 20, and 32 kHz ranged from 17 to 31 dB SPL with the largest amplitudes at 16 kHz (Fig. 2). Treatment with 1 mM ouabain resulted in roughly a 9 dB reduction in DPOAE amplitude at 32 kHz which was statistically significant (repeated measures, two-way analysis of variance, significant effect of ouabain, p<0.01, frequency, p<0.0001, ouabain and frequency interaction p<0.001; Bonferroni post-hoc analysis, p<0.001 at 32 kHz). Treatment with 10 mM ouabain caused a large, statistically significant decrease in DPOAE amplitude at all frequencies with the largest decreases occurring at the high frequencies (repeated measures, two-way analysis of variance; significant effect of ouabain, p<0.01, frequency, p<0.0001; ouabain and frequency interaction, p<0.0002; Bonferroni post hoc analysis, significant difference at all frequencies, p<0.05).
Figure 2.
Mean (+/−, n=8/group) DPOAE amplitudes plotted as a function of f2 frequency; L1/L2 levels of 65/55 dB SPL. Data shown before and after treatment with 1 mM or 10 mM ouabain (figure legend). Noise floor (NF) of measurement system.
Hair Cell Loss
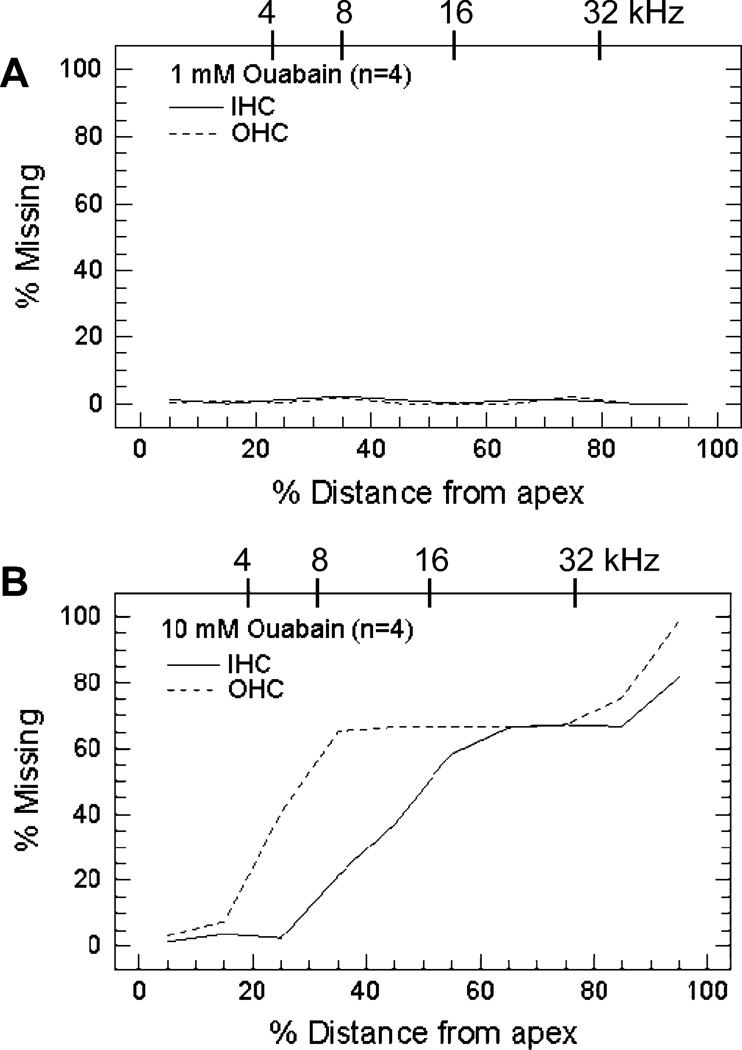
Cytocochleograms were prepared from the control and experimental ears of half the rats group I treated with 1 mM (n=4) and half the rats in group II treated with 10 mM (n=4) ouabain. Individual cochleograms from the untreated control ears showed no obvious signs of damage (data not shown). Likewise, cochleograms from inner ears treated with 1 mM ouabain showed little or no hair cell loss. In contrast, inner ears treated with 10 mM ouabain showed extensive loss of OHC and IHC over much of the cochlea except for the most apical regions. Average cochleograms from ears treated with 1 mM (n=4 cochleas) and 10 mM (n=4 cochlea) ouabain are shown in Figure 3. Percent IHC and OHC loss is plotted as a function of percent distance from the apex (lower x-axis); cochlear position is related to frequency (upper x-axis) using the tonotopic map for the rat (Muller, 1991). The 1 mM dose of ouabain caused little or no IHC or OHC loss (Fig. 3A). However, more than 60% of OHC were missing 30–100% distance from the apex and 60% or more IHC were missing from 60–100% distance from the apex (Fig. 3B). Hair cell loss was more severe in the extreme base of the cochlea while little or no hair cell loss was observed in the apical 10–20% of the cochlea.
Figure 3.
Mean (n=4) cochleograms obtained 7 days after treatment with (A) 1 mM (group I) and (B) 10 mM (group II) ouabain. Percent inner hair cell (IHC) and outer hair cell (OHC) loss plotted as a function of percent distance from the apex. Upper x-axis shows location of 4, 8, 16 and 32 kHz on the frequency-place map for the rat cochlea (Muller, 1991).
