Abstract
Background
FLT3 mutations (FLT3/Mut) are prevalent in de novo AML and are associated with early relapse. The prevalence and prognostic significance of FLT3/Mut have not been well defined in childhood acute promyelocytic leukemia (APL).
Procedure
Diagnostic specimens from 104 pediatric APL patients were screened for FLT3/Mut (FLT3/ITD or FLT3/ALM). FLT3/Mut status was correlated with disease characteristics and clinical outcome for patients treated on CALGB C9710 (N=50).
Results
Forty-two of the 104 patients (40%) had either FLT3/ITD (N=28, 27%) or FLT3/ALM (N=15, 14%). Median diagnostic WBC count was 23,400 cells/uL vs. 3,600 cells/uL for those with and without FLT3/Mut (p <0.001), and similar results for the cohort of 50 patients treated on C9710 (p <0.001). In patients treated on C9710, presence of a FLT3 mutation was highly correlated with diagnostic WBC count >10,000 (p=0.004), microgranular variant histology (p=0.035), and a lower remission rate (p=0.009). In patients who received ATRA (C9710 or CCG-2911, n=58), those with FLT3/Mut had an induction death rate of 30% (7/23) compared to 3% (1/35) in FLT3/WT patients (p=0.005). In patients with high WBC counts (>10,000), those with FLT3/Mut had a significantly higher risk of induction death versus FLT3/WT patients (47% vs. 0%, p=0.05). FLT3/Mut was not associated with adverse outcome in those who survived induction therapy.
Conclusions
FLT3/Mut are prevalent in pediatric APL and are associated with high WBC count and increased induction death. This study provides further evidence for testing APL patients for FLT3/Mut and the potential role for FLT3 inhibitors in this disease.
Keywords: APL, Acute Promyelocytic Leukemia, FLT3 mutation, Pediatric
Introduction
Somatic mutations of the FLT3 gene (FLT3/Mut) are commonly present in acute myeloid leukemia (AML) blasts including both internal tandem duplications of the juxtamembrane domain coding sequence (FLT3/ITD) and missense mutations in the activation loop domain of the tyrosine kinase domain (FLT3/ALM).[1-3] Both mutations lead to autonomous phosphorylation and constitutive activation of the receptor.[4,5] The majority of studies in de novo AML in children and young adults demonstrate that only FLT3/ITD is associated with adverse outcome[1,6] but others have also reported worse outcomes in adults with FLT3/ALM.[7] Together FLT3/ITD and FLT3/ALM are one of the most common genetic abnormalities in AML, and these mutations may be even more prevalent in acute promyelocytic leukemia (APL).[8-10] Mouse models have demonstrated that FLT3/Mut cooperate with RARα translocations by conferring a proliferative advantage to cells in maturation arrest.[11,12] It remains unclear whether FLT3/Mut are predictive of clinical outcome in pediatric patients with APL.
Studies of APL patients (mostly adults) have shown 20-30% of APL patients are FLT3/ITD positive and another 10-20% harbor FLT3/ALM. [8-10,13] Evaluations of the prognostic significance of FLT3/Mut in APL are more variable. An analysis of 119 adults with APL by the European cooperative APL Group found that there was a trend toward shorter overall survival in patients with FLT3/ITD (but not FLT3/ALM) due to very poor post-relapse survival.[14] Stock, et al examined a subset of 78 adult patients treated on CALGB C9710 and found no correlation between FLT3/Mut and survival.[15] The MRC trials AML10 and AML12 evaluated 203 adult and pediatric patients with APL, and patients with FLT3/Mut (both FLT3/ITD and FLT3/ALM) had a higher rate of induction death but no difference in relapse risk or overall survival.[10] Their analysis did not separate pediatric and adult patients. A study of 75 adult APL patients in Korea also found an association of FLT3/ITD (but not FLT3/ALM) with early deaths and this resulted in an inferior prognosis.[16] Only one previous study, by Arrigoni, et al, has examined FLT3/Mut in an exclusively pediatric APL population.[17] Among 29 pediatric APL patients they found 10 patients (34.5%) with FLT3/Mut. However, only a small subset of these 29 patients was treated with current ATRA based therapy and thus they were unable to analyze clinical outcome. Here we present the largest study of FLT3/Mut restricted to pediatric patients with APL and the first study to analyze the prognostic significance of these mutations in a pediatric population.
