Abstract
Estrogen plays important roles in hormone receptor-positive breast cancer. Endocrine therapies, such as the antiestrogen tamoxifen, antagonize the binding of estrogen to estrogen receptor (ER), whereas aromatase inhibitors (AIs) directly inhibit the production of estrogen. Understanding the mechanisms of endocrine resistance and the ways in which we may better treat these types of resistance has been aided by the development of cellular models for resistant breast cancers. In this review, we will discuss what is known thus far regarding both de novo and acquired resistance to tamoxifen or AIs. Our laboratory has generated a collection of AI- and tamoxifen-resistant cell lines in order to comprehensively study the individual types of resistance mechanisms. Through the use of microarray analysis, we have determined that our cell lines resistant to a particular AI (anastrozole, letrozole, or exemestane) or tamoxifen are distinct from each other, indicating that these mechanisms can be quite complex. Furthermore, we will describe two novel de novo AI-resistant cell lines that were generated from our laboratory. Initial characterization of these cells reveals that they are distinct from our acquired AI-resistant cell models. In addition, we will review potential therapies which may be useful for overcoming resistant breast cancers through studies using endocrine resistant cell lines. Finally, we will discuss the benefits and shortcomings of cell models. Together, the information presented in this review will provide us a better understanding of acquired and de novo resistance to tamoxifen and AI therapies, the use of appropriate cell models to better study these types of breast cancer, which are valuable for identifying novel treatments and strategies for overcoming both tamoxifen and AI-resistant breast cancers.
Keywords: Models, endocrine resistance, tamoxifen, aromatase inhibitors
1. Introduction
Breast cancers are characterized based on whether they express several protein receptors: estrogen receptor α (ERα), progesterone receptor (PR), and ErbB2/HER2 receptor. There are two estrogen receptors: α and β [1]. While ERα has been implicated to play a role in breast cancer, the role of ERβ in breast cancer is not fully defined [2–3]. Further discussion of ERα in this review will be referred to as ER. Approximately 70% of breast cancers are ER positive and/or PR positive (ER+/PR+) [4], about 20% overexpress HER2 [5], and about 10% do not express ER, PR, or HER2 (triple negative) [6]. Breast cancer is also characterized by an overexpression and increased activity of the cytochrome P450 aromatase enzyme [7–10]. Aromatase is predominantly expressed in the ovaries in premenopausal women under the regulation by the gonadotropins follicle-stimulating hormone (FSH) and lutenizing hormone (LH). Both FSH and LH are players in a feedback regulatory mechanism in which they stimulate the synthesis of aromatase and subsequent secretion of estrogen in the ovaries. However, in postmenopausal women, aromatase is primarily expressed in the adipose tissue, which is stimulated by glucocorticoids, and in cancer cells, which is regulated through a cAMP-mediated mechanism [11]. Thus, aromatase expression in postmenopausal women is not regulated in a gonadotropic manner and makes aromatase inhibitors (AIs) useful for the treatment of estrogen-dependent breast cancers in postmenopausal patients. Unlike in postmenopausal women, aromatase expression is regulated by FSH and LH in premenopausal women and use of AIs would be rendered ineffective.
ER+ breast cancers require the hormone estrogen for their growth. The standard mode of treatment of ER+ breast cancers in postmenopausal women is estrogen deprivation or blockade of the estrogen-regulated pathways. This is through the use of ovarian ablation, or more commonly through the use of endocrine therapies such as tamoxifen or fulvestrant (ICI 182,780) to block the ER action, or by directly inhibiting the production of estrogen by AIs. The efficiency of AIs over tamoxifen, demonstrated by a number of clinical trials such as ATAC [12–13], IMPACT [14], and BIG 1–98 [15], has resulted in AIs becoming the first line of therapy for ER+ breast cancer.
While the use of antiestrogens and AIs is effective in the clinic, resistance towards these therapies has been observed. There are two types of resistance: de novo/intrinsic and acquired. De novo resistance occurs when the patient commences on a treatment but does not respond to the therapy. Acquired resistance is when the patient responds to the prescribed treatment, but after some time, no longer responds and breast cancer recurs. The occurrence of resistance to endocrine therapy presents a difficult challenge for the treatment of breast cancer, particularly determining the next line of treatment when all options have been exhausted. A number of laboratories have taken up the aim of elucidating and learning more about the mechanisms of resistance in order to identify new avenues of treating these types of breast cancers.
2. Cell Models
A collection of breast cancer cell lines derived from patients to serve as simplified in vitro models to study the inner workings of breast cancers are readily available and used by a number of laboratories. The growth of ER+ breast cancer is promoted via the ER-mediated pathway. Endocrine therapies are those which target and disrupt the ER pathway. Therefore, endocrine therapies are administered to breast cancer patients whose cancer cells express ERα. Since ERα is absolutely required for estrogen-dependent breast cancer, studies on hormone-responsive and endocrine resistant breast cancers have utilized a number of ERα+ breast cancer cell lines. ER-negative (ER−) cell lines do not express ERα. Therefore, ER− cells do not respond to endocrine therapies and should not be considered models of endocrine resistance. Furthermore, the roles of ERβ in endocrine resistance are not totally understood. Therefore, studies using ERβ+ cell lines require clear physiologically relevant justifications. Many endocrine therapies target the hormone activation of the ER signaling pathway, including AIs and anti-estrogens. One ERα+ line predominantly used is the MCF-7 cell line that responds well to estrogen stimulation and to anti-estrogen-mediated suppression. MCF-7 cells express aromatase at very low levels [16]. To engineer an aromatase-positive breast cancer cell model for the evaluation of AI responses, our laboratory stably overexpressed aromatase in MCF-7 cells, which were referred to as MCF-7aro [16–17]. The MCF-7aro cell line has been shown in several laboratories to be a suitable cell model to study AI-responsive breast cancer. Two additional ERα+ cell lines, T47D and ZR75-1, are commonly used to study ER-mediated response. Aromatase-expressing versions of these lines, T47Daro and ZR75-1aro, have also been generated to study AI response. Through the use of these cell lines we can study resistance mechanisms and test new therapeutic agents for their ability to overcome endocrine therapy resistance.
3. Acquired Endocrine Resistance
3.1 Acquired tamoxifen resistance models
Tamoxifen was the first approved line of endocrine therapy to treat postmenopausal women before AIs were proven to be superior and superseded tamoxifen for this spot. Tamoxifen is a SERM, which demonstrates selective antagonist activity in the breast and agonist activity in the endometrium and bone [18–19]. While tamoxifen has increased survivorship from breast cancer, recurrence still occurs. Due to its dual agonist/antagonist activity, tamoxifen can demonstrate weak estrogenic effect, which can prevent a total blockade of estrogen-stimulated breast cancer cell growth [20]. To study other mechanisms involving acquired tamoxifen-resistance, cells were generated by culturing the MCF-7 ER+ breast cancer cell line long-term in the presence of tamoxifen until growth was achieved in the presence of this anti-estrogen [21–22], such as LCC2 [23] and MCF-7/TAMR-1[24].
Increased expression of EGFR [25] or HER2 [26–27] in cancer patients have been observed, potentially leading to tamoxifen-stimulated cancer cell growth. Furthermore, several groups have proposed that resistance to endocrine therapies such as tamoxifen may be due to the cross-talk between ER and growth factor signaling proteins, especially EGFR/HER2. This has been determined through the use of several tamoxifen-resistant cell lines which have been generated by overexpressing a specific protein, including growth factor signaling proteins and kinases [28–31]. In these events, growth factor signaling proteins such as EGFR and mitogen-activated protein kinase (MAPK) display increased activity. These kinases can phosphorylate and induce ER activation in the absence of ligand or in the presence of tamoxifen [32–34].
Considering the ratio of coactivators and corepressors in the cells may explain another possible mechanism for acquired tamoxifen resistance. It has been shown that the differential expression or activity of coactivators and corepressors in the cell can modulate the agonist versus antagonist activity of drugs [35]. Coactivators and corepressors may play a part in acquired tamoxifen resistance. Tamoxifen displays both agonist and antagonist activities, which may be due to the activation and recruitment of coactivators or corepressors to the ER. For example, the ER coactivator AIB-1 is overexpressed in more than 50% of breast tumors and its gene is amplified in 5–10% of breast tumors [36]. In cell culture, AIB-1 enhances tamoxifen agonist activity [37]. Moreover, AIB-1 is phosphorylated and activated by a number of different kinases, such as p42/44 MAPK, which can be activated by HER2 [38]. Therefore, in cells overexpressing HER2 the role of tamoxifen may depend on the activation of AIB-1, which confers tamoxifen agonist activity.