SGNs
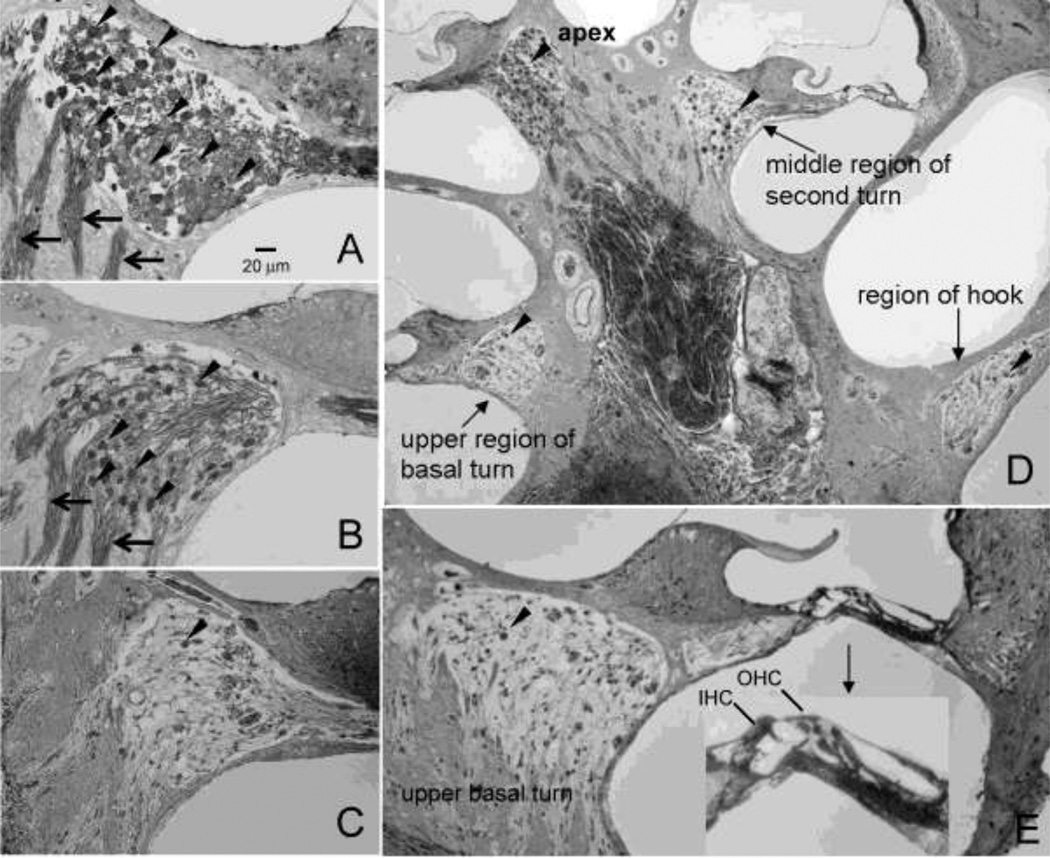
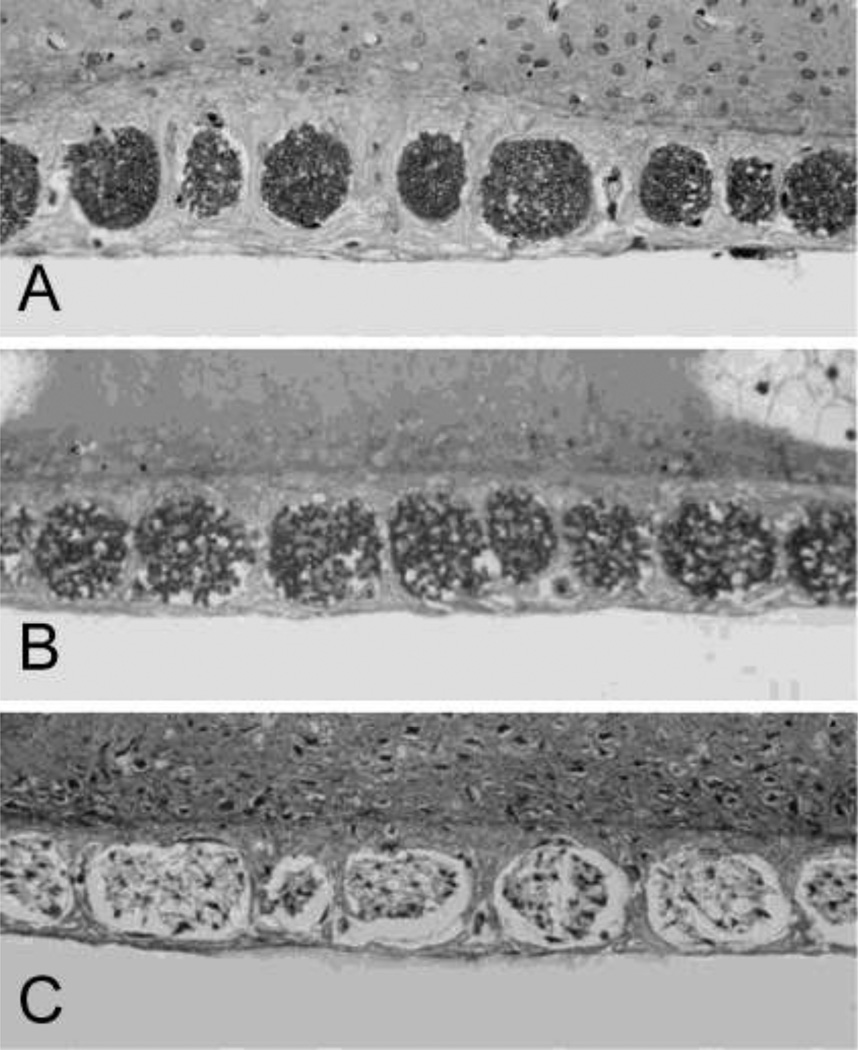
Figure 4A–C shows representative photomicrographs of SGNs in Rosenthal’s canal from the upper half of the basal turn in a normal ear and other cochlear treated with 1 mM (group I) or 10 mM ouabain (group II). In normal controls, Rosenthal’s canal was densely packed with SGNs and thick fascicles of auditory nerve fibers (Fig. 4A). The number of SGNs in Rosenthal’s canal was reduced slightly seven days after treatment with 1 mM ouabain (Fig. 4B). In contrast, nearly all SGNs and nerve fibers were missing from Rosenthal’s canal seven days after treatment with 10 mM ouabain (Fig. 4C). SGN loss varied with cochlear location as shown in Figure 4D. After treatment with 10 mM ouabain, nearly all SGNs were missing from the hook region and upper basal turn; some SGNs were present in the second turn and most were present in the apex. Figure 4E shows a cross section through the modiolus, organ of Corti and lateral wall in the upper basal turn of an ear that had been treated with 10 mM ouabain (group II). Despite the fact that SGN density in Rosenthal’s canal was greatly reduced, the organ of Corti appeared relatively normal and the OHCs and three rows of IHCs were present. Thus, the SGNs generally appear to be more vulnerable ouabain than the hair cells as illustrated by this section from the upper basal turn of an ear that had been treated with 10 mM ouabain.
Figure 4.