Methods
Patients and Treatment
Genomic DNA was available from 104 children (age <21 years) with diagnosis of APL for FLT3 mutation profiling. This cohort consisted of 81 patients treated on cooperative group studies CCG-2891 (n=13), CCG-2911 (n=18) and CALGB C9710 (n=50) and 23 patients treated per institutional standard therapy. All patients were confirmed to have acute promyelocytic leukemia as FAB M3 morphology and the characteristic t(15;17) by cytogenetics, PCR or FISH. On CCG-2891 and CCG-2911, FAB M3 morphology and cytogenetics were centrally reviewed. For the 23 patients treated per institutional standards, these patients had local diagnostic confirmation. Outcome correlations were restricted to pediatric patients treated on the intergroup APL trial CALGB C9710. CALGB C9710 required diagnosis confirmation centrally by RTPCR and most patients had PML breakpoint characterized as the long versus short isoform. Patient specimens were classified by FAB criteria as M3 or M3v (microgranular variant). In CALGB C9710, all patients received ATRA, cytarabine and daunorubicin for remission induction. Patients less than 15 years old were non-randomly assigned to treatment without arsenic trioxide (ATO) while patients aged 15 or older were randomly assigned to receive two consolidation cycles with ATO immediately after entering remission. Both arms also received two consolidation courses with ATRA plus daunorubicin. In our cohort of 50 patients aged 2-20 years old, 19 were age 15 or older, and 12 of these received ATO. Patients were also randomized to one year of maintenance therapy with intermittent ATRA alone or ATRA plus oral 6-mercaptopurine and oral methotrexate. Further details of this trial have previously been published.[18]
FLT3 mutation analysis
In all patients, mutations of the FLT3 gene were determined including both internal tandem duplications (FLT3/ITD) of the juxtamembrane domain and mutations of the activating loop of the tyrosine kinase domain (FLT3/ALM). Genomic DNA was extracted from diagnostic marrow specimens. The methods for FLT3 mutation analysis and ITD allelic ratio determination have been previously described.[1] Consent was obtained from all participants for evaluation of tissue samples in accordance with the Declaration of Helsinki.
Statistical Methods
Clinical outcome analyses were performed on 50 patients treated on CALGB C9710. These outcomes were correlated with FLT3 mutation status and high WBC count at diagnosis (>10,000 cells/uL). Three of the 50 patients were missing WBC counts from diagnosis. Kaplan Meier estimates were determined for 5 year overall survival and event free survival rates. Correlations between dichotomous endpoints such as FLT3 mutation or wild type, high WBC or low, were analyzed using contingency tables and Fisher's exact test. The relationship between dichotomous factors and time to event outcomes was determined using the log-rank statistic. Survival rates were estimated from Kaplan-Meier plots and their variances were estimated using Greenhouse estimators. Comparisons between 5 year survival rates were made using t-tests. Event free survival was defined as time from study entry until relapse or death. Relapse free survival was defined as the time from first clinical remission (CR) until relapse of disease and patients were censored at time of death or last follow-up.
Results
FLT3 mutation prevalence in APL
FLT3 mutation status was determined on available DNA from diagnostic marrow specimens from 104 pediatric APL patients treated on 3 different clinical trials (N=81) or institutional protocols (N=23) as described under Methods. Forty-two of the 104 patients (40%) harbored either FLT3/ITD (n=28, 27%) or FLT3/ALM (n=15, 14%). One patient in our cohort had both FLT3/ITD and FLT3/ALM. Diagnostic WBC count was available from all but 3 patients tested for FLT3/Mut on the three pediatric trials. Patients with FLT3/Mut had a median WBC count of 23,400 cells/uL while FLT3 wild type (FLT3/WT) patients had a median WBC count of 3,600 cells/uL (p< 0.001). Similarly when considering patients with high WBC count (>10,000 cells/uL), 68% of FLT3/Mut patients had high WBC counts compared to only 26% of FLT3/WT patients (p< 0.001). Of note, patients with available diagnostic specimens in our tissue bank had higher diagnostic WBC counts (41% had WBC count >10,000 cells/uL compared to 18% of patients who did not have an available diagnostic specimen for FLT3 testing, p< 0.001) which may lead to overestimation of the prevalence of FLT3/Mut due to their association with high WBC count.
In pediatric AML, FLT3/ITD allelic ratio (ITD-AR) has been shown to be associated with clinical outcome. Patients with high ITD-AR (AR>0.4) have poor outcome.[1] The median ITD-AR was 0.58 in APL patients, which is similar to the median of 0.56 in non-APL AML. Sixty two percent of the FLT3/ITD APL patients had an ITD-AR greater than 0.4, which is also similar to the rate of high ITD-AR reported in pediatric non-APL AML patients with FLT3/ITD (67%).
