Summary
Long chain n3 fatty acids appear to have beneficial effects in several domains of health relevant to breast cancer survivors. This study evaluated inter-individual differences in serum levels of n3 fatty acids in 40 breast cancer patients who were participating in a pilot study for prevention of weight gain. Significant predictors of baseline n3 fatty acid levels in serum were dietary intakes of n3 fatty acids, BMI, serum levels of carotenoids and TV hours watched/day, accounting for 43% of the variance. Counseling for prevention of weight gain also tended to increase n3 fatty acid blood levels over time during chemotherapy.
Keywords: obesity, breast cancer, diet, physical activity, omega 3 fatty acids, n3 fatty acids
Introduction
Maintenance of n3 fatty acid levels is of particular relevance to breast cancer patients and survivors. Increased n3 fatty acid levels have been linked with lower rates of breast cancer recurrence and mortality.1 Fish oils appear to sensitize breast tumors to chemotherapy. 2–4 In addition to having anti-tumor effects, n3 fatty acids may have a role in improving other aspects of health in breast cancer survivorship. Fish oils are known to reduce blood triglyceride levels, to improve immunity, decrease inflammation, decrease cardiovascular risks and maintain mental health. 5–9
Interestingly, better quality diets and higher flavonoid intakes can increase n3 fatty acid levels independently of dietary n3 fatty acid intakes. 10, 11 Overweight/obesity also is important, and high body mass index (BMI) attenuated the response of tissue and serum fatty acids to supplementation with the fish oil n3 fatty acids eicosapentanoic acid (EPA, 20:5 n3) and docosahexaenoic acid (DHA, 22:6 n3) in women at high risk for breast cancer. 12 Conversely, weight loss increased n3 fatty acid levels independently of changes in dietary intakes. 13
The aim of the present study was to evaluate the factors that contribute to n3 fatty acid blood levels in breast cancer patients who were participating in a randomized trial to prevent weight gain during chemotherapy. We examined the dietary and non-dietary factors that contributed to n3 fatty acid blood at study entry, which was at the start of chemotherapy, and changes over time.
Methods
Subjects and Assessments
Women at the start of chemotherapy for stage I-IIIA breast cancer were eligible, as previously described. 14 The study was approved by the University of Michigan Institutional Review Board (HUM000012524, Clinical Trials registration NCT00583726). All participants received written materials on diet and exercise, a pedometer and a bimonthly study newsletter on healthy living. Subjects randomized to the intervention arm received educational materials developed for the study and telephone counseling from a registered dietitian for increasing fruit and vegetable intakes, decreasing fat intake and increasing physical activity to prevent weight gain during treatment. 14
All study participants were asked to complete assessment visits at baseline, six months and twelve months. At these visits, subjects were weighed, and filled out questionnaires.14 Physical activity was assessed using a validated questionnaire from the Women’s Health Initiative 15. Dietary intakes of fatty acids were assessed at each study visit by 24-hour recalls using a modified USDA five-pass method. 16, 17 The recalls were analyzed for nutrient intakes using the Nutrition Data System Research Software (University of Minnesota, Nutrition Coordinating Center, database and software version 2008). Fasting blood samples also were drawn. Diagnosis and treatment information was abstracted from patient medical charts.
Blood Measures
For analysis of fat-soluble micronutrients in serum, HPLC analysis was used, as described previously.18 Fatty acid analysis was performed by GC-MS of fatty acid methyl esters (FAME) using METH-PREP II derivatization reagent (Alltech, Deerfield, IL). Quantitation was done using standard curves 19. Levels of n3 fatty acids were calculated either as total n3 fatty acids (the sum of 18:3, 20:5 and 22:6), long-chain n3 fatty acids (20:5, 22:6) or the n3 highly unsaturated fatty acids (HUFA) index that is a ratio of n3 fatty acids to all fatty acids 20 carbons or longer. 20
Statistical Methods
All analyses were done with SPSS version 17.0. Potential predictors of baseline n3 fatty acid levels were initially identified from Spearman correlation coefficients. Predictors that remained statistically significant in the regression model were retained in the final model. Spearman correlations were also used to evaluate if change in n3 fatty acids over time was related to changes in body weight, blood measures of health risks, or well-being from the FACT questionnaire. Changes over time by the study arms for n3 fatty acids were evaluated using a mixed linear regression model with time-varying serum n3 fatty acid as output and study arm assignment as the primary between-subject predictor. The primary within-subject predictors were the visit indices and visit-arm interaction. The model was further controlled for using the time-varying effects of BMI, dietary intakes of n3 fatty acid, total serum carotenoids, and the baseline measure of hours of TV watched per day. Clustering within repeated measurements on a subject was accounted for using a random intercept due to subject. Model diagnostics including residual plots and Box-Cox transformations on the residuals were carried out. Logarithm of serum n3 fatty acid was used as the outcome in the final model as suggested by Box-Cox transformation approach.
