Abstract
Salt-sensitivity is a key risk factor for cardiovascular disease and renal injury. Alterations in renal nitric oxide may contribute to salt-dependent increases in blood pressure and tissue damage. Therefore, we assessed the expression of nitric oxide synthase (NOS) isoforms in the kidney, as well as the effects of nNOS inhibition on renal injury, inflammation and oxidative stress in the female mRen2.Lewis rat (mRen), a model of salt-sensitive hypertension. We find that a high salt diet (HS, 4% sodium) significantly reduced endothelial NOS mRNA (2.6 fold) and protein (1.5 fold) but increased nNOS mRNA (2.4 fold) and protein (1.9 fold) in the renal cortex of these animals. Immunostaining for nNOS also appeared higher in macula densa and cortical tubules of the HS rats. Circulating nitrate and nitrite levels were reduced, as well as the tissue levels of the NOS co-factor tetrahydrobiopterin (BH4). Cortical markers of oxidative stress (4HNE, 8-OH-deoxyguanosine) and fibrosis were increased; however, mRNA levels of the NAD(P)H oxidase components NOX4, p22phox and p47phox were reduced. Chronic treatment with the nNOS inhibitor L-VNIO did not influence SBP after 4 weeks but significantly attenuated albuminuria, renal fibrosis, inflammation and indices of oxidative stress. We conclude that an increase in nNOS expression in conjunction with reduced levels of cortical BH4 may stimulate oxidative stress and renal injury in the salt-sensitive female mRen2.Lewis rat.
Keywords: albuminuria, renal inflammation, renal cortex, tetrahydrobiopterin, 8-hydroxy-deoxyguanosine
Introduction
Salt-sensitivity is a risk factor for cardiovascular disease and renal injury (1). Although the mechanisms underlying salt-dependent increases in blood pressure and tissue damage remain equivocal, dysregulation of the nitric oxide (NO) system may be a contributing element (2). Typically nitric oxide (NO) formation increases in response to high salt intake to facilitate sodium excretion and maintain normal blood pressure in the salt resistance state (3,4). However, both clinical and experimental data reveal that a salt-sensitive increase in blood pressure is associated with a failure to increase NO that may promote sodium retention, hypertension and renal damage (5-7). The formation of NO is catalyzed by at least three NO synthase (NOS) isoforms that include type I (neuronal or nNOS), type II (inducible or iNOS) and type III (endothelial or eNOS). All three NOS isoforms have been detected in the kidney and their expression is influenced by a high salt diet in several salt-sensitive models, although the expression of iNOS is more variable (8-9). Chronic eNOS inhibition or eNOS knockout mice express a salt-sensitive phenotype typified by an increase in blood pressure and renal damage in response to a high salt diet (11). In comparison, inhibition of renal nNOS enhances the tubuloglomerular feedback response in animals maintained on a normal salt diet (5,12). The nNOS isoform is reported to exert a tonic inhibitory influence on renin release from juxatglomerular cells (5,12). In addition to the regulation of NOS expression, the bioavailability of NO is influenced by endogenous tissue levels of the NOS inhibitor asymmetric dimethylarginine (ADMA), posttranslational modifications of NOS and the availability of the substrate L-arginine, as well as coupling to the co-factor tetrahydrobiopterin (BH4) which determines the extent of NO versus superoxide (SO) production (13).
Our previous studies in the female hypertensive mRen2.Lewis, a model of tissue renin expression and angiotensin II (Ang II)-dependent hypertension, demonstrated differential expression of eNOS and nNOS isoforms following estrogen depletion (ovariectomy, OVX); renal expression of iNOS was not detected. In both the kidney and heart, nNOS was increased in the OVX-mRen2.Lewis and chronic treatment with the selective nNOS inhibitor N5-(1-Imino-3-butenyl)-L-ornithine (L-VNIO) significantly reduced blood pressure (14,15). Administration of L-VNIO in the OVX-mRen2.Lewis also improved cardiac diastolic dysfunction and attenuated oxidative stress within the heart (16). Moreover, exogenous supplementation with the co-factor BH4 yielded similar cardiac effects (17). Taken together with the reduction in BH4 levels in the heart, increased nNOS may contribute to oxidative stress rather than NO via an uncoupling mechanism in estrogen-depleted mRen2.Lewis rats.
In addition to estrogen sensitivity, the female mRen2.Lewis is characterized as a salt-sensitive strain and chronic maintenance on a high salt diet markedly exacerbates the hypertension, increases cardiac fibrosis and promotes diastolic dysfunction, as well as proteinuria and albuminuria (15,18). In contrast, the male mRen2.Lewis does not exhibit an increase in systolic blood pressure in response to salt although proteinuria and angiotensinogen excretion are increased (19,20). Since few studies have addressed the role of NOS isoforms, particularly nNOS in female salt-sensitive rats, we investigated the influence of a high salt on the expression of the NOS enzymes in the kidney of the intact female mRen2.Lewis. In addition, we determined the influence of long-term treatment with the nNOS inhibitor L-VNIO on blood pressure, renal injury, and oxidative stress within the kidney of the intact female congenics.