As previously mentioned, ERα cross-talks with a number of growth factor receptors and kinases, which activate ERα in a ligand independent manner. This suggests that ERα is an important factor for tamoxifen resistance. Studies have demonstrated that expression of ERα in ER− breast cancer cells resulted in inhibition of growth [39–41]. To determine the response of increased ERα protein levels in an ER+ breast cells, ERα was overexpressed in MCF-7 cells, which are referred to as MLET5 [42]. Microarray analysis comparing the parental MCF-7 cells to MLET5 revealed that genes involved in the apoptosis mechanism were differentially expressed between the two cell lines, such that MLET5 cells could evade apoptosis and survive. These findings support the possibility that breast cancer tumors which display elevated levels of ERα may develop estrogen-independent mechanisms of growth, likely through activation by growth factor receptors and kinases, and devise ways to circumvent apoptosis.
Models for acquired resistance to ICI 182,780 have also been developed [43–44]. MCF-7 cells were cultured for several months in the presence of ICI 182,780 until the cells gained the ability to proliferate in the presence of the antiestrogen. Work from the Lykkesfeldt group has demonstrated that ICI 182,780-resistant cells retain ER protein expression [43] and growth factor proteins ErbB3, EGFR, and kinase ERK are necessary for growth [45]. Both mRNA and protein levels as well as their downstream kinases pAKT and pERK are elevated. The Nephew lab has shown that ICI 182,780-resistant cells are also cross-resistant to tamoxifen [46]. In addition, these cells contain an increased number of growth-stimulatory pathways, including EGFR, HER2, and Wnt/β-catenin which may drive resistant cell growth in an estrogen-independent manner [44]. Clark and colleagues also reported that their ICI 182,780-resistant cells, MCF-7/LCC9, retain their ER expression and that upregulation of PR mRNA and protein was observed independent of estrogen [47]. Interestingly, in ICI 1827,780 resistant cells, NF-κB p65 was upregulated while interferon regulatory factor-1 (IRF-1) was downregulated [48]. Interferon gamma induction of interferon regulatory factor-1 resulted in suppression of NF-κB p65 activity and antiestrogen response in ICI 182,780 resistant cells via inhibiting prosurvival gene expression and inducing proapoptotic genes [49]. These results suggest new treatment options which may include those that induce IRF-1.
3.2 Acquired AI resistance models - LTED
AIs, another type of treatment for breast cancer have shown efficacy in the clinic, however, acquired resistance to these inhibitors have also been observed. In order to investigate the mechanisms governing acquired AI resistance, cell models were generated. Several groups used the MCF-7 cell line and cultured cells long-term in the absence of hormone until the cells acquired the ability to proliferate without estrogen. In these culture conditions, a majority of the cells died, but a few remained and proliferated. This fraction of proliferating cells was considered resistant and termed long-term estrogen deprived (LTED). Characterization and mechanistic studies using these cells were performed in order to elucidate the mechanisms of acquired resistance [17, 50–53]. LTED cells appear to undergo three phases: a quiescent phase; a hypersensitive phase in which growth is induced at lower concentrations of estradiol compared to the MCF-7 control; and the final estrogen-independent phase [53]. This last phase occurs when the cells no longer require estrogen for growth. The process from responsiveness to resistance has been the subject of numerous studies. It has been observed that hypersensitivity of ER to estrogen may result from the action of different kinases, especially p42/44 MAPK [52, 54]. Kinases such as MEK1/2, c-Raf, p42/44 MAPK, p90RSK, c-MYC, display increased expression levels of both their unphosphorylated and phosphorylated forms in resistant cells [51]. Moreover, these activated growth factors and kinases influence the action of transcription factors to augment estrogen action through ER. For example, the AIB1 coactivator, a ligand-dependent ER coactivator, is enhanced by p42/44 MAPK phosphorylation leading to the recruitment of CBP and an associated increase in acetyl transferase activity [38]. In contrast to the thought that estrogen may still induce growth in AI-resistant cells, it has been reported that after 24 months, LTED cells evolved into estrogen-inhibitory cells [55]. Estrogen treatment of these cells resulted in a marked increase in Fas ligand and activation of Bcl-2 family protein [56].
It is regarded that when a cell is responsive to endocrine therapy, the ER signaling pathway is the most important mode of growth. During periods of estrogen deprivation, the estrogen-induced ER pathway is no longer viable. As a result, the cell develops an alternative method of ER activation. This is through increased expression and activation of growth factors such as HER2, Akt [57–58], and MAPK [51, 59], which can phosphorylate ER. Phosphorylation of ER at serine 118 by MAPK [51] or serine 167 by Akt [59] has been shown to induce transcription of growth-promoting genes. To confirm the importance of the ER pathway in AI resistance, LTED cells were treated with the pure antiestrogen fulvestrant, which prevents the activation of AF-1 and AF-2 and reduces the ERα half-life [54]. Fulvestrant markedly inhibits LTED cell growth, while excess estradiol restores cell growth [54]. Based on these results, it could be concluded that the ER-mediated pathway is solely responsible for proliferation.
Work from the Brodie laboratory has focused on acquired AI resistance and potential treatment options for AI resistance. Two AI-resistant cell models have been described. UMB-1Ca cells, an LTED model for acquired AI resistance, was generated by culturing MCF-7aro cells established by the Chen laboratory [16], in estrogen-free medium for several months until cells acquired the ability to grow in the absence of hormone [57]. Long-term letrozole-treated (LTLT-Ca) cells, a model for acquired letrozole resistance [59] was also generated from the parental MCF-7aro cells. A tumor xenograft was grown using MCF-7aro cells injected into ovariectomized mice, which were treated with letrozole for up to 56 weeks. Afterwards, cells from this tumor were extracted and maintained as a cell line. Studies using these cell lines have revealed changes in the protein levels of ERα [57, 59], increased protein expression and enhanced activation of growth factor receptor pathways, in particular HER2 and MAPK [59]. UMB-1Ca cells were characterized by increased protein levels of epidermal growth factor receptor 2, ERα, and phosphorylated levels of Akt [57]. In addition, these cells were less sensitive to AIs, tamoxifen, and ICI 182,780 compared to the parental cells.
UMB-1Ca and LTLT-Ca treated with inhibitors against the activated growth factor receptors and their downstream kinase targets showed abrogated activation of these proteins [57, 59]. Furthermore, ERα protein levels in the resistant cells were restored to levels seen in the AI responsive cells, such that they regained responsiveness to tamoxifen [59] and letrozole [60]. Combination treatment using signal transduction inhibitors for the activated kinases and an AI resulted in regained sensitivity to AIs. Interestingly, the use of an ERβ agonist DPN has been shown to overcome acquired AI resistance in vivo [61]. Combination usage of an ERβ agonist and letrozole resensitized acquired letrozole resistance in a LTLT-Ca xenograft model. Inhibition of tumor growth was associated with decreased ERα/ERβ ratio and blocked G1/S phase progression. Additionally, interesting results have been also generated through the use of histone deacetylase inhibitors.
The Gee group has also developed an acquired AI resistant cell line referred to as MCF-7X [58]. Studies using these cells suggest an important role for the PI3K/Akt pathway in promoting growth via phosphorylation and activation of ER. Single treatment of ICI 182,780 or an inhibitor for MAPK or PI3K inhibited growth to a degree. Cotreatment resulted in an enhanced inhibitory effect, indicating that a combination therapy to block both ER and kinases which modulate it should be used for greater suppression of AI resistant cell growth.
Findings from the Dowett laboratory have shown that the HER2/MAPK pathways were activated and IGF-1R was upregulated in LTED cells [51, 53]. Furthermore, these pathways crosstalk with ER to phosphorylate and subsequently activate it. Santen and colleagues have reported that long-term hormone deprivation causes cells to develop hypersensitivity to estrogen [62–63]. These cells display a 4–10 fold increase in ER expression. Furthermore, insulin growth factor receptor (IGF-1R) activates nongenomic ER signaling and downstream MAPK and PI3K/Akt/mTOR pathways, which induce proliferation in an estrogen hypersensitive state.
3.3. Alternative methods of estrogen biosynthesis
Resistance to AIs may also result from incomplete inhibition of estrogen production. In addition to aromatase, estrone sulfatase, and 17β-hydroxysteroid dehydrogenase (17 β -HSD) are enzymes involved in estrogen biosynthesis in human breast cancer tissues. Estrone sulfatase converts estrone sulfate to estrone. Estrone can then be converted into estradiol by 17β-HSD. This route of estradiol synthesis is independent of aromatase and may be a source for AI resistance. It is known that estrogen sulfate concentrations are several times higher in breast cancer tissue compared to normal breast tissue [64–66]. Therefore, the high concentration of estrogen sulfate in the tissue has potential for producing estradiol, which can then activate ER and promote cancer cell growth regardless of whether AIs are present.