Results
Subject Characteristics
Breast cancer stage was not significantly associated with blood levels of total n3 fatty acids at baseline, although there was a trend for levels to be lower with advancing stage of cancer: Stage I 4.34% (SD 1.34, n=7), Stage II 4.19 (SD 1.55, n=23), Stage III 3.17 (SD 0.76, n=10). All the subjects received chemotherapy and steroidal medications. Women who had (n=20) or had not started chemotherapy (n=20) did not have significantly different dietary intakes of n3 fatty acids (p=0.49), blood levels of n3 fatty acids (p=0.80) or any other blood measures by the two-sample t-test. At the 12 month visit, subjects not taking any form of breast cancer therapy did not have significantly different levels of serum n3 fatty acids than those who were (p=0.40).
Baseline Predictors of n3 fatty acid blood levels
Both dietary and supplement intakes of n3 fatty acids were correlated with blood levels of n3 fatty acids (p<0.05 in each case), and we therefore chose total n3 fatty acids intakes as the dietary variable for the model. Fish oils have good bioavailability.12 Fish oil supplements were used by eight women at any time during the study.
The second potential predictor evaluated was body weight and associated measures. Both BMI and body fat from DXA measures were significantly correlated with n3 fatty acid blood levels. In the regression models, results were similar with either measure but were generally stronger with BMI; therefore, BMI was used in the final model (Table 1).
Table 1.
Predictors of baseline n3 fatty acid levels in breast cancer patients from hierarchical regression analysis
| Predictor a | β Coefficient | Adjusted R square b | Significance c |
|---|---|---|---|
| Dietary intake n3 fatty acids | 0.342 | 0.170 | 0.006 |
| BMI (kg/m2) | −0.217 | 0.259 | 0.027 |
| Blood levels of carotenoids d | 0.214 | 0.327 | 0.041 |
| Hours TV/day | −0.356 | 0.435 | 0.010 |
All variables were at study entry (baseline). Dietary intake of n3 fatty acids included that from 24-hour recalls of diet as well as from all supplements reported as taken in the past two weeks.
The adjusted R square from the hierarchical regression analysis increases as variables are entered in the model, accounting for 43.5% of the inter-individual variance in blood levels of n3 fatty acids when all four variables are present.
Significance is the p-value for the F change.
Blood levels of carotenoids were calculated the sum of lutein, zeaxanthin, lycopene, β-carotene, α-carotene and β-cryptoxanthin.
Both total physical activity and moderate-vigorous physical activity, in hours/week, also were significantly correlated with n3 fatty acid blood levels at baseline. Hours per day spent watching TV, however, was correlated to a stronger extent with serum levels of n3 fatty acids and was a stronger predictor in the regression model than hours per day of physical activity. In the model that included TV hours/day (Table 1), the addition of physical activity was not significant predictor and did not improve the regression coefficient.
Finally, fruit and vegetable intakes were significantly associated with n3 fatty acid blood levels, and the correlation with blood carotenoid levels was even stronger. Blood carotenoids can be used as an unbiased measure of fruit and vegetable intakes 21. We therefore evaluated blood carotenoids as a predictive variable.
In the model shown in Table 1, 44% of the inter-individual variation in total n3 fatty acid blood levels was explained by dietary intake, BMI, hours of TV watched/day and blood carotenoid levels. The same predictors explained a similar percentage of the variance in serum levels of n3HUFA (41%), long chain n3 fatty acids (40%) and the n3/n6 ratio (39%).
Changes in n3 fatty acid blood levels over 12 months
During 12 months of study, there was a trend for n3 fatty acid blood levels to increase in the more intensive intervention arm but not in the group that received written materials only (Figure 1). This, however, did not reach statistical significance in the ANOVA model. Similar to the situation at baseline, levels of total n3 fatty acids at 12 months were significantly correlated with total dietary intakes of n3 fatty acids, BMI, percent body fat and total levels of serum carotenoids (p<0.025 in each case).
Fig. 1.