Methods
Experimental animals
Hemizygous female mRen2.Lewis rats weighing 90-100 g at the age of 4 weeks were obtained from the congenic colony of the Hypertension and Vascular Research Center of Wake Forest School of Medicine. Rats were kept in an AALAC-approved facility with a 12 h light: dark cycle (lights on 6:00 am to 6:00 pm) and were randomly assigned to study groups with five to seven animals per group. Animals were fed a high salt diet (8% sodium chloride, Harlan Teklab, Madison, WI) starting at 5 weeks of age. To investigate the role of nNOS, high salt fed rats were divided into two groups: untreated or systemically treated with the selective nNOS inhibitor, N5-(1-Imino-3-butenyl)-L-ornithine (L-VNIO) to achieve a target dose of 0.5 mg/kg/day (Alexis Biochemicals, San Diego, CA) (14); the treatment started at 11 weeks of age and continued for 4 weeks. L-VNIO was diluted in saline and administered intraperitoneally via an osmotic minipump (28 day, 2.5μl/h; model 2ML4, ALZA Corp. Palo Alto, CA, USA). The dose of the L-VNIO was chosen based on several previous in vivo studies that demonstrated functional in vivo effects of the inhibitor (14,21). Drug delivery was verified by measuring the residual volume of each pump at the end of the study. L-VNIO is a selective and potent nNOS inhibitor (Ki of 100 nM) exhibiting over 100-fold lower affinity for eNOS and iNOS isoforms (Ki of 12 μM and 60 μM, respectively) (22). Urine was collected in metabolic cages over a 24-h period at the end of the study. At 15 weeks of age, rats were decapitated and the trunk blood was collected for serum. Renal cortical tissue was used to quantify the mRNA and protein of NOS isoforms, biopterin profile, and the mRNA of NAD(P)H oxidase subunits. For all experiments, right kidney was used for mRNA and protein detection by reverse transcriptase real-time polymerase chain reaction (RT real-time PCR) and Western blot hybridization, respectively, while left kidney was used for immunohistochemical staining. All procedures were approved by the Wake Forest School of Medicine IACUC for animal care.
Blood pressures
Systolic blood pressure (SBP) was measured weekly in trained rats (mean of 5 determinations per data point) using the automated tail-cuff system (Narco Bio-systems, Houston, TX, USA) after warming the rats under slight restraint.
RNA isolation and RT real-time PCR assay
RNA was isolated from renal cortex tissue and RT real-time PCR was performed as previously described (14). The primer/probe sets for all genes used in the study were purchased from Applied Biosystems (Lexington, KY). All reactions were performed in triplicate and 18S ribosomal RNA, amplified using the TaqMan Ribosomal RNA Control Kit (Applied Biosystems, Lexington, KY) served as an internal control. The results were quantified as Ct values, where Ct is defined as the threshold cycle of PCR at which amplified product is first detected, and expressed as the ratio of target to control.
Western blot hybridization
Soluble and membrane fractions of renal cortex (50 μg of the total protein) were prepared for the detection of NOS isoforms by Western blot hybridization as described 14. Purified mouse nNOS (1:2000), eNOS (1:500), and iNOS (1:2000) antibodies were purchased from Transduction Laboratories (Lexington, KY). The data were quantified using densitometry (MCID software); the results were expressed as arbitrary units of intensity, and normalized to EF1-α protein intensities as described (14). The appropriate positive controls: human endothelial cells lysates for eNOS, rat cerebrum cell lysate for nNOS, and mouse macrophage IFNλ/LPS lysate for iNOS were used.
Masson's trichrome staining and pathology scoring
Paraffin-embedded kidney sections (5μm) were stained with Masson's trichrome method for identifying collagen fibers. The total area of collagen deposition was identified in 12 sections of the digitized images from each kidney (n=3-4 in each group) and measured as the collagen-to-field ratio using Adobe Photoshop 7.0. The same tissue sections were scored by a pathologist from the Experimental Pathology Laboratories, Inc. (Charlottesville, VA) blind to the individual groups (23). The following criteria were used to diagnose renal injury: for glomerulosclerosis - increased collagen deposition within the glomerular or periglomerular area; for vascular smooth muscle cell hyperplasia – changes in the cellularity/organization of layers observed in arteries; for interstitial fibrosis- an increased amount of collagen between tubules; greater degrees of fibrosis were graded higher if more parenchyma was involved; for interstitial inflammation - the increased number of mononuclear cells within interstitium; for tubular dilation - tubules considerably dilated beyond the normal diameter. Each lesion was given a grade using a 5-point grading scale: grade 1 = minimal, grade 2 = mild, grade 3 = moderate, grade 4 = marked, grade 5 = severe. The data are reported as injury scores calculated as a product of a lesion's severity grade and the incidence.
Immunohistochemical methods
Kidney paraffin-embedded 5-μm sections were stained using the Avidin Biotin Complex (ABC) method. The staining for all proteins required antigen retrieval treatment with sodium citrate buffer (pH 6.0) at 90-95°C for 30 min. Sections were incubated with the following primary antibodies: rabbit polyclonal affinity purified nNOS (dilution: 1:400; BD Biosciences, San Diego, CA); rabbit polyclonal 4-hydroxy-2-nonenal Michael Adducts (4-HNE; dilution: 1:15,000; Calbiochem, La Jolla, CA); monoclonal CD68 (dilution: 1:100; Chemicon, Temecula, CA); monoclonal 8-hydroxy-2-deoxy guanosine (8-OHdG; dilution: 1:100; JaICA, Japan); and secondary biotinylated goat anti-rabbit or anti-mouse antibody (dilution: 1:500; Vector Laboratories, Burlingame, CA). For increased visualization of nuclei for 8-OHdG immunostaining, DAB nickel enhancement kit was followed by eosin staining. The stained sections were examined under a light microscope and photographed with a 1394 FireWare™ Digital camera and SimplePCI (Version 6) software. Images of 4-HNE staining were analyzed using Adobe Photoshop 7.0 in 12 sections from each kidney (n=3-4 in each group) and the data are reported as relative intensity units. The CD68- or 8-OHdG-positive cells were counted using automated counting software (Image Pro 6.3; MadiaCybernatics, Bethesda, MD, USA) in 7 sections from each kidney (n=4 in each group). The data for CD68 and 8-OHdG are reported as a number of positive cells per 200× area.