3.4 ER-independent mechanism
Few investigators have suggested that the ER may not be important for the proliferation of resistant cells. Several clinical studies have observed cases in which patients who are initially ER+ lose the expression of ER, and develop resistance to endocrine therapy [67–68]. In addition, breast tumors can be categorized based on their expression of HER2, ER and PR protein receptors. Luminal A tumors are highly ER+ and PR+ and have a better response to antiestrogen and AI therapy. Luminal B tumors are those which are ER+ and display high cellular proliferation. These tumors typically display poor response to antiestrogen and AI therapy. It is possible during antiestrogen or AI therapy, tumors lack the estrogen-induced pathway and can evolve from luminal A to luminal B tumors which become entirely ER-independent. In these instances, resistance to antiestrogen or AI therapies may be the result of selection for cells which have lost the expression of ER.
3.5 Suppression of apoptotic pathways in endocrine resistant cells
Endocrine resistant cell lines generated by overexpression of certain growth factor signaling proteins and kinases have shown that increased levels and activity of these proteins may lead to pathways which block apoptosis and promote cell growth via estrogen-independent mechanisms [28–29, 69]. Growth factor signaling proteins overexpressed in cells cross-talk and activate ER and can circumvent tamoxifen-induced apoptosis through the upregulation of antiapoptotic proteins [70] and induce growth independently of estrogen-dependent pathways. In addition, serum- and glucocorticoid-inducible kinase 3 (SGK3) kinase, an ER regulated protein, was stably overexpressed in MCF-7 cells and conferred resistance to ICI 182,780-induced apoptosis [71]. Moreover, the anti-apoptotic proteins BCL2 and BCL-W have also been implicated in contributing to resistance [72]. Inhibitors to these proteins indicate that sensitive and resistant cells survive by different mechanisms. The mechanism of resistance may have developed by altering the programmed cell death pathway [72]. Moreover, these results further support the thought that sensitive and resistant cells grow and survive by different mechanisms.
3.6 Differential gene expression between tamoxifen and AI resistant cells
It is well recognized that there is a lack of complete cross-resistance among three FDA approved AIs [73]. Therefore, we believed that resistance mechanisms for each AI and tamoxifen may differ. Therefore, in order to study the mechanisms of tamoxifen and AI resistance, our laboratory also developed a set of acquired endocrine resistant cell lines. We generated several replicates of the MCF-7aro LTED cell line, in addition to several replicates of MCF-7aro derived cell lines specifically resistant to a particular FDA approved AI (exemestane, anastrozole, or letrozole): T+EXE R, T+ANA R and T+LET R, or tamoxifen: T+TAM R. Inhibitor-only resistant cell lines were also generated [17]. This was performed by culturing MCF-7aro cells with the appropriate inhibitor with or without testosterone for several months. Initially, many cells died, while few remained. These cells proliferated and took several months for complete resistance to be achieved. Using these cell lines, we sought to identify genes and pathways involved in the mechanisms of AI-resistance through the use of microarray analysis [17]. The significance of our microarray study compared to others is two-fold: first, we are using true resistant cell lines which were generated after several months of AI-treatment or hormone deprivation. Second, we utilized a nonbiased genome-wide approach in order to identify genes and pathways involved in the mechanisms of AI-resistance from these individual cell lines. To check the quality of our microarray results, we carried out hierarchical clustering analysis. We were pleased to see that our nonbiased genome-wide approach resulted in the replicates for each AI-resistance clustering together. Based on the gene expression profiles T+LET R, T+ANA R, and T+EXE R cells are similar to each other, and distinct from T+TAM R and LTEDaro cells, which display similarities [74]. Moreover, results from our microarray study revealed that cells resistant to a specific AI possess unique gene expression signatures and are different from the LTEDaro cell model, suggesting that the LTED model alone is not an accurate representation of AI resistance. Rather, we must take into consideration the uniqueness of each AI and study these cells which have acquired resistance to the particular AI in order to obtain a better understanding of AI resistance.
Through the use of these cell lines as models of acquired AI or tamoxifen resistance, we can determine that the ER remains important for cell growth. This is demonstrated by their growth inhibition to the pure antiestrogen inhibitor ICI 182,780 [17]. These results are similar to those reported previously [54] and support our cell lines as true models of endocrine resistance. Results from our studies using these cells also revealed that ER in T+ANA R, T+LET R, and LTEDaro cells is constitutively activated through its phosphorylation on serine 118 in the absence of ligand, confirmed by luciferase reporter assays and western blot analysis [17]. However, the ER activity in T+EXE R and T+TAM R cells remained estrogen-dependent. The steroidal AI exemestane has been reported to display mild estrogenic activity [74–75]. Our microarray results also indicated that most of the upregulated genes from EXE-only resistant cells were estrogen-dependent. In addition, we discovered that there are notable differences between the expression profiles of the upregulated genes, such as PgR, in the AI- and T+TAM R cell lines. While PgR is upregulated in testosterone-only, T+ANA R, T+LET R, T+EXE R, EXE R, and T+TAM R, it is not in LTEDaro and ANA R. Taking the data together, it delineates four types of AI-resistant cell lines. The first type is LTEDaro and ANA R, in which ER is overexpressed and is constitutively active. The second type consists of T+ANA R and T+LET R, which have constitutively active ER. The third type consists of EXE R and T+EXE R, which contain ER that depends on estrogen for its activation and exemestane which acts as a weak estrogen [74]. The fourth type includes T+TAM R, which has a unique gene expression profile compared to LTEDaro and the individual AI-resistant cell lines [76].
3.7 Cross-resistance studies
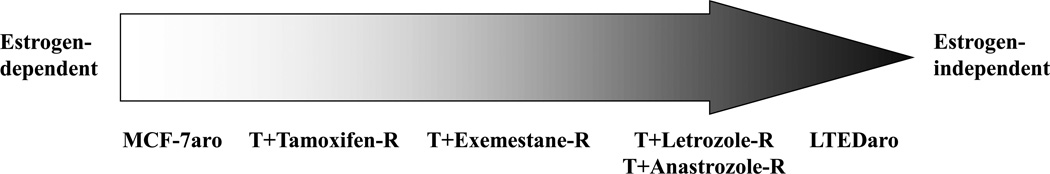
Determining which secondary therapy to use once a tumor develops resistance is extremely important. Using our cell lines as models of tamoxifen-resistance or a specific AI resistance, we conducted cross-resistance studies to determine whether cells resistant to tamoxifen or a particular AI, are responsive to a different AI. These studies are important as it has the potential to identify possible alternative treatment options once a breast cancer patient develops resistance to a particular AI therapy. We found that tamoxifen-resistant cells are growth inhibited by all three AIs. Cells resistant to a particular AI were still responsive to treatment with a different AI. However, LTEDaro cells were unaffected by either tamoxifen or AI. These results, together with our previous findings that exemestane displays estrogenic activity [74], suggest that our cell lines may be models for what occurs in cells as they progress towards acquired resistance. Cells begin as estrogen-dependent cells (MCF-7aro) which are responsive to both tamoxifen and AIs. During prolonged treatment with AIs, cells become less dependent on estrogen and develop alternative pathways to activate ER. These involve activation of ER by growth factor signaling proteins in a ligand-independent manner. Cells which begin to develop this phenotype are represented by the tamoxifen-resistant and exemestane-resistant cells. In these resistant cells, estrogen-mediated ERα-signaling is blocked; however, tamoxifen and exemestane may function as weak estrogens which drive cell growth. This is the first stage of resistance. Over time, the growth pathways become more estrogen-independent and rely on an increased number of growth factor signaling pathways. It is believed that cells at this stage are demonstrated by the anastrozole-resistant and letrozole-resistant cells. Finally, once the cells have achieved total estrogen-independence, they no longer respond to any AI or tamoxifen. These cells are exemplified by the LTEDaro cells [17].
4. De novo resistance
Most ER+ patients respond well to ER-targeted therapy; however, there is a number of patients who do not respond to endocrine therapy (de novo/intrinsic resistance) [77]. For example, approximately 30% of ER+ breast cancers do not respond to tamoxifen treatment [78]. Several proteins are being considered as markers for de novo resistance. Increased expression of type 1 growth factor receptors (e.g. EGFR and HER2) and kinases such as Akt have been reported to be associated with indices of reduced likelihood of responding to or benefiting from tamoxifen.
4.1 HER2
HER2 overexpression in clinical tumor samples has been shown to positively correlate with resistance to tamoxifen. Studies using clinical samples have revealed that tumors with high circulating levels of HER2 or overexpression of HER2 as observed in luminal B cancers are less responsive to endocrine therapy with lower response rate and shorter duration of response to antiestrogen therapy [79–81] than luminal A subtype which overexpress ER but not HER2.
To date, there is a paucity of in vitro models for de novo endocrine therapy resistance. Those which exist typically overexpress the HER2 protein to mimic the cancer cells observed in the clinic [30–31, 69]. In vitro studies correlate with findings from the clinic. In cell-based studies, HER2 has been implicated in playing a role in resistance. In these studies, cell lines used have generally been an ER+ MCF-7 line which has been transfected to overexpress HER2. Evidence suggests that overexpressed HER2 confers tamoxifen resistance in ER+ breast cancer [29, 69]. Whether HER2 overexpression results in estrogen-dependent or -independent growth is unclear [30–31, 82]. Moreover, increased EGFR/HER2 signaling is associated with tamoxifen resistance, in which case tamoxifen may function as an agonist and stimulate cell growth [69, 83].
