Abstract
The integrin α9β1 binds a number of extracellular matrix components to mediate cell adhesion, migration and tissue invasion. Although expressed in variety of normal human cells including endothelium, it is also expressed in cancer cells. We have previously shown that α9β1 binds VEGF-A to facilitate angiogenesis, an important component of the tumor microenvironment. Since α9β1 induces accelerated cancer cell migration, we wished to determine what role it played in cancer growth and metastasis. In this study we show that α9β1 expression induces molecular changes consistent with epithelial-mesenchymal transition. In addition, we found that α9β1 forms a tri-partite protein complex with β-catenin and E-cadherin, which dissociates following integrin activation and subsequent src and β-catenin phosphorylation. These findings were consistent in cells in which: α9β1 was exogenously over-expressed, or when its expression was suppressed in cancer cells endogenously expressing α9β1. These in vitro results are biologically significant since α9β1 expressing cancer cells induce greater tumor growth and metastases in mice as compared to cells without α9β1 expression or when integrin expression is suppressed. Furthermore, integrin α9β1 is expressed in primary human small cell lung cancer and patients having a high expression of α9β1 demonstrated significantly worse long term survival compared to patients with low α9β1 expression. These findings highlight a novel mechanism of integrin α9β1 function in human cancer.
Keywords: EMT, alpha9beta1 integrin, lung cancer, metastasis, cell migration
Introduction
Epithelial-mesenchymal transition (EMT) represents a well co-ordinated series of molecular changes in epithelial cells including loss of E-cadherin expression, increased vimentin and N-cadherin1 and rearrangement of cortical actin, imparting an increased migratory capacity to the resulting mesenchymal cells.2 The timing and extent of EMT is essential for fetal development but is also associated with diseases such as fibrosis and cancer metastasis.3,4 In tumors, EMT imparts a more aggressive phenotype, ultimately resulting in metastasis by direct local tissue invasion or distantly through blood vessels and lymphatics.5 The prototypic stimulus for EMT is TGF-β, which induces phosphorylation of β-catenin and subsequent activation of transcription factors including Snail.6 TGF-β is not alone in its ability to induce EMT and various other growth factors and transmembrane receptors have been implicated, including the integrins.1,5,7
Integrins are matrix adhesion receptors that can transduce bi-directional signals to regulate cancer cell migration and invasion facilitating mechanisms such as EMT, depending upon available ligand and their interactions with membrane receptors.8-12 Integrins α3β1, α5β1, and αvβ6 have all been shown to modulate TGF-β induced EMT through distinct mechanisms in a variety of tumor cell lines.3,13-15 EMT induces an increase in both αvβ6 and α5β1 expression to facilitate cell migration: αvβ6 through autocrine TGF-β activation,13 and α5β1 through binding of autocrine fibronectin.15 Integrin α3β1 facilitates cell-cell adhesion by formation of a complex with E-cadherin and β-catenin.16 In kidney and lung epithelium α3β1 complexes with E-cadherin and TGF-βR1 and its activation is required for β-catenin phosphorylation.3,14
Integrin α9β1 is expressed in human cancers and appears to correlate with higher grade tumors.17-19 Although we have recently shown that integrin α9β1 plays a role in lymphangiogenesis,20-23 and angiogenesis 24,25 the exact mechanisms by which it transduces a pro-cancerous effect is unclear. Since integrins have been implicated in EMT4 and having shown that α9β1 induces accelerated cancer cell migration through src, a signaling intermediate implicated in cancer metastasis,26,27 we postulated that α9β1 might promote carcinoma growth and metastasis via src mediated EMT.
Results
Exogenously expressed α9β1 promotes molecular and cytoskeletal changes consistent with EMT
We have previously used SW480 colon carcinoma cells7 transfected with integrin α9β125 and observed that, compared to mock cells, α9-transfected SW480 (Fig 1A) displayed a more spindle shape, suggestive of a mesenchymal phenotype. Immunoflourescent staining (Fig 1A) and immunoblots of cell lysates (Fig 1B) showed that compared to mock, SW480-α9 expressed less E-cadherin, more α-SMA and vimentin and demonstrated loss of cortical F-actin; all molecular changes consistent with EMT. These molecular changes increased significantly with α9β1 activation, and were associated with increased expression of the transcriptional repressor Snail (Fig. 1C), further supporting the notion that α9β1-induces EMT. In contrast, SW480-mock did not demonstrate these changes when activated by the common integrin ligand, plasma fibronectin (pFN). Transient α9-overexpression in other cancer cell lines also resulted in similar EMT changes measured by immunoblotting (Suppl. Fig-S1A) and immunocytochemistry (Suppl. Fig-S1B); helping to rule out the confounding effect of cell type.
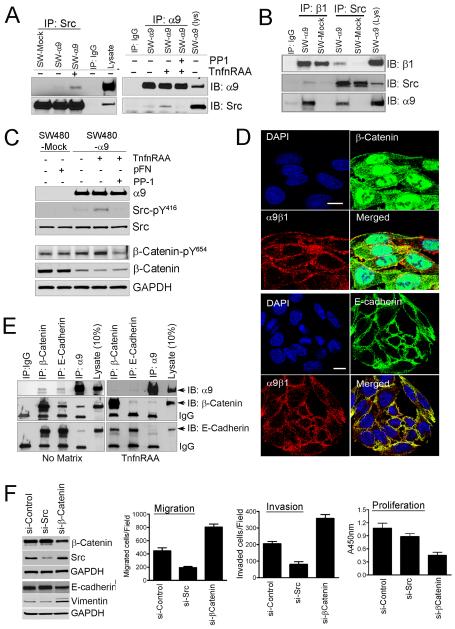
Figure-1. α9β1 promotes phenotypic and functional changes of EMT.