(A–C) Representative photomicrographs of Rosenthal’s canal taken from the upper half of the basal turn showing the density of SGNs in (A) a control rat and a rat treated with (B) 1 mM (group I) or (C) 10 mM (group II) ouabain. Arrowheads point to SGNs, arrow point to fascicles of auditory nerve fibers. (D) Cross section through the modiolus showing the SGN (arrowheads) in the hook region, upper basal turn, middle region of the second turn and apex 7 days after treatment with 10 mM ouabain. Note the massive loss of SGNs in the hook and upper basal turn, moderate number of SGN in the middle of the second turn and large number of SGN in the apex. (E) Cross section through the modiolus, organ of Corti and lateral wall showing the condition of the SGNs and IHC and OHC in the organ of Corti (insert) in the upper basal turn after 10 mM ouabain. Only a few SGNs (arrowhead) present in Rosenthal’s canal; IHC and OHCs in organ of Corti are still present despite the massive loss of SGNs.
SGNs in Rosenthal’s canal were counted in the hook region, upper basal turn and second turn of the cochlea of control ears (30 canals/region from 4 ears) and inner ears treated with 1 mM (16 canals/region from 4 ears) or 10 mM (16 canals/region from 4 ears) ouabain (Table 1). Mean numbers (+/− SD) of SGNs in the hook region, upper basal turn and middle turn were approximately 42, 56 and 42 respectively. The effects of ouabain dose, cochlear location and the interaction of dose × cochlear location were statistically significant (Two-way ANOVA, p<0.001). Treatment with 1 mM ouabain caused a statistically significant decrease in the number of SGNs relative to controls in the hook region (~37% reduction) and upper basal turn (~32% reduction) (Tukey post-hoc, p<0.05). Treatment with 10 mM ouabain caused a statistically significant decrease in the number SGNs relative to controls in the hook region (~95% reduction), upper basal turn (~96% reduction) and second turn (~55% reduction) (Tukey post-hoc, p<0.05).
Table 1.
Mean (+/− SD) Numbers SGNs vs. Cochlear Location
| Treatment | Hook | Upper Basal Turn | Second Turn |
|---|---|---|---|
| Control | 42.07 +/− 0.43 | 56.17 +/− 4.09 | 41.70 +/− 2.88 |
| 1 mM Ouabain | 26.56 +/− 2.99 * | 38.25 +/− 2.82 * | 40.31 +/− 3.16 |
| 10 mM Ouabain | 2.75 +/− 1.06 ** | 3.00 +/− 1.36 ** | 18.75 +/− 2.62 ** |
p<0.05
p<0.01
Nerve Fibers
Auditory nerve fiber densities in the habenula perforata were measured in control ears and ears treated with 1 mM or 10 mM ouabain. The photomicrographs in Figure 5 show the nerve fibers passing through 7–9 habenular openings in the upper basal turn of the rat cochlea. The nerve fibers were densely packed in the habenular openings of control ears (Fig. 5A). After treatment with 1 mM ouabain, a slight decrease in nerve fiber density was evident and some empty spaces were present (Fig. 5B). In contrast, treatment with 10 mM ouabain resulted in a dramatic decrease in nerve fiber density, pale staining and a large vacant ring surrounding the residual core of nerve fibers (Fig. 5C).
Figure 5.
Representative photomicrographs showing the nerve fibers in the habenula perforata in the upper basal turn in (A) a normal rat and in rats treated with (B) 1 mM and (C) 10 mM ouabain and allowed to survive 7 days. Habenular openings in normal control ear densely packed with nerve fibers. Note slight reduction in nerve fiber density after treatment with 1 mM ouabain and massive loss of nerve fibers after 10 mM ouabain treatment.
Nerve fibers were counted by placing a 100 µm2 grid over the center of each habenular opening. Mean values (+/− SD) were computed for the hook, upper basal turn and middle turn of the rat cochlea (Table 2) from control ears (65 openings/region from 4 ears) and ears treated with 1 mM (34 openings/region from 4 ears) and 10 mM (34 openings/region from 4 ears). Mean nerve fiber densities per 100 µm2 were 7.35, 23.54 and 16.03 in the hook, upper basal turn and second turn respectively. The effects of ouabain dose, cochlear location and the interaction of dose and cochlear location were statistically significant (Two-way ANOVA, p<0.001). Treatment with 1 mM ouabain significantly reduced nerve fiber density by approximately 58% in the hook region and 32% and upper basal turn (Tukey post-hoc, p<0.05). After treatment with 10 mM ouabain, nerve fiber density decreased significantly by 82% in hook region, 89% in the upper basal turn and 63% in the second turn (Tukey post-hoc analysis, p<0.05).
Table 1.
Mean (+/− SD) Number Nerve Fibers per 100 µM2
| Treatment | Hook | Upper Basal Turn | Second Turn |
|---|---|---|---|
| Control | 7.35 +/− 1.68 | 23.54 +/− 1.13 | 16.03 +/− 2.27 |
| 1 mM Ouabain | 3.12 +/− 1.87 * | 15.97 +/− 2.61 ** | 16.38 +/− 2.98 |
| 10 mM Ouabain | 2.06 +/− 1.41 ** | 2.59 +/− 1.33 ** | 5.94 +/− 1.98 ** |
p<0.05
p<0.01
Apoptotic in SGNs and Hair Cells
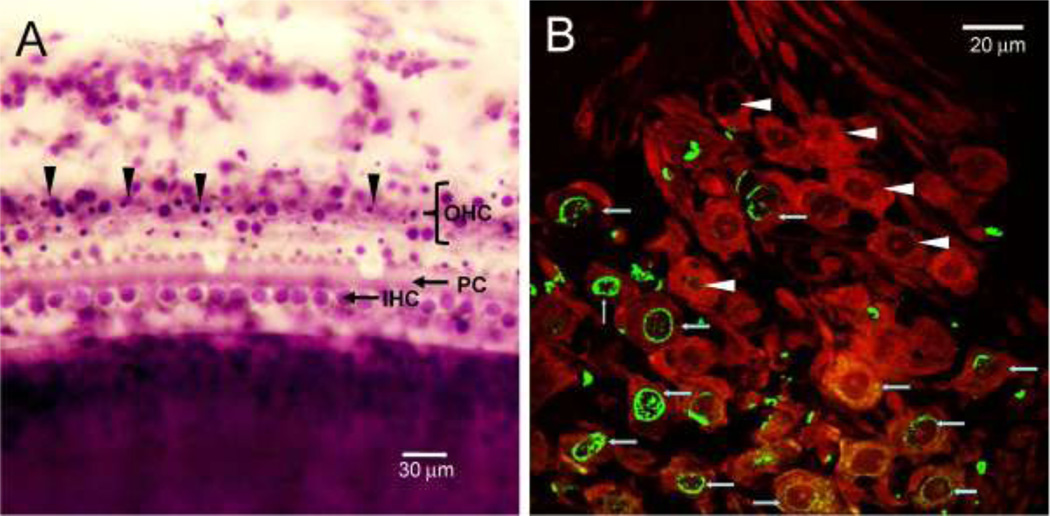
To evaluate how SGNs and hair cells were degenerating during the early stage of ouabain treatment, some rats were sacrificed 2 days after applying 10 mM ouabain to the round window; these data were obtained 5 days earlier than anatomical data shown in Figure 3–5. Figure 6A is photomicrograph of a surface preparation from the second turn of the cochlea obtained 2 days following ouabain treatment. Many OHCs were missing, most IHC were present and a few pillar cells were also absent. A few OHC with large, round nuclei were present; however, the nuclei of many OHC were shrunken and condensed, morphological features of cells dying by apoptosis.