We have previously demonstrated that FLT3/ALM may involve D835 or I836 residues.[1] In the 15 patients with FLT3/ALM in this study, all mutations involved the D835 residue (8 patients with D835Y, 2 patients each with D835H, D835E and D835A and one patient with D835V). In contrast to non-APL AML in which 18% of activating loop mutations involve the I836 codon [1], no mutations of the I836 codon were observed in our cohort of pediatric APL patients.
Disease Characteristics of the Study Population
Clinical correlation was performed on pediatric patients who were treated on CALGB C9710. Results of the adult Intergroup study CALGB C9710 have been previously reported.[18] A total of 95 eligible patients less than 21 years of age enrolled on CALGB C9710 with evaluable clinical data. Diagnostic specimens were available from 50 of 95 (53%) for FLT3 mutation profiling. Disease characteristics and clinical outcome was compared between those with (n=50) and without (n=45) available diagnostic specimens. (Table I) Those without diagnostic specimens differed from the study population in regard to gender (64% male in the analyzed group versus 40% male in the non-analyzed group, p=0.024). They also differed in percent of patients with WBC count >10,000 cells/uL (45% versus 14%, P=0.0024) for those analyzed versus those not analyzed, respectively. Complete remission (CR) rate was 80% for those analyzed compared to 91% for those not analyzed (p=0.15). Other clinical outcomes where not significantly different including 5-year overall survival (p=0.11) and event free survival (p=0.88).
Table I.
Comparing disease characteristics and clinical outcome of patients with and without available diagnostic specimens
| FLT3 Measured N=50 | FLT3 Not Measured N=45 | P-value | |
|---|---|---|---|
| Age (mean), years | 11.86 | 12.27 | 0.731 |
| Gender | |||
| Male | 32 (64%) | 18 (40%) | 0.0242 |
| Female | 18 (36%) | 27 (60%) | |
| WBC | |||
| ≤10 | 26 (55%) | 37 (86%) | 0.00242 |
| >10 | 21 (45%) | 6 (14%) | |
| Achieved CR? | |||
| Yes | 39 (80%) | 41 (91%) | 0.152 |
| No | 10 (20%) | 4 (9%) |
T-test Pooled p-value and Satterwaite p-value both 0.73
2-sided Fisher's exact test.
Note: Five patients were missing data on WBC count and one patient was missing data on CR.
This cohort of 50 pediatric patients had a similar prevalence of FLT3/Mut as our larger cohort of 104 patients with a prevalence of 46% FLT3/Mut (28% FLT3/ITD and 18% FLT3/ALM). There was not a significant difference in gender, median age, or race for patients with FLT3/Mut versus FLT3/WT. (Table II)
Table II.
Clinical Features of 50 pediatric APL patients treated on CALGB C9710.
| FLT3/WT (n=29) | FLT3/Mut (n=21) | p-value comparing FLT3/WT vs. FLT3/Mut | FLT3/ITD (n=12) | FLT3/ALM (n=9) | |
|---|---|---|---|---|---|
| Sex | |||||
| -Male | 21 (72%) | 11 (52%) | p=0.23 | 6 (50%) | 5 (56%) |
| -Female | 8 (28%) | 10 (48%) | 6 (50%) | 4 (44%) | |
| Age | Rank sum | ||||
| -Mean | 12.66 | 10.76 | p=0.24 | 12.17 | 8.89 |
| Race | |||||
| -White | 20 (69%) | 17 (81%) | 9 (75%) | 8 (89%) | |
| -Non-white | 9 (31%) | 4 (19%) | p=0.52 | 3 (25%) | 1 (11%) |
| Dx WBC1 | Rank sum | ||||
| -Median | 3.6 | 32.95 | p=0.0004 | 32.95 | 36.75 |
| -Range | 0.7 - 109.4 | 1.3 - 138.6 | 6.9 - 138.6 | 1.3 - 82.7 | |
| ->10,000 | 7 (26%) | 14 (70%) | p=0.0036 | 9 (75%) | 5 (63%) |
| -<10,000 | 20 (74%) | 6 (30%) | 3 (25%) | 3 (37%) | |
| FAB2 | |||||
| -M3 | 23 (85%) | 9 (53%) | 5 (50%) | 4 (57%) | |
| -M3v (microgranular variant) | 4 (15%) | 8 (47%) | p=0.035 | 5 (50%) | 3 (43%) |
| Isoform3 | |||||
| -Long | 14 (70%) | 6 (46%) | 2 (29%) | 4 (67%) | |
| -Short | 6 (30%) | 7 (54%) | p=0.28 | 5 (71%) | 2 (33%) |
| ATRA syndrome4 | |||||
| -Yes | 7 (44%) | 6 (43%) | p=1.00 | 3 (50%) | 3 (37%) |
| -No | 9 (56%) | 8 (57%) | 3 (50%) | 5 (63%) | |
| Induction Death | |||||
| -Yes | 0 (0%) | 7 (33%) | p=0.0012 | 4 (33%) | 3 (33%) |
| -No | 29 (100%) | 14 (67%) | 8 (67%) | 6 (67%) | |
| CR5 | |||||
| -Yes | 27 (93%) | 12 (60%) | p=0.0093 | 6 (54.6%) | 6 (66.7%) |
| -No | 2 (7%) | 8 (40%) | 5 (45.4%) | 3 (33.3%) | |
| OS at 5 years | 68% | 62% | log rank test p=0.057 | 67% | 56% |
| EFS at 5 years | 43% | 42% | log rank test p=0.23 | 49% | 42% |
Abbreviations: WT= Wild type; ITD = internal tandem duplication; PM= point mutation.