A, Top: Phase contrast photomicrographs comparing SW480-Mock and SW480-α9 cells following 36h of serum depletion; 2nd-4th panels: immunoflourescent photomicrographs of SW480-Mock and α9 cells for E-cadherin, α-SMA or cytoskeletal F-actin. Cell nuclei stained blue with DAPI. B, Immunoblots of SW480-mock and α9 lysates for α9 and markers of EMT: E-cadherin, α-SMA and vimentin. C, Immunoblots of SW480-mock and α9 lysates grown in the absence or presence of activating matrix, as indicated, demonstrating the relative expression of the α9 integrin subunit and markers of EMT: E-cadherin, N-cadherin, α-SMA, vimentin and Snail. D, Chemotaxis and haptotaxis assays with activating matrices: pFN or TnfnRAA; using SW480-mock (grey) or SW480-α9 (black). E, Invasion assays: Left panel, SW480-mock or α9 cells after 24h or 36h growth; Right panel, SW480-α9 cells in absence (grey) or presence of TnfnRAA (black), with or without α9β1 inhibitors Y9A2 or VLO5.
α9β1 expressing cancer cells demonstrate increased migration and invasion in vitro
The functional consequences of EMT include increased cell migration, invasion and anchorage independent growth; all of which were increased with expression of α9β1. Figure 1D shows that α9β1 expression increases both haptotaxis and chemotaxis of SW-480 cells; which were associated with an increased cellular expression of N-cadherin and loss of cortical actin, both markers of increased migratory capacity (Figure 1C). Furthermore, expression of α9β1 was associated with a time dependent increase in invasion, increasing further when cells were treated with α9β1 ligand and significantly decreased by α9-specific inhibitors (Fig 1E). Lending further support to the notion that α9β1 induces a transformed phenotype, SW480-α9 were found to have increased anchorage independent growth (Suppl. Fig S1-C). Taken together, these results suggest that α9β1 can induce a more aggressive cancer cell phenotype by inducing EMT.
Activation of α9β1 results in src dependent phosphorylation of β-catenin
Having recently shown src is essential for α9β1 signaling28 and knowing src is implicated in EMT29 we investigated its role in α9β1-associated EMT. Fig 2A shows that association of src with integrin α9β1 is facilitated by α9β1 ligand and depends on the α9 subunit and not β1 (Fig 2B). During EMT there is loss of cell-cell contact resulting from β-catenin phosphorylation and disruption of the β-catenin/E-cadherin complex at the adherens junction.13 Fig 2C shows that ligation of α9β1 induces phosphorylation of src-Y416 and also β-catenin-Y654, the tyrosine phosphorylation site associated with EMT.14 Src also mediated α9β1-induced β-catenin phosphorylation and changes in E-cadherin and vimentin expression consistent with EMT (Suppl. Fig S2-A). In non-α9 expressing SW480 neither src nor β-catenin phosphorylation were significantly induced by pFN, suggesting other β1-integrins are not playing a role (Fig 2C).
Figure-2. α9β1 co-associates with E-cadherin and β-catenin inducing EMT through src signaling.
A, Immunoblots for α9 or src from immunoprecipitates of SW480 cells cultured with or without the α9β1 specific ligand, TnfnRAA and/or the src inhibitor, PP1. B, Immunoblots for β1, α9 or Src from immunoprecipitates of matrix activated SW-480-mock (pFN) or SW480-α9 cells (TnfnRAA). C, Immunoblots for α9, Src or β-catenin in lysates from SW480-mock or α9 cells grown with pFN or TnfnRAA and/or PP1. D, Confocal images of SW480-α9 cells to determine co-localization of α9β1 (red) with β-catenin (green, top panel) or E-cadherin (green, bottom panel); nuclei stained blue with DAPI. E, Immunoblots, of immunoprecipitates from SW480-α9 grown on plastic (no matrix) or TnfnRAA (right panel), to determine co-association of α9 with β-catenin or E-cadherin. F, Left panel, Immunoblots for β-catenin, src, E-cadherin or vimentin in lysates from SW480-α9 cells transfected with non-targeted siRNA or siRNA targeted to src or β-catenin; Right panel: Migration (left), invasion (middle) or proliferation (right) assays in SW480-α9 cells following transfection with siRNA as indicated.
α9β1 forms a complex with E-cadherin and β-catenin, which dissociates following α9β1 activation
Similar to other integrins,3,14 we found that α9β1 co-localizes with both β-catenin and E-cadherin on the cell surface (Fig 2D). This co-localization is disrupted following α9β1 activation in SW480-α9 cells (Fig 2E) and appears to be mediated by src (Suppl. Fig S2-C). Although the dissociation of this tripartite protein complex may be secondary to loss of E-cadherin function, when taken together our results appear to support a direct effect of α9β1-associated EMT. Furthermore, targeted knockdown of β-catenin in SW480-α9 cells decreased E-cadherin and increased vimentin expression and were associated with functional changes consistent with EMT: increased cell migration, invasiveness and decreased proliferation (Fig 2F). In contrast, expression of E-cadherin and vimentin did not change significantly when cells were exposed to src-targeted siRNA, and migration and invasion were significantly less (Fig 2F, left panel).