Figure 6.
(A) Representative photomicrograph of surface of the organ of Corti from the middle of a cochlea from a rat treated with 10 mM ouabain and evaluated 2 days post-treatment, i.e., during the early stage of degeneration. Many OHCs are missing and numerous OHC have condensed nuclei (arrowhead), morphological characteristic of apoptosis. (B) Photomicrograph of SGN labeled (10 mM ouabain, 2 d post-treatment) with an antibody against neuronal class III β-tubulin and secondary antibody labeled with Alexa Fluor 555 (red). Specimens also labeled with TUNEL (green) to identify cells dying by apoptosis. Note strong green or yellow (overlap of green and red) TUNEL labeling in the nuclei of SGNs.
TUNEL staining was used to identify apoptotic cells in the Rosenthal’s canal. The photomicrograph in Figure 6B shows SGNs labeled with neuronal class III β-tubulin and a secondary antibody conjugated to Alexa Fluor 555. At this time, Rosenthal’s canal was filled with many large diameter SGNs intensely labeled with β-tubulin. However, many SGNs showed intense green or yellow (overall green plus red) TUNEL labeling within and around the nucleus and to a lesser extent the surrounding cytoplasm.
Discussion
Rats are widely used in studies of ototoxicity, noise-induced hearing loss and neural regeneration and repair (Chen and Fechter, 2003, Crofton et al., 1990, Dormans et al., 1996, Hu et al., 2004, 2005, Kamiya et al., 2007, Rybak et al., 1999). However, the toxic effects of ouabain have yet to be examined in this commonly used species. The current study shows that when ouabain is acutely applied to the round window membrane of the rat, the hair cells and SGNs are damaged in a dose and location dependent manner. Application of 1 mM ouabain for 30 minutes caused little or no damage to the hair cell (Fig. 3). Consistent with these anatomical findings, DPOAEs, which reflect the functional status of the OHCs, remained normal except for a slight amplitude reduction at 32 kHz (Fig. 2) (Hofstetter et al., 1997, Li et al., 2011). Despite the lack of hair cell damage, the 1 mM dose caused a significant loss of SGNs and nerve fibers in the hook and upper basal turn of the rat cochlea (Fig. 4–5; Table 1 and 2). This neuronal loss was associated with a significant increase in ABR thresholds at 20 and 32 kHz and a slight thresholds increase at 16 kHz. Thus, 30 minute treatment with 1 mM ouabain selectively damages SGNs and nerve fibers in the base and middle turn of the rat cochlea; however, at this concentration, only about a third of SGNs are destroyed. On the other hand, when 10 mM ouabain was applied to the round window, there was a significant loss of SGNs and hair cells. Hair cell loss was greatest near the base of the cochlea and decreased towards the apex. OHC loss was generally greater than IHC loss (Fig. 3), a pattern similar to that seen with other ototoxic drugs (Crofton et al., 1994, Forge, 1985). Ouabain-induced OHC loss was associated with a significant decline in DPOAE amplitude; this decrease was most severe at the high frequencies and less at low frequencies consistent with the base to apex gradient of OHC loss (Fig. 2–3). The 10 mM dose of ouabain destroyed nearly all the SGNs and nerve fibers in the hook and upper basal turn and more than half of those in the second turn (Table 1 and 2; Fig. 4D). Finally, ouabain-induced cell death appeared to be largely apoptotic as reflected by condensation of many hair cell nuclei (Fig. 5A) and TUNEL positive labeling of SGNs (Fig. 5B).
Species Differences
The literature and current results suggest that the exact nature of the cochlear lesion and hearing impairments induced by ouabain vary substantially across species, dose and duration of drug treatment, and method of drug delivery. When ouabain is continuously perfused into the guinea pig cochlea, it causes a sudden and significant drop in the EP, cochlear microphonic potential (CM) and CAP (Konishi and Mendelsohn, 1970, Kuijpers et al., 1967). In cases where ouabain is applied to the chinchilla round window membrane, it also causes a decline in the EP (Rybak et al., 1984). Low concentrations of ouabain (1 m) applied to the gerbil round window membrane mainly depress the EP and CAP while higher concentrations (10 mM) cause a decline in the EP, DPOAE and CAP (Schmiedt et al., 2002). When ouabain is applied for a limited time, the EP generally recovers; recovery most likely occurs because of drug diffusion (Schmiedt et al., 2002). The effects of prolonged, low-dose application of ouabain vary with species. If ouabain is slowly infused onto the gerbil round window membrane for 12–24 h (0.25 µl/h; 1 mM ouabain), DPOAEs, EPs and hair cells remain normal, but high-frequency CAP thresholds are elevated significantly and many basal turn SGNs are damaged (Schmiedt et al., 2002). If the same treatment is continued for 4–8 days, DPOAEs, EPs and hair cells again remain normal, but now CAPs are abolished at all frequencies and nearly all SGNs were missing. Results similar to this have been reported when ouabain-soaked Gelfoam was applied to the round window membrane of the gerbil for 24–96 h or if ouabain was applied to the round window of gerbils or mice for at least 1 h (Lang et al., 2011, Matsuoka et al., 2007, Wang et al., 2006). In contrast, when ouabain was applied to the guinea pig round window membrane once or twice a day for 2–3 days, damage was largely confined to the OHC and spiral limbus fibrocytes with occasional loss of IHC and some edema of nerve fibers beneath the IHC (Hamada and Kimura, 1999). Our new findings for the rat fall somewhere between those reported for gerbils and mice versus guinea pigs. The 1mM dose of ouabain mainly destroyed SGNs in the base of the rat cochlea similar to what has been seen in gerbils and mice; however, the damage was smaller and less widespread than that seen in gerbils and mice. Possible explanations for this include the fact that (1) ouabain was not replaced at regular intervals thereby reducing its long-term average concentration and (2) the duration of treatment was shorter, limiting the range of diffusion and duration of trauma. On the other hand, the 10 mM dose of ouabain caused extensive damage to hair cells and SGNs, mimicking the hair cell damage seen in guinea pigs as well as that seen in gerbils at very high concentration. Future parametric studies in rat that seek to cause greater SGN loss without damaging hair cells might benefit from low concentration treatments lasting an hour or more since these seem to be most effective in preferentially damaging SGNs in mice and gerbils (Corrales et al., 2006, Lang et al., 2011, Schmiedt et al., 2002).