Metabolic urinary parameters
Serum and urinary creatinine was measured by a Quantichrom™ Creatinine Assay Kit (Bioassay Systems, Hayward, CA). Creatinine clearance was calculated from its urinary excretion rate divided by serum concentration and the data were expressed as ml per min. Urinary albumin was determined by an enzyme immunoassay (SPI-BIO), and expressed as daily excretion.
Nitrate and nitrite concentrations (NOx)
The levels of NOx were measured in the serum using a Griess assay (Nitrate/nitrite colorimetric assay kit; Alexis Biomedicals, San Diego, CA).
Tetrahydrobiopterin (BH4) levels
BH4 content was determined by HPLC with fluorescence detection in renal cortical tissue from NS and HS rats as described by Chen and colleagues (24). Dihydrobiopterin (BH2) and oxidized biopterin were subtracted from total biopterins to calculate BH4 concentration.
Statistical Analysis
All measurements were expressed as the mean ± standard error of the mean (SE). Changes between NS and HS groups or HS and HS-L-VNIO were calculated by the unpaired t-test (GraphPad Prism IV plotting and statistical software, San Diego, CA).
Results
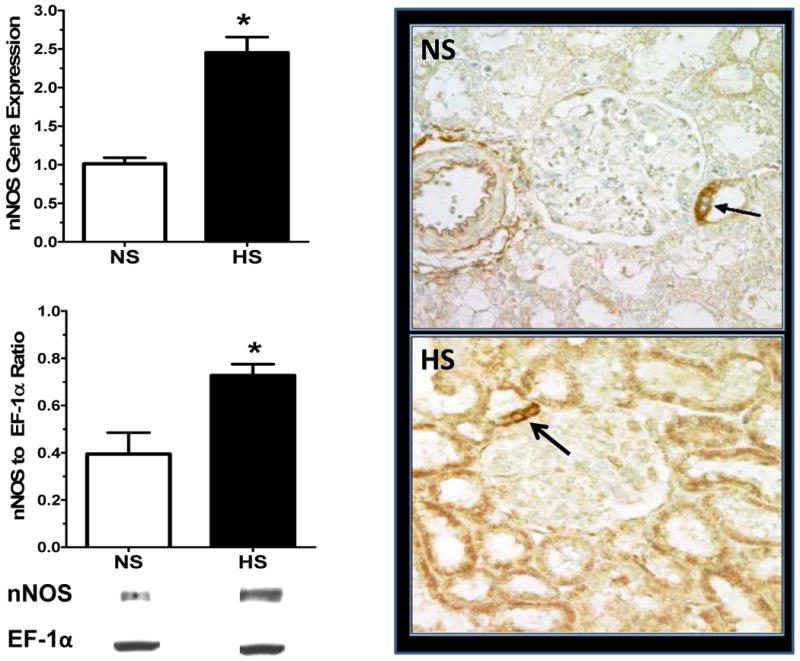
As reported previously in intact female mRen2.Lewis rats, chronic feeding of a HS diet was associated with a significant increase in systolic blood pressure [137 ± 3 vs. 203 ± 4 mmHg, p<0.05] proteinuria [3.5 ± 0.3 vs. 6.4 ± 0.7 mg/day, p<0.05] and albuminuria [1.8 ± 0.1 vs. 2.4 ± 0.3 mg/day, p<0.05], but reduced creatinine clearance [0.89 ± 0.15 vs. 0.54 ± 0.06 ml/min, p<0.05] (18). The biochemical assessment of this cohort revealed a significant reduction in cortical eNOS protein expression and eNOS mRNA levels (Figure 1). However, nNOS protein expression and nNOS mRNA levels were significantly increased in the HS-fed mRen2.Lewis rats (Figure 2). Immunohistochemical staining for nNOS also appeared higher in the HS group with staining evident in the macula densa and cortical proximal and distal tubules (Figure 2). Both mRNA and protein for the iNOS were undetectable in the cortex of either the NS or HS mRen2.Lewis rats (data not shown). Circulating nitric oxide metabolites (NOx) were significantly reduced in the HS rats [NS: 22.8 ± 2.9 vs. HS: 8.9 ± 1.8; p<0.05, n=5-7].
Figure 1. The effect of high salt (HS) on renal cortical eNOS in the female mRen2.Lewis rat.
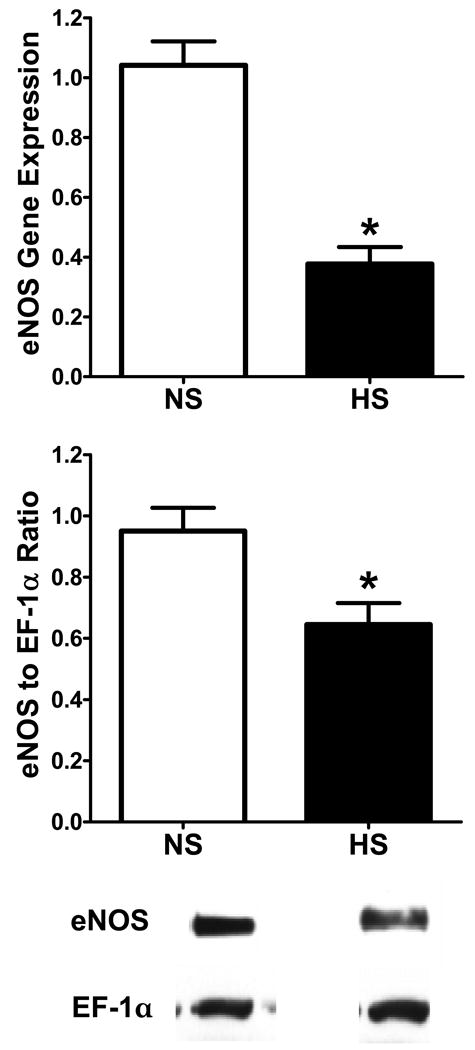
The mRNA data were expressed as the ratio of the target gene to 18 S rRNA. Data are mean ± SE; *p<0.05 compared to NS group; n=6-7. Western blot analysis data are expressed as the ratio of protein to EF-1α. Data are mean ± SE; *p<0.05 compared to normal salt (NS) group; n=6-7.