β-catenin is also a component of the Wnt signaling pathway which is associated with EMT.30,31 Our data shows that wnt signaling does not appear to play a role in α9β1-associated EMT (Suppl Fig S3). Blocking wnt signaling either by Dickkof1 (Dkk1), a Wnt signaling antagonist32 or by siRNA-mediated knockdown of lipoprotein receptor-related protein-6 (LRP6), the major wnt co-receptor, did not show any effect on α9β1 mediated pro-EMT changes in E-cadherin or vimentin (Suppl Fig S3A,B,D) or on the invasiveness of cancer cells (Suppl Fig S3C,E).
α9β1-associated EMT is not modulated by other EMT-associated integrins or TGF-β
Since integrins α3β1 and αvβ6 are known to regulate EMT through β-catenin phosphorylation13,14 we next determined if these integrins might influence α9β1-associated EMT. Using flow cytometry we found minimal expression of αVβ6, no differential expression between SW480-mock and α9 cells (Suppl. Fig S4-A) and that expression was unaltered by culture conditions, type of matrix (Suppl. Fig S4-A) or cell culture time (data not shown). Integrins α3β1 and αvβ6 modulate EMT through their interactions with TGF-β, and modulating downstream SMAD2 signaling.13,14 However, we found no difference in SMAD2 phosphorylation in SW480-mock or SW480-α9 at baseline or following stimulation by ligand: pFN (common ligand to α3β1 and αvβ6) or α9-specifc ligand, TnfnRAA (Suppl. Fig S4-B). Furthermore, α9β1 expression in cancer cells did not alter TGF-β induced EMT (Suppl Fig S4-C) nor did TGF-β change cellular expression of α9β1 (Suppl Fig4D). Taken together these results suggest that unique to the integrins, α9β1 can induce EMT independent of TGF-β.
In cells expressing α9β1, total β-catenin is significantly decreased (Fig 2C) suggesting that β-catenin is either trafficking to the nucleus or undergoing proteasomal degradation. Supporting this notion, we found proportionally greater accumulation of nuclear β-catenin in SW480-α9 compared to mock cells suggesting that at least in part, there is increased transcriptional activity (Suppl. Fig S5-A). This is further supported by the increased proliferative capacity of SW480-α9, which can be blocked by inhibiting α9β1 (Suppl. Fig S5-B,C).
α9β1 promotes tumor growth, vasculogenesis, and metastasis
We next performed tumor experiments to determine the pro-carcinogenic effects of α9β1 in-vivo. Tumors formed in mice from flank injection of SW480-α9 cells were up to 3 times larger than tumors formed from SW480-mock cells (Fig 3A), were associated with increased intra-tumoral angiogenesis and lymphangiogenesis (Fig 3B) and displayed altered cadherin and vimentin expression consistent with EMT (Fig 3C).
Figure-3. α9β1 is associated with increased tumor growth, EMT and cancer metastasis.
A, Volume of mouse flank tumors formed following subcutaneous injection of SW480-mock (n=5) or SW480-α9 (n=5) cells. B, Top panel: representative photomicrographs of tumor sections stained for the endothelial cell marker CD31 or the lymphatic marker, D2-40; Bottom panel: quantitative analysis of intra-tumoral blood vessels counted in 1 view field (10X) (n=3 per cell type). C, Immunoblots showing relative expression of α9, CD31, EMT-associated cadherin proteins and vimentin in 3 separate tumors derived from SW480-mock or α9 cells. D, Left panel: representative images of lymph nodes from mice injected with SW480-mock or α9 cells; Right top panels: photomicrographs showing lung metastases grossly (arrow) or H&E stained; Bottom right panels: H&E stained liver sections demonstrating metastases at low (40x) and high power (400X).
Also consistent with an EMT phenotype, animals injected with SW480-α9 cells demonstrated significantly greater metastasis with marked inguinal lymphadenopathy and widespread lung and liver metastases (Fig 3D). In contrast, only infrequent metastases could be detected in mice injected with SW480-mock cells (data not shown).
Endogenous α9β1 mediates EMT in lung carcinoma cells and increases cell migration and invasiveness
Having shown that EMT and metastatic cell behavior can be induced by the exogenous over-expression of α9β1, we next determined whether endogenously expressed integrin in lung cancer cells had a comparable action. Using flow cytometry (Fig 4A) and immunoblotting (Fig 4B) we found that α9β1 expression was variable but appeared more robust in human SCLC compared to NSCLC. In addition, LLC1, a mouse model cell line for lung carcinoma, also expressed integrin α9β1. Compared to normal bronchial epithelium (NHBE), these cancer cell lines showed evidence of EMT, as measured by E and N-cadherin expression. However, those cells expressing α9β1 demonstrated relatively less expression of E-cadherin and higher expression of N-cadherin and the extent of colony formation by these cancer cells on soft agar was dependent on the extent of α9β1 expression (Suppl. Fig S6-A,B). Integrin α9β1 appears to induce a functionally relevant EMT phenotype in these lung cancer cells as demonstrated by increased α9β1-dependent adhesion (Fig 4C), migration (Fig 4D) and Matrigel invasion (Fig 4E). Although the overall extent of the response varied, they were mostly proportional to the magnitude of α9β1 expression.
Figure-4. α9β1 induces cellular changes of EMT and enhanced cancer cell migration and invasion.