It has been proposed that HER2-mediated tamoxifen resistance is through the MAPK pathway. In vitro studies using ER+ MCF-7 cells overexpressing HER2 showed that treatment with the EGFR inhibitor AG1478 + 4OH tamoxifen or MAPK inhibitor U0126 + 4OH tamoxifen enhances the inhibitory effect of 4OH tamoxifen on ER transcriptional activity [83–84]. This was the result of restoration of the interaction between ER and nuclear corepressor (N-CoR) with tamoxifen treatment. This in turn, resulted in cell growth inhibition [84] and decreased colony formation [83]. HER2-overexpressing MCF-7 xenografts in athymic mice also confirmed the findings that the combination of both AG1478 and tamoxifen arrested tumor growth relative to untreated controls. This arrest in growth was due to the anti-proliferative effect of AG1478 and apoptosis [83]. Resistance to tamoxifen may be a result of anti-apoptotic mechanisms employed by the cell. Tamoxifen resistance mediated by HER2 was correlated with overexpression of the anti-apoptotic protein, Bcl-2, in MCF-7 cells [70].
4.2 Akt
Akt has also been demonstrated to play a role in de novo endocrine resistance. Akt overexpression is associated with HER2 overexpression and aberrantly activated Akt has been reported in many tumors and associated with resistance to endocrine therapy. MCF-7 cells overexpressing constitutively active Akt display activation of ER in a ligand-independent manner through phosphorylation of ERα at serine 167 by Akt. These cells demonstrate reduced sensitivity to tamoxifen, yet are growth inhibited by the pure-antiestrogen ICI 182,780 [28]. This suggests that AKT is responsible for 4OH tamoxifen resistance by modulating the ERα activity. Further studies showed that AKT expression prevents apoptosis by increasing the expression of the anti-apoptotic Bcl-2 gene, which is regulated by ERα [28]. Akt regulation of ERα on a post-translational level, may lead to increased Bcl-2 expression. This may result in the cells ability to resist endocrine therapy via its capability of evading apoptosis.
4.3 De novo AI resistance model
To our knowledge, currently there are no cell models for de novo AI resistance. Moreover, little is known about the mechanisms governing de novo AI resistance and suitable therapeutic options available for this type of resistant breast cancer. Therefore, as proof-of-concept studies, our laboratory has generated de novo AI therapy resistant cell lines. In an effort to model breast cancer patients who are thought to be suitable candidates for endocrine therapy, however, later discover that their breast cancer cells are intrinsically resistant to endocrine therapies, we generated cells which stably overexpress both aromatase and HER2 or Akt at high levels. These cells are denoted MCF-7HER2aro and MCF-7Aktaro. Our de novo AI-resistant cells are the first of its kind. To address the lack of information regarding de novo AI resistant breast cancer cell models, we treated our MCF-7HER2aro and MCF-7Aktaro cells with AIs and ICI. Both cell lines did not respond to the AI treatment and were mildly growth inhibited by the ICI treatment. These results indicated that these cell lines are models for de novo endocrine therapy resistance. We further characterized these cells and learned that the overexpression of aromatase had no role in contributing to the de novo AI resistant phenotype. The ER activity remained intact; however, the cells were not heavily dependent on the ER activation for proliferation (unpublished data). We are currently investigating with these cell lines to elucidate their mechanisms of resistance, how they differ from acquired AI-resistance, and to determine possible treatment options for de novo AI resistance. Currently, there are no good targeted therapeutic options for de novo AI resistant breast cancer. It is imperative that we invest our time and effort into studying this type of resistance and identifying novel treatment options for this type of cancer.
5. Importance of cell models
In vitro cell models are important tools for studying both tamoxifen and AI responsive and resistant breast cancer. Through the use of these simplified disease models, we can elucidate and better understand the mechanisms governing breast cancer. Clearly, these cells are valuable in that we may rapidly test a number of novel drugs for their anti-cancer abilities and their modes of action. One such novel drug to treat AI resistant breast cancers is an inhibitor for the heat shock protein 90 (HSP90) [85]. HSP90 is a chaperone protein that assists a number of “client” proteins in assuming a conformationally active form. Many of these client proteins are factors and key players in the promotion of oncogenesis. Inhibition of this critical chaperone protein results in proteasome-meditated degradation of these client proteins. The finding from our laboratory is the first report which determines the efficacy of HSP90 inhibitors on acquired AI-resistant breast cancer cells [85]. In our studies we used the HSP90 inhibitor 17-DMAG to treat our AI-responsive MCF-7aro cells and acquired AI-resistant LTEDaro cells. We found that 17- DMAG inhibits both AI-responsive and AI-resistant cell growth at low nanomolar concentrations and was specific at inhibiting cancer cells rather than normal breast cells. HSP90 inhibitor treatment resulted in induced apoptosis and cell cycle arrest. Importantly, a number of client proteins thought to be important for cancer cell growth promotion, including ERα, HER2, and Akt were degraded upon HSP90 inhibitor treatment. Notably, HER2 and Akt are important proteins believed to crosstalk with ERα and activate it in a hormone-independent manner in AI-resistant breast cancers. Collectively, our results provide evidence for further examination of HSP90 inhibitors as a second line therapy for acquired AI resistance. The use of cell models has also enabled novel treatment regimens to be considered. Continuous treatment with an anticancer therapy forces the cell to adapt and depend on alternative pathways for promoting growth. Studies have demonstrated that drug treatment in an intermittent fashion may delay resistance from occurring and reduce the experience of side-effects [50].
Moreover, cross-talk between steroid hormone receptors such as ER and a number of kinases has been demonstrated in the presence of tamoxifen and AI therapy which results in the activation of ER in the absence of hormone. The discovery of cross-talk between these key proteins involved in cancer promotion has resulted in both single therapy to block kinase action as well as combination therapy treatment to simultaneously inhibit ER activation as well as the signaling proteins which cross-talks with ER. The HER2 antibody inhibitor, trastuzumab (Herceptin), is currently used for the treatment of HER2-postive metastatic breast cancer. Combination therapy involving endocrine therapy (i.e. tamoxifen) and the EGFR tyrosine kinase inhibitor (gefinitib) is being evaluated as a therapeutic option to overcome de novo resistance and delay acquired resistance. Results from a phase II study trial combining gefitinib with and without anastrozole did not show significant biological or clinical differences between outcomes for gefinitib with anastrozole or anastrozole alone [86]. Lapatinib, a dual EGFR and HER2 inhibitor, blocks the autophosphorylation of both receptors. A phase III trial comparing lapatinib with or without letrozole in ER+, HER2-positive metastatic cancer concluded that lapatinib with letrozole was superior to letrozole alone in terms of the progression-free survival as well as clinical benefit rate [87]. These findings resulted in the accelerated approval of lapatinib with letrozole as a therapy for hormone receptor-positive, HER2-overexpressing breast cancer in postmenopausal women. Others have proposed that inhibiting the function and activity of growth factor signaling pathways through the use of inhibitors may force the cell to revert to the initial hormone-dependent ER regulated pathway to promote growth. Tamoxifen-resistant and LTED cells were treated with lapatinib and were growth suppressed. Interestingly, LTED cells demonstrated resensitization to tamoxifen and estrogen deprivation, while tamoxifen-resistant cells were resensitized to tamoxifen [88]. These exciting findings suggest new treatment options, but require further testing in animal models as well as in the clinic. Combination therapy appears to be effective in overcoming resistance. Moreover, if signal transduction inhibitor treatment can reverse cells to their former endocrine therapy responsive state, this type of therapy is promising and may be a method to combat resistance.
Drug testing can also be conducted in a more complex model than cell lines through the use of xenograft models developed using these cell models. Testing in vivo is necessary to determine whether a drug’s efficacy can be translatable to the clinic. Determining the proper dosage, the pharmacodynamics of the drug, clearance from the body, and retention in the tumor are important factors to consider when deciding whether to proceed with the investigational drug.
6. Limitations of cell culture studies for endocrine resistance
In vitro cell models for various types of breast cancers are valuable tools which can be used to study the different mechanisms of oncogenesis and resistance, as well as to test novel therapeutic agents for their ability to block cancer cell growth and the development of resistance. As important as these cell models are, they are not without limitations.
While various cell lines allow us to study differing types of breast cancers, the population of breast cancer patients is quite diverse and the number of cell lines available to study the variety of breast cancers is limited. To study AI resistance, many laboratories have used the MCF-7 cell line in the past to generate models for acquired resistance. Only recently are multiple models of acquired resistance being developed using other ER+ breast cancer cell lines [89]. Currently, our laboratory is generating other ER+ AI-resistant cell lines which overexpress aromatase in order to obtain a more comprehensive collection of AI-resistant breast cancer cell lines that reflect the diverse ER+ breast cancer patient population. These cell lines will be characterized and examined for their mechanisms of resistance in order for us to gain a better understanding of different types of acquired resistance in breast cancer patients. It will be interesting and informative to detect differences in resistance mechanisms among the cell lines, which may provide clues as to how ER+ breast cancer patients respond differently to various endocrine therapies. Moreover, these valuable cell lines will enable us to test a variety of novel inhibitors with the hope of identifying new alternative therapies which can overcome resistance.