The lack of ouabain-induced SGN loss in guinea pigs may be related to the intermittent nature of ouabain application or the location of the hair cells and SGNs with respect to the round window membrane. We speculate that major anatomical differences between these two species are likely to play a major role. In particular, the round window membrane in the gerbil is recessed and lies against the external wall of the modiolus (Chamberlain, 1977, Sokolich et al., 1976). Because of the close proximity of the round window membrane to the auditory nerve, the concentration of ouabain at the auditory nerve would be expected to be much higher than at the hair cells since the drug must diffuse through the perilymph to reach the organ of Corti. However, in guinea pigs, ouabain would have to pass through the round window membrane into the fluid space of scala tympani, leading to a decrease in its concentration, and then in diffuse in parallel towards the hair cells in the organ of Corti and the SGNs ensconced within the bony modiolus. Another factor that could contribute to these differences are variations in Na+/K+-ATPase abundance or the subtypes of Na+/K+-ATPase expressed in different species, cell types or locations along the cochlea. Cells that highly express Na+/K+-ATPase, especially those containing the α3 subunit, may be most sensitive to ouabain (Betts et al., 1997, Hara et al., 1988, Schmiedt et al., 2002, Urayama and Sweadner, 1988).
Our results as well as those in the gerbil indicate the SGNs and hair cells in the base of the cochlea are more easily damaged than those in the apex of the cochlea. A major factor underlying this gradient of cell loss is diffusion of ouabain through round window and the resulting decline in its concentration from the base towards the apex. However, another potential contributing factor is the intrinsic gradient of protective antioxidant enzymes which are lower in the base of the cochlea than the apex, making the basal hair cells, and possibly SGNs, more vulnerable to ototoxic injury (Sha et al., 2001).
Ouabain and Cell Death
Morphologically, necrotic death is characterized by swelling of the cell body and mitochondria content, appearance of vacuoles in the cytoplasm and rupture of the cell membrane. Apoptotic cell death, on the other hand, is characterized by shrinkage of the cell body, nuclear condensation and fragmentation and DNA cleavage. The signaling mechanisms underlying ouabain-induced apoptosis are not fully understood and may vary with cell type and drug concentration. Previous studies indicate that ouabain-induced death of cultured neurons occurs through a combination of apoptosis and necrosis (Xiao et al., 2002). On the other hand our results (Fig. 6B), and those of others, indicate that SGN damage induced by ouabain clearly involves apoptotic mechanisms as reflected by TUNEL labeling (Lang et al., 2005, Schmiedt et al., 2002). The current study also shows that apoptosis plays an important role in ouabain-induced hair cell death as illustrated by their highly condensed and fragment nuclei (Fig 6A). We found little evidence of swollen SGNs or hair cells in our material (Fig. 4, 6); however, necrotic features such as this could have been missed since we did not examine the inner ears until two days or more following ouabain treatment. Therefore, we cannot rule out the possibility of necrotic cell death in the inner ear since one report mentions necrosis of SGNs following acute application of ouabain to gerbil round window membrane (Wang et al., 2006).
In summary, the use of ouabain to partially (rat) or completely (gerbil) eliminate SGNs while retaining the hair cells provides auditory neuroscientists with an extremely powerful model to study the neural or perceptual correlates of auditory neuropathy, a hearing disorder that involves the loss of SGNs (Harrison, 1998) and to test the ability of transplanted stem cells to differentiate into neurons that reconnect the sensory hair cells in the cochlea with second order neurons in the brainstem (Lang et al., 2008, Matsuoka et al., 2007). The partial SGNs lesions seen in rats treated with 1 mM could prove to be especially useful to those interested in studying the perceptual deficits in animal model that mimics the human condition of auditory neuropathy characterized functionally by near normal hearing thresholds, cochlear microphonic potential and otoacoustic emissions, but abnormal temporal resolution, impaired discrimination in noise and low amplitude or absent ABR beginning with wave I (Starr et al., 2003, Starr et al., 1996, Starr et al., 2001, Zeng et al., 1999). Interestingly, some auditory neuropathy patients exhibit severe loss of spiral ganglion neurons with r etention of IHC and most OHC (Starr et al., 2003). Since there is a partial loss of SGNs in the high frequencies, hearing performance in the high frequency regions where SGNs are missing could be compared to those in the low frequencies where SGNs remain intact. While many patients have been diagnosed with auditory neuropathy, the histopathologic conditions that can give rise to this functional classification are poorly understood in both humans and animal models (Harrison, 1998, Palmgren et al., 2010, Rapin and Gravel, 2003, Salvi et al., 1999, Shaia et al., 2005). Further work is needed to better characterized histopathological changes that occur in SGN, the surrounding myelin sheath and the synaptic complex between IHC and SGN in humans and animal models of auditory neuropathy (El-Badry et al., 2007, Tang et al., 2006, Tekin et al., 2005).