A, Flow cytometry analysis showing the extent of α9β1 surface expression (shaded histograms) on cells as indicated; and isotype antibody control (clear histogram). B, Immunoblots for α9, β1, E-cadherin or N-cadherin in lysates from mouse (LLC-1) or human NSCLC and SCLC cell lines. C, Cell adhesion on plastic (no matrix) or TnfnRAA with (black) or without (grey) α9β1 inhibitor, VLO5. D, Cell migration in the presence (black) or absence (grey) of TnfnRAA and/or VLO5. E, Cell invasion using cells stimulated by TnfnRAA and treated with (black) or without (grey) VLO5. F, Immunoblots to detect the EMT markers E-cadherin and vimentin in lysates from cells grown on plastic (no matrix) or TnfnRAA or with TGF-β.
α9β1-associated EMT was dependent on the expression level of the integrin. The change in expression of E-cadherin and vimentin following α9β1 activation was comparable to that induced by TGF-β in high α9β1-expressing LLC-1, intermediate in the minimally expressing A549, while NHBE or H69 cells showed no significant change in expression of E-cadherin and vimentin (Fig 4F). Taken together these findings support a physiologically relevant role for endogenous α9β1 in the induction of EMT in human cancer cells in vitro.
Suppression of α9 expression decreases tumor growth and metastasis
Having shown that endogenously expressed α9β1 confers an EMT phenotype and more aggressive cancer attributes, we next assessed EMT when α9 expression was suppressed using α9-targeted lentiviral shRNA (Fig 5A). As expected α9 depleted LLC-1 demonstrated significantly decreased adhesion on TnfnRAA (Fig 5B) whereas cell adhesion to pFN, a non-α9β1 ligand, was not affected. Similarly, migration and invasion (Fig 5C, D) were significantly decreased in cells expressing α9-targeted shRNA suggesting an inhibitory effect on EMT.
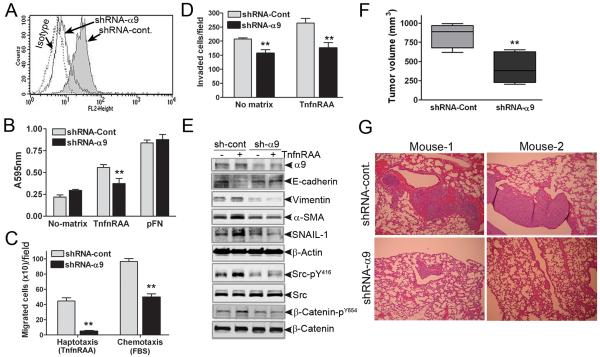
Figure-5. α9β1-induced malignant tumor growth and metastasis is inhibited followingα9 knockdown.
A, Flow cytometry for expression of α9β1 in LLC-1 cells transduced with non-targeted (shRNA-Cont) or α9β1-targeted (shRNA-α9) shRNA. B, Cell adhesion C, Haptotaxis (using α9β1-specific ligand, TnfnRAA) and chemotaxis (using 10% FBS) and D, Cell invasion assays in shRNA-Cont (grey bars) or shRNA-α9 (black bars) LLC-1 grown on plastic (no matrix) or TnfnRAA or pFN as indicated. E, Immunoblots to detect EMT and signaling proteins as indicated in lysates from LLC-1 cells expressing shRNA-cont (grey) or shRNA-α9, and grown with (+) or without (−) TnfnRAA. F, Volume of mouse flank tumors from subcutaneous injection of LLC-1 cells expressing shRNA-Cont (grey) or shRNA-α9 (black) ** p<0.01. G, Representative photomicrographs of H&E stained lung (2 mice) showing metastatic lesions derived from LLC-1 cells expressing shRNA-cont or shRNA-α9.
This inhibition of adhesion, migration and invasion was accompanied by changes in EMT markers. Compared to control LLC-1, α9-depleted cells grown on α9β1-specific ligand, demonstrated neither a decrease in E-cadherin nor an increase in vimentin, αSMA or Snail, suggesting that the process of α9β1 induced EMT was blocked (Fig 5E). Furthermore, the α9β1 specific ligand, TnfnRAA, did not significantly activate the pro-EMT signaling intermediates, Src or β-catenin. Taken together these findings provide further support for α9β1 inducing physiologically relevant EMT through src signaling.
The relevance of α9β1-associated EMT in vitro was confirmed by in vivo tumor studies using shRNA to deplete LLC1 cells of α9. Fig 5F demonstrates that tumors formed by α9-depleted cells (shRNA-α9), were at least half the size of those formed from cells with endogenous expression of α9β1 (shRNA-Cont). Furthermore, cancer cell metastasis was negligible in mice injected with α9-depleted LLC-1 compared to those injected with cells expressing α9β1 (Fig 5G).