To study a specific protein or type of resistant cancer, cells have been generated to stably overexpress or silence a particular protein or gene. While these are useful and important models to gain better insight into the roles of these proteins of interest and disease models, we must keep in mind that these cell lines are simply models and not true representations of the disease itself. Overexpression of a protein or silencing a gene may be a simplified version of the disease model and does not account for other alterations that may be present in the disease state which we are unaware of and may lead us to incorrect conclusions and not allow us to obtain a comprehensive understanding of the disease.
Most AI-resistant cell models are epithelial breast cancer cells which express high levels of aromatase. However, in breast tumor tissue, the stromal cells surrounding the epithelial cells also express aromatase mRNA at high levels [9–10]. By focusing our in vitro studies solely with epithelial cells, the paracrine effects contributed by stromal cells in the breast could be overlooked. The current models would be improved once stable aromatase-expressing human stromal cell lines become available.
Testing various therapeutic agents in cell models may not translate in the whole body system. On occasion, a therapy may show strong potency in the cell, but may not have an effect on the tumor in the animal model. In other instances, the drug may demonstrate potency in in vivo models, which is due in part to the high toxicity of the therapy in the animal system, thus rendering the drug unusable in the clinic. Animal models provide a platform which allows us to take into consideration the tumor microenvironment and the entire system of the organism that cell models cannot. For example, a small population of women carry the inactive allele for cytochrome P450 2D6 (CYP2D6) which converts tamoxifen to its active metabolite, endoxifene [90]. As a result, these women are unable to metabolize tamoxifen properly and are less responsive to tamoxifen. In this era of science, simply designing a study using solely cell models is not enough. To fully determine the significance of the science, our findings from cell models must be compared to those conducted in animal models, and then finally correlated with the data from the clinic. However, we must also realize that cell models are important and provide useful information in a timely manner for the design of animal experiments and clinical trials.
Ideally, in order to obtain useful data from the clinic, procedures in the clinic must be modified to allow the collection of breast tumor samples at different stages of the treatment; such as before and after therapy, pre- and post-resistance to therapy and during therapy which would allow us to compare and confirm molecular targets from both in vitro and clinical studies. However, it is not easy to justify obtaining tumor biopsies at different stages of disease or to monitor patients over extended periods of time until relapse occurs. Moreover, it is not feasible to collect tissue samples pre- and post-resistance to therapy from the same patient.
Finally, it is currently difficult to obtain clinical samples relevant to laboratory studies. Laboratory and clinical groups should collaborate and carefully design studies to incorporate clinical samples in the laboratory data analysis in order to provide more convincing and substantial conclusions. Likewise, it is important for clinical and laboratory groups to work together in order to determine the mechanism of action of an effective drug and to identify the cancer growth mechanisms.
7. Summary
Despite the obvious limitations associated with cell lines as models of disease, cell models have been extremely important tools in helping us dissect the mechanisms which govern both endocrine responsive as well as resistant breast cancer cell growth. These in vitro models have allowed us to test a variety of novel endocrine therapies, small molecule inhibitors to block proteins which cross-talk with ER and inhibitors that can simultaneously block multiple proteins in an effort to shut-down a number of pathways involved in promoting growth and survival. Furthermore, different treatment regimens and combinations of therapies have been tested using these cell lines which have yielded interesting and potentially promising results, including several currently being used in the clinic.
Based on the available literature, it is important to note several points. First, regarding cell models for AI resistance, it is absolutely necessary that the cell line express aromatase. This allows us to test the effect of AIs in ER+ breast cancers as well as inhibitors that may modulate aromatase activity or aromatase expression. Second, new models for antiestrogens and AI resistance are strongly needed in order to gain a better understanding of the disease of resistance. Using these models derived from various ER+ breast cancer cells we must compare the results obtained from them to those from the MCF-7aro LTED model in order to verify whether our current knowledge of endocrine resistance is consistent in other ER+ antiestrogen- or AI-resistant breast cancers. Moreover, it is interesting to note the parallels between the mechanisms proposed for de novo and acquired resistance, in particular for tamoxifen. In both of these types of resistance, growth factor signaling proteins are upregulated and activated, which can modulate ER activity and downstream ER-regulated genes. However, there are differences between the two types of resistance. De novo resistance is characterized by inherent upregulation of growth factor signaling pathways while acquired resistance is an adaptation process of the cell to activate these pathways in order to bypass the suppression of ER-mediated pathways. Therefore, more studies are required to distinguish the differences between de novo and acquired resistance.
Highlights.
Two types of endocrine resistance: de novo and acquired.
Resistance involves activation of ERα by growth factor receptors and kinases.
Cross-resistance studies show resistance mechanisms are different.
Focus on treatment therapies with multiple targets to overcome resistance.
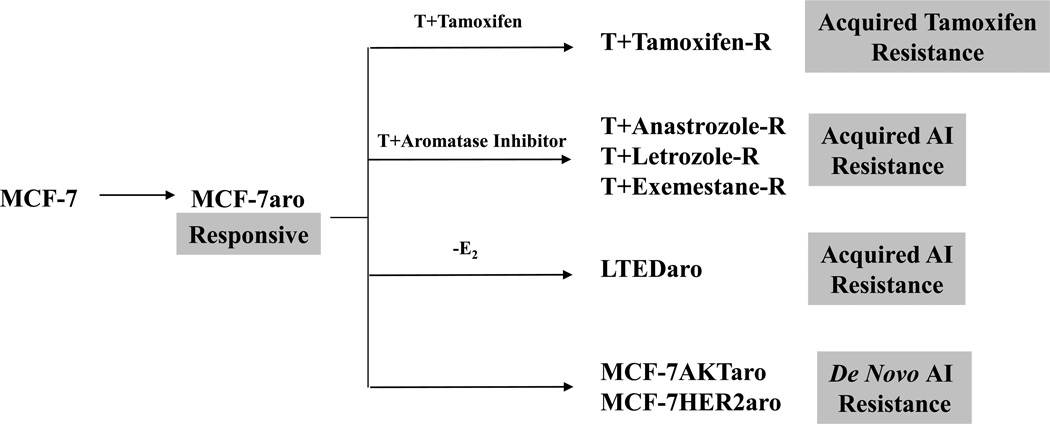
Figure 1.
Generation of Tamoxifen and Aromatase inhibitor resistant cell lines. AI and Tamoxifen responsive cells were generated by engineering MCF-7 cells to overexpress aromatase. MCF-7aro cells were cultured long-term in the presence of tamoxifen or in the absence of estrogen until cells gained the ability to proliferate in these conditions. These cells are considered cells which have acquired either tamoxifen resistance (Tamoxifen-R) or AI resistance (LTEDaro). Anastrozole resistant (Anastrozole-R), Letrozole resistant (Letrozole-R), and Exemestane resistant (Exemestane-R) cells that have acquired resistance to a specific AI were generated by culturing MCF-7aro cells in the presence of testosterone and the appropriate inhibitor until cells gained the ability to proliferate in these conditions. Finally, models for de novo AI resistance were generated by overexpressing aromatase and HER2 or Akt.
Figure 2.
Estrogen dependency of growth pathways in endocrine-resistant cell lines.
Table 1.