Finally, there is growing evidence that the central auditory system undergoes neuroplastic changes to compensate for the loss of neural inputs from the periphery (Kraus et al., 2009, Salvi et al., 2000). The rat ouabain model described here could serve as a useful model for studying the physiological changes that occur at different stages of the central auditory pathway after eliminating only some of the SGNs, which provide the only pathway for delivering acoustic information to the brain.
Acknowledgments
Research supported in part from by NIH grants R01DC009091 and R01DC009219, Natural Science Foundation of China (No. 81070782), Qianjiang Talents Project of the Technology Office in the Zhejiang province (No. 2011R10014) and Natural Science Foundation of Ningbo (No. 2011A610042).
References
- Betts DH, MacPhee DJ, Kidder GM, Watson AJ. Ouabain sensitivity and expression of Na/K-ATPase alpha-and beta-subunit isoform genes during bovine early development. Mol Reprod Dev. 1997;46:114–126. doi: 10.1002/(SICI)1098-2795(199702)46:2<114::AID-MRD2>3.0.CO;2-T. (1997) [DOI] [PubMed] [Google Scholar]
- Chamberlain SC. Neuroanatomical aspects of the gerbil inner ear: light microscope observations. J Comp Neurol. 1977;171:193–204. doi: 10.1002/cne.901710205. (1977) [DOI] [PubMed] [Google Scholar]
- Chen GD, Fechter LD. The relationship between noise-induced hearing loss and hair cell loss in rats. Hear Res. 2003;177:81–90. doi: 10.1016/s0378-5955(02)00802-x. (2003) [DOI] [PubMed] [Google Scholar]
- Chen GD, Kermany MH, D'Elia A, Ralli M, Tanaka C, Bielefeld EC, et al. Too much of a good thing: long-term treatment with salicylate strengthens outer hair cell function but impairs auditory neural activity. Hear Res. 2010;265:63–69. doi: 10.1016/j.heares.2010.02.010. (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales CE, Pan L, Li H, Liberman MC, Heller S, Edge AS. Engraftment and differentiation of embryonic stem cell-derived neural progenitor cells in the cochlear nerve trunk: growth of processes into the organ of Corti. J Neurobiol. 2006;66:1489–1500. doi: 10.1002/neu.20310. (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofton KM, Dean KF, Menache MG, Janssen R. Trimethyltin effects on auditory function and cochlear morphology. Toxicol Appl Pharmacol. 1990;105:123–132. doi: 10.1016/0041-008x(90)90364-z. (1990) [DOI] [PubMed] [Google Scholar]
- Crofton KM, Janssen R, Prazma J, Pulver S, Barone S., Jr The ototoxicity of 3,3'-iminodipropionitrile: functional and morphological evidence of cochlear damage. Hear Res. 1994;80:129–140. doi: 10.1016/0378-5955(94)90104-x. (1994) [DOI] [PubMed] [Google Scholar]
- Ding D, McFadden SL, Salvi R. Cochlear Hair Cell Densities and Inner-Ear Staining Techniques. In: Willott JF, editor. Handbook of Mouse auditory Research from Behavior to Molecular Biology. Boca Raton: CRC Press; 2001. pp. 189–204. [Google Scholar]
- Ding D, Roth J, Salvi R. Manganese is toxic to spiral ganglion neurons and hair cells in vitro. Neurotoxicology. 2011;32:233–241. doi: 10.1016/j.neuro.2010.12.003. (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Stracher A, Salvi RJ. Leupeptin protects cochlear and vestibular hair cells from gentamicin ototoxicity. Hear Res. 2002;164:115–126. doi: 10.1016/s0378-5955(01)00417-8. (2002) [DOI] [PubMed] [Google Scholar]
- Ding DL, McFadden SL, Wang J, Hu BH, Salvi RJ. Age- and strain-related differences in dehydrogenase activity and glycogen levels in CBA and C57 mouse cochleas. Audiology and Neuro-Otology. 1999a;4:55–63. doi: 10.1159/000013822. (1999a) [DOI] [PubMed] [Google Scholar]
- Ding DL, Wang J, Salvi R, Henderson D, Hu BH, McFadden SL, et al. Selective loss of inner hair cells and type-I ganglion neurons in carboplatin-treated chinchillas. Mechanisms of damage and protection. Ann N Y Acad Sci. 1999b;884:152–170. doi: 10.1111/j.1749-6632.1999.tb08640.x. (1999b) [DOI] [PubMed] [Google Scholar]
- Dormans JA, Peters-Volleberg GW, Dortant PM, Speijers GJ. Ototoxicity of tobramycin in young adult and old rats. Toxicology and Applied Pharmacology. 1996;136:179–185. doi: 10.1006/taap.1996.0022. (1996) [DOI] [PubMed] [Google Scholar]
- El-Badry MM, Ding DL, McFadden SL, Eddins AC. Physiological effects of auditory nerve myelinopathy in chinchillas. Eur J Neurosci. 2007;25:1437–1446. doi: 10.1111/j.1460-9568.2007.05401.x. (2007) [DOI] [PubMed] [Google Scholar]
- Forge A. Outer hair cell loss and supporting cell expansion following chronic gentamicin treatment. Hear Res. 1985;19:171–182. doi: 10.1016/0378-5955(85)90121-2. (1985) [DOI] [PubMed] [Google Scholar]
- Hamada M, Kimura RS. Morphological changes induced by administration of a Na+,K+-ATPase inhibitor in normal and hydropic inner ears of the guinea pig. Acta Otolaryngol. 1999;119:778–786. doi: 10.1080/00016489950180423. (1999) [DOI] [PubMed] [Google Scholar]
- Hara Y, Urayama O, Kawakami K, Nojima H, Nagamune H, Kojima T, et al. The third type of alpha-subunit of Na,K-ATPase. Prog Clin Biol Res. 1988;268A:73–78. (1988) [PubMed] [Google Scholar]
- Harrison RV. An animal model of auditory neuropathy. Ear Hear. 1998;19:355–361. doi: 10.1097/00003446-199810000-00002. (1998) [DOI] [PubMed] [Google Scholar]