Three patients were missing diagnostic WBC data.
Six patients were missing histology subtype classification.
Isoform data were available for 33 patients.
ATRA syndrome data were available for 30 patients.
One FLT3/ITD patient was missing CR data.
FLT3 mutations associated with high WBC count at diagnosis
White blood cell count at diagnosis is the most powerful known prognostic factor in APL, where those with high diagnostic WBC count (>10,000 cells/uL) have a worse outcome.[19,20] We evaluated the correlation of FLT3/Mut with diagnostic WBC count. Median diagnostic WBC count for those with FLT3/Mut was 32,950 cells/uL compared to 3,600 cells/uL in those without FLT3/Mut (p=0.004). Median diagnostic WBC counts in those with FLT3/ITD and FLT3/ALM were 32,950 cells/uL and 36,750 cells/uL, respectively. Conversely, for those patients with high WBC counts the prevalence of FLT3/Mut was 67% vs. 23% in those with lower diagnostic WBC counts. Seventy percent (14/20) of patients with FLT3/Mut had high diagnostic WBC count compared to 26% (7/27) of FLT3/WT patients (p=0.004). The prevalence of high WBC count was similar in the FLT3/ITD group (75%) and the FLT3/ALM group (63%). (Table II)
FLT3 mutations and APL characteristics
Morphologic assessment of diagnostic specimens can distinguish the common subtype of M3 leukemia from the microgranular variant (M3v) based on the presence of cytoplasmic microgranules. The microgranular variant was seen in a significantly higher proportion of FLT3 mutated patients compared to FLT3/WT patients (47% versus 15%, p=0.035). (Table II) We further compared the PCR isoforms in 33 patients with and without FLT3/Mut. Short isoform (bcr 3) was seen in 30% of those without FLT3/Mut compared to that of 54% in FLT3/Mut patients (p=0.28). Limited data on incidence of coagulopathy associated events other than induction deaths (such as DIC) precluded meaningful correlation of non-fatal coagulopathy with FLT3/Mut in the C9710 study.
Clinical outcome
The morphologic complete remission (CR) rate was determined for patients on CALGB C9710 following the first induction course of therapy. Patients with FLT3/Mut had a significantly lower CR rate (60%) compared to FLT3/WT patients (93%, p=0.009). CR rates for patients with FLT3/ITD and FLT3/ALM were 55% and 67%, respectively.
While the use of ATRA has improved overall survival in APL patients, coagulopathy remains an important cause of early death. In our cohort of 50 pediatric patients on CALGB C9710, 7 had an early induction death. (Table II) To fully analyze induction death, we included an additional 8 patients from CCG-2911 randomized to receive ATRA in induction. In total, there were 8 induction deaths among this group of 58 patients. Cause of death was intracranial hemorrhage in 5 patients, ischemic stroke in one patient, both ischemic stroke and pulmonary hemorrhage in one patient and cardio-respiratory arrest (not otherwise specified) in one patient. Seven of the 8 deaths occurred in the first 5 days of treatment with two patients dying prior to initiation of ATRA. Days of ATRA treatment among patients who died in induction ranged from 0 to 7. Those with FLT3/Mut had an induction death rate of 30% (7/23) compared to 3% (1/35) in FLT3/WT patients (p=0.005). Induction death rates in patients with FLT3/ITD and FLT3/ALM were similar (4 of 13, 31% and 3 of 10, 30% respectively). (Figure 1) We examined the role of ITD-AR in induction death among the 13 patients with FLT3/ITD. Three of 4 (75%) patients who died in induction had a high ITD-AR which was similar to the 6 of 9 (67%) patients who survived induction and had a high ITD-AR (p=1.0).