Summary of de novo and acquired resistance
| Growth factors, kinases |
ER protein levels |
ER activity | Response to ICI | ||
|---|---|---|---|---|---|
| De novo resistance | Tamoxifen | HER2, Akt | Unaltered | Unaltered | Responsive |
| AI | HER2, Akt | Unaltered | Unaltered | Nonresponsive | |
| Acquired resistance | Tamoxifen | EGFR, HER2 | Changeda | Ligand-independent | Responsive |
| AI | HER2, PI3K/Akt, MAPK | Changedb | Ligand independent | Responsive |
unaltered, decreased;
increased, decreased according to different reports
Acknowledgements
The research findings discussed in this article were generated by Selma Masri, Ph.D., Sheryl Phung, M.S., Xin Wang, Ph.D., and Cynthie Wong, Ph.D. This work was supported by NIH Grants CA44735 (to S. Chen), ES08258 (to S. Chen), NIH predoctoral training fellowship CA123691 (to S. Masri), and California Breast Cancer Research Program 13GB-0157 (to C.Wong)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 2.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- 4.Hanstein B, Djahansouzi S, Dall P, Beckmann MW, Bender HG. Insights into the molecular biology of the estrogen receptor define novel therapeutic targets for breast cancer. Eur J Endocrinol. 2004;150:243–255. doi: 10.1530/eje.0.1500243. [DOI] [PubMed] [Google Scholar]
- 5.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 6.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 7.Esteban JM, Warsi Z, Haniu M, Hall P, Shively JE, Chen S. Detection of intratumoral aromatase in breast carcinomas. An immunohistochemical study with clinicopathologic correlation. Am J Pathol. 1992;140:337–343. [PMC free article] [PubMed] [Google Scholar]
- 8.Miller WR, O'Neill J. The importance of local synthesis of estrogen within the breast. Steroids. 1987;50:537–548. doi: 10.1016/0039-128x(87)90037-7. [DOI] [PubMed] [Google Scholar]
- 9.Bulun SE, Price TM, Aitken J, Mahendroo MS, Simpson ER. A link between breast cancer and local estrogen biosynthesis suggested by quantification of breast adipose tissue aromatase cytochrome P450 transcripts using competitive polymerase chain reaction after reverse transcription. J Clin Endocrinol Metab. 1993;77:1622–1628. doi: 10.1210/jcem.77.6.8117355. [DOI] [PubMed] [Google Scholar]
- 10.Harada N. Aberrant expression of aromatase in breast cancer tissues. J Steroid Biochem Mol Biol. 1997;61:175–184. [PubMed] [Google Scholar]
- 11.Zhou D, Chen S. Identification and characterization of a cAMP-responsive element in the region upstream from promoter 1.3 of the human aromatase gene. Arch Biochem Biophys. 1999;371:179–190. doi: 10.1006/abbi.1999.1454. [DOI] [PubMed] [Google Scholar]
- 12.Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, Sahmoud T. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359:2131–2139. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 13.Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 14.Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, Griffith C, Boeddinghaus I, Salter J, Detre S, Hills M, Ashley S, Francis S, Walsh G. Short-term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival. Clin Cancer Res. 2005;11:951s–958s. [PubMed] [Google Scholar]
- 15.Coates AS, Keshaviah A, Thurlimann B, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Colleoni M, Lang I, Del Mastro L, Smith I, Chirgwin J, Nogaret JM, Pienkowski T, Wardley A, Jakobsen EH, Price KN, Goldhirsch A. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol. 2007;25:486–492. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 16.Zhou DJ, Pompon D, Chen SA. Stable expression of human aromatase complementary DNA in mammalian cells: a useful system for aromatase inhibitor screening. Cancer Res. 1990;50:6949–6954. [PubMed] [Google Scholar]
- 17.Masri S, Phung S, Wang X, Wu X, Yuan YC, Wagman L, Chen S. Genome-wide analysis of aromatase inhibitor-resistant, tamoxifen-resistant, and long-term estrogen-deprived cells reveals a role for estrogen receptor. Cancer Res. 2008;68:4910–4918. doi: 10.1158/0008-5472.CAN-08-0303. [DOI] [PubMed] [Google Scholar]
- 18.Gottardis MM, Robinson SP, Satyaswaroop PG, Jordan VC. Contrasting actions of tamoxifen on endometrial and breast tumor growth in the athymic mouse. Cancer Res. 1988;48:812–815. [PubMed] [Google Scholar]
- 19.Jordan VC, Phelps E, Lindgren JU. Effects of anti-estrogens on bone in castrated and intact female rats. Breast Cancer Res Treat. 1987;10:31–35. doi: 10.1007/BF01806132. [DOI] [PubMed] [Google Scholar]
- 20.Jordan VC. Selective estrogen receptor modulation: concept and consequences in cancer. Cancer Cell. 2004;5:207–213. doi: 10.1016/s1535-6108(04)00059-5. [DOI] [PubMed] [Google Scholar]
- 21.Nawata H, Bronzert D, Lippman ME. Isolation and characterization of a tamoxifen-resistant cell line derived from MCF-7 human breast cancer cells. J Biol Chem. 1981;256:5016–5021. [PubMed] [Google Scholar]
- 22.Knowlden JM, Hutcheson IR, Jones HE, Madden T, Gee JM, Harper ME, Barrow D, Wakeling AE, Nicholson RI. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology. 2003;144:1032–1044. doi: 10.1210/en.2002-220620. [DOI] [PubMed] [Google Scholar]
- 23.Brunner N, Frandsen TL, Holst-Hansen C, Bei M, Thompson EW, Wakeling AE, Lippman ME, Clarke R. MCF7/LCC2: a 4-hydroxytamoxifen resistant human breast cancer variant that retains sensitivity to the steroidal antiestrogen ICI 182,780. Cancer Res. 1993;53:3229–3232. [PubMed] [Google Scholar]
- 24.Lykkesfeldt AE, Madsen MW, Briand P. Altered expression of estrogen-regulated genes in a tamoxifen-resistant and ICI 164,384 and ICI 182,780 sensitive human breast cancer cell line, MCF-7/TAMR-1. Cancer Res. 1994;54:1587–1595. [PubMed] [Google Scholar]
- 25.Massarweh S, Osborne CK, Creighton CJ, Qin L, Tsimelzon A, Huang S, Weiss H, Rimawi M, Schiff R. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 2008;68:826–833. doi: 10.1158/0008-5472.CAN-07-2707. [DOI] [PubMed] [Google Scholar]
- 26.Meng S, Tripathy D, Shete S, Ashfaq R, Haley B, Perkins S, Beitsch P, Khan A, Euhus D, Osborne C, Frenkel E, Hoover S, Leitch M, Clifford E, Vitetta E, Morrison L, Herlyn D, Terstappen LW, Fleming T, Fehm T, Tucker T, Lane N, Wang J, Uhr J. HER-2 gene amplification can be acquired as breast cancer progresses. Proc Natl Acad Sci U S A. 2004;101:9393–9398. doi: 10.1073/pnas.0402993101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipton A, Leitzel K, Ali SM, Demers L, Harvey HA, Chaudri-Ross HA, Evans D, Lang R, Hackl W, Hamer P, Carney W. Serum HER-2/neu conversion to positive at the time of disease progression in patients with breast carcinoma on hormone therapy. Cancer. 2005;104:257–263. doi: 10.1002/cncr.21202. [DOI] [PubMed] [Google Scholar]
- 28.Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem. 2001;276:9817–9824. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- 29.Chung YL, Sheu ML, Yang SC, Lin CH, Yen SH. Resistance to tamoxifen-induced apoptosis is associated with direct interaction between Her2/neu and cell membrane estrogen receptor in breast cancer. Int J Cancer. 2002;97:306–312. doi: 10.1002/ijc.1614. [DOI] [PubMed] [Google Scholar]
- 30.Benz CC, Scott GK, Sarup JC, Johnson RM, Tripathy D, Coronado E, Shepard HM, Osborne CK. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat. 1992;24:85–95. doi: 10.1007/BF01961241. [DOI] [PubMed] [Google Scholar]
- 31.Pietras RJ, Arboleda J, Reese DM, Wongvipat N, Pegram MD, Ramos L, Gorman CM, Parker MG, Sliwkowski MX, Slamon DJ. HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene. 1995;10:2435–2446. [PubMed] [Google Scholar]
- 32.Le Goff P, Montano MM, Schodin DJ, Katzenellenbogen BS. Phosphorylation of the human estrogen receptor. Identification of hormone-regulated sites and examination of their influence on transcriptional activity. J Biol Chem. 1994;269:4458–4466. [PubMed] [Google Scholar]
- 33.Arnold SF, Vorojeikina DP, Notides AC. Phosphorylation of tyrosine 537 on the human estrogen receptor is required for binding to an estrogen response element. J Biol Chem. 1995;270:30205–30212. doi: 10.1074/jbc.270.50.30205. [DOI] [PubMed] [Google Scholar]