High expression of integrin α9β1 in human small cell lung cancer (SCLC) is a prognostic indicator of poor survival
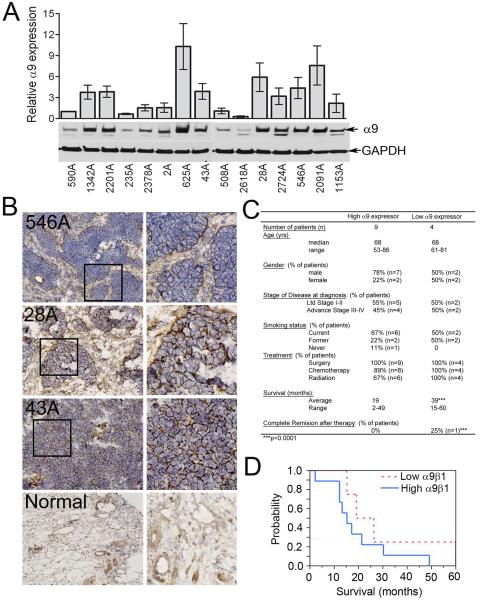
Based on our in vitro and in vivo data, we postulated that the extent of α9β1 expression in SCLC might correlate with the severity of disease. We analyzed SCLC tissue from fifteen patients and found variable α9β1 expression both by immunoblot (Fig 6A) and RT-PCR (data not shown). Tumors from three separate patients, were also used for immunohistochemistry which confirmed the expression of α9β1 on the surface of SCLC cells (Fig 6B). Subsequently, we were able to separate patients based on the extent of α9β1 expression in their tumor tissue: eleven high expressers and four low expressers. Fig 6C tables the characteristics of the patients in relation to the degree of α9β1 activity and is highlighted by significant differences in stage of disease and survival time. The average survival for patients with high α9β1 was 19 mo compared to 30 mo in patients with low α9β1 expression (p<0.001), (Fig 6D).
Figure-6. High expression of α9β1 in human SCLC predicts shorter patient survival.
A, Immunoblot of lysates from fresh frozen SCLC samples for detection of α9β1; densitometric quantification from 3 separate immunoblots. B, Representative photomicrographs (20X) of SCLC samples (3 patients) stained to detect α9β1. Bottom panels are normal lung controls. C, Patient characteristics of tumors with high or low α9β1 expression. D, Kaplan-Meyer plot comparing survival in patients with high or low α9β1 expression.
Discussion
The cellular mechanisms that induce EMT are complex3,5,26 and a role of integrins has been well established under the paradigm of TGF-β induced EMT.33 In contrast, our findings show that in cancer cells, α9β1 expressed exogenously or endogenously can trigger EMT, in a TGF-β independent fashion and that expression of α9β1 and its pro-EMT effects are not modulated by TGF-β. These results suggest there is at least one distinct integrin pathway regulating EMT; which is not surprising since tumor stroma provides a rich supply of integrin ligands.34 Although we did not examine expression of all integrins, our findings also suggest that α9β1 does not require or promote the increased expression of other known pro-EMT integrins such as αvβ6 or α3β1. Other transmembrane receptors are known to partner with integrins to induce EMT without a growth factor stimulus7 such as the serine protease TMPRSS4, which requires the synergistic action of active integrin α5β1 (ref. 35). Whether α9β1 functions in concert with other transmembrane proteins to facilitate EMT is yet to be determined.
Integrin α9β1 also distinguishes itself from other integrins such as α5β1 and α3β1 since its pro-EMT effect is transduced without a requirement for a change in expression. Following ras transformation of mammary epithelial cells the resulting increased expression of α5β1 does not initiate EMT,15 and expression of α3β1 alone is not sufficient to induce EMT as suggested by α3-null alveolar cells which express similar amounts of E-cadherin to α3 expressing cells.14 Although TGF-β does induce increased expression of αvβ6, it is unknown whether the exogenous expression of αvβ6 might itself induce EMT. However, this would seem unlikely since the function of increased αvβ6 is to activate autocrine derived TGF-β.13 Whether integrin α9β1 induces autocrine TGF-β production and presumably activation of TGF-β receptor is yet to be determined but seems unlikely since we found no significant SMAD2 activation under our experimental conditions.
Our findings implicate src as a signaling intermediate for α9β1-associated EMT acting by facilitating disassociation of E-cadherin and β-catenin, which is consistent with previous reports showing src induced effects on the cytoskeleton and E-cadherin/β-catenin.29 The relevance of these molecular effects was confirmed by the inhibition of cancer cell migration and invasion, both functional EMT effects. In human carcinomas the role of src is well established with more than half of lung carcinomas demonstrating aberrantly activated src.36. Further cancer research should focus on determining whether co-expression of α9β1 directly contributes to the deregulation of src activity or if α9β1 can induce EMT in cells lacking activated src.
The importance of α9β1 in human cancer is supported by reports of its expression in breast, brain and lung cancer.18,37,38 We show for the first time a clinical relevance for high expression of α9β1 in SCLC namely a decreased patient survival by 11 months. These findings are tempered by the low number of patients included in our study which reflects clinical practice in which few patients undergo surgical procedures for diagnosis or treatment. This not withstanding, our results suggest that future research should focus on confirming these findings. Taken together with our previous work it appears α9β1 may promote the cancer process through multiple mechanisms including effects on cell migration and invasion, induction of nitric oxide production,28 angiogenesis24 and lymphangiogenesis20,22 and now promotion of EMT. Our findings present cancer researchers with novel approaches to not only deciphering the carcinogenic trigger but also to better understand the interplay between the seemingly separate EMT mechanisms of TGF-β and integrin α9β1. Furthermore, our findings highlight the need for developing novel therapies targeting more than one pathogenic pathway and identify integrin α9β1 as a strong candidate for pharmacotherapy in human cancer.