Figure 1.
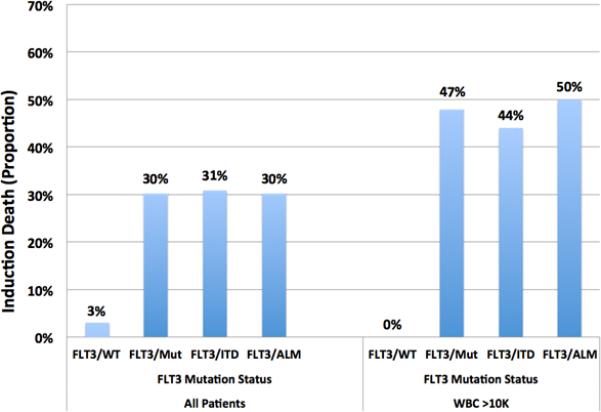
Induction Death in all patients (left) and in those with high WBC count (right) based on FLT3 mutation status.
High WBC count has been established as a risk factor for induction death. In our study, patients with high WBC count had an induction death rate of 30% (7/23) compared to 3% (1/32) in the low WBC count group (p=0.007). We inquired whether FLT3/Mut has clinical implications in patients with high WBC count. Twenty three patients had WBC count >10,000 cells/uL of whom 15 (65%) had FLT3/Mut (9 FLT3/ITD and 6 FLT3/ALM). In the high WBC count cohort, patients with FLT3/Mut had an induction death rate of 47% (7/15) compared to 0% (0/8) in FLT3/WT patients (p=0.052). (Figure 1)
ATRA syndrome is characterized by increasing WBC counts, fever, and weight gain from fluid retention including complications of pulmonary infiltrates and pleural effusions. Data on ATRA syndrome were available for 30 patients in the cohort, and 13 (43%) developed ATRA syndrome. ATRA syndrome was observed in 43% of patients with FLT3/Mut compared to 44% in FLT3/WT patients. (p=1.0, Table II).
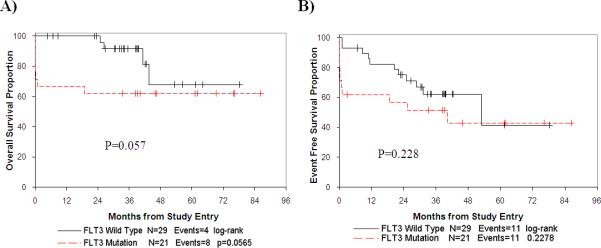
We evaluated the overall survival and event free survival for C9710 APL patients with and without FLT3/Mut. Patients with FLT3/ITD and FLT3/ALM had similar clinical characteristics including the percentage with high WBC counts and similar outcomes including induction death rate (Table II). Therefore, patients with both types of FLT3/Mut were considered together in comparison to those with FLT3 wild type alleles when analyzing survival data. The overall survival at 5 years from study entry for those with and without FLT3/Mut was 62% and 68%, respectively (log rank p=0.057; Table II and Figure 2A). The event free survival at 5 years from study entry for those with and without FLT3/Mut was 43% and 42%, respectively (log rank p=0.23; Table II and Figure 2B).
Figure 2.
Overall survival from study entry by FLT3 mutation status (A) and event free survival by FLT3 mutation status (B).
We further evaluated the clinical impact of FLT3/Mut in patients who achieve CR at end of induction. Kaplan Meier analysis for OS and EFS showed no difference between patients stratified by FLT3 mutation status (log rank p=0.39 and p=0.76, respectively). Kaplan Meier analysis of relapse free survival (RFS) was performed where patients who died were censored. In patients who achieved an initial CR, RFS from end of the induction course was 69% at 5 years for FLT3/Mut patients compared to 48% for FLT3/WT patients (log rank p=0.45). (Figure 3)
Figure 3.
