Summary
Repeated social defeat (RSD) activates neuroendocrine pathways that have a significant influence on immunity and behavior. Previous studies from our lab indicate that social defeat enhances the inflammatory capacity of CD11b+ cells in the brain and promotes anxiety-like behavior in an interleukin (IL)-1 and β-adrenergic receptor-dependent manner. The purpose of this study was to determine the degree to which mice subjected to RSD were more responsive to a secondary immune challenge. Therefore, RSD or control (HCC) mice were injected with saline or lipopolysaccharide (LPS) and activation of brain CD11b+ cells and behavioral responses were determined. Peripheral LPS (0.5 mg/kg) injection caused an extended sickness response with exaggerated weight loss and prolonged social withdrawal in socially defeated mice. LPS injection also amplified mRNA expression of IL-1β, tumor necrosis factor (TNF)-α, inducible nitric oxide synthase (iNOS), and CD14 in enriched CD11b+ cells isolated from socially defeated mice. In addition, IL-1β mRNA levels in enriched CD11b+ cells remained elevated in socially defeated mice 24 h and 72 h after LPS. Moreover, microglia and CNS macrophages isolated from socially defeated mice had the highest CD14 expression after LPS injection. Both social defeat and LPS injection increased the percentage of CD11b+/CD45high macrophages in the brain and the number of inflammatory macrophages (CD11b+/CD45high/CCR2+) was highest in RSD-LPS mice. Anxiety-like behavior was increased by social defeat, but was not exacerbated by the LPS challenge. Nonetheless, reduced locomotor activity and increased social withdrawal were still present in socially defeated mice 72 h after LPS. Last, LPS-induced microglia activation was most evident in the hippocampus of socially defeated mice. Taken together, these findings demonstrate that repeated social defeat enhanced the neuroinflammatory response and caused prolonged sickness following innate immune challenge.
Keywords: Stress, Microglia, LPS, Cytokines, Anxiety, Sickness
1. Introduction
In clinical studies, psychosocial stress increases inflammation that is associated with increased morbidity and mortality (Kiecolt-Glaser and Glaser 2002; Kiecolt-Glaser et al. 2003; Glaser and Kiecolt-Glaser 2005; Miller et al. 2008; Cole et al. 2010). Coinciding with increased inflammation, individuals who report high levels of stress have a greater incidence of mental health disorders including anxiety and depression (Suarez et al. 2003; Suarez et al. 2004). In rodent models, our lab and others have demonstrated that social stressors increase systemic levels of IL-6 and IL-1β in mice and these cytokine levels are elevated further after LPS injection (Johnson et al. 2002; Stark et al. 2002; Johnson et al. 2004). In addition, peripheral CD11b+ cells isolated from socially defeated mice have enhanced production of IL-6, IL-1β, and TNF-α following ex vivo immune stimulation (Stark et al. 2001; Avitsur et al. 2005; Bailey et al. 2009; Powell et al. 2009). Related to enhanced inflammatory cytokine production socially defeated mice are more susceptible to endotoxic shock (Quan et al. 2001). Similar to clinical studies the increased inflammatory capacity corresponds to reduced sensitivity to glucocorticoid regulation and increased gene transcription driven by NF-κB activation (Stark et al. 2001; Avitsur et al. 2002; Quan et al. 2003; Miller et al. 2009; Cole et al. 2010).
An amplified inflammatory response following stress is not limited to the periphery. The central nervous system (CNS) is also vulnerable to stress-induced inflammation. For example, social defeat increases IL-1β, IL-6, and TNF-α mRNA in the pre-frontal cortex (Audet et al. 2011). The enhanced neuroinflammatory status is likely mediated by activated CD11b+ cells within the brain (microglia and CNS macrophages) because they are the primary regulators of inflammatory processes (Davalos et al. 2005; Nimmerjahn et al. 2005; Serrats et al. 2010; Yirmiya and Goshen 2011). This idea is consistent with our previous report that social defeat increased mRNA levels of IL-1β in brain CD11b + cells (Wohleb et al. 2011). In addition, microglia isolated from socially defeated mice had increased surface expression of several inflammatory proteins including CD14 and TLR4, which is the receptor complex that recognizes LPS. The enhanced reactivity of microglia following social defeat coincided with a marked increase in CNS macrophages that also expressed higher levels of CD14 and CD86 (Wohleb et al. 2011). Other stressors, including inescapable footshock and restraint stress, also increase the inflammatory profile of CD11b+ cells. For instance, footshock increased MHCII and reduced CD200 receptor mRNA levels in CD11b+ cells from the hippocampus of rats (Frank et al. 2007). In addition, social defeat or restraint stress enhanced the activated morphology of microglia (Tynan et al. 2010; Hinwood et al. 2011; Wohleb et al. 2011) and increased their proliferative capacity (Nair and Bonneau 2006).
The stress-induced changes in brain CD11b+ cells are relevant because these cells may be more reactive to a secondary immune stimulation. In support of this notion, brain CD11b+ cells isolated from stressed mice or rats produced higher levels of inflammatory cytokines, including IL-1β, TNF-α, MCP-1 (CCL2), and IL-6, when stimulated ex vivo with LPS (Frank et al. 2007; Wohleb et al. 2011). Furthermore, inescapable footshock increased IL-1β mRNA in the hypothalamus and hippocampus and these levels were further enhanced following peripheral LPS injection in vivo (Johnson et al. 2002). Microglia were implicated in this model because increased IL-1β mRNA levels in the hypothalamus following footshock were reduced following administration of minocycline, a putative microglial inhibitor (Blandino et al. 2009). In models of aging in which brain CD11b+ cells show a similar inflammatory profile, peripheral immune stimulation causes amplified neuroinflammation that leads to behavioral and cognitive deficits, including prolonged sickness, depressive-like behavior, and memory impairment (Godbout et al. 2005; Chen et al. 2008; Henry et al. 2009; Wynne et al. 2010; Corona et al. 2011). Moreover, in our work with repeated social defeat, stress-induced neuroinflammation contributed to prolonged anxiety-like behavior (Kinsey et al. 2007; Krishnan et al. 2007; Wohleb et al. 2011). Thus, it is plausible that peripheral LPS challenge will cause exaggerated neuroinflammation and corresponding behavioral deficits in socially defeated mice.
Therefore, the purpose of this study was to determine the extent to which stress-induced reactivity of brain CD11b+ cells (microglia and CNS macrophages) influences the neuroinflammatory and behavioral response to a peripheral LPS injection. Our data indicate that social defeat alone had a significant effect on the inflammatory environment in the CNS. In addition, this increased neuroinflammatory profile was further amplified following a peripheral LPS challenge and was associated with exaggerated weight loss and prolonged social withdrawal.
2. Methods
2.1 Animals
Six week old male C57BL/6 and 12 mo male CD-1 (retired breeders) mice were purchased from Charles River Laboratories (Wilmington, MA). Mice were allowed to acclimate to their surroundings for 7–10 days before any experimental procedures. C57BL/6 mice were housed in cohorts of 3 and CD-1 mice were singly housed. Both groups were housed in 11.5”× 7.5”× 6” polypropylene cages. Rooms were maintained at 21°C under a 12 h light: 12 h dark cycle with ad libitum access to water and rodent chow. All procedures were in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the Ohio State University Institutional Laboratory Animal Care and Use Committee.
2.2 Social Defeat
Repeated social defeat (RSD) was performed as previously described (Wohleb et al. 2011). It is important to note that repeated social defeat is similar to social disruption stress (SDR) previously reported (Avitsur et al. 2001; Stark et al. 2001). In brief, an aggressive CD-1 intruder mouse was introduced into cages of established male cohorts (3 per cage) of C57BL/6 mice six consecutive nights for 2 h between 1700h and 1900h. During each cycle, submissive behavior including upright posture, fleeing, and crouching (Avitsur et al., 2001; Stark et al., 2001) and wounding patterns were observed to ensure that the resident mice were exhibiting subordinate behavior. If the intruder did not initiate an attack within 5–10 minutes or was defeated by any of the resident mice then a new intruder was introduced. At the end of the 2 h period, the intruder was removed and the residents were left undisturbed until the following day when the paradigm was repeated. Different intruders were used on consecutive nights. The health status of the mice was carefully examined throughout the paradigm. Mice that were injured or moribund were removed from the study. Consistent with previous studies using social defeat, less than 5% of mice (n=4) met the early removal criteria for the current study. Control mice (HCC) were left undisturbed in a separate room until sacrificed.