- Hofstetter P, Ding D, Powers N, Salvi RJ. Quantitative relationship of carboplatin dose to magnitude of inner and outer hair cell loss and the reduction in distortion product otoacoustic emission amplitude in chinchillas. Hear Res. 1997;112:199–215. doi: 10.1016/s0378-5955(97)00123-8. (1997) [DOI] [PubMed] [Google Scholar]
- Hu Z, Ulfendahl M, Olivius NP. Central migration of neuronal tissue and embryonic stem cells following transplantation along the adult auditory nerve. Brain Res. 2004;1026:68–73. doi: 10.1016/j.brainres.2004.08.013. (2004) [DOI] [PubMed] [Google Scholar]
- Hu Z, Ulfendahl M, Olivius NP. NGF stimulates extensive neurite outgrowth from implanted dorsal root ganglion neurons following transplantation into the adult rat inner ear. Neurobiology of disease. 2005;18:184–192. doi: 10.1016/j.nbd.2004.09.010. (2005) [DOI] [PubMed] [Google Scholar]
- Ichimiya I, Adams JC, Kimura RS. Immunolocalization of Na+, K(+)-ATPase, Ca(++)-ATPase, calcium-binding proteins, and carbonic anhydrase in the guinea pig inner ear. Acta Otolaryngol. 1994;114:167–176. doi: 10.3109/00016489409126037. (1994) [DOI] [PubMed] [Google Scholar]
- Jamesdaniel S, Ding D, Kermany MH, Davidson BA, Knight PR, 3rd, Salvi R, et al. Proteomic analysis of the balance between survival and cell death responses in cisplatin-mediated ototoxicity. J Proteome Res. 2008;7:3516–3524. doi: 10.1021/pr8002479. (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya K, Fujinami Y, Hoya N, Okamoto Y, Kouike H, Komatsuzaki R, et al. Mesenchymal stem cell transplantation accelerates hearing recovery through the repair of injured cochlear fibrocytes. Am J Pathol. 2007;171:214–226. doi: 10.2353/ajpath.2007.060948. (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi T, Mendelsohn M. Effect of ouabain on cochlear potentials and endolymph composition in guinea pigs. Acta Oto-laryngologica. 1970;69:192–199. doi: 10.3109/00016487009123353. (1970) [DOI] [PubMed] [Google Scholar]
- Kraus KS, Ding D, Zhou Y, Salvi RJ. Central auditory plasticity after carboplatin-induced unilateral inner ear damage in the chinchilla: up-regulation of GAP-43 in the ventral cochlear nucleus. Hear Res. 2009;255:33–43. doi: 10.1016/j.heares.2009.05.001. (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers W, Bonting SL. Studies on (Na+-K+)-activated ATPase. XXIV. Localization and properties of ATPase in the inner ear of the guinea pig. Biochim Biophys Acta. 1969;173:477–485. doi: 10.1016/0005-2736(69)90012-1. (1969) [DOI] [PubMed] [Google Scholar]
- Kuijpers W, Van der Vleuten AC, Bonting SL. Cochlear function and sodium and potassium activated adenosine triphosphatase. Science. 1967;157:949–950. doi: 10.1126/science.157.3791.949. (1967) [DOI] [PubMed] [Google Scholar]
- Lang H, Li M, Kilpatrick LA, Zhu J, Samuvel DJ, Krug EL, et al. Sox2 up-regulation and glial cell proliferation following degeneration of spiral ganglion neurons in the adult mouse inner ear. J Assoc Res Otolaryngol. 2011;12:151–171. doi: 10.1007/s10162-010-0244-1. (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang H, Schulte BA, Goddard JC, Hedrick M, Schulte JB, Wei L, et al. Transplantation of mouse embryonic stem cells into the cochlea of an auditory-neuropathy animal model: effects of timing after injury. J Assoc Res Otolaryngol. 2008;9:225–240. doi: 10.1007/s10162-008-0119-x. (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang H, Schulte BA, Schmiedt RA. Ouabain induces apoptotic cell death in type I spiral ganglion neurons, but not type II neurons. J Assoc Res Otolaryngol. 2005;6:63–74. doi: 10.1007/s10162-004-5021-6. (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ding D, Jiang H, Fu Y, Salvi R. Co-administration of Cisplatin and Furosemide Causes Rapid and Massive Loss of Cochlear Hair Cells in Mice. Neurotox Res. 2011 doi: 10.1007/s12640-011-9244-0. (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka AJ, Kondo T, Miyamoto RT, Hashino E. Enhanced survival of bone-marrow-derived pluripotent stem cells in an animal model of auditory neuropathy. Laryngoscope. 2007;117:1629–1635. doi: 10.1097/MLG.0b013e31806bf282. (2007) [DOI] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Burkard RF, Jiang H, Reaume AG, Flood DG, et al. Cu/Zn SOD deficiency potentiates hearing loss and cochlear pathology in aged 129,CD-1 mice. J Comp Neurol. 1999;413:101–112. (1999) [PubMed] [Google Scholar]
- McLean WJ, Smith KA, Glowatzki E, Pyott SJ. Distribution of the Na,K-ATPase alpha subunit in the rat spiral ganglion and organ of corti. J Assoc Res Otolaryngol. 2009;10:37–49. doi: 10.1007/s10162-008-0152-9. (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M. Frequency representation in the rat cochlea. Hear Res. 1991;51:247–254. doi: 10.1016/0378-5955(91)90041-7. (1991) [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Spicer SS, Schulte BA. Ultrastructural localization of Na,K-ATPase in the gerbil cochlea. J Histochem Cytochem. 1995;43:981–991. doi: 10.1177/43.10.7560888. (1995) [DOI] [PubMed] [Google Scholar]
- Palmgren B, Jin Z, Ma H, Jiao Y, Olivius P. beta-Bungarotoxin application to the round window: an in vivo deafferentation model of the inner ear. Hear Res. 2010;265:70–76. doi: 10.1016/j.heares.2010.02.009. (2010) [DOI] [PubMed] [Google Scholar]
- Qi W, Ding D, Salvi RJ. Cytotoxic effects of dimethyl sulphoxide (DMSO) on cochlear organotypic cultures. Hear Res. 2008;236:52–60. doi: 10.1016/j.heares.2007.12.002. (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran AK, Gopal J, Rajasekaran SA. Na,K-ATPase in the regulation of epithelial cell structure. Ann N Y Acad Sci. 2003;986:649–651. doi: 10.1111/j.1749-6632.2003.tb07276.x. (2003) [DOI] [PubMed] [Google Scholar]