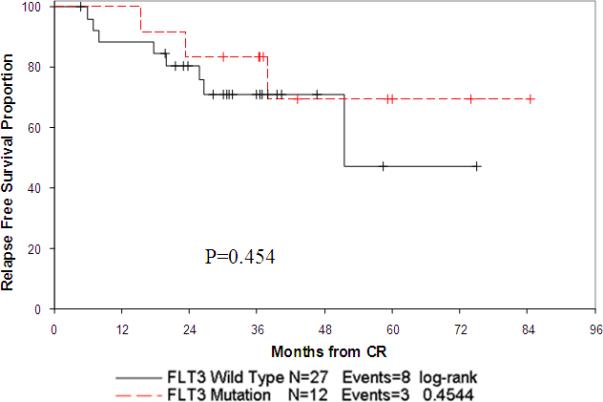
Relapse free survival from end of induction 1 by FLT3 mutation status.
Multivariate Cox proportional hazard model analyses of OS were able to distinguish FLT3/Mut status (p=0.043) and high WBC count (p=0.041) each as independent prognostic factors, after the effect of treatment was removed from the model. For EFS, neither FLT3/Mut status (p=0.28) nor high WBC count (p=0.10) had a significant effect.
We examined the role of allelic ratio in outcome among the 12 patients with FLT3-ITD treated on C9710. Eight of the 12 patients (67%) had high-AR (AR≥0.4). The 5 year OS for patients with high-AR was 62.5% compared to 75% for patients with low-AR (P=0.65). Similarly, the 5 year EFS was 50% versus 50% for patients with high and low allelic ratio, respectively (P= 1.0).
Discussion
Despite improvements in overall outcome with ATRA therapy in APL, a subset of patients die from rapid and catastrophic hemorrhagic or ischemic events during induction or prior to initiation of therapy. Induction death rates reported in clinical trials likely underestimate this complication as some patients die before enrollment on clinical trials. Death is more frequent in patients with higher diagnostic WBC counts, but attempts to identify specific markers predictive of induction death have not identified useful markers. In this study we present data on the high prevalence of FLT3/Mut in APL and their significant association with high diagnostic WBC count and induction death. We further demonstrate that although FLT3/Mut are associated with high diagnostic WBC count, within those with diagnostic WBC count >10,000 cells/uL, presence of FLT3/Mut remains highly associated with induction death.
In non-APL AML only high-AR FLT3/ITD is associated with outcome in pediatric patients, but our analysis in pediatric APL patients showed FLT3/ITD and FLT3/ALM patients have similar outcomes including induction death. Also FLT3/ITD patients with low and high allelic ratios had similar outcomes although this analysis was limited by number of patients (9 high-AR and only 4 low-AR patients). The overall outcome of the patients in our study cohort (5 year EFS 42%) is inferior to other reported trials. This may be due in part to the high percentage of patients in our cohort with high WBC. However, the role of FLT3 mutations on outcome will need to be further validated when clinical outcome data is available from the currently accruing pediatric APL study within the Children's Oncology Group.
Based on our study cohort, the prevalence of both FLT3/ITD and FLT3/ALM is higher in APL than in non-APL AML pediatric patients.[1] In our study, diagnostic WBC counts in patients with available specimens were higher than in patients not tested, which may have led to overestimation of the true prevalence of FLT3/Mut. However, the prevalence reported in this study is similar to those reported in previous studies.[8-10] The high prevalence of FLT3/Mut in t(15;17) suggests a common molecular mechanism of leukemogenesis. The differentiation arrest caused by t(15;17) likely cooperates with the proliferative advantage conferred by FLT3/Mut to result in promyelocytic leukemia. This oncogenic cooperation has been demonstrated in mouse models.[11,12]
Due to the high correlation of FLT3/Mut and high WBC count, it may be difficult to differentiate between the contribution of high WBC count and FLT3/Mut to induction death. Analysis of induction death by FLT3/Mut status in the high WBC count group showed that 47% of the FLT3 mutant patients experienced an early death, whereas none of those without FLT3/Mut had an induction death (p=0.052). In contrast, in the low WBC count group, only one early death occurred in a FLT3/WT patient. The association of FLT3/Mut with induction death in patients with higher WBC count may indicate direct contribution of FLT3 activation to coagulation dysregulation, however, our data are limited by the number of patients evaluable in this trial (8 induction deaths among 58 analyzed patients).
Gene expression profiling in APL has shown a distinct signature for FLT3/Mut APL.[21] Genes associated with cell proliferation were up-regulated consistent with the leukocytosis seen in FLT3/Mut leukemia. There was also a cluster of genes associated with inflammation and coagulation that are uniquely up-regulated in FLT3/Mut APL samples, suggesting that activation of FLT3 in APL may lead to up-regulation of genes involved in coagulation. As similar complications are not observed in de novo (non-APL) AML, a unique combination of activated FLT3 in a background of t(15;17) may be required for derangement of coagulation.