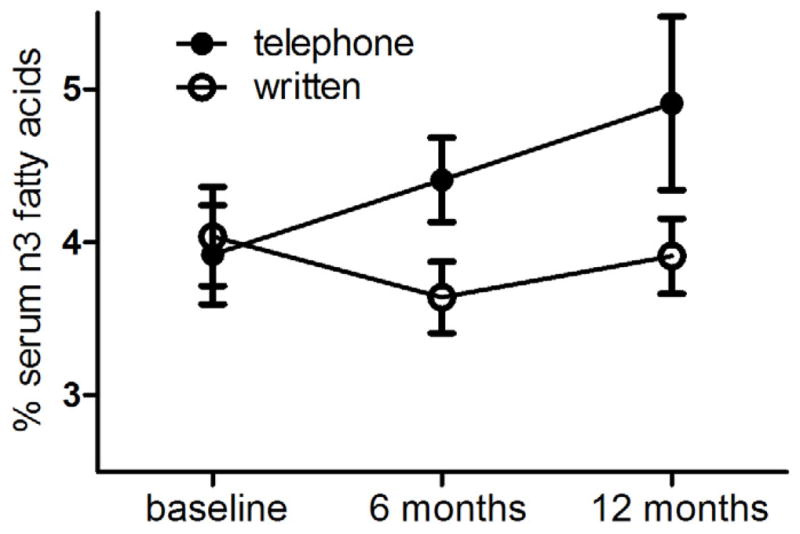
Total n3 fatty acids in serum over time in breast cancer patients who started on study at the beginning of chemotherapy. Subjects in the telephone arm received dietary and exercise counseling from a registered dietitian for prevention of body weight gain. The n3 fatty acids were not significantly different between diet arms in this pilot study at baseline (p=0.643), 6 months (p=0.082) or 12 months (p=0.117). The p-values are from a mixed linear regression model of log n3 fatty acid in serum adjusted for BMI, hours of TV, dietary intake of n3 fatty acid, and total carotenoid in serum. Results were similar for fatty acids expressed as n3 HUFA or n3/n6 ratios.
Discussion
The n3 fatty acids EPA and DHA, as well as linolenic acid (18:3 n3) that can be elongated and desaturated to form EPA and DHA, are of interest for health because of their anti-inflammatory effects. 22 This is of particular relevance to breast cancer survivors because of the links between inflammation and adverse breast cancer outcomes. 23 Several of the lifestyle factors that have been shown to influence breast cancer outcomes, including obesity, diet quality and physical activity, could exert their anti-inflammatory effects in part by affecting n3 fatty acids levels.
Better quality diets have been shown to increase n3 fatty acid levels independently of dietary n3 intakes. 24, 25 This could be due to effects of phytochemicals on fatty acid metabolism. In rats, fish oil increased plasma n3 fatty acids to a significantly greater extent when given concurrently with an anthocyanin-rich diet, perhaps resulting from the effects of flavonoids on desaturase activity 26. This mechanism also appears to contribute to the effects of wine on increasing n3 fatty acid levels since wine is high in flavonoids. 27, 28
Another aspect of a healthy lifestyle, namely physical activity, also has been positively associated with n3 fatty acid levels. 29, 30 Conversely, obesity negatively affected n3 fatty acid blood levels in healthy adults. 31 Given that an important concern after a breast cancer diagnosis is weight gain, this data supports fatty acid metabolism as one mechanism by which a combined diet and physical activity intervention could improve outcomes in breast cancer. 32–34
Conclusions
These results indicate that counseling for an active lifestyle with control of weight gain and good diet quality can contribute to increased n3 fatty acid blood levels in the absence of specific advice to increase dietary intakes of n3 fatty acids. Time spent watching TV was a stronger predictor of n3 fatty acid levels than physical activity. The study was small, but the results indicate that several aspects of a healthy lifestyle can work together to increase n3 fatty acid blood levels in breast cancer patients.
Acknowledgments
We thank all the women who volunteered to participate in this study. We thank Mary Rapai, M.A., for assisting with study recruitment and follow-up and Anne Weldon, B.S. for assistance with data collection. The study was supported by the University of Michigan Metabolomics and Obesity Center (grant DK 089503), the University of Michigan Comprehensive Cancer Center Core Grant (grant P30 CA46592) and the Barbara Padnos Research Fund at the University of Michigan. The study utilized the Chemistry Core of the Michigan Diabetes Research and Training Center (grant DK020572) and the Michigan Clinical Research Unit (grant UL1 RR024986).