MATERIALS AND METHODS
Materials
Mouse monoclonal antibodies to α9β1: Y9A2, NC4, polyclonal anti α9-serum and TNfnRAA (gift from Dr. Dean Sheppard, UCSF); mAb against murine α9β1 (R&D), β-Actin and GAPDH (Sigma), E-cadherin, N-cadherin and β-catenin (BD Biosciences), β-catenin-Y654 (Invitrogen). Rabbit polyclonal antibodies: Src-Y416, SMAD2-ser245/250/255, Src, vimentin, Snail, HDAC1, Erk2 and LRP6 (Cell Signaling), anti α3β1, αvβ6, anti-SMAD2 (Millipore); anti-αSMA (Santa Cruz Biotechnology), anti-CD31 (Dako), anti-podoplanin (D2-40, Covance). Phycoerythrin conjugated goat anti-mouse (Jackson Immunoresearch); Horseradish peroxidase conjugated anti-mouse and anti-rabbit antibodies (Amersham Biosciences). Plasma fibronectin (Chemicon), wnt3A and Dkk1 (R&D); TGFβ (Dr. E. Leof, Mayo Clinic), src inhibitor, PP1 (BioMol International), VLO5 (Dr. Cezary Marcinkiewicz, Temple University).
Cell culture
SW480-α9 or mock cells were cultured as previously described.24,28 A549 and Lewis lung carcinoma cells (LLC-1) cultured in F12K/DMEM medium with10% FBS. All other human lung carcinoma cell lines (ATCC) cultured in RPMI with 10% FBS. Primary NHBE cells: specialized medium (Lonza).
siRNA, plasmids and cell transfection
Integrin α9-EGFP-N3 expression vector (Addgene, Cambridge-MA), siRNA oligonucleotides, negative control (Ambion) and siRNA to human β-Catenin, c-Src (Santa Cruz Biotechnology) and LRP6 (Cell Signaling) performed as described previously.28 Lentivirus packaging; ViraPower Lentiviral Expression System (Invitrogen, Life Technologies). Using Lipofectamine-2000, 293FT cells were co-transfected with ViraPower DNA mix and plasmid (pLKO.1-puro) encoding shRNA targeting murine α9-subunit (Mayo Clinic RNA Resource) and supernatants collected 48-72 h after transfection. LLC-1 cells were transduced in the presence of 6 μg/mL polybrene (Sigma-Aldrich), and stableg clones selected in 10 μg/mL puromycin.
Immunoprecipitation, SDS-PAGE and immunoblot analysis
Serum starved cells were trypsinized, washed with PBS and suspended in DMEM. In a 6-well plate, 1×106 cells were added to wells coated or not with 10 μg/ml TnfnRAA or pFN, incubated for various times and lysed in RIPA buffer as previously described.28 Protein lysates from fresh frozen tissue ~50mg were obtained by pulverizing in RIPA buffer. After 20 min incubation on ice, lysates were cleared by centrifugation and immunoprecipitation and immunoblotting performed as previously described.28
To detect nuclear translocation of β-catenin, nuclear and cytoplasmic proteins were extracted using ‘NE-PER Extraction Reagents (Thermo Scientific) and immunoblotted for β-catenin. Extraction purity was determined by immunobloting for Erk2 and HDAC1.
Fluorescence microscopy
Cells cultured on sterile glass cover slips were washed with PBS, fixed, and incubated with antibody in 5% normal goat serum (Upstate) at 4°C overnight. Cells were washed, incubated with anti mouse Alexa Fluor-594 (or -488) antibody (BD Biosciences) for 1 hr. The cytoskeleton of fixed cells was immunostained with Texas-Red phalloidin and coverslips mounted with Slowfade containing DAPI (Invitrogen). Images were acquired as previously described.28 Co-localization of integrin α9β1 with β-catenin (or E-cadherin) was determined using appropriate primary antibodies followed by incubation with Alexa-Fluor labeled secondary antibodies. Immunostained cells were analyzed by confocal microscopy (Zeiss/SM510); 100x oil DIC objective with ~0.5 μm optical sectioning.
Flow cytometry
Single cell suspensions were rinsed and suspended in PBS at a density of 0.5-1×106 per ml, incubated with appropriate primary antibody for 30 min. Subsequent staining with phycoerythrin conjugated secondary antibody, acquisition and data analysis was performed as previously described.28
Cell adhesion assays
Cell adhesion assays were performed as previously described28 in flat bottom 96 well microtiter plates (ICN, Linbro/Titertek) coated with 10 μg/ml TnfnRAA or FN at 37°C for 1 hr, blocked with 1%BSA (Sigma) for 30 min. 5×104 cells suspended in basal medium were plated in each well and when indicated incubated with α9β1 inhibitors; Y9A2 (20 μg/ml) or 50 μM VLO5 for 30 min.
Cell migration and invasion assays
Cell migration assays were performed as previously described.24,28 Haptotaxis: 10 μg/ml of TnfnRAA or FN for 1 hr at 37°C; chemotaxis: medium with 10% FBS. 5×104 cells were added to the top chamber, with 30min inhibitor incubation. Migration time of 12-16 hrs at 37°C. Invasion assays were performed using BD BioCoat (8 μm pore size) Matrigel chambers (Becton Dickinson-BD) as per the manufacturer’s instructions. Briefly, cells treated with or without inhibitors were placed in the top chamber and 10% FBS for chemoattractant. After 14-16-hrs (A549 and LLC-1) or 24-36 hrs (SW480, SCLC lines), invaded cells were fixed, stained (Hema-3 kit, Fisher Scientific) and counted in four separate view fields by light microscopy (Axiostar, Zeiss).
Cell proliferation assay
2×103 SW480-mock or SW480-α9 cells in full growth medium were added to 12-well plates (Costar), trypsinized and counted using a hemocytometer. For BrdU assays cells were grown overnight in 96-well plates, in the absence or presence of 50 μM VLO5 for 16 hrs. Medium containing BrdU was added for 2 hrs and uptake measured with ELISA (RPN 250, Amarsham Biosciences) as per the manufacturer’s instructions.
Soft agar assay
To prevent cell settling, bottom plugs (0.5 mL, 0.8% Sea Plaque-agarose, 10% FBS/DMEM; FMC Bioproducts) were casted in 12-well plates (Costar). Top plugs (1 mL, 0.4% agarose, 10% FBS/DMEM), and 103 cells with or without 50 μM VLO5 were incubated for14 d at 37°C. The number of colonies >50 μm diameter were scored using Gelcount (Oxford Optronics).
Animal tumor studies
All experiments were IACUC approved and animals cared for in accordance with IACUC guidelines. 1×106 SW480-mock or α9 cells were injected subcutaneously in the flank of Nude mice (n=5). After 35 days, tumor volume was measured (lxwxh/2). To study metastasis, cells were injected through the tail vein and on day 45 tissues harvested for histology. In other experiments 0.5×106 LLC-1 cells were transduced with lentivirus carrying control or α9-specific shRNA were injected subcutaneously in the flank (n=5) or tail vein (n=3) of nude mice.
Human lung cancer tissues
The Mayo Clinic IRB approved all human studies. Fresh frozen lung cancer specimens were from the Mayo Clinic Lung Cancer Specimen Registry. Fifteen SCLC samples were analyzed for expression of α9β1 and patient clinical characteristics. Histology was verified by a Board Certified lung pathologist (MCA). The patient clinical data was analyzed in a blinded fashion (BPW). Survival (months) was from diagnosis to death.
Histology and Immunohistochemistry
Mouse tissue was fixed in 10% formalin, embedded in paraffin and 5 μm sections stained with H&E or immunostained for PECAM1 (CD31) or podoplanin (D2-40). 5 Human SCLC samples and matched normal lung were immunostained with α9β1 specific rabbit antibody (1:250) overnight at 4°C followed by biotinylated goat anti-rabbit secondary antibody (1:200), streptavidin-peroxidase (1:100), then 3,3′-diaminobenzidine (Amresco) and peroxide. Slides were rinsed, counterstained with hematoxylin, dehydrated and mounted. Images were obtained by scanning laser microscopy (Axiostar, Zeiss) or with NanoZoomer Digital Pathology software (Hamamatsu).
Statistical methods
Unless otherwise indicated data presented as mean ± SE from 3 or more experiments; p values determined using paired student’s T-tests. Immunoblots were performed at least 3 times and representative examples presented. Statistical analysis of patient survival and clinical characteristics was performed using multivariate analysis with a p-value <0.05.
Supplementary Material
Acknowledgements
We thank Drs. Dean Sheppard (UCSF, CA), Debabrata Mukhopadhyay and Edward Leof (Mayo Clinic, Rochester, MN) for helpful discussions; Mark A. Schroeder for help with animal studies; Aaron Bungam for the lung tissue registry. This work was supported by NHLBI Research Grant K08HL076455-05 and Mayo Foundation Research Grants to N.E.V.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website
References
- 1.Radisky DC. Epithelial-mesenchymal transition. J Cell Sci. 2005;118:4325–4326. doi: 10.1242/jcs.02552. [DOI] [PubMed] [Google Scholar]
- 2.Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 2006;1796:75–90. doi: 10.1016/j.bbcan.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Kim KK, Wei Y, Szekeres C, Kugler MC, Wolters PJ, Hill ML, et al. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest. 2009;119:213–224. doi: 10.1172/JCI36940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007;39:305–318. doi: 10.1080/00313020701329914. [DOI] [PubMed] [Google Scholar]
- 5.Tsuji T, Ibaragi S, Hu GF. Epithelial-mesenchymal transition and cell cooperativity in metastasis. Cancer Res. 2009;69:7135–7139. doi: 10.1158/0008-5472.CAN-09-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 7.Jung H, Lee KP, Park SJ, Park JH, Jang YS, Choi SY, et al. TMPRSS4 promotes invasion, migration and metastasis of human tumor cells by facilitating an epithelial-mesenchymal transition. Oncogene. 2008;27:2635–2647. doi: 10.1038/sj.onc.1210914. [DOI] [PubMed] [Google Scholar]
- 8.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 9.Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh P, Chen C, Pal-Ghosh S, Stepp MA, Sheppard D, Van De Water L. Loss of integrin alpha9beta1 results in defects in proliferation, causing poor re-epithelialization during cutaneous wound healing. J Invest Dermatol. 2009;129:217–228. doi: 10.1038/jid.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rathinam R, Alahari SK. Important role of integrins in the cancer biology. Cancer Metastasis Rev. 2010;29:223–237. doi: 10.1007/s10555-010-9211-x. [DOI] [PubMed] [Google Scholar]
- 13.Bates RC, Bellovin DI, Brown C, Maynard E, Wu B, Kawakatsu H, et al. Transcriptional activation of integrin beta6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J Clin Invest. 2005;115:339–347. doi: 10.1172/JCI23183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y, Kugler MC, Wei Y, Kim KK, Li X, Brumwell AN, et al. Integrin alpha3beta1- dependent beta-catenin phosphorylation links epithelial Smad signaling to cell contacts. J Cell Biol. 2009;184:309–322. doi: 10.1083/jcb.200806067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maschler S, Wirl G, Spring H, Bredow DV, Sordat I, Beug H, et al. Tumor cell invasiveness correlates with changes in integrin expression and localization. Oncogene. 2005;24:2032–2041. doi: 10.1038/sj.onc.1208423. [DOI] [PubMed] [Google Scholar]