- Rajasekaran AK, Rajasekaran SA. Role of Na-K-ATPase in the assembly of tight junctions. Am J Physiol Renal Physiol. 2003;285:F388–F396. doi: 10.1152/ajprenal.00439.2002. (2003) [DOI] [PubMed] [Google Scholar]
- Rapin I, Gravel J. "Auditory neuropathy": physiologic and pathologic evidence calls for more diagnostic specificity. International journal of pediatric otorhinolaryngology. 2003;67:707–728. doi: 10.1016/s0165-5876(03)00103-4. (2003) [DOI] [PubMed] [Google Scholar]
- Rybak LP, Husain K, Whitworth C, Somani SM. Dose dependent protection by lipoic acid against cisplatin-induced ototoxicity in rats: antioxidant defense system. Toxicol Sci. 1999;47:195–202. doi: 10.1093/toxsci/47.2.195. (1999) [DOI] [PubMed] [Google Scholar]
- Rybak LP, Wright LB, Whitworth C. Cochlear effects of locally applied inhibitors. Arch Otorhinolaryngol. 1984;240:207–213. doi: 10.1007/BF00453479. (1984) [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D-L, Stecker N, Arnold S. Auditory deprivation of the central auditory system resulting from selective inner hair cell loss: animal model of auditory neuropathy. Scandinavian Audiology. 1999;(Suppl. 51):1–12. (1999) [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000;147:261–274. doi: 10.1016/s0378-5955(00)00136-2. (2000) [DOI] [PubMed] [Google Scholar]
- Santarelli R, Cama E, Scimemi P, Dal Monte E, Genovese E, Arslan E. Audiological and electrocochleography findings in hearing-impaired children with connexin 26 mutations and otoacoustic emissions. Eur Arch Otorhinolaryngol. 2008;265:43–51. doi: 10.1007/s00405-007-0412-z. (2008) [DOI] [PubMed] [Google Scholar]
- Schmiedt RA, Okamura HO, Lang H, Schulte BA. Ouabain application to the round window of the gerbil cochlea: a model of auditory neuropathy and apoptosis. J Assoc Res Otolaryngol. 2002;3:223–233. doi: 10.1007/s1016200220017. (2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha SH, Taylor R, Forge A, Schacht J. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear Res. 2001;155:1–8. doi: 10.1016/s0378-5955(01)00224-6. (2001) [DOI] [PubMed] [Google Scholar]
- Shaia WT, Shapiro SM, Spencer RF. The jaundiced gunn rat model of auditory neuropathy/dyssynchrony. Laryngoscope. 2005;115:2167–2173. doi: 10.1097/01.MLG.0000181501.80291.05. (2005) [DOI] [PubMed] [Google Scholar]
- Sokolich WG, Hamernik RP, Zwislocki JJ, Schmiedt RA. Inferred response polarities of cochlear hair cells. J Acoust Soc Am. 1976;59:963–974. doi: 10.1121/1.380955. (1976) [DOI] [PubMed] [Google Scholar]
- Starr A, Michalewski HJ, Zeng FG, Fujikawa-Brooks S, Linthicum F, Kim CS, et al. Pathology and physiology of auditory neuropathy with a novel mutation in the MPZ gene (Tyr145->Ser) Brain. 2003;126:1604–1619. doi: 10.1093/brain/awg156. (2003) [DOI] [PubMed] [Google Scholar]
- Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain. 1996;119:741–753. doi: 10.1093/brain/119.3.741. (1996) [DOI] [PubMed] [Google Scholar]
- Starr A, Sininger Y, Nguyen T, Michalewski HJ, Oba S, Abdala C. Cochlear receptor (microphonic and summating potentials, otoacoustic emissions) and auditory pathway (auditory brain stem potentials) activity in auditory neuropathy. Ear Hear. 2001;22:91–99. doi: 10.1097/00003446-200104000-00002. (2001) [DOI] [PubMed] [Google Scholar]
- Tang W, Zhang Y, Chang Q, Ahmad S, Dahlke I, Yi H, et al. Connexin29 is highly expressed in cochlear Schwann cells, and it is required for the normal development and function of the auditory nerve of mice. J Neurosci. 2006;26:1991–1999. doi: 10.1523/JNEUROSCI.5055-05.2006. (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekin M, Akcayoz D, Incesulu A. A novel missense mutation in a C2 domain of OTOF results in autosomal recessive auditory neuropathy. Am J Med Genet A. 2005;138:6–10. doi: 10.1002/ajmg.a.30907. (2005) [DOI] [PubMed] [Google Scholar]
- ten Cate WJ, Curtis LM, Rarey KE. Na,K-ATPase alpha and beta subunit isoform distribution in the rat cochlear and vestibular tissues. Hear Res. 1994;75:151–160. doi: 10.1016/0378-5955(94)90066-3. (1994) [DOI] [PubMed] [Google Scholar]
- Urayama O, Sweadner KJ. Ouabain sensitivity of the alpha 3 isozyme of rat Na,K-ATPase. Biochem Biophys Res Commun. 1988;156:796–800. doi: 10.1016/s0006-291x(88)80914-8. (1988) [DOI] [PubMed] [Google Scholar]
- Wang J, Ding D, Salvi RJ. Carboplatin-induced early cochlear lesion in chinchillas. Hear Res. 2003;181:65–72. doi: 10.1016/s0378-5955(03)00176-x. (2003) [DOI] [PubMed] [Google Scholar]
- Wang LE, Cao KL, Yin SK, Wang Z, Chen ZN. Cochlear function after selective spiral ganglion cells degeneration induced by ouabain. Chin Med J (Engl) 2006;119:974–979. (2006) [PubMed] [Google Scholar]
- Xiao AY, Wei L, Xia S, Rothman S, Yu SP. Ionic mechanism of ouabain-induced concurrent apoptosis and necrosis in individual cultured cortical neurons. J Neurosci. 2002;22:1350–1362. doi: 10.1523/JNEUROSCI.22-04-01350.2002. (2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng FG, Oba S, Garde S, Sininger Y, Starr A. Temporal and speech processing deficits in auditory neuropathy. Neuroreport. 1999;10:3429–3435. doi: 10.1097/00001756-199911080-00031. (1999) [DOI] [PubMed] [Google Scholar]