Abbreviations
- BMI
body mass index
- DHA
docosahexaenoic acid (22:6 n3)
- EPA
eicosapentanoic acid (20:5 n3)
- FAME
fatty acid methyl esters
- HUFA
highly unsaturated fatty acids
Footnotes
The authors declare no conflicts of interest with the research presented.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Patterson RE, Flatt SW, Newman VA, et al. Marine Fatty Acid intake is associated with breast cancer prognosis. J Nutr. 2011;141:201–6. doi: 10.3945/jn.110.128777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bougnoux P, Hajjaji N, Ferrasson MN, Giraudeau B, Couet C, Le Floch O. Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: a phase II trial. Br J Cancer. 2009;101:1978–85. doi: 10.1038/sj.bjc.6605441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menendez JA, Lupu R, Colomer R. Exogenous supplementation with omega-3 polyunsaturated fatty acid docosahexaenoic acid (DHA; 22:6n-3) synergistically enhances taxane cytotoxicity and downregulates Her-2/neu (c-erbB-2) oncogene expression in human breast cancer cells. Eur J Cancer Prev. 2005;14:263–70. doi: 10.1097/00008469-200506000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Rose DP, Connolly JM. Regulation of tumor angiogenesis by dietary fatty acids and eicosanoids. Nutr Cancer. 2000;37:119–27. doi: 10.1207/S15327914NC372_1. [DOI] [PubMed] [Google Scholar]
- 5.Ebrahimi M, Ghayour-Mobarhan M, Rezaiean S, et al. Omega-3 fatty acid supplements improve the cardiovascular risk profile of subjects with metabolic syndrome, including markers of inflammation and auto-immunity. Acta Cardiol. 2009;64:321–7. doi: 10.2143/AC.64.3.2038016. [DOI] [PubMed] [Google Scholar]
- 6.Riediger ND, Othman RA, Suh M, Moghadasian MH. A systemic review of the roles of n-3 fatty acids in health and disease. J Am Diet Assoc. 2009;109:668–79. doi: 10.1016/j.jada.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Saldeen P, Saldeen T. Women and omega-3 Fatty acids. Obstet Gynecol Surv. 2004;59:722–30. doi: 10.1097/01.ogx.0000140038.70473.96. quiz 45–6. [DOI] [PubMed] [Google Scholar]
- 8.Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr. 2010;91:757–70. doi: 10.3945/ajcn.2009.28313. [DOI] [PubMed] [Google Scholar]
- 9.Weinberger T, Forrester A, Markov D, Chism K, Kunkel EJ. Women at a dangerous intersection: diagnosis and treatment of depression and related disorders in patients with breast cancer. Psychiatr Clin North Am. 2010;33:409–22. doi: 10.1016/j.psc.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 10.O’Sullivan TA, Ambrosini GL, Mori TA, Beilin LJ, Oddy WH. Omega-3 Index correlates with healthier food consumption in adolescents and with reduced cardiovascular disease risk factors in adolescent boys. Lipids. 2011;46:59–67. doi: 10.1007/s11745-010-3499-8. [DOI] [PubMed] [Google Scholar]
- 11.Toufektsian MC, Salen P, Laporte F, Tonelli C, de Lorgeril M. Dietary flavonoids increase plasma very long-chain (n-3) fatty acids in rats. J Nutr. 2011;141:37–41. doi: 10.3945/jn.110.127225. [DOI] [PubMed] [Google Scholar]
- 12.Yee LD, Lester JL, Cole RM, et al. Omega-3 fatty acid supplements in women at high risk of breast cancer have dose-dependent effects on breast adipose tissue fatty acid composition. Am J Clin Nutr. 2010;91:1185–94. doi: 10.3945/ajcn.2009.29036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ouyang P, Jiang Y, Doan HM, et al. Weight Loss via exercise with controlled dietary intake may affect phospholipid profile for cancer prevention in murine skin tissues. Cancer Prev Res (Phila) 2010;3:466–77. doi: 10.1158/1940-6207.CAPR-09-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Djuric Z, Ellsworth JS, Weldon AL, et al. A Diet and Exercise Intervention during Chemotherapy for Breast Cancer Open Obesity Journal. 2011 doi: 10.2174/1876823701103010087. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson-Kozlow M, Rock CL, Gilpin EA, Hollenbach KA, Pierce JP. Validation of the WHI brief physical activity questionnaire among women diagnosed with breast cancer. Am J Health Behav. 2007;31:193–202. doi: 10.5555/ajhb.2007.31.2.193. [DOI] [PubMed] [Google Scholar]
- 16.Conway JM, Ingwersen LA, Moshfegh AJ. Accuracy of dietary recall using the USDA five-step multiple-pass method in men: an observational validation study. J Am Diet Assoc. 2004;104:595–603. doi: 10.1016/j.jada.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am J Clin Nutr. 2003;77:1171–8. doi: 10.1093/ajcn/77.5.1171. [DOI] [PubMed] [Google Scholar]