- 16.Chattopadhyay N, Wang Z, Ashman LK, Brady-Kalnay SM, Kreidberg JA. alpha3beta1 integrin-CD151, a component of the cadherin-catenin complex, regulates PTPmu expression and cell-cell adhesion. J Cell Biol. 2003;163:1351–1362. doi: 10.1083/jcb.200306067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Timoshenko AV, Rastogi S, Lala PK. Migration-promoting role of VEGF-C and VEGF-C binding receptors in human breast cancer cells. Br J Cancer. 2007;97:1090–1098. doi: 10.1038/sj.bjc.6603993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown MC, Staniszewska I, Lazarovici P, Tuszynski GP, Del Valle L, Marcinkiewicz C. Regulatory effect of nerve growth factor in alpha9beta1 integrin-dependent progression of glioblastoma. Neuro Oncol. 2008;10:968–980. doi: 10.1215/15228517-2008-047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lydolph MC, Morgan-Fisher M, Hoye AM, Couchman JR, Wewer UM, Yoneda A. Alpha9beta1 integrin in melanoma cells can signal different adhesion states for migration and anchorage. Exp Cell Res. 2009;315:3312–3324. doi: 10.1016/j.yexcr.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 20.Huang XZ, Wu JF, Ferrando R, Lee JH, Wang YL, Farese RV, Jr., et al. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol Cell Biol. 2000;20:5208–5215. doi: 10.1128/mcb.20.14.5208-5215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kajiya K, Hirakawa S, Ma B, Drinnenberg I, Detmar M. Hepatocyte growth factor promotes lymphatic vessel formation and function. EMBO J. 2005;24:2885–2895. doi: 10.1038/sj.emboj.7600763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vlahakis NE, Young BA, Atakilit A, Sheppard D. The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin alpha9beta1. J Biol Chem. 2005;280:4544–4552. doi: 10.1074/jbc.M412816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bazigou E, Xie S, Chen C, Weston A, Miura N, Sorokin L, et al. Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell. 2009;17:175–186. doi: 10.1016/j.devcel.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vlahakis NE, Young BA, Atakilit A, Hawkridge AE, Issaka RB, Boudreau N, et al. Integrin alpha9beta1 directly binds to vascular endothelial growth factor (VEGF)-A and contributes to VEGF-A-induced angiogenesis. J Biol Chem. 2007;282:15187–15196. doi: 10.1074/jbc.M609323200. [DOI] [PubMed] [Google Scholar]
- 25.Oommen S, Gupta SK, Vlahakis NE. Vascular endothelial growth factor A (VEGF-A) induces endothelial and cancer cell migration through direct binding to integrin {alpha}9{beta}1: identification of a specific {alpha}9{beta}1 binding site. J Biol Chem. 2011;286:1083–1092. doi: 10.1074/jbc.M110.175158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wheeler DL, Iida M, Dunn EF. The role of Src in solid tumors. Oncologist. 2009;14:667–678. doi: 10.1634/theoncologist.2009-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aleshin A, Finn RS. SRC: a century of science brought to the clinic. Neoplasia. 2010;12:599–607. doi: 10.1593/neo.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta SK, Vlahakis NE. Integrin alpha9beta1 mediates enhanced cell migration through nitric oxide synthase activity regulated by Src tyrosine kinase. J Cell Sci. 2009;122:2043–2054. doi: 10.1242/jcs.041632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nam JS, Ino Y, Sakamoto M, Hirohashi S. Src family kinase inhibitor PP2 restores the E- cadherin/catenin cell adhesion system in human cancer cells and reduces cancer metastasis. Clin Cancer Res. 2002;8:2430–2436. [PubMed] [Google Scholar]
- 30.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 31.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signaling: Disease and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 32.Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nature Cell Biology. 2001;3:683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- 33.Borok Z. Role for alpha3 integrin in EMT and pulmonary fibrosis. J Clin Invest. 2009;119:7–10. doi: 10.1172/JCI38084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alphonso A, Alahari SK. Stromal cells and integrins: conforming to the needs of the tumor microenvironment. Neoplasia. 2009;11:1264–1271. doi: 10.1593/neo.91302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim S, Kang HY, Nam EH, Choi MS, Zhao XF, Hong CS, et al. TMPRSS4 induces invasion and epithelial-mesenchymal transition through upregulation of integrin alpha5 and its signaling pathways. Carcinogenesis. 2010;31:597–606. doi: 10.1093/carcin/bgq024. [DOI] [PubMed] [Google Scholar]
- 36.Song L, Morris M, Bagui T, Lee FY, Jove R, Haura EB. Dasatinib (BMS-354825) selectively induces apoptosis in lung cancer cells dependent on epidermal growth factor receptor signaling for survival. Cancer Res. 2006;66:5542–5548. doi: 10.1158/0008-5472.CAN-05-4620. [DOI] [PubMed] [Google Scholar]
- 37.Allen MD, Vaziri R, Green M, Chelala C, Brentnall AR, Dreger S, et al. Clinical and functional significance of alpha9beta1 integrin expression in breast cancer: a novel cell- surface marker of the basal phenotype that promotes tumour cell invasion. J Pathol. 2011;223:646–658. doi: 10.1002/path.2833. [DOI] [PubMed] [Google Scholar]
- 38.Hibi K, Yamakawa K, Ueda R, Horio Y, Murata Y, Tamari M, et al. Aberrant upregulation of a novel integrin alpha subunit gene at 3p21.3 in small cell lung cancer. Oncogene. 1994;9:611–619. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.