- 34.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 35.Szapary D, Huang Y, Simons SS., Jr Opposing effects of corepressor and coactivators in determining the dose-response curve of agonists, and residual agonist activity of antagonists, for glucocorticoid receptor-regulated gene expression. Mol Endocrinol. 1999;13:2108–2121. doi: 10.1210/mend.13.12.0384. [DOI] [PubMed] [Google Scholar]
- 36.Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 37.Webb P, Nguyen P, Shinsako J, Anderson C, Feng W, Nguyen MP, Chen D, Huang SM, Subramanian S, McKinerney E, Katzenellenbogen BS, Stallcup MR, Kushner PJ. Estrogen receptor activation function 1 works by binding p160 coactivator proteins. Mol Endocrinol. 1998;12:1605–1618. doi: 10.1210/mend.12.10.0185. [DOI] [PubMed] [Google Scholar]
- 38.Font de Mora J, Brown M. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol. 2000;20:5041–5047. doi: 10.1128/mcb.20.14.5041-5047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang SY, Jordan VC. Growth regulation of estrogen receptor-negative breast cancer cells transfected with complementary DNAs for estrogen receptor. J Natl Cancer Inst. 1992;84:580–591. doi: 10.1093/jnci/84.8.580. [DOI] [PubMed] [Google Scholar]
- 40.Zajchowski DA, Sager R, Webster L. Estrogen inhibits the growth of estrogen receptor-negative, but not estrogen receptor-positive, human mammary epithelial cells expressing a recombinant estrogen receptor. Cancer Res. 1993;53:5004–5011. [PubMed] [Google Scholar]
- 41.Lazennec G, Katzenellenbogen BS. Expression of human estrogen receptor using an efficient adenoviral gene delivery system is able to restore hormone-dependent features to estrogen receptor-negative breast carcinoma cells. Mol Cell Endocrinol. 1999;149:93–105. doi: 10.1016/s0303-7207(98)00254-8. [DOI] [PubMed] [Google Scholar]
- 42.Tolhurst RS, Thomas RS, Kyle FJ, Patel H, Periyasamy M, Photiou A, Thiruchelvam PT, Lai CF, Al-Sabbagh M, Fisher RA, Barry S, Crnogorac-Jurcevic T, Martin LA, Dowsett M, Charles Coombes R, Kamalati T, Ali S, Buluwela L. Transient over-expression of estrogen receptor-alpha in breast cancer cells promotes cell survival and estrogen-independent growth. Breast Cancer Res Treat. 2011;128:357–368. doi: 10.1007/s10549-010-1122-6. [DOI] [PubMed] [Google Scholar]
- 43.Lykkesfeldt AE, Larsen SS, Briand P. Human breast cancer cell lines resistant to pure anti-estrogens are sensitive to tamoxifen treatment. Int J Cancer. 1995;61:529–534. doi: 10.1002/ijc.2910610417. [DOI] [PubMed] [Google Scholar]
- 44.Fan M, Yan PS, Hartman-Frey C, Chen L, Paik H, Oyer SL, Salisbury JD, Cheng AS, Li L, Abbosh PH, Huang TH, Nephew KP. Diverse gene expression and DNA methylation profiles correlate with differential adaptation of breast cancer cells to the antiestrogens tamoxifen and fulvestrant. Cancer Res. 2006;66:11954–11966. doi: 10.1158/0008-5472.CAN-06-1666. [DOI] [PubMed] [Google Scholar]
- 45.Frogne T, Benjaminsen RV, Sonne-Hansen K, Sorensen BS, Nexo E, Laenkholm AV, Rasmussen LM, Riese DJ, 2nd, de Cremoux P, Stenvang J, Lykkesfeldt AE. Activation of ErbB3, EGFR and Erk is essential for growth of human breast cancer cell lines with acquired resistance to fulvestrant. Breast Cancer Res Treat. 2009;114:263–275. doi: 10.1007/s10549-008-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rao X, Di Leva G, Li M, Fang F, Devlin C, Hartman-Frey C, Burow ME, Ivan M, Croce CM, Nephew KP. MicroRNA-221/222 confers breast cancer fulvestrant resistance by regulating multiple signaling pathways. Oncogene. 2011;30:1082–1097. doi: 10.1038/onc.2010.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brunner N, Boysen B, Jirus S, Skaar TC, Holst-Hansen C, Lippman J, Frandsen T, Spang-Thomsen M, Fuqua SA, Clarke R. MCF7/LCC9: an antiestrogen-resistant MCF-7 variant in which acquired resistance to the steroidal antiestrogen ICI 182,780 confers an early cross-resistance to the nonsteroidal antiestrogen tamoxifen. Cancer Res. 1997;57:3486–3493. [PubMed] [Google Scholar]
- 48.Gu Z, Lee RY, Skaar TC, Bouker KB, Welch JN, Lu J, Liu A, Zhu Y, Davis N, Leonessa F, Brunner N, Wang Y, Clarke R. Association of interferon regulatory factor-1, nucleophosmin, nuclear factor-kappaB, and cyclic AMP response element binding with acquired resistance to Faslodex (ICI 182,780) Cancer Res. 2002;62:3428–3437. [PubMed] [Google Scholar]
- 49.Ning Y, Riggins RB, Mulla JE, Chung H, Zwart A, Clarke R. IFNgamma restores breast cancer sensitivity to fulvestrant by regulating STAT1, IFN regulatory factor 1, NF-kappaB, BCL2 family members, and signaling to caspase-dependent apoptosis. Mol Cancer Ther. 2010;9:1274–1285. doi: 10.1158/1535-7163.MCT-09-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masri S, Phung S, Wang X, Chen S. Molecular characterization of aromatase inhibitor-resistant, tamoxifen-resistant and LTEDaro cell lines. J Steroid Biochem Mol Biol. 2010;118:277–282. doi: 10.1016/j.jsbmb.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin LA, Farmer I, Johnston SR, Ali S, Marshall C, Dowsett M. Enhanced estrogen receptor (ER) alpha, ERBB2, and MAPK signal transduction pathways operate during the adaptation of MCF-7 cells to long term estrogen deprivation. J Biol Chem. 2003;278:30458–30468. doi: 10.1074/jbc.M305226200. [DOI] [PubMed] [Google Scholar]
- 52.Santen RJ, Song RX, McPherson R, Kumar R, Adam L, Jeng MH, Yue W. The role of mitogen-activated protein (MAP) kinase in breast cancer. J Steroid Biochem Mol Biol. 2002;80:239–256. doi: 10.1016/s0960-0760(01)00189-3. [DOI] [PubMed] [Google Scholar]
- 53.Chan CM, Martin LA, Johnston SR, Ali S, Dowsett M. Molecular changes associated with the acquisition of oestrogen hypersensitivity in MCF-7 breast cancer cells on long-term oestrogen deprivation. J Steroid Biochem Mol Biol. 2002;81:333–341. doi: 10.1016/s0960-0760(02)00074-2. [DOI] [PubMed] [Google Scholar]
- 54.Dowsett M, Martin LA, Smith I, Johnston S. Mechanisms of resistance to aromatase inhibitors. J Steroid Biochem Mol Biol. 2005;95:167–172. doi: 10.1016/j.jsbmb.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 55.Song RX, Mor G, Naftolin F, McPherson RA, Song J, Zhang Z, Yue W, Wang J, Santen RJ. Effect of long-term estrogen deprivation on apoptotic responses of breast cancer cells to 17beta-estradiol. J Natl Cancer Inst. 2001;93:1714–1723. doi: 10.1093/jnci/93.22.1714. [DOI] [PubMed] [Google Scholar]
- 56.Maximov PY, Lewis-Wambi JS, Jordan VC. The Paradox of Oestradiol-Induced Breast Cancer Cell Growth and Apoptosis. Curr Signal Transduct Ther. 2009;4:88–102. doi: 10.2174/157436209788167484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sabnis GJ, Jelovac D, Long B, Brodie A. The role of growth factor receptor pathways in human breast cancer cells adapted to long-term estrogen deprivation. Cancer Res. 2005;65:3903–3910. doi: 10.1158/0008-5472.CAN-04-4092. [DOI] [PubMed] [Google Scholar]
- 58.Staka CM, Nicholson RI, Gee JM. Acquired resistance to oestrogen deprivation: role for growth factor signalling kinases/oestrogen receptor cross-talk revealed in new MCF-7X model. Endocr Relat Cancer. 2005;12(Suppl 1):S85–S97. doi: 10.1677/erc.1.01006. [DOI] [PubMed] [Google Scholar]
- 59.Jelovac D, Sabnis G, Long BJ, Macedo L, Goloubeva OG, Brodie AM. Activation of mitogen-activated protein kinase in xenografts and cells during prolonged treatment with aromatase inhibitor letrozole. Cancer Res. 2005;65:5380–5389. doi: 10.1158/0008-5472.CAN-04-4502. [DOI] [PubMed] [Google Scholar]
- 60.Sabnis G, Schayowitz A, Goloubeva O, Macedo L, Brodie A. Trastuzumab reverses letrozole resistance and amplifies the sensitivity of breast cancer cells to estrogen. Cancer Res. 2009;69:1416–1428. doi: 10.1158/0008-5472.CAN-08-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nair HB, Kirma NB, Ganapathy M, Vadlamudi RK, Tekmal RR. Estrogen receptor-beta activation in combination with letrozole blocks the growth of breast cancer tumors resistant to letrozole therapy. Steroids. 2011;76:792–796. doi: 10.1016/j.steroids.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 62.Santen RJ, Song RX, Zhang Z, Kumar R, Jeng MH, Masamura S, Lawrence J, Jr, MacMahon LP, Yue W, Berstein L. Adaptive hypersensitivity to estrogen: mechanisms and clinical relevance to aromatase inhibitor therapy in breast cancer treatment. J Steroid Biochem Mol Biol. 2005;95:155–165. doi: 10.1016/j.jsbmb.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 63.Santen RJ, Song RX, Zhang Z, Kumar R, Jeng MH, Masamura A, Lawrence J, Jr, Berstein L, Yue W. Long-term estradiol deprivation in breast cancer cells up-regulates growth factor signaling and enhances estrogen sensitivity. Endocr Relat Cancer. 2005;12(Suppl 1):S61–S73. doi: 10.1677/erc.1.01018. [DOI] [PubMed] [Google Scholar]