- 18.Djuric Z, Ren J, Blythe J, VanLoon G, Sen A. A Mediterranean dietary intervention in healthy American women changes plasma carotenoids and fatty acids in distinct clusters. Nutr Res. 2009;29:156–63. doi: 10.1016/j.nutres.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Djuric Z, Ren JW, Blythe J, VanLoon G, Sen A. A Mediterranean dietary intervention in healthy American women changes plasma carotenoids and fatty acids in distinct clusters. Nutrition Research. 2009;29:156–63. doi: 10.1016/j.nutres.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metherel AH, Armstrong JM, Patterson AC, Stark KD. Assessment of blood measures of n-3 polyunsaturated fatty acids with acute fish oil supplementation and washout in men and women. Prostaglandins Leukot Essent Fatty Acids. 2009;81:23–9. doi: 10.1016/j.plefa.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 21.Maskarinec G, Chan CL, Meng L, Franke AA, Cooney RV. Exploring the feasibility and effects of a high-fruit and -vegetable diet in healthy women. Cancer Epidemiol Biomarkers Prev. 1999;8:919–24. [PubMed] [Google Scholar]
- 22.Li Y, Kang JX, Leaf A. Differential effects of various eicosanoids on the production or prevention of arrhythmias in cultured neonatal rat cardiac myocytes. Prostaglandins. 1997;54:511–30. doi: 10.1016/s0090-6980(97)00122-6. [DOI] [PubMed] [Google Scholar]
- 23.Pierce BL, Ballard-Barbash R, Bernstein L, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27:3437–44. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerber MJ, Scali JD, Michaud A, et al. Profiles of a healthful diet and its relationship to biomarkers in a population sample from Mediterranean southern France. J Am Diet Assoc. 2000;100:1164–71. doi: 10.1016/S0002-8223(00)00340-0. [DOI] [PubMed] [Google Scholar]
- 25.O’Sullivan TA, Bremner AP, Beilin LJ, et al. Polyunsaturated fatty acid intake and blood pressure in adolescents. J Hum Hypertens. 2011;93:314–21. doi: 10.1038/jhh.2011.7. [DOI] [PubMed] [Google Scholar]
- 26.Toufektsian MC, Salen P, Laporte F, Tonelli C, de Lorgeril M. Dietary flavonoids increase plasma very long-chain (n-3) fatty acids in rats. J Nutr. 2010;141:37–41. doi: 10.3945/jn.110.127225. [DOI] [PubMed] [Google Scholar]
- 27.di Giuseppe R, de Lorgeril M, Salen P, et al. Alcohol consumption and n-3 polyunsaturated fatty acids in healthy men and women from 3 European populations. Am J Clin Nutr. 2009;89:354–62. doi: 10.3945/ajcn.2008.26661. [DOI] [PubMed] [Google Scholar]
- 28.de Lorgeril M, Salen P, Martin JL, Boucher F, de Leiris J. Interactions of wine drinking with omega-3 fatty acids in patients with coronary heart disease: a fish-like effect of moderate wine drinking. Am Heart J. 2008;155:175–81. doi: 10.1016/j.ahj.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Itomura M, Fujioka S, Hamazaki K, et al. Factors influencing EPA+DHA levels in red blood cells in Japan. In Vivo. 2008;22:131–5. [PubMed] [Google Scholar]
- 30.Magnusardottir AR, Steingrimsdottir L, Thorgeirsdottir H, Gunnlaugsson G, Skuladottir GV. Docosahexaenoic acid in red blood cells of women of reproductive age is positively associated with oral contraceptive use and physical activity. Prostaglandins Leukot Essent Fatty Acids. 2009;80:27–32. doi: 10.1016/j.plefa.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Micallef M, Munro I, Phang M, Garg M. Plasma n-3 polyunsaturated fatty acids are negatively associated with obesity. Br J Nutr. 2009;102:1370–4. doi: 10.1017/S0007114509382173. [DOI] [PubMed] [Google Scholar]
- 32.Chlebowski RT, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clin Oncol. 2002;20:1128–43. doi: 10.1200/JCO.2002.20.4.1128. [DOI] [PubMed] [Google Scholar]
- 33.Ballard-Barbash R, Hunsberger S, Alciati MH, et al. Physical activity, weight control, and breast cancer risk and survival: clinical trial rationale and design considerations. J Natl Cancer Inst. 2009;101:630–43. doi: 10.1093/jnci/djp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sestak I, Distler W, Forbes JF, Dowsett M, Howell A, Cuzick J. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: an exploratory analysis from the ATAC trial. J Clin Oncol. 2010;28:3411–5. doi: 10.1200/JCO.2009.27.2021. [DOI] [PubMed] [Google Scholar]