2.3 Experimental Protocols
In the first set of studies, adult male C57BL/6 mice were subjected to repeated social defeat. Fourteen hours later mice received an intraperitoneal (i.p.) injection of saline or Escherichia coli LPS (0.5 mg/kg; serotype 0127:B8, Sigma, St. Louis, MO) and social exploratory behavior was determined 0, 4, 8, and 24 h after injection (n=8–9). The 14 h time point after social defeat was selected because brain CD11b+ cells isolated from RSD mice have an increased inflammatory phenotype and are more reactive to ex vivo LPS stimulation than HCC controls (Wohleb et al. 2011). In addition, repeated social defeat promotes anxiety-like behavior that is present at this time (Kinsey et al. 2007; Wohleb et al. 2011). This LPS dosage was selected because it elicits a pro-inflammatory cytokine response in the brain resulting in a transient sickness response in adult C57BL/6 mice (Corona et al. 2010). At 4 or 24 h after injections, mice were sacrificed and enriched CD11b+ cells were isolated by discontinuous Percoll density gradient. Enriched CD11b+ cells were used for total RNA isolation/quantitative PCR (n=6–10) or flow cytometric analyses (n=8–11).
In the second set of studies, adult male C57BL/6 mice were subjected to repeated social defeat and 14 h later mice were injected with saline or LPS (0.5 mg/kg). Four or 72 h later, mice were deeply anesthetized and transcardially perfused with sterile phosphate-buffered saline (PBS, pH 7.4 w/EDTA) followed by fixation with 4% formaldehyde. Brains were removed, processed, and labeled with anti-Iba-1 (n=3–5).
In the third study, adult male C57BL/6 mice were subjected to repeated social defeat and 14 h later mice were injected with saline or LPS. At 72 h after injection, social exploratory behavior and open-field activity were determined. Following behavior testing enriched CD11b+ cells were isolated by discontinuous Percoll density gradient and used for total RNA isolation/quantitative PCR (n=6–10). In addition, plasma was collected from mice at each time point (4 h, 24 h, 72 h) and IL-6 or corticosterone concentrations were determined (n=8–12).
2.4 Behavior
Social exploratory behavior was determined as previously described (Corona et al. 2010). In brief, experimental mice were introduced individually into the home cage of a novel juvenile (3 weeks old) for a ten minute period. Behavior was videotaped and the cumulative amount of time the experimental mouse engaged in social investigation was determined. Social exploration tests were conducted during the light phase (between 0900h and 1700h) of the photoperiod. Behavior was scored by trained observers who were blind to the experimental treatments. Baseline social behavior was measured immediately before experimental treatment (time 0). Social behavior was determined as the amount of time that the experimental subject spent actively investigating (e.g., anogenital sniffing or trailing) the juvenile. Results are expressed as percent decrease in social behavior compared to respective baseline measures.
Anxiety-like behavior was determined as previously described (Kinsey et al. 2007; Wohleb et al. 2011). In brief, experimental mice were placed in the test apparatus that consisted of a 40×40×25 cm Plexiglas box that was illuminated with standard lighting (~300 lux). Open-field tests were conducted during the light phase (between 0900h and 1000h) of the photoperiod. To initiate testing, mice were placed into the corner of the open-field and locomotor activity was recorded for 5 min using an automated system and analyzed using VersaMap software (AccuScan Instruments, Columbus, OH).
2.7 Isolation of brain CD11b+ cells
Enriched CD11b+ cells were isolated from whole brain homogenates as described previously (Wynne et al. 2010; Wohleb et al. 2011). In brief, brains were homogenized in phosphate-buffered saline (PBS, pH 7.4) by passing through a 70 µm nylon cell strainer. Resulting homogenates were centrifuged at 600×g for 6 min. Supernatants were removed and cell pellets were re-suspended in 70% isotonic Percoll (GE-healthcare, Uppsala, Sweden) at room temperature. A discontinuous Percoll density gradient was layered as follows: 70%, 50%, 35%, and 0% isotonic Percoll. The gradient was centrifuged for 20 min at 2000×g and microglia were collected from the interphase between the 70% and 50% Percoll layers. Cells were washed and then re-suspended in sterile PBS. We have previously characterized these cells as approximately 90% CD11b+/CD45+ (Henry et al. 2009; Wohleb et al. 2011). Based on this characterization, cells isolated by Percoll density separation will be referred to as “enriched CD11b+ cells”.
2.8 RNA isolation and real-time PCR
RNA was isolated from enriched brain CD11b+ cells using the PrepEase RNA Spin Kit (USB Corportation, Cleveland, OH), RNA was reverse transcribed to cDNA using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) and RNA concentration was determined by spectrophotometry (Eppendorf, Hauppauge, NY). Quantitative PCR was performed using the Applied Biosystems (Foster, CA) Assay-on-Demand Gene Expression protocol as previously described (Godbout et al. 2005). In brief, microglia isolated cDNA was amplified by real-time PCR where a target cDNA (e.g., IL-1β, TNF-α, iNOS, and CD14) and a reference cDNA (glyceraldehyde-3-phosphate dehydrogenase; GAPDH) were amplified simultaneously using an oligonucleotide probe with a 5' fluorescent reporter dye (6-FAM) and a 3' quencher dye (NFQ or TAMRA). Fluorescence was determined on an ABI PRISM 7300-sequence detection system (Applied Biosystems, Foster, CA). Data were analyzed using the comparative threshold cycle (Ct) method and results are expressed as fold difference from home cage control mice injected with saline.
2.10 Flow cytometry
Staining of microglia surface antigens was performed as previously described (Henry et al. 2008; Henry et al. 2009; Wohleb et al. 2011). In brief, Fc receptors were blocked with anti-CD16/CD32 antibody (eBioscience, San Diego, CA). Cells were washed and then incubated with the appropriate antibodies (CD45, CD11b, CCR2 CD14, MHCII (eBioscience, San Diego, CA), or Ly6C (BD Biosciences, San Jose, CA) for 45 min. Cells were washed and then re-suspended in FACS buffer (2% FBS in HBSS with 1 mg/ml sodium azide) for analysis. Because the CCR2 primary antibody is not conjugated, cells labeled with CCR2 were incubated in a secondary antibody (FITC; goat anti-rabbit F’ab, eBioscience). Non-specific binding was assessed by using non-specific, isotype-matched antibodies. Antigen expression was determined using a Becton-Dickinson FACSCaliber four color cytometer. Ten thousand events were recorded for each sample and isotype matched-conjugate. Data were analyzed using FlowJo software (Ashland, OR) and gating for each antibody was determined based on non-specific binding of appropriate negative isotype stained controls.
2.11 Morphological analysis of microglia
To analyze the morphology of microglia tissue was labeled with ionized calcium binding adapter molecule-1 (Iba-1) as previously described(Corona et al. 2010; Wohleb et al. 2011). In brief, mice were deeply anesthetized and transcardially perfused with sterile phosphate-buffered saline (PBS, pH 7.4 w/EDTA) and 4% formaldehyde. Brains were post-fixed in 4% formaldehyde for 24 h and incubated in 20% sucrose for an additional 24 h. Fixed brains were frozen using isopentane (−78° C) and sectioned (20 µm) using a Microm HM550 cryostat (Mikron Instruments). Brain regions were identified by reference markers in accordance with the stereotaxic mouse brain atlas (Paxinos and Franklin 2004). Sections were mounted on slides and blocked with 5% normal goat serum. Next, sections were washed in PBS with 1% BSA and incubated with a rabbit anti-mouse Iba-1 antibody (Wako Chemicals, Richmond, VA). Sections were washed, then incubated in a biotinylated goat anti-rabbit secondary antibody and developed using the 3,3'-Diaminobenzidine (DAB) protocol. These sections were cover-slipped with Permount (Fischer-Scientific, Pittsburgh, PA). DAB stained sections were visualized using an epi-fluorescent Leica DM5000B microscope. Images were captured using a Leica DFC300 FX camera and imaging software. To quantify the phenotypic changes of microglia, digital image analysis (DIA) (Donnelly et al. 2009; Wohleb et al. 2011) of Iba-1 staining was performed. Six representative images were taken from each brain region at 20× magnification. A threshold for positive staining was determined for each image and was processed by densitometric scanning of the threshold targets using ImageJ software (NIH). Proportional area was reported as the average percent area in the positive threshold for all representative pictures.
2.12 Statistical analysis
To ensure a normal distribution, data were subjected to Shapiro-Wilk test using Statistical Analysis Systems (SAS) statistical software (Cary, NC). Observations greater than 3 interquartile ranges from the first and third quartile were considered outliers and were excluded in the subsequent analysis. Overall, less than 1% of the total observations were determined to be outliers. To determine significant main effects and interactions between factors, data were analyzed using one- (stress,), two- (stress × LPS), or three- way (stress × LPS × time) ANOVA using the General Linear Model procedures of SAS. When appropriate, differences between treatment means were evaluated by an F-protected Student’s t-test. All data are expressed as treatment means ± standard error of the mean (SEM).
3. Results
3.1 Peripheral LPS injection caused extended weight loss, social withdrawal, and elevated plasma IL-6 levels in socially defeated mice
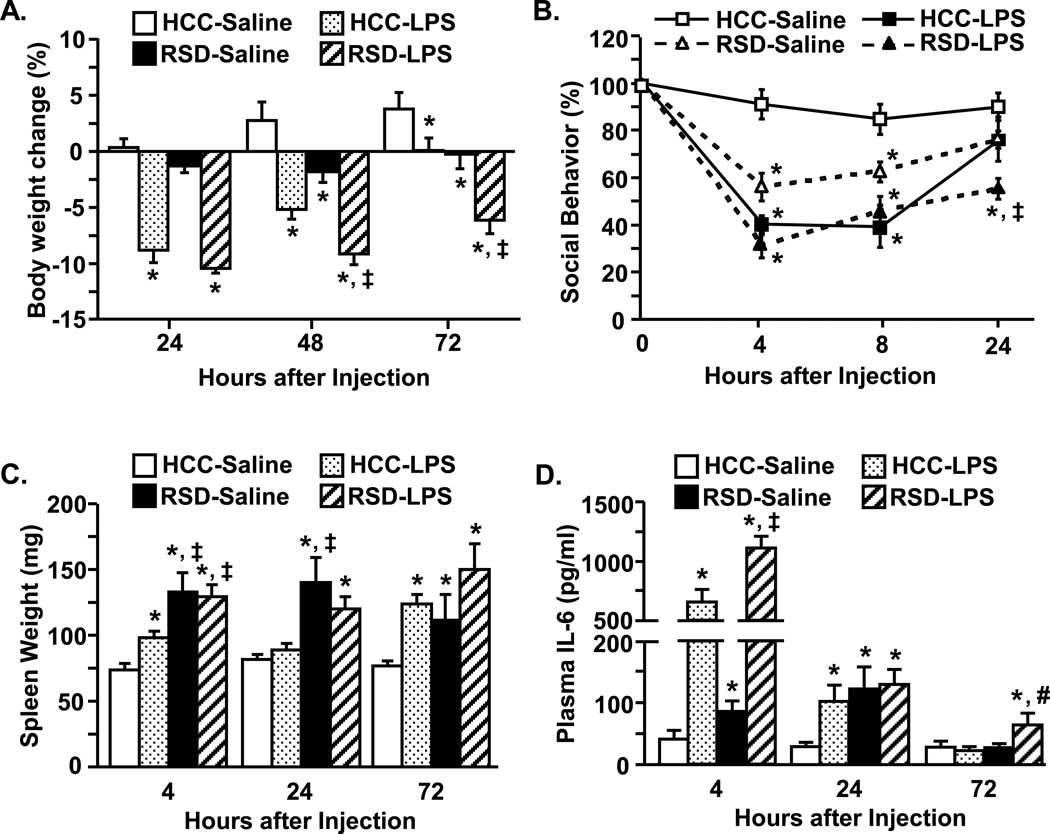
Peripheral injection of lipopolysaccharide (LPS) causes a transient sickness response with anorexia and decreased social exploratory behavior (i.e., social withdrawal) (Dantzer et al. 2008; Godbout et al. 2008; Henry et al. 2008; Corona et al. 2010). To determine the extent to which repeated social defeat altered the behavioral response to peripheral LPS injection, body weight and social exploratory behavior were determined at several times after LPS injection. In the first experiment, mice were subjected to repeated social defeat (RSD) or left undisturbed (HCC) and 14 h later were injected i.p. with saline or LPS. Body weight was determined 0, 24, 48, and 72 h after LPS injection. Fig.1A shows that LPS injection decreased body weight in a time-dependent manner (LPS × time interaction; F(1,46)=4.18, p<0.01). Moreover, control mice (HCC-LPS) returned to baseline body weight within 72 h after LPS injection, but socially defeated mice (RSD-LPS) had exaggerated weight loss (p<0.0003).
Figure 1. Peripheral LPS injection caused extended weight loss, social withdrawal, and elevated plasma IL-6 levels in socially defeated mice.
Male C57BL/6 mice were subjected to repeated social defeat (RSD) or left undisturbed as controls (HCC). Fourteen hours after RSD, mice were injected with saline or LPS (0.5 mg/kg). A) Body weight was determined at 0 (baseline), 24, 48, and 72 h after injection. Graph represents percent total weight loss from baseline (n=8–12). B) Social exploratory behavior was determined at 0 (baseline), 4, 8, and 24 h after injection and percent was calculated from baseline (n = 8–9). C) Average spleen weight (n = 8–18) and D) plasma IL-6 concentrations were determined at 4, 24, and 72 h after injection (n = 8–12). Bars or points represent the mean ± SEM. Means marked with * are significantly different from HCC-Saline (p<0.06) and means marked with ‡ are significantly different from HCC-LPS (p<0.05). Means marked with # tend to be different from all other experimental groups (p<0.08).
In the next experiment, social exploratory behavior was determined 0, 4, 8, and 24 h after experimental treatments. Fig.1B shows that RSD (F(1,34)=20.25, p<0.0001) and LPS (F(1,34)=66.46, p<0.0001) reduced social exploratory behavior. Moreover, the LPS-induced reduction of social behavior was more profound in socially defeated mice (RSD) compared to control mice (HCC) (stress × LPS interaction; F(1,34)=4.94, p<0.03). This interaction tended to be time dependent (stress × LPS × time interaction, F(2,99)=2.69, p=0.07). For instance, control mice (HCC-LPS) returned to baseline exploratory behavior by 24 h after LPS injection while socially defeated mice (RSD-LPS) had a significant reduction in social exploratory behavior at this time (p<0.002). Taken together, these data indicate that LPS-induced weight loss and social withdrawal were extended in mice subjected to RSD compared to HCC mice.
Repeated social defeat increases spleen weight (i.e., splenomegaly) (Avitsur et al. 2003) and plasma levels of interleukin(IL)-6 and corticosterone (Stark et al. 2001; Stark et al. 2002). Therefore, spleen weight and plasma IL-6 or corticosterone levels were determined 4, 24, and 72 h after injection. Consistent with previous findings social defeat increased spleen weight (F(1,160)=26.33, p<0.0001; Fig.1C). In addition, LPS injection also increased spleen weight (F(1,160)=7.47, p<0.007), but LPS injection did not further increase stress-induced splenomegaly. Fig.1D shows that social defeat (F(1,110)=13.38, p<0.0004) and LPS (F(1,110)=96.38, p<0.0001) increased IL-6 levels in the plasma. At 4 h after injection, plasma IL-6 levels were highest in the socially defeated mice injected with LPS (stress × LPS interaction; F(1,110)=4.47, p<0.04). Furthermore, elevated plasma IL-6 levels were still detectable in socially defeated mice 72 h after LPS injection (stress × LPS × time interaction; F(2,110)=6.17, p<0.003). Corticosterone levels showed a similar pattern with highest levels in socially defeated mice injected with LPS at 4 h after injection (p<0.05). The elevated levels of corticosterone induced by social defeat and LPS returned to baseline by 24 and 72 h after injection (data not shown).
3.2 Peripheral LPS injection caused amplified and prolonged IL-1β and TNF-α mRNA expression in enriched brain CD11b+ cells from socially defeated mice
We previously reported that enriched CD11b+ cells from brains of socially defeated mice secreted higher protein levels of TNF-α, IL-6, and MCP-1 (CCL2) following ex vivo LPS stimulation (Wohleb et al. 2011). Therefore, we sought to determine the degree to which in vivo LPS challenge caused amplified activation of microglia/macrophages in socially defeated mice. In these experiments, mice were subjected to repeated social defeat (RSD) or left undisturbed (HCC) and 14 h later mice were injected i.p. with saline or LPS. Next, mRNA levels of several inflammatory genes, IL-1β, TNF-α, iNOS and CD14, were determined in enriched CD11b+ cells 4 h and 24 h after saline or LPS injection (Fig.2A).
Figure 2. Peripheral LPS injection caused amplified and prolonged IL-1β and TNF-α mRNA expression in enriched brain CD11b+ cells from socially defeated mice.
Male C57BL/6 mice were subjected to repeated social defeat (RSD) or left undisturbed as controls (HCC). Fourteen hours after RSD, mice were injected with saline or LPS (0.5 mg/kg). A) IL-1β, TNF-α, iNOS and CD14 mRNA levels were determined in enriched CD11b+ cells 4 h or 24 h later (n=6–10). A subset of brain CD11b+ cells were used to determine the surface expression of CD11b, CD45, and CD14. B) Representative bivariate dot plots of CD11b/CD14 staining on microglia 4 h after injection. C–D) Average percent of CD14+ microglia (CD11b+/CD45low) collected 4 or 24 h later (n=5–8). Bars represent the mean ± SEM. Means with different letters (a, b, c, or d) are significantly different (p<0.06) from each other.
Consistent with our previous studies, social defeat alone increased mRNA levels of IL-1β(F(1,33)=4.51, p<0.05), TNF-α (F(1,34)=4.72, p<0.04), iNOS (F(1,30)=4.50, p<0.04) and CD14 (F(1,35)=3.91, p=0.06). As expected, these genes were also increased by LPS (IL-1β F(1,33)=12.86, p<0.001; TNF-α F(1,34)=6.45, p<0.01; iNOS F(1,30)=3.66, p<0.008; CD14 F(1,35)=10.16, p<0.003). The most robust increases in LPS-induced mRNA expression was in enriched CD11b+ cells isolated from socially defeated mice injected with LPS (stress × LPS interaction; IL-1β F(1,33)=3.37, p=0.07; TNF-α F(1,34)=3.21, p=0.08; iNOS F(1,32)=1.79, p=0.06; CD14 F(1,35)=2.58, p=0.10). For example, post hoc analysis confirmed that the highest induction of IL-1β, TNF-α, iNOS, and CD14 mRNA was in socially defeated mice injected with LPS (RSD-LPS) compared to all other treatment groups (p<0.01, for each).
A similar pattern of mRNA expression was apparent in HCC and RSD mice 24 h after injections. Fig.2A shows that IL-1β (F(1,28)=3.62, p=0.06), TNF-α, (F(1,29)=5.08, p<0.03), iNOS (F(1,29)=6.24, p<0.02), and CD14 (F(1,29)=2.70, p=0.10) mRNA levels were elevated in mice subjected to social defeat. Increased mRNA levels of IL-1β (F(1,28)=7.47, p<0.01), TNF-α (F(1,29)=5.41, p<0.03), iNOS (F(1,29)=3.31, p=0.08), and CD14 (F(1,29)=6.62, p<0.02) were also detected 24 h after LPS. Furthermore, the highest expression of IL-1β (stress × LPS interaction; F(1,28)=3.20, p=0.08) and TNF-α (stress × LPS interaction; F(1,29)=3.57, p=0.07) mRNA 24 h after LPS injection tended to be in socially defeated mice injected with LPS. Post hoc analysis showed that LPS-induced a significant increase in IL-1β and TNF-α mRNA levels in mice subjected to social defeat (RSD-LPS) (p<0.01, for each). These data indicate that LPS injection caused an amplified inflammatory cytokine response in CD11b+ cells isolated from socially defeated mice compared to control mice. Moreover, IL-1β and TNF-α mRNA levels remained significantly elevated in enriched CD11b+ cells isolated from socially defeated mice injected i.p. with LPS.
To confirm the reactive microglia (CD11b+/CD45low) phenotype, flow cytometric analysis of CD14 was performed 4 h and 24 h after injection. As expected, social defeat (F(1,27)=13.94, p<0.001) and LPS (F(1,27)=15.08, p<0.0007) increased CD14 expression on microglia (Fig.2C). Although there was not an interaction between stress and LPS, post hoc analysis revealed that CD14 protein levels were highest on socially defeated mice injected with LPS (p<0.005) (Fig.2C). The increased CD14 expression on microglia induced by social defeat (F(1,22)=11.89, p<0.003) and LPS (F(1,22)=33.57, p<0.0001) was still detected 24 h after injection (Fig. 2D). Similar to the 4 h time-point, CD14 expression was enhanced on socially defeated mice injected with LPS (p<0.03) compared to all other groups. Taken together these results indicate that social defeat and LPS injection increased CD14 expression on microglia and CD14 expression was highest on microglia from socially defeated mice injected with LPS.
3.3 Enhanced microglial activation in the hippocampus (HPC) of socially defeated mice injected with LPS
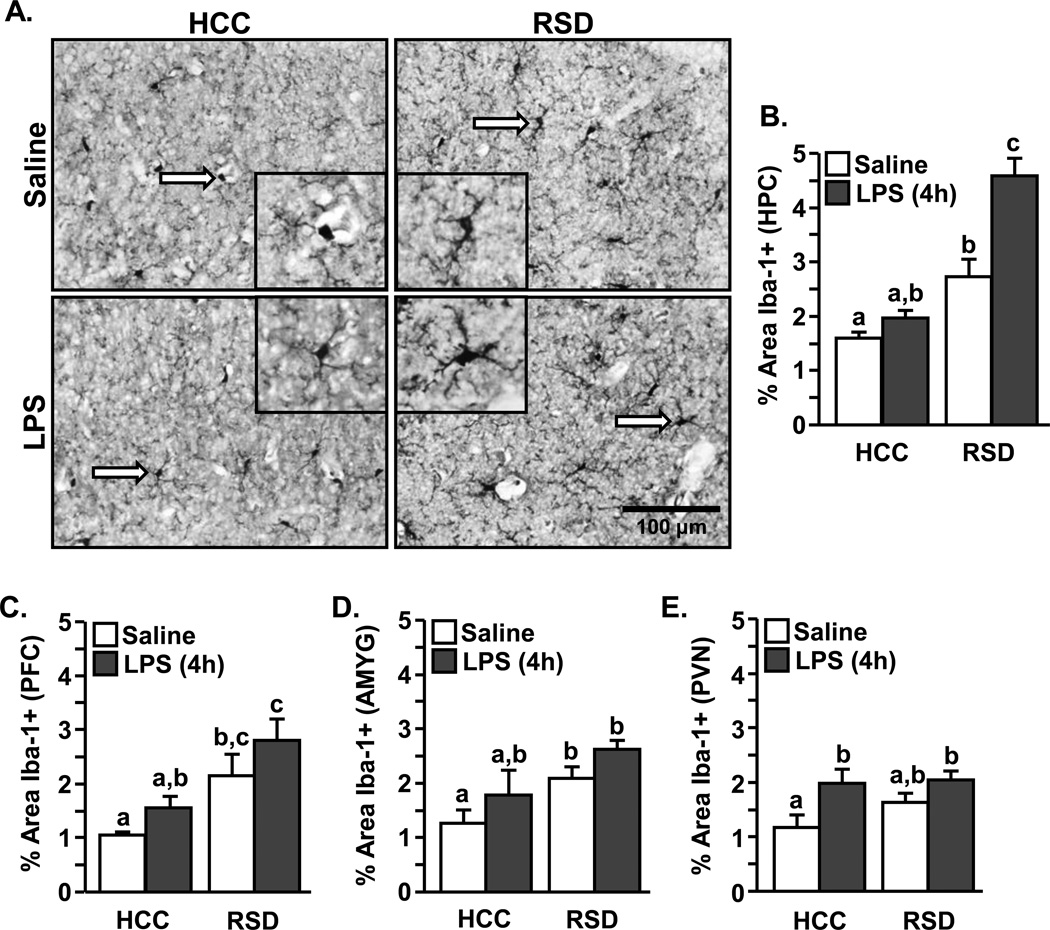
Next, we sought to determine the degree to which the interaction between social defeat and LPS injection caused brain region dependent differences in microglia activation. Following restraint stress or social defeat, microglia in stress-responsive regions have a more de-ramified or activated morphology detected by Iba-1 immunohistology (Tynan et al. 2010; Hinwood et al. 2011; Wohleb et al. 2011). Therefore, Iba-1proportional area in the pre-frontal cortex (PFC), amygdala (AMYG), paraventricular nucleus (PV N), and hippocampus (HPC) was determined. Fig.3A shows representative images of Iba-1 staining 4 h after injection in the HPC of control (HCC) or socially defeated (RSD) mice. Overall, microglia from socially defeated mice were hypertrophic with shorter and thicker processes, while microglia from control mice had small and round cell bodies with longer and thinner processes. Digital image analysis (DIA)(Corona et al. 2010; Wohleb et al. 2011) (Fig.3B–E) confirmed that repeated social defeat significantly increased the proportional area of Iba-1+ cells in the HPC (F(1,14)=39.33, p<0.001, Fig.3A&B), PFC (F(1,15)=11.61, p<0.01, Fig.3C), and AMYG (F(1,15)=8.22, p<0.01, Fig.3D). Iba-1 immunoreactivity, however, was not increased in the PVN following social defeat (Fig.3E). LPS injection alone significantly enhanced Iba-1 proportional area in the HPC (F(1,14)=13.78, p<0.003, Fig.3A&B) and PVN (F(1,15)=8.67, p<0.01, Fig.3E), while the PFC (p=0.10) and AMYG (p=0.08) tended to have increased Iba-1+ proportional area (Fig.3C&D). Moreover, LPS injection markedly enhanced the stress-induced Iba-1 immunoreactivity in the HPC (stress × LPS interaction, F(1,14)=6.26, p<0.03). Taken together, these data indicate that social defeat and LPS injection increased the activated morphology of microglia in the PFC, AMYG, PVN, and HPC. Moreover, LPS injection exaggerated the stress-induced increase in microglial activation specifically in the hippocampus.
Figure 3. Enhanced microglia activation in the HPC of socially defeated mice injected with LPS.
Male C57BL/6 mice were subjected to repeated social defeat (RSD) or left undisturbed as controls (HCC). Fourteen hours after RSD, mice were injected with saline or LPS (0.5 mg/kg). A) Representative images of Iba-1 staining in the hippocampus of brains collected 4 h after injection (40×). Inset includes enlarged image of Iba-1+ cell indicated by white arrow. B–E) Proportional area for Iba-1 staining in the B) hippocampus (HPC), C) pre-frontal cortex (PFC), D) amygdala, and E) paraventricular nucleus (PVN) (n=3–5). Bars represent the mean ± SEM. Means with different letters (a, b, or c) are significantly different (p<0.06) from each other.
3.4 Peripheral LPS injection increased the number of CCR2+ macrophages in the brain of socially defeated mice
We have previously reported that social defeat increased the percentage of macrophages (CD11b+/CD45high) in the brain (Wohleb et al. 2011). Thus, we next determined the extent to which peripheral LPS injection altered the proportion of CNS macrophages in HCC and RSD mice. In these experiments, mice were subjected to repeated social defeat (RSD) or left undisturbed (HCC) and 14 h later mice were injected i.p. with saline or LPS. Enriched brain CD11b+ cells were collected 4 h after injection and microglia (CD11b+/CD45low) or CNS macrophages (CD11b+/CD45high) were differentiated using flow cytometry based on CD45 expression (Nair and Bonneau 2006; Wohleb et al. 2011).
Fig.4A shows representative bivariate dot plots of CD11b and CD45 staining for each treatment group 4 h after injection. The percentage of CNS macrophages (CD11b+/CD45high) increased with social defeat (F(1,52)=12.00, p<0.001) and LPS (F(1,52)=21.11, p<0.0001) (Fig.4B). Although there was not a significant interaction between stress and LPS, post hoc analysis indicates that the highest percentage of CNS macrophages was in the RSD-LPS group (p<0.04) compared to all other groups. The stress-induced increase (F(1,21)=14.52, p<0.001) in CNS macrophages was still present 24 h after injection, but the LPS-induced increase was resolved by 24 h (data not shown). In addition, surface expression of CD14 on CNS macrophages was increased by social defeat (F(1,27)=25.93 p<0.0001) and LPS injection (F(1,27)= 1016.37, p<0.0001). Stress-induced CD14 expression on CNS macrophages was further amplified following LPS injection (stress × LPS interaction, F(1,27)=11.38, p<0.003) (Fig.3C). These results indicate that stress and LPS injection increased the percentage of CNS macrophages and LPS caused amplified CD14 expression on CNS macrophages in socially defeated mice.
Figure 4. Peripheral LPS injection increased the number of CCR2+ macrophages in the brain of socially defeated mice.
Male C57BL/6 mice were subjected to repeated social defeat (RSD) or left undisturbed as controls (HCC). Fourteen hours after RSD, mice were injected with saline or LPS (0.5 mg/kg). A) Representative bivariate dot plots of CD11b/CD45 staining of enriched brain CD11b+ collected 4 h after injection. B) Average percentage of macrophages (CD11b+/CD45high) that were present in the brain 4 h after injection (n=6–12). C) Average percentage of CD14+ macrophages (n=6–8). Relative number of D) Ly6Chigh or E) CCR2+ macrophages are shown (n=5–8). Bars represent the mean ± SEM. Means with different letters (a, b, c, or d) are significantly different (p<0.05) from each other.
Because repeated social defeat increased the percentage of CNS macrophages, markers of macrophage reactivity and recruitment, Ly6C and CCR2 (Mildner et al. 2007; Prinz and Priller 2010; Wohleb et al. 2011) were determined 4 and 24 h after LPS injection. Fig.4D shows that LPS injection significantly increased the number of Ly6Chigh CNS macrophages (F(1,27)=27.05, p<0.0001), while social defeat tended to increase the number of Ly6Chigh macrophages in the brain (p=0.10). There was no interaction between stress and LPS injection on the number of Ly6Chigh macrophages. Fig.4E shows that social defeat significantly increased the number of CCR2+ macrophages (F(1,18)=25.36, p<0.0001), and the number of CCR2+ macrophages was further amplified by LPS injection at 4 h (stress × LPS interaction; F(1,18)=5.12, p<0.04). These data indicate that LPS injection enhanced the number of Ly6Chigh macrophages in the brain. Moreover, social defeat increased the number of CCR2+ macrophages in the brain which were significantly enhanced by LPS injection.
3.5 Socially defeated mice have persistent sickness behavior 72 h after LPS injection
Repeated social defeat promotes anxiety-like behavior (Kinsey et al. 2007) that is associated with increased levels of IL-1β in the brain (Wohleb et al. 2011). Because LPS injection caused prolonged expression of IL-1β in brain CD11b+ cells from socially defeated mice, we determined the degree to which LPS injection exaggerated anxiety-like behavior in HCC and RSD mice. Open-field activity was used to examined anxiety-like behavior by time spent in the center of the open-field and time to enter the center (Kinsey et al. 2007; Wohleb et al. 2011). Fig.5A shows that either LPS injection (F(1,20)=14.78; p<0.001) or social defeat (F(1,20)= 10.24, p<0.005) decreased time spent in the center of the open field. Fig.5B shows that socially defeated mice have an overall increased time to enter the open field (F(1,20)=6.04, p<0.03). In addition, socially defeated mice injected with LPS had a marked increase in time to enter the open field compared to all groups (p<0.01).
Figure 5. Socially defeated mice have residual sickness behavior 72 h after LPS injection.
Male C57BL/6 mice were subjected to repeated social defeat (RSD) or left undisturbed as controls (HCC). Fourteen hours after RSD, mice were injected with saline or LPS (0.5 mg/kg) and open-field activity was determined 72 h later. A) Average time to enter the center of the open-field, B) average number of entries into the center, C) total time spent in the center of the open-field, and D) total distance traveled were determined (n=5–6). E) Social exploratory behavior was determined at 0 (baseline), 24, and 72 h after injections and percent was determined from baseline (n=3). Bars represent the mean ± SEM. Means marked with * are significantly different (p<0.05) from each other. Means marked with # tend to be different from RSD-Saline (p=0.07).
While LPS injection increased the time to enter the center of the open field in RSD mice compared to HCC mice, it is important to point out that entry into the center of the open-field was significantly delayed in RSD-LPS mice. These mice also tended to have a reduction in total distance traveled (Fig.5C, p=0.08). Because decreased locomotor activity is indicative of residual sickness-like behavior (Godbout et al. 2005; Dantzer and Kelley 2007), this may confound the results of anxiety-like behavior analysis. To address this issue, a cohort of mice was used in a social exploratory behavioral paradigm similar to Fig.1B, but mice were only tested at baseline and then again at 72 h after injection. Consistent with reduced locomotor activity (Fig.5B&C) in the open-field, socially defeated mice injected with LPS still had a significant reduction in social exploratory behavior compared to all experimental groups (stress × LPS interaction; F(1,11)=5.31, p<0.05). Taken together these results indicate that the LPS-induced sickness response is prolonged in socially defeated mice.
3.6 Elevated IL-1β mRNA in enriched CD11b+ cells and enhanced microglia activation in the HPC of socially defeated mice 72 h after LPS injection
Socially defeated mice injected with LPS had extended deficits in social exploration and locomotor activity that were apparent 72 h after peripheral challenge. Therefore, mRNA analysis of inflammatory genes in enriched brain CD11b+ cells and Iba-1 immunohistology were performed 72 h after injection. Similar to the previous time-points, either social defeat increased mRNA expression of IL-1β (F(1,30)=8.54, p<0.006), TNF-α (F(1,31)=6.60, p<0.02), iNOS (F(1,31)=3.50, p=0.07), and CD14 (F(1,29)=6.11, p<0.02). Moreover, LPS injection caused a significant increase in IL-1β (F(1,30)=11.16, p<0.003). Although there was not a significant interaction between stress and LPS, post hoc analysis indicates that the highest expression of IL-1β mRNA was in socially defeated mice injected with LPS (RSD-LPS) (p<0.01) compared to all other treatment groups (Fig.6A). Consistent with Fig.3A, Fig.6B–E shows that stress-induced Iba-1 proportional area was markedly enhanced in the HPC (F(1,16)=119.48, p<0.0001), PFC (F(1,16)=3.81, p=0.07), AMYG (F(1,16)=10.07, p<0.007), and PVN (F(1,16)=22.94, p<0.0004). Furthermore, LPS injection increased Iba-1 proportional area in the HPC (F(1,16)=129.03, p<0.0001), PFC (F(1,16)=6.98, p<0.02), AMYG (F(1,16)=13.11, p<0.003), and PVN (F(1,16)=18.66, p<0.0008). The activated morphology of Iba-1+ cells was most pronounced in the HPC of socially defeated mice injected with LPS (stress × LPS interaction, F(1,16)=49.46, p<0.0001). Taken together, these data indicate that LPS injection extended and enhanced stress-induced activation of microglia in the HPC.
Figure 6. Elevated IL-1β mRNA in enriched CD11b+ cells and enhanced microglia activation in the HPC of socially defeated mice 72 h after LPS.
Male C57BL/6 mice were subjected to repeated social defeat (RSD) or left undisturbed as controls (HCC). Fourteen hours after RSD, mice were injected with saline or LPS (0.5 mg/kg). A) IL-1β, TNF-α, iNOS and CD14 mRNA levels were determined from enriched CD11b+ cells collected 72 h later (n=7–9). B) Representative images of Iba-1 staining in the hippocampus of brains collected 72 h after injections (40×). Inset includes enlarged image of Iba-1+ cell indicated by white arrow. C–F) Proportional area for Iba-1 staining in the C) hippocampus (HPC), D) pre-frontal cortex (PFC), E) medial amygdala (MeA), and F) paraventricular nucleus (PVN) (n=3–5). Bars represent the mean ± SEM. Means with different letters (a, b, or c) are significantly different (p<0.05) from each other.
4. Discussion
Previous studies indicate that repeated social defeat increases the inflammatory phenotype of peripheral and brain CD11b+ cells (Stark et al. 2001; Avitsur et al. 2005; Bailey et al. 2009; Powell et al. 2009; Wohleb et al. 2011). The present study demonstrates that repeated social defeat enhanced the inflammatory response of brain CD11b+ cells following a peripheral LPS challenge. Socially defeated mice injected with LPS had a prolonged sickness response with extended social withdrawal and exaggerated weight loss. Moreover, enriched CD11b+ cells from the brains of socially defeated mice injected with LPS had exaggerated expression of inflammatory mediators including IL-1β, TNFα, and CD14. There was also a stress-induced increase in activated morphology of microglia (Iba-1+) in the PFC, AMYG, PVN, and HPC. The activated morphology of microglia was markedly amplified in the HPC of socially defeated mice injected with LPS. In addition, social defeat and LPS injection caused a significant increase in CNS macrophages with the most pronounced increase in the number of CCR2+ macrophages in socially defeated mice injected with LPS. Furthermore, enriched CD11b+ cells from socially defeated mice had an extended increase in IL-1β mRNA that corresponded to decreased social exploratory behavior 72 h after LPS injection.
An important finding in this study was that stress-induced activation of brain CD11b+ cells (microglia and CNS macrophages) caused an amplified and prolonged inflammatory response following peripheral LPS challenge. We confirmed our previous data that repeated social defeat increased IL-1β expression in brain CD11b+ cells (Wohleb et al. 2011). In addition, we show that 4 h after LPS injection enriched brain CD11b+ cells from socially defeated mice had exaggerated expression of IL-1β, TNF-α, iNOS and CD14 (Fig.2A). This is consistent with our previous findings that ex vivo treatment of enriched brain CD11b+ cells from socially defeated mice have increased production of IL-6, TNF-α, and MCP-1(Wohleb et al. 2011). These findings are also consistent with inescapable footshock and restraint stress that promote ex vivo reactivity of brain CD11b+ cells to secondary immune stimulation. For instance, microglia isolated from the hippocampus of stressed rats had enhanced IL-1β mRNA expression after ex vivo LPS stimulation (Frank et al. 2007). In addition to initial amplified expression, mRNA levels of IL-1β and TNF-α were also prolonged 24 h after LPS injection in brain CD11b+ cells from socially defeated mice compared to controls (Fig.2). Furthermore, enhanced mRNA expression of IL-1β was still detected 72 h after LPS in socially defeated mice. Thus, stress-induced priming of brain CD11b+ cells leads to an amplified and protracted neuroinflammatory response following peripheral innate immune activation.
In support of an amplified inflammatory response by brain CD11b+ cells, both microglia (CD11b+/CD45low) and CNS macrophages (CD11b+/CD45high) from socially defeated mice had amplified surface expression of CD14 after LPS injection (Fig.2 & Fig.4). Because CD14 is an activation marker of CD11b+ cells, the enhanced expression with stress and LPS supports the amplified inflammatory mRNA levels obtained. It is important to point out that there was not a significant interaction between stress and LPS on CD14 expression in microglia (Fig.2C&D). This may represent an additive effect between stress and LPS, but RSD-LPS mice had the highest level of CD14 protein expression at both 4 and 24 h compared to all other groups. Moreover, it is plausible that the high levels of CD14 expression on microglia (approximately 80% CD14+ microglia) in the RSD-LPS group represents a maximum bound. In this instance the maximum possible expression is 100% CD14+ microglia and 80% CD14+ microglia is the highest level we have observed with any treatment in C57BL/6 mice. The higher protein expression of CD14 at 4 h after LPS in the RSD mice corresponds with the amplified mRNA levels of CD14 and inflammatory cytokines.
Another important finding was that the proportion of CNS macrophages was increased with RSD and these CNS macrophages were further increased by LPS injection (Fig.4A&B). The increased percentage of CNS macrophages with social defeat and LPS injection is relevant because these cells have a significant role in propagating neuroinflammatory responses (Serrats et al. 2010; Moon et al. 2011). Coinciding with the increased percentage of CNS macrophages there was a significant increase in the number of CCR2+ macrophages in social defeated mice injected with LPS (Fig.4E). The increased number of CCR2+ macrophages following social defeat and LPS injection is important because CCR2 expression is indicative of a reactive subset of monocytes that traffic to sites of inflammation (Mildner et al. 2007; Getts et al. 2008; King et al. 2009; Wohleb et al. 2011). Furthermore, the stress-induced increase of CCR2+ macrophages is relevant because enriched brain CD11b+ cells from socially defeated mice have enhanced production of MCP-1 (CCL2) after ex vivo LPS stimulation, which is the primary ligand for CCR2 (Mahad et al. 2006; Wohleb et al. 2011). These results are similar to previous findings in which social defeat increased CCL2 mRNA expression and trafficking of peripheral CD11b+ cells in the lung (Curry et al. 2010). Thus, it is plausible that the increased number of CCR2+ macrophages gain access to the CNS following social defeat and LPS injection.
Moreover, these trafficking CNS macrophages are likely derived from circulating CD11b+ cells. This is relevant because peripheral CD11b+ cells have a more reactive phenotype following repeated social defeat. For example, peripheral CD11b+ cells from socially defeated mice have enhanced IL-6, TNF-α, and IL-1β production following ex vivo LPS stimulation (Stark et al. 2001; Avitsur et al. 2005; Bailey et al. 2009; Powell et al. 2009). The increased reactivity of peripheral CD11b+ cells is related to impaired glucocorticoid anti-inflammatory feedback and enhanced NF-κB transcription factor activation (Stark et al. 2001; Quan et al. 2003). There is relevant because NF-κB gene transcription in the nucleus accumbens can regulate development of anxiety-like behavior following chronic social defeat (Christoffel et al. 2011).
Microglia activation was apparent in several brain regions after LPS injection or social defeat, but the most striking difference was in the HPC. For example, LPS injection caused amplified (Fig.3A&B) and prolonged activation of microglia in the HPC of socially defeated mice (Fig.6A&B). This is important because extended activation of CD11b+ cells in the HPC is associated with decreased neurogenesis and cognitive impairment (Tanaka et al. 2006; Goshen and Yirmiya 2009; Yirmiya and Goshen 2011). Consistent with previous work (Martinez et al. 2002; Hinwood et al. 2011; Wohleb et al. 2011), the present study shows that Iba-1 proportional area increased in the PFC, AMYG, PVN, and HPC following with stress. Several brain regions, including the PFC and AMYG, showed additive effects of social defeat and LPS injection. This may reflect different stress- and LPS-induced neuroimmune pathways that activate brain CD11b+ cells (Quan and Banks 2007; Quan 2008). While social defeat increased microglial activation in several stress-responsive brain regions, our data indicate that the convergence between stress and LPS injection on microglia activation occurred primarily in the HPC.
Another key finding of this study was that stress-induced reactivity of brain CD11b+ cells caused prolonged physiological and behavioral consequences. For example, LPS injection caused extended social withdrawal and exaggerated weight loss in socially defeated mice compared to HCC mice (Fig.1 and Fig.7). Consistent with a heightened inflammatory state, IL-6 plasma levels were amplified and tended to be prolonged in socially defeated mice injected with LPS (Fig.1D). The connection between amplified neuroinflammation and behavioral deficits in socially defeated mice are consistent with our work in aged mice (Corona et al. 2011). Age-related priming of microglia (Henry et al. 2009) is associated with long-lasting sickness and depressive complications following central or peripheral immune challenge (Godbout et al. 2005; Abraham et al. 2008; Godbout et al. 2008; Huang et al. 2008; Wynne et al. 2010). The mechanism that primes CD11b+ cell in the brain may differ, but nevertheless the exaggerated neuroinflammatory response following LPS injection results in similar behavioral deficits.
It was expected that LPS would exaggerate anxiety-like behavior induced by repeated social defeat but this was not the case. Consistent with our previous findings RSD caused prolonged anxiety-like behavior (Fig.5) (Kinsey et al. 2007; Wohleb et al. 2011). LPS injection also caused anxiety-like behavior that was present 72 h after injection (Fig.5). This coincides with previous studies that show the anxiogenic effects of LPS injection (Lacosta et al. 1999; Kohman et al. 2008). Because social defeat and LPS injection alone caused anxiety-like behavior, open-field testing may not have been sensitive enough to detect interactions between stress and LPS. It is also possible that LPS-induced anxiety represents a different behavioral subtype than anxiety caused by social defeat. Thus, a limitation of this study was that other measures of anxiety, including stimulus-dependent anxiety (Berton et al. 2006; Christoffel et al. 2011) and hippocampal-dependent anxiety/fear responses (Tsetsenis et al. 2007), were not examined. Although open-field activity may be confounded in this context, social withdrawal was still evident 72 h after LPS injection in socially defeated mice (Fig.5). Therefore, we provide evidence that an exaggerated neuroinflammatory response in socially defeated mice resulted in a long-lasting sickness response.
In conclusion, we provide evidence that repeated social defeat enhances the neuroinflammatory capacity of CD11b+ cells following a secondary immune challenge. Moreover, there are physiological and behavioral consequences as a result of stress-induced activation of brain CD11b+ cells. It is important to note that brain CD11b+ cells exhibit a similar reactive phenotype as peripheral CD11b+ cells after social defeat. In the periphery increased inflammation may be advantageous in the clearance of bacterial or viral pathogens (Bailey et al. 2007; Mays et al. 2010), however this is often associated with increased tissue damage (Bailey et al. 2009; Curry et al. 2010; Dong-Newsom et al. 2010). This is relevant in the present study because the brain is particularly sensitive to inflammation, thus stress-induced exacerbation of inflammatory responses can lead to long-lasting behavioral and cognitive deficits.
Acknowledgements
The authors thank Dr. Ronald Glaser (OSU, IBMR) for the use of the Applied Biosystems PRISM 7300 sequence detection system. We also thank Brenda Reader for her help with the corticosterone enzyme immunoassay (EIA).
Role of Funding Source:
This research was supported by a NIA grant R01AG033028 to J.P.G. E.S.W. is supported by an NIDCR training grant T32DE014320 to J.F.S. A.M.F. is supported by a HHMI Med to Grad scholarship. The NIA, NIDCR, and HHMI had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. sources
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
The authors declare that they do not have any actual or potential conflicts of interest. This includes any financial, personal, or other relationships with other people or organizations that inappropriately influenced or perceived to have influenced their work.
Contributors:
E.S.W., N.D.P., J.F.S., and J.P.G. designed research; E.S.W., A.M.F., and A.M.P. performed research; N.D.P., J.F.S., and J.P.G. contributed unpublished reagents and analytical tools; E.S.W., A.M.F., A.M.P., N.D.P., J.F.S., and J.P.G. analyzed data; E.S.W., J.F.S., and J.P.G. wrote the paper.
References
- Abraham J, Jang S, Godbout JP, Chen J, Kelley KW, Dantzer R, Johnson RW. Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiol. Aging. 2008;29(4):614–621. doi: 10.1016/j.neurobiolaging.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audet MC, Jacobson-Pick S, Wann BP, Anisman H. Social defeat promotes specific cytokine variations within the prefrontal cortex upon subsequent aggressive or endotoxin challenges. Brain Behav. Immun. 2011;25(6):1197–1205. doi: 10.1016/j.bbi.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Kavelaars A, Heijnen C, Sheridan JF. Social stress and the regulation of tumor necrosis factor-alpha secretion. Brain Behav Immun. 2005;19(4):311–317. doi: 10.1016/j.bbi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Padgett DA, Dhabhar FS, Stark JL, Kramer KA, Engler H, Sheridan JF. Expression of glucocorticoid resistance following social stress requires a second signal. J. Leukoc. Biol. 2003;74(4):507–513. doi: 10.1189/jlb.0303090. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Dhabhar FS, Padgett DA, Sheridan JF. Social disruption-induced glucocorticoid resistance: kinetics and site specificity. J. Neuroimmunol. 2002;124(1–2):54–61. doi: 10.1016/s0165-5728(02)00010-3. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Sheridan JF. Social stress induces glucocorticoid resistance in subordinate animals. Horm. Behav. 2001;39(4):247–257. doi: 10.1006/hbeh.2001.1653. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Engler H, Powell ND, Padgett DA, Sheridan JF. Repeated social defeat increases the bactericidal activity of splenic macrophages through a Toll-like receptor-dependent pathway. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293(3):R1180–R1190. doi: 10.1152/ajpregu.00307.2007. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Kierstein S, Sharma S, Spaits M, Kinsey SG, Tliba O, Amrani Y, Sheridan JF, Panettieri RA, Haczku A. Social stress enhances allergen-induced airway inflammation in mice and inhibits corticosteroid responsiveness of cytokine production. J. Immunol. 2009;182(12):7888–7896. doi: 10.4049/jimmunol.0800891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MT, Kinsey SG, Padgett DA, Sheridan JF, Leblebicioglu B. Social stress enhances IL-1beta and TNF-alpha production by Porphyromonas gingivalis lipopolysaccharide-stimulated CD11b+ cells. Physiol. Behav. 2009;98(3):351–358. doi: 10.1016/j.physbeh.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311(5762):864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Blandino P, Jr., Barnum CJ, Solomon LG, Larish Y, Lankow BS, Deak T. Gene expression changes in the hypothalamus provide evidence for regionally-selective changes in IL-1 and microglial markers after acute stress. Brain Behav. Immun. 2009;23(7):958–968. doi: 10.1016/j.bbi.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav. Immun. 2008;22(3):301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, Krishnan V, Reyes CM, Han MH, Ables JL, Eisch AJ, Dietz DM, Ferguson D, Neve RL, Greengard P, Kim Y, Morrison JH, Russo SJ. IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J. Neurosci. 2011;31(1):314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Arevalo JM, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK, Sheridan JF, Seeman TE. Computational identification of gene-social environment interaction at the human IL6 locus. Proc. Natl. Acad. Sci. U.S.A. 2010;107(12):5681–5686. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona AW, Fenn AM, Godbout JP. Cognitive and Behavioral Consequences of Impaired Immunoregulation in Aging. J Neuroimmune Pharmacol. 2011 doi: 10.1007/s11481-011-9313-4. (DOI: 9-20-11). [DOI] [PubMed] [Google Scholar]
- Corona AW, Huang Y, O'Connor JC, Dantzer R, Kelley KW, Popovich PG, Godbout JP. Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. J. Neuroinflammation. 2010;7:93. doi: 10.1186/1742-2094-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry JM, Hanke ML, Piper MG, Bailey MT, Bringardner BD, Sheridan JF, Marsh CB. Social disruption induces lung inflammation. Brain Behav. Immun. 2010;24(3):394–402. doi: 10.1016/j.bbi.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav. Immun. 2007;21(2):153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Dong-Newsom P, Powell ND, Bailey MT, Padgett DA, Sheridan JF. Repeated social stress enhances the innate immune response to a primary HSV-1 infection in the cornea and trigeminal ganglia of Balb/c mice. Brain Behav. Immun. 2010;24(2):273–280. doi: 10.1016/j.bbi.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DJ, Gensel JC, Ankeny DP, van Rooijen N, Popovich PG. An efficient and reproducible method for quantifying macrophages in different experimental models of central nervous system pathology. J. Neurosci. Methods. 2009;181(1):36–44. doi: 10.1016/j.jneumeth.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav. Immun. 2007;21(1):47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Getts DR, Terry RL, Getts MT, Muller M, Rana S, Shrestha B, Radford J, Van Rooijen N, Campbell IL, King NJ. Ly6c+ "inflammatory monocytes" are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. J. Exp. Med. 2008;205(10):2319–2337. doi: 10.1084/jem.20080421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat. Rev. Immunol. 2005;5(3):243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb. J. 2005;19(10):1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, J OC, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33(10):2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I, Yirmiya R. Interleukin-1 (IL-1): a central regulator of stress responses. Front. Neuroendocrinol. 2009;30(1):30–45. doi: 10.1016/j.yfrne.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J. Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav. Immun. 2009;23(3):309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinwood M, Morandini J, Day TA, Walker FR. Evidence that Microglia Mediate the Neurobiological Effects of Chronic Psychological Stress on the Medial Prefrontal Cortex. Cereb. Cortex. 2011 doi: 10.1093/cercor/bhr229. (DOI: 8-30-11). [DOI] [PubMed] [Google Scholar]
- Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol. Aging. 2008;29(11):1744–1753. doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, O'Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav. Immun. 2002;16(4):461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O'Connor KA, Watkins LR, Maier SF. The role of IL-1beta in stress-induced sensitization of proinflammatory cytokine and corticosterone responses. Neuroscience. 2004;127(3):569–577. doi: 10.1016/j.neuroscience.2004.05.046. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R. Depression and immune function: central pathways to morbidity and mortality. J. Psychosom. Res. 2002;53(4):873–876. doi: 10.1016/s0022-3999(02)00309-4. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc. Natl. Acad. Sci. U.S.A. 2003;100(15):9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113(14):3190–3197. doi: 10.1182/blood-2008-07-168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Bailey MT, Sheridan JF, Padgett DA, Avitsur R. Repeated social defeat causes increased anxiety-like behavior and alters splenocyte function in C57BL/6 and CD-1 mice. Brain Behav. Immun. 2007;21(4):458–466. doi: 10.1016/j.bbi.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, Tarr AJ, Day CE, McLinden KA, Boehm GW. Influence of prenatal stress on behavioral, endocrine, and cytokine responses to adulthood bacterial endotoxin exposure. Behav. Brain Res. 2008;193(2):257–268. doi: 10.1016/j.bbr.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131(2):391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Lacosta S, Merali Z, Anisman H. Behavioral and neurochemical consequences of lipopolysaccharide in mice: anxiogenic-like effects. Brain Res. 1999;818(2):291–303. doi: 10.1016/s0006-8993(98)01288-8. [DOI] [PubMed] [Google Scholar]
- Mahad D, Callahan MK, Williams KA, Ubogu EE, Kivisakk P, Tucky B, Kidd G, Kingsbury GA, Chang A, Fox RJ, Mack M, Sniderman MB, Ravid R, Staugaitis SM, Stins MF, Ransohoff RM. Modulating CCR2 and CCL2 at the blood-brain barrier: relevance for multiple sclerosis pathogenesis. Brain. 2006;129(Pt 1):212–223. doi: 10.1093/brain/awh655. [DOI] [PubMed] [Google Scholar]
- Martinez M, Calvo-Torrent A, Herbert J. Mapping brain response to social stress in rodents with c-fos expression: a review. Stress. 2002;5(1):3–13. doi: 10.1080/102538902900012369. [DOI] [PubMed] [Google Scholar]
- Mays JW, Bailey MT, Hunzeker JT, Powell ND, Papenfuss T, Karlsson EA, Padgett DA, Sheridan JF. Influenza virus-specific immunological memory is enhanced by repeated social defeat. J. Immunol. 2010;184(4):2014–2025. doi: 10.4049/jimmunol.0900183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat. Neurosci. 2007;10(12):1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc. Natl. Acad. Sci. U.S.A. 2009;106(34):14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, Ma R, Cole SW. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol. Psychiatry. 2008;64(4):266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon ML, McNeil LK, Freund GG. Macrophages make me sick: How macrophage activation states influence sickness behavior. Psychoneuroendocrinology. 2011 doi: 10.1016/j.psyneuen.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Bonneau RH. Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J. Neuroimmunol. 2006;171(1–2):72–85. doi: 10.1016/j.jneuroim.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. 2nd edition 2004. [Google Scholar]
- Powell ND, Bailey MT, Mays JW, Stiner-Jones LM, Hanke ML, Padgett DA, Sheridan JF. Repeated social defeat activates dendritic cells and enhances Toll-like receptor dependent cytokine secretion. Brain Behav. Immun. 2009;23(2):225–231. doi: 10.1016/j.bbi.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M, Priller J. Tickets to the brain: role of CCR2 and CX3CR1 in myeloid cell entry in the CNS. J. Neuroimmunol. 2010;224(1–2):80–84. doi: 10.1016/j.jneuroim.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Quan N. Immune-to-brain signaling: how important are the blood-brain barrier-independent pathways? Mol. Neurobiol. 2008;37(2–3):142–152. doi: 10.1007/s12035-008-8026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N, Avitsur R, Stark JL, He L, Lai W, Dhabhar F, Sheridan JF. Molecular mechanisms of glucocorticoid resistance in splenocytes of socially stressed male mice. J. Neuroimmunol. 2003;137(1–2):51–58. doi: 10.1016/s0165-5728(03)00042-0. [DOI] [PubMed] [Google Scholar]
- Quan N, Avitsur R, Stark JL, He L, Shah M, Caligiuri M, Padgett DA, Marucha PT, Sheridan JF. Social stress increases the susceptibility to endotoxic shock. J Neuroimmunol. 2001;115(1–2):36–45. doi: 10.1016/s0165-5728(01)00273-9. [DOI] [PubMed] [Google Scholar]
- Quan N, Banks WA. Brain-immune communication pathways. Brain Behav. Immun. 2007;21(6):727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Serrats J, Schiltz JC, Garcia-Bueno B, van Rooijen N, Reyes TM, Sawchenko PE. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron. 2010;65(1):94–106. doi: 10.1016/j.neuron.2009.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark JL, Avitsur R, Hunzeker J, Padgett DA, Sheridan JF. Interleukin-6 and the development of social disruption-induced glucocorticoid resistance. J. Neuroimmunol. 2002;124(1–2):9–15. doi: 10.1016/s0165-5728(02)00004-8. [DOI] [PubMed] [Google Scholar]
- Stark JL, Avitsur R, Padgett DA, Campbell KA, Beck FM, Sheridan JF. Social stress induces glucocorticoid resistance in macrophages. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280(6):R1799–R1805. doi: 10.1152/ajpregu.2001.280.6.R1799. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Krishnan RR, Lewis JG. The relation of severity of depressive symptoms to monocyte-associated proinflammatory cytokines and chemokines in apparently healthy men. Psychosom. Med. 2003;65(3):362–368. doi: 10.1097/01.psy.0000035719.79068.2b. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Lewis JG, Krishnan RR, Young KH. Enhanced expression of cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide stimulation are associated with hostility and severity of depressive symptoms in healthy women. Psychoneuroendocrinology. 2004;29(9):1119–1128. doi: 10.1016/j.psyneuen.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Ide M, Shibutani T, Ohtaki H, Numazawa S, Shioda S, Yoshida T. Lipopolysaccharide-induced microglial activation induces learning and memory deficits without neuronal cell death in rats. J. Neurosci. Res. 2006;83(4):557–566. doi: 10.1002/jnr.20752. [DOI] [PubMed] [Google Scholar]
- Tsetsenis T, Ma XH, Lo Iacono L, Beck SG, Gross C. Suppression of conditioning to ambiguous cues by pharmacogenetic inhibition of the dentate gyrus. Nat. Neurosci. 2007;10(7):896–902. doi: 10.1038/nn1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynan RJ, Naicker S, Hinwood M, Nalivaiko E, Buller KM, Pow DV, Day TA, Walker FR. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav. Immun. 2010;24(7):1058–1068. doi: 10.1016/j.bbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. β-Adrenergic Receptor Antagonism Prevents Anxiety-Like Behavior and Microglial Reactivity Induced by Repeated Social Defeat. J. Neurosci. 2011;31(17):6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne AM, Henry CJ, Huang Y, Cleland A, Godbout JP. Protracted downregulation of CX(3)CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behav. Immun. 2010;24(7):1190–1201. doi: 10.1016/j.bbi.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 2011;25(2):181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]