- 64.Edery M, Goussard J, Dehennin L, Scholler R, Reiffsteck J, Drosdowsky MA. Endogenous oestradiol-17beta concentration in breast tumours determined by mass fragmentography and by radioimmunoassay: relationship to receptor content. Eur J Cancer. 1981;17:115–120. doi: 10.1016/0014-2964(81)90220-6. [DOI] [PubMed] [Google Scholar]
- 65.Millington DS. Determination of hormonal steroid concentrations in biological extracts by high resolution mass fragmentography. J Steroid Biochem. 1975;6:239–245. doi: 10.1016/0022-4731(75)90139-9. [DOI] [PubMed] [Google Scholar]
- 66.Pasqualini JR, Chetrite G, Blacker C, Feinstein MC, Delalonde L, Talbi M, Maloche C. Concentrations of estrone, estradiol, and estrone sulfate and evaluation of sulfatase and aromatase activities in pre- and postmenopausal breast cancer patients. J Clin Endocrinol Metab. 1996;81:1460–1464. doi: 10.1210/jcem.81.4.8636351. [DOI] [PubMed] [Google Scholar]
- 67.Johnston SR, Saccani-Jotti G, Smith IE, Salter J, Newby J, Coppen M, Ebbs SR, Dowsett M. Changes in estrogen receptor, progesterone receptor, and pS2 expression in tamoxifen-resistant human breast cancer. Cancer Res. 1995;55:3331–3338. [PubMed] [Google Scholar]
- 68.Kuukasjarvi T, Kononen J, Helin H, Holli K, Isola J. Loss of estrogen receptor in recurrent breast cancer is associated with poor response to endocrine therapy. J Clin Oncol. 1996;14:2584–2589. doi: 10.1200/JCO.1996.14.9.2584. [DOI] [PubMed] [Google Scholar]
- 69.Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 70.Kumar R, Mandal M, Lipton A, Harvey H, Thompson CB. Overexpression of HER2 modulates bcl-2, bcl-XL, and tamoxifen-induced apoptosis in human MCF-7 breast cancer cells. Clin Cancer Res. 1996;2:1215–1219. [PubMed] [Google Scholar]
- 71.Wang Y, Zhou D, Phung S, Masri S, Smith D, Chen S. SGK3 is an estrogen-inducible kinase promoting estrogen-mediated survival of breast cancer cells. Mol Endocrinol. 2011;25:72–82. doi: 10.1210/me.2010-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Crawford AC, Riggins RB, Shajahan AN, Zwart A, Clarke R. Co-inhibition of BCL-W and BCL2 restores antiestrogen sensitivity through BECN1 and promotes an autophagy-associated necrosis. PLoS One. 2010;5:e8604. doi: 10.1371/journal.pone.0008604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lonning PE. Exploring the lack of cross-resistance between aromatase inhibitors: evidence for a difference? Anticancer Drugs. 2008;19(Suppl 2):S11–S13. doi: 10.1097/01.cad.0000277875.81122.25. [DOI] [PubMed] [Google Scholar]
- 74.Masri S, Lui K, Phung S, Ye J, Zhou D, Wang X, Chen S. Characterization of the weak estrogen receptor alpha agonistic activity of exemestane. Breast Cancer Res Treat. 2009;116:461–470. doi: 10.1007/s10549-008-0151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ariazi EA, Leitao A, Oprea TI, Chen B, Louis T, Bertucci AM, Sharma CG, Gill SD, Kim HR, Shupp HA, Pyle JR, Madrack A, Donato AL, Cheng D, Paige JR, Jordan VC. Exemestane's 17-hydroxylated metabolite exerts biological effects as an androgen. Mol Cancer Ther. 2007;6:2817–2827. doi: 10.1158/1535-7163.MCT-07-0312. [DOI] [PubMed] [Google Scholar]
- 76.Chen S. An "omics" approach to determine the mechanisms of acquired aromatase inhibitor resistance. OMICS. 2011;15:347–352. doi: 10.1089/omi.2010.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miller WR, Larionov A. Changes in expression of oestrogen regulated and proliferation genes with neoadjuvant treatment highlight heterogeneity of clinical resistance to the aromatase inhibitor, letrozole. Breast Cancer Res. 2010;12:R52. doi: 10.1186/bcr2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Riggins RB, Schrecengost RS, Guerrero MS, Bouton AH. Pathways to tamoxifen resistance. Cancer Lett. 2007;256:1–24. doi: 10.1016/j.canlet.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harris AL, Nicholson S, Sainsbury JR, Farndon J, Wright C. Epidermal growth factor receptors in breast cancer: association with early relapse and death, poor response to hormones and interactions with neu. J Steroid Biochem. 1989;34:123–131. doi: 10.1016/0022-4731(89)90072-1. [DOI] [PubMed] [Google Scholar]
- 80.Yamauchi H, O'Neill A, Gelman R, Carney W, Tenney DY, Hosch S, Hayes DF. Prediction of response to antiestrogen therapy in advanced breast cancer patients by pretreatment circulating levels of extracellular domain of the HER-2/c-neu protein. J Clin Oncol. 1997;15:2518–2525. doi: 10.1200/JCO.1997.15.7.2518. [DOI] [PubMed] [Google Scholar]
- 81.Leitzel K, Teramoto Y, Konrad K, Chinchilli VM, Volas G, Grossberg H, Harvey H, Demers L, Lipton A. Elevated serum c-erbB-2 antigen levels and decreased response to hormone therapy of breast cancer. J Clin Oncol. 1995;13:1129–1135. doi: 10.1200/JCO.1995.13.5.1129. [DOI] [PubMed] [Google Scholar]
- 82.Liu Y, el-Ashry D, Chen D, Ding IY, Kern FG. MCF-7 breast cancer cells overexpressing transfected c-erbB-2 have an in vitro growth advantage in estrogen-depleted conditions and reduced estrogen-dependence and tamoxifen-sensitivity in vivo. Breast Cancer Res Treat. 1995;34:97–117. doi: 10.1007/BF00665783. [DOI] [PubMed] [Google Scholar]
- 83.Kurokawa H, Lenferink AE, Simpson JF, Pisacane PI, Sliwkowski MX, Forbes JT, Arteaga CL. Inhibition of HER2/neu (erbB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-overexpressing, tamoxifen-resistant breast cancer cells. Cancer Res. 2000;60:5887–5894. [PubMed] [Google Scholar]
- 84.Dowsett M. Overexpression of HER-2 as a resistance mechanism to hormonal therapy for breast cancer. Endocr Relat Cancer. 2001;8:191–195. doi: 10.1677/erc.0.0080191. [DOI] [PubMed] [Google Scholar]
- 85.Wong C, Chen S. Heat shock protein 90 inhibitors: new mode of therapy to overcome endocrine resistance. Cancer Res. 2009;69:8670–8677. doi: 10.1158/0008-5472.CAN-09-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Polychronis A, Sinnett HD, Hadjiminas D, Singhal H, Mansi JL, Shivapatham D, Shousha S, Jiang J, Peston D, Barrett N, Vigushin D, Morrison K, Beresford E, Ali S, Slade MJ, Coombes RC. Preoperative gefitinib versus gefitinib and anastrozole in postmenopausal patients with oestrogen-receptor positive and epidermal-growth-factor-receptor-positive primary breast cancer: a double-blind placebo-controlled phase II randomised trial. Lancet Oncol. 2005;6:383–391. doi: 10.1016/S1470-2045(05)70176-5. [DOI] [PubMed] [Google Scholar]
- 87.Schwartzberg LS, Franco SX, Florance A, O'Rourke L, Maltzman J, Johnston S. Lapatinib plus letrozole as first-line therapy for HER-2+ hormone receptor-positive metastatic breast cancer. Oncologist. 2010;15:122–129. doi: 10.1634/theoncologist.2009-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leary AF, Drury S, Detre S, Pancholi S, Lykkesfeldt AE, Martin LA, Dowsett M, Johnston SR. Lapatinib restores hormone sensitivity with differential effects on estrogen receptor signaling in cell models of human epidermal growth factor receptor 2-negative breast cancer with acquired endocrine resistance. Clin Cancer Res. 2010;16:1486–1497. doi: 10.1158/1078-0432.CCR-09-1764. [DOI] [PubMed] [Google Scholar]
- 89.Miller TW, Hennessy BT, Gonzalez-Angulo AM, Fox EM, Mills GB, Chen H, Higham C, Garcia-Echeverria C, Shyr Y, Arteaga CL. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120:2406–2413. doi: 10.1172/JCI41680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoskins JM, Carey LA, McLeod HL. CYP2D6 and tamoxifen: DNA matters in breast cancer. Nat Rev Cancer. 2009;9:576–586. doi: 10.1038/nrc2683. [DOI] [PubMed] [Google Scholar]