Prior studies have examined the impact of FLT3/Mut on induction death with variable results. The MRC AML 10 and AML 12 studies demonstrated increased induction death and elevated WBC count for APL patients with FLT3/Mut.[10] An association between early death and FLT3/ITD was also reported in an APL study of 75 adult and adolescent patients treated in Korea.[16] They found an early death rate of 33.3% in FLT3/ITD positive patients versus 4.5% in other patients (P=0.020). Adult patients treated on CALGB C9710 had a high prevalence of FLT3/Mut (54%) which was associated with increased WBC count, however, FLT3/Mut did not demonstrate association with outcome.[15] Although patients aged 15 or older treated on C9710 were randomized to receive ATO as part of their therapy and those who received ATO had a substantially improved long term survival, ATO was introduced during consolidation therapy. ATO treatment could not have impacted induction death, thus not addressing the discrepancy between the adult and pediatric studies.
This is the largest study devoted to pediatric patients with APL that examines the prevalence and impact of FLT3/Mut. Our data show that FLT3/Mut are common in pediatric APL and they are associated with elevated diagnostic WBC count and induction death. If the link between FLT3/Mut status, coagulopathy and induction death observed in this study is further substantiated, interruption of FLT3 signal transduction pathway by FLT3 inhibitors may ameliorate the rapidly progressive coagulopathy and death. This provides further evidence that FLT3 inhibitors should be studied within clinical trials. This treatment would need to be initiated early during induction therapy to have a possibility of impacting induction deaths which may occur in the first days of therapy. While FLT3 testing results may be delayed, there is a very high association of FLT3 mutations with high WBC. Thus a clinical trial could be designed to use high WBC as an initial surrogate criterion for initiating FLT3 inhibitor therapy. This approach needs to be closely studied in the setting of a clinical trial to monitor for complications including chemotherapy resistance and possible coagulopathy[22] from the FLT3 inhibitors themselves.
Acknowledgments
We would like to thank the patients and their families who consented to use of the specimens used in the conduct of this research and the COG AML Reference Laboratory for providing these specimens. This work was supported by the National Institutes of Health grants including the Ruth L. Kirschstein National Research Service Award T32CA009351 (M.A.K.) and R01-CA114563 (S.M.).
Supported by Grants No. U10-CA98543 (Chair's Grant), U10-CA98413 (Statistics and Data Center Grant), U24-CA114766 (Children's Oncology Group), NCI R01-CA114563 (S.M.), and NCI R21 CA 104964-02 (SM). Dr. Matthew Kutny was supported in part by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award T32CA009351. The research for CALGB 9710 was supported, in part, by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (Richard L. Schilsky, MD, Chairman) and to the CALGB Statistical Center (Stephen George, PhD, CA33601). The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
A complete listing of grant support for research conducted by CCG and POG before initiation of the COG grant in 2003 is available online at: http://www.childrensoncologygroup.org/admin/grantinfo.html
Footnotes
Note to Journal Editor: The number of authors is greater than the recommended limit of 6 due to the significant contributions of authors to the multiple clinical trials included in this report.
Conflict of Interest Statement: The authors report no pertinent conflicts of interest.
References
- 1.Meshinchi S, Alonzo T, Stirewalt D, et al. Clinical implications of FLT3 mutations in pediatric AML. Blood. 2006;108(12):3654–3661. doi: 10.1182/blood-2006-03-009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99(12):4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 3.Kottaridis P, Gale R, Frew M, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98(6):1752–1759. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 4.Mizuki M, Fenski R, Halfter H, et al. Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood. 2000;96(12):3907–3914. [PubMed] [Google Scholar]
- 5.Yamamoto Y, Kiyoi H, Nakano Y, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97(8):2434–2439. doi: 10.1182/blood.v97.8.2434. [DOI] [PubMed] [Google Scholar]
- 6.Gale RE, Green C, Allen C, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. 2008;111(5):2776–2784. doi: 10.1182/blood-2007-08-109090. [DOI] [PubMed] [Google Scholar]
- 7.Whitman SP, Ruppert AS, Radmacher MD, et al. FLT3 D835/I836 mutations are associated with poor disease-free survival and a distinct gene-expression signature among younger adults with de novo cytogenetically normal acute myeloid leukemia lacking FLT3 internal tandem duplications. Blood. 2008;111(3):1552–1559. doi: 10.1182/blood-2007-08-107946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shih L, Kuo M, Liang D, et al. Internal tandem duplication and Asp835 mutations of the FMS-like tyrosine kinase 3 (FLT3) gene in acute promyelocytic leukemia. Cancer. 2003;98(6):1206–1216. doi: 10.1002/cncr.11636. [DOI] [PubMed] [Google Scholar]
- 9.Au W, Fung A, Chim C, et al. FLT-3 aberrations in acute promyelocytic leukaemia: clinicopathological associations and prognostic impact. Br J Haematol. 2004;125(4):463–469. doi: 10.1111/j.1365-2141.2004.04935.x. [DOI] [PubMed] [Google Scholar]
- 10.Gale R, Hills R, Pizzey A, et al. Relationship between FLT3 mutation status, biologic characteristics, and response to targeted therapy in acute promyelocytic leukemia. Blood. 2005;106(12):3768–3776. doi: 10.1182/blood-2005-04-1746. [DOI] [PubMed] [Google Scholar]
- 11.Kelly L, Kutok J, Williams I, et al. PML/RARalpha and FLT3-ITD induce an APL-like disease in a mouse model. Proc Natl Acad Sci U S A. 2002;99(12):8283–8288. doi: 10.1073/pnas.122233699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Beau MM, Davis EM, Patel B, et al. Recurring chromosomal abnormalities in leukemia in PML-RARA transgenic mice identify cooperating events and genetic pathways to acute promyelocytic leukemia. Blood. 2003;102(3):1072–1074. doi: 10.1182/blood-2003-01-0155. [DOI] [PubMed] [Google Scholar]
- 13.Mathews V, Thomas M, Srivastava V, et al. Impact of FLT3 mutations and secondary cytogenetic changes on the outcome of patients with newly diagnosed acute promyelocytic leukemia treated with a single agent arsenic trioxide regimen. Haematologica. 2007;92(7):994–995. doi: 10.3324/haematol.10802. [DOI] [PubMed] [Google Scholar]
- 14.Callens C, Chevret S, Cayuela J, et al. Prognostic implication of FLT3 and Ras gene mutations in patients with acute promyelocytic leukemia (APL): a retrospective study from the European APL Group. Leukemia. 2005;19(7):1153–1160. doi: 10.1038/sj.leu.2403790. [DOI] [PubMed] [Google Scholar]
- 15.Stock W, Najib K, Moser BK, et al. High incidence of FLT3 mutations in adults with Acute Promyelocytic Leukemia (APL): Correlation with diagnostic features and treatment outcome (CALGB 9710). J Clin Oncol. 2008 May;26(20 suppl) abstr 7002. [Google Scholar]
- 16.Yoo SJ, Park CJ, Jang S, et al. Inferior prognostic outcome in acute promyelocytic leukemia with alterations of FLT3 gene. Leuk Lymphoma. 2006;47(9):1788–1793. doi: 10.1080/10428190600687927. [DOI] [PubMed] [Google Scholar]
- 17.Arrigoni P, Beretta C, Silvestri D, et al. FLT3 internal tandem duplication in childhood acute myeloid leukaemia: association with hyperleucocytosis in acute promyelocytic leukaemia. Br J Haematol. 2003;120(1):89–92. doi: 10.1046/j.1365-2141.2003.04032.x. [DOI] [PubMed] [Google Scholar]
- 18.Powell BL, Moser B, Stock W, et al. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood. 2010;116(19):3751–3757. doi: 10.1182/blood-2010-02-269621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burnett A, Grimwade D, Solomon E, et al. Presenting white blood cell count and kinetics of molecular remission predict prognosis in acute promyelocytic leukemia treated with all-trans retinoic acid: result of the Randomized MRC Trial. Blood. 1999;93(12):4131–4143. [PubMed] [Google Scholar]
- 20.Sanz M, Lo Coco F, Martín G, et al. Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups. Blood. 2000;96(4):1247–1253. [PubMed] [Google Scholar]
- 21.Marasca R, Maffei R, Zucchini P, et al. Gene expression profiling of acute promyelocytic leukaemia identifies two subtypes mainly associated with flt3 mutational status. Leukemia. 2006;20(1):103–114. doi: 10.1038/sj.leu.2404000. [DOI] [PubMed] [Google Scholar]
- 22.Je Y, Schutz FA, Choueiri TK. Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. Lancet Oncol. 2009;10(10):967–974. doi: 10.1016/S1470-2045(09)70222-0. [DOI] [PubMed] [Google Scholar]