Figure 2. The effect of high salt (HS) on renal cortical nNOS in the female mRen2.Lewis rat.
Left panel: The mRNA data were expressed as the ratio of the target gene to 18 S rRNA. Data are mean ± SE; *p<0.05 compared to NS group; n=6-7. Western blot analysis data are expressed as the ratio of protein to EF-1α. Data are mean ± SE; *p<0.05 compared to NS group; n=3-4. Right panel: Immunohistochemical distribution of nNOS in the renal cortex from the normal salt (NS) and the high salt (HS) fed groups. The nNOS immunoreactivity was localized to macula densa (MD) in the NS group and to MD (black arrow) and cortical tubules in the HS group. Magnification: 400×.
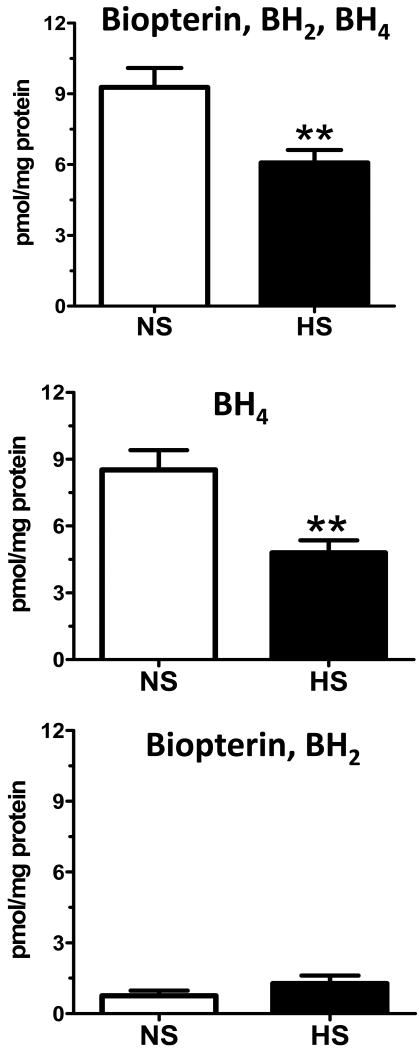
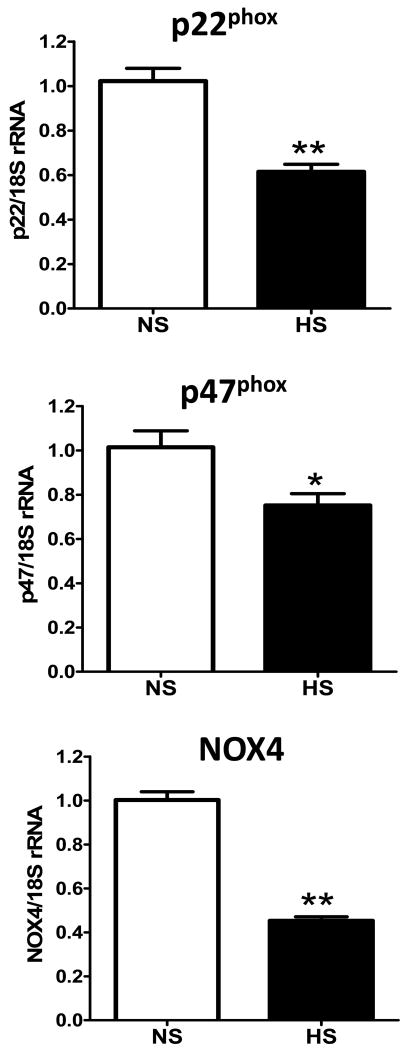
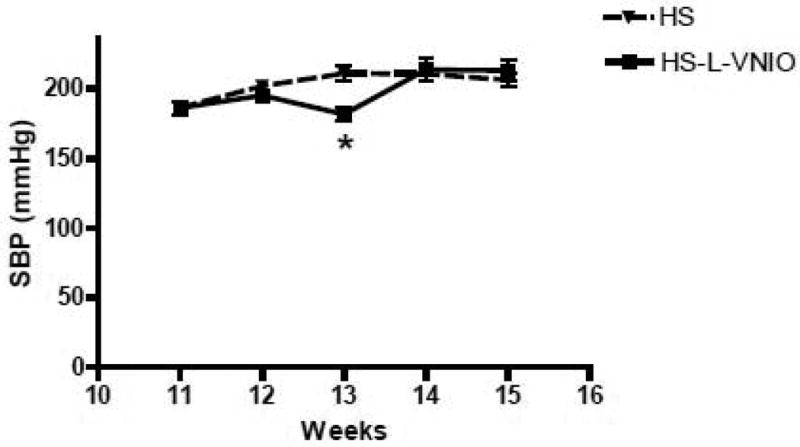
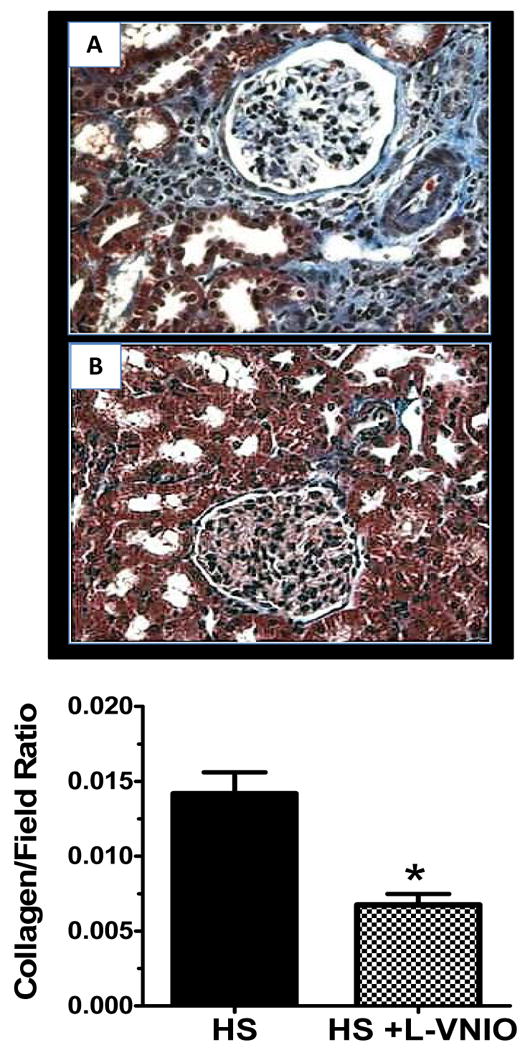
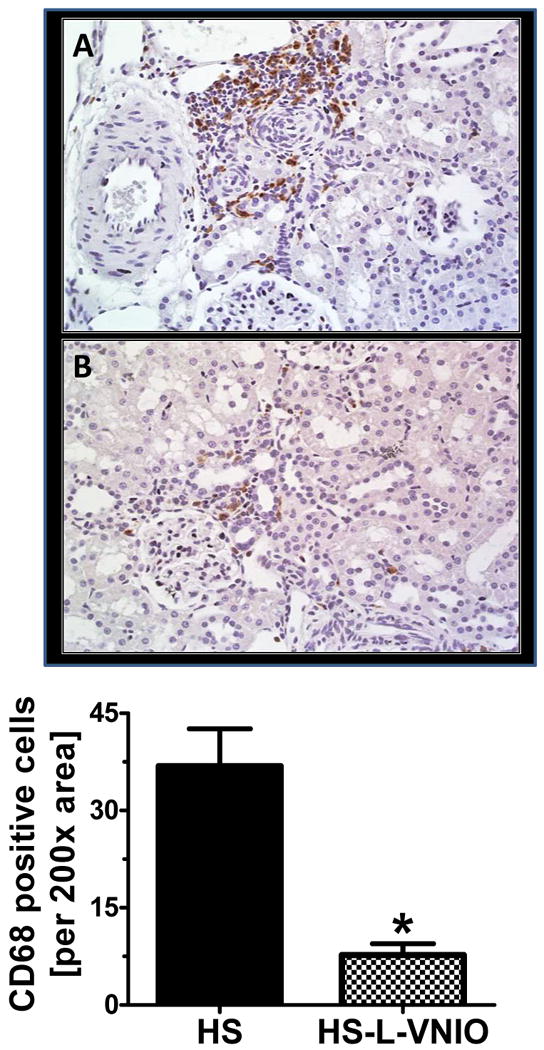
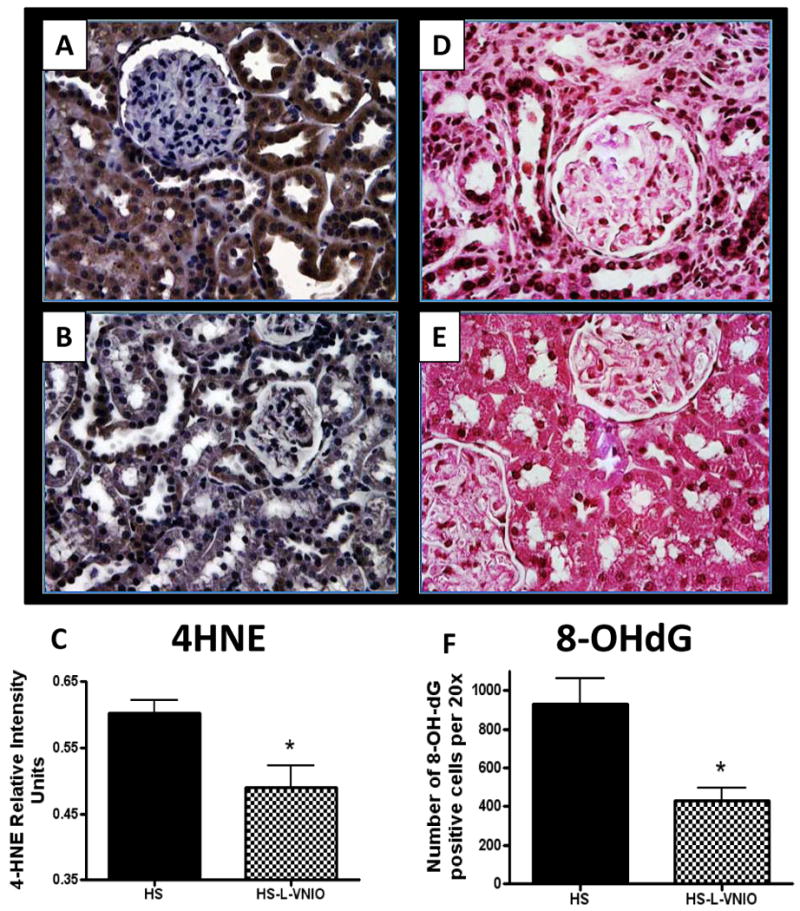
In lieu of the increase in renal nNOS expression, we administered the selective nNOS inhibitor L-VNIO to a second cohort of HS-fed mRen2.Lewis rats by osmotic minipump for 4 weeks and compared the responses to an untreated HS group of littermates. The time-course of systolic blood pressure in response to the L-VNIO in the HS group versus an untreated HS group is shown on Figure 3. At 11 weeks of age there was no difference in blood pressure between the HS and HS-L-VNIO groups. L-VNIO significantly reduced blood pressure at week 13 (*p<0.05), but did not alter systolic blood pressure at week 12, 14 or at the end of the study at week 15 [week 15, HS-L-VNIO: 212 ± 8 vs. HS: 206 ± 5 mmHg; p>0.05, n=5-7]. L-VNIO significantly reduced the extent of albuminuria [HS-L-VNIO: 7.9 ± 0.8 vs. HS: 109.4 ± 37.6 mg/kg/day; p<0.05, n=6] and normalized creatinine clearance [HS-L-VNIO: 0.9 ± 0.1 vs. HS: 0.4 ± 0.1 ml/min; p<0.05, n=5] by the end of the study. Circulating NOx did not change following L-VNIO administration [HS-L-VNIO: 5.6 ± 0.8 vs. HS: 8.9 ± 1.8 μM; p>0.05, n=5]. The administration of L-VNIO was associated with a significant improvement in renal injury (Table 1), reduced total collagen area (Figure 4) and lowered the kidney-to-body weight ratio [HS-L-VNIO: 4.4 ± 0.2 vs. HS: 5.3 ± 0.3 g, p<0.05, n=5]. Associated with the reduction in fibrosis, CD68-positive (monocytes and macrophages) cell abundance was significantly reduced by L-VNIO in the HS group [HS-L-VNIO: 7.7 ± 1.8 vs. HS: 36.8 ± 5.8; p<0.05] (Figure 5). The nNOS inhibitor markedly reduced the intensity of the lipid peroxidation product, 4-HNE [HS-L-VNIO: 0.49 ± 0.03 vs. HS: 0.60 ± 0.01; p<0.05, n=4] (Figure 6A-C) and the number of cells positive for the oxidative DNA marker, 8-OHdG [HS-L-VNIO: 432 ± 62 vs. HS: 929 ± 135.5; p<0.05, n=4] (Figure 6D-F) in the renal cortex of HS rats. Since a high salt diet promotes the uncoupling of NOS and the generation of reactive oxygen species (13), we assessed the NOS co-factor BH4 in the renal cortex of the HS mRen2.Lewis. Total cortical levels [biopterin, BH2, BH4] as well as BH4 alone were significantly reduced in the HS fed mRen2.Lewis rats (Figure 7). The analysis also reveals that BH4 is the predominant form in the cortex and that other species [biopterin, BH2] were not altered by the salt diet (Figure 7). Finally, we determined whether components of the NAD(P)H oxidase system were altered following the HS diet as an alternative source of reactive oxygen species within the kidney. As shown in Figure 8, the mRNA levels for, p22phox, p47phox and NOX4 were significantly lower in the HS mRen2.Lewis as compared to the NS group.
Figure 3. The effect of the nNOS inhibitor L-VNIO on systolic blood pressure in the high salt (HS) female mRen2.Lewis rats.
L-VNIO was given via intraperitoneal osmotic mini-pumps for 4 weeks starting at 11 week of age. The systolic blood pressure (SBP) data are shown as mean ± SE; *p<0.05 compared to HS group; n=6 in each group.
Table 1. The nNOS Inhibitor L-VNIO Reduces Renal Injury Scores of the High Salt Female mRen2.Lewis Rats.
| Renal Indices | High Salt | High Salt + L-VNIO |
|---|---|---|
| Interstitial fibrosis | 150 ± 28 | 56 ± 19* |
| Glomerulosclerosis | 150 ± 28 | 6.3 ± 6.3* |
| Tubular dilation | 125 ± 25 | 6.3 ± 6.3* |
| VSMC hyperplasia | 25 ± 14 | 25 ± 14 |
| Interstitial Inflammation | 150 ± 28 | 56 ± 19* |
Lesions were given a graded by a 5-point scale: grade 1 = minimal, grade 2 = mild, grade 3 = moderate, grade 4 = marked, grade 5 = severe. The data are reported as injury scores calculated as a product of a lesion's severity grade and the incidence;
p<0.05 vs. HS group.
Figure 4. Collagen deposition in the renal cortex of the female mRen2.Lewis rat.
Collagen shown in light blue color was evident primarily in the periglomerular, glomerular, perivascular as well as interstitial areas of the renal cortex of the high salt (HS) female mRen2.Lewis rat (panel A) in comparison to L-VNIO (panel B). Magnification: 400×. Panel C: The analysis of total collagen deposition in the renal cortex. Data are mean ± SE; n=4 in each group; *p<0.05 vs. high salt (HS) group.
Figure 5. The nNOS inhibitor L-VNIO reduces CD68-positive cells in the renal cortex of the high salt (HS) female mRen2.Lewis rat.
The CD68-positive cells were localized predominantly to the tubulointerstitial space of the renal cortex. The cell abundance was determined by counting the number of positively stained cells per 200× area using an automated software analysis (Image Pro 6.3; MadiaCybernatics, Bethesda, MD, USA) (Panel C). Data are mean ± SE; *p<0.05 vs. HS group; n=4 per group. Pictures shown as magnification 400×.
Figure 6. The nNOS inhibitor L-VNIO reduces oxidative stress markers within the renal cortex of the high salt (HS) female mRen2.Lewis rat.
Panel markers: 4-hydroxy-2-nonenal Michael adducts (4-HNE) (panels A, B, and C) and 8-hydroxy-2-deoxy guanosine (8-OHdG) (panel D, E, and F) in the renal cortex of the female mRen2.Lewis rat. The intensity of 4-HNE staining was quantified by Adobe Photoshop. Data are mean ± SE; *p<0.05 vs. HS; n=3-4. The number of 8-OHdG-positive nuclei in the renal cortex was counted with the automated counting software (Image Pro 6.3; MadiaCybernatics, Bethesda, MD, USA). Data are mean ± SE; *p<0.05 vs. HS group; n=4 per group.
Figure 7. Renal cortical levels of tetrahydrobiopterin (BH4) are reduced in the high salt (HS) fed female mRen2.Lewis rat.
Top panel: total biopterin levels [Biopterin, BH2, BH4]. Middle panel B: BH4 content. Bottom panel: Biopterin, BH2. Data are mean ± SE; *p<0.05 compared to normal salt (NS) group; n=5 in each group.
Figure 8. Gene expression of NAD(P)H oxidase components are reduced in the high salt (HS) fed female mRen2.Lewis rat.
The mRNA for p22phox (top panel), p47phox (middle panel) and NOX4 (bottom panel) in the renal cortex are expressed as the ratio of the target gene to 18S ribosomal RNA (rRNA). Data are mean ± SE; *p<0.05 vs. normal salt (NS) group; n=5-7.
Discussion
The results of the present study suggest that an increase in nNOS expression in conjunction with reduced BH4 content within the kidney may promote oxidative stress in the intact female mRen2.Lewis rat. Treatment with the nNOS inhibitor L-VNIO was associated with reduced glomerular, tubular, and interstitial renal injury in the HS-fed female congenic rats, as well as attenuated the degree of renal inflammation and oxidative stress independent of significant changes in systolic blood pressure. Although we cannot discount other direct actions of L-VNIO such as potential antioxidant effects, these data suggest that the development of renal injury in the salt-sensitive female mRen2.Lewis may reflect an uncoupling of nNOS and subsequent production of oxidative stress accompanied by renal inflammation.
The female mRen2.Lewis rat maintained on a chronic high salt diet exhibit a marked increase in blood pressure, proteinuria and albuminuria (15,18). The mechanisms of salt-induced hypertension and renal injury in the female mRen2.Lewis rat are currently unknown; however, similar to other salt-sensitive models, renal cortical eNOS was significantly reduced. The downregulation of eNOS may contribute to the decrease in NO and exacerbation of blood pressure by mechanisms that include reduced vasodilation and enhanced Ang II-dependent vasoconstriction in the kidney (25). Indeed, eNOS null mice are hypertensive and exhibit salt-sensitive increases in blood pressure (11). Reduced eNOS may also contribute to renal injury due to its role in tubular survival and response to tubular injury (26-28). Furthermore, deletion of eNOS in mice results in glomerular abnormalities and tubular cell death (26).
In contrast to the reduction in eNOS, the high salt diet increased nNOS in the renal cortex. The increase in nNOS immunostaining was most evident in macula densa and renal cortical tubules of the kidneys of the female mRen2.Lewis. Our findings differ with previous studies in which high salt attenuated nNOS expression in the renal cortex of male normotensive rats (29). Apart from sex differences, the salt influence on cortical nNOS may reflect the salt-sensitive phenotype of the mRen2.Lewis strain in conjunction with an activated or maintained RAS (18). The increase in nNOS in the female mRen2.Lewis does not appear to be a compensatory response as chronic administration of the nNOS inhibitor for 4 weeks markedly reduced renal injury and inflammation in the absence of changes in systolic blood pressure at the end of the study. The attenuated renal inflammation was evident by the reduction in the number of CD68 positive cells in the renal cortex. The infiltration of inflammatory cells may not only initiate but also sustain the degree of injury in the kidney, in part through the stimulation of Ang II and oxidative stress (30).
As depicted in Figure 9, we speculate that the mechanism for the renoprotective effects of the nNOS inhibitor may reflect a reduction in oxidative stress. Renal 4-HNE and 8-OHdG staining were reduced in the renal cortex following nNOS inhibitor treatment. The BH4 content was also markedly reduced in the renal cortex of the female mRen2.Lewis maintained on the HS diet. Vasquez-Vivar and colleagues report that nNOS generates SO rather than NO in a process that is tightly regulated by the availability of the co-factor BH4 (31). In the female mRen2.Lewis, nNOS-induced SO may well contribute to oxidative stress in the renal cortex. Although there are potentially various sources of ROS within the cell, the assessment of the NAD(P)H oxidase system within the renal cortex revealed reduced mRNA levels of NOX4, p22phox and p47phox following HS intake. NOX4 is the major NAD(P)H isoform in the kidney while p22phox and p47phox are required components for the assembly/activation of the NAD(P)H oxidase complex to form SO or H2O2 (32,33). We cannot completely exclude the possibility of renal eNOS inhibition by the administration of L-VNIO, particularly if eNOS is uncoupled as well by high salt. Taylor et al. (13) find that the depletion of BH4 by high salt may uncouple eNOS in the renal medulla of the male Dahl salt-sensitive rat. However in our study, eNOS protein expression was reduced with high salt and its contribution to NO or SO (provided eNOS is uncoupled as well) is likely to be attenuated. The lack of an effect on blood pressure by L-VNIO may also reflect the selective inhibition of nNOS and attenuation of oxidative stress-induced ROS formation (Figure 9). L-VNIO exhibits a 100-fold lower affinity for eNOS and iNOS in comparison to nNOS (22). In male Sprague-Dawley rats on a normal salt diet, acute administration of L-VNIO significantly attenuated NO levels (30–40%) in cortical and medullary regions of the kidney (34). Sears et al (35) report that the increase in sarcoplasmic reticulum calcium was similar between L-VNIO-treated cardiomyocytes from wild-type mice and myocytes isolated from nNOS knockout mice using a dose comparable to the Ki value for nNOS. Altogether, these findings argue for the specificity of L-VNIO to attenuate nNOS; however, we cannot rule out the possibility that the inhibitor may exhibit other actions in vivo, albeit these appear to be renoprotective in this salt-sensitive strain. Furthermore, we did not assess BH4 content following L-VNIO treatment which, if increased, may contribute to NO production via coupled eNOS. It is important to emphasize that we did not directly measure NOS-dependent generation of SO and NO in the kidney of the HS rats or following chronic administration of the nNOS inhibitor; additional studies are required to validate our hypothesis on the salt-induced uncoupling of nNOS and ROS generation. Moreover, the use of other nNOS inhibitors or antioxidants may be warranted to compare their in vivo effects, although nNOS inhibitors such as 7-nitroindazole and S-methyl-thiocitrulline exhibit less selectivity than L-VNIO (36).
Figure 9. Putative scheme for the influence of high salt on nNOS and oxidative stress within the renal cortex of the mRen2.Lewis rat.
High salt may induce oxidative stress in the renal cortex by increasing nNOS expression but reducing the cofactor BH4 which may uncouple nNOS and increase superoxide production (O2-). High salt also reduces eNOS expression which may contribute to lower levels of nitric oxide (NO) and salt-dependent hypertension. The nNOS inhibitor L-VNIO may attenuate the nNOS-dependent formation of O2- to reduce renal fibrosis, inflammation, albuminuria, and renal hypertrophy without altering blood pressure.
Our data support evidence that the extent of tissue or renal injury at the end of the study may be independent from changes in blood pressure. Tinel (37) reported that losartan reduced blood pressure but not renal hypertrophy, TGF-β, or collagen expression in the kidneys of the Dahl salt-sensitive rat. Nishiyama et al. (38) found that reduction in cortical collagen content following candesartan treatment was not associated with lower blood pressure in the male Dahl salt-sensitive rats. Reckelhoff and colleagues also note significant male-female differences on the contribution of oxidative stress to an increase in blood pressure in various hypertensive strains (39). However, the slight reduction in systolic blood pressure at week 13 may contribute to the overall reduction in albuminuria and renal injury following administration of L-VNIO in female mRen2.Lewis. Additional studies using in vivo telemetry assessment of 24 hour blood pressure are necessary to distinguish potential changes in pressure and renal injury. Finally, the present studies with the nNOS inhibitor in the salt-sensitive mRen2.Lewis rat were performed only in intact females and the response in the male congenics is not known. There are marked sex differences in this hypertensive strain, particularly regarding the salt-dependent exacerbation of blood pressure and the extent of renal injury (18, 20). Additional studies are required to establish whether the male mRen2.Lewis exhibit similar renoprotective effects to the nNOS inhibitor.
Conclusions
The present findings demonstrate that chronic treatment with the nNOS inhibitor L-VNIO conveys renoprotection independent of changes in blood pressure in the salt-sensitive female mRen2.Lewis strain. In this model, salt-sensitivity is associated with the differential expression of NOS isoforms within the kidney, as well as reduced tissue levels of BH4. The increase in cortical nNOS coupled with lower BH4 content may lead to increased oxidative stress and inflammation within the kidney. The demonstration of the renal protective effects of the nNOS inhibitor may provide an additional treatment regimen in salt-dependent tissue damage. We conclude that the development and maintenance of high salt-induced renal injury is likely a multi-factorial event that may not be amenable to a single therapeutic regimen. In the high salt fed female mRen2.Lewis rat, the regulatory mechanisms involved in the exacerbation of hypertension and renal injury appear distinct. However, renal inflammation and tubulointerstitial injury may reflect a significant contribution of nNOS and formation of ROS within the kidney.
Acknowledgments
Portions of this study were presented at the 61st Annual Fall Conference and Scientific Sessions of the Council for High Blood Pressure Research-2007, Washington DC. This work was supported by grants from the National Heart Lung and Blood Institute, NIH to M.C. Chappell (HL-56973, HL-51952, HL-112237); S.H. Lindsey (HL-103974); the American Heart Association Mid-Atlantic Affiliate to M.C. Chappell (AHA-151521) and to L.M. Yamaleyeva (AHA-525586U); the National Institute of General Medical Science, NIH (R01GM077352) and the American Diabetes Association (7-08-RA-23) to A.F. Chen.
Source of support: This work was supported by grants from the National Heart Lung and Blood Institute, NIH to M.C. Chappell (HL-56973, HL-51952); the American Heart Association Mid-Atlantic Affiliate to M.C. Chappell (AHA-151521) and to L.M. Yamaleyeva (AHA-525586U); the National Institute of General Medical Science, NIH (R01GM077352) and the American Diabetes Association (7-08-RA-23) to A.F. Chen.
Footnotes
Disclosure of funding: NIH to M.C. Chappell (HL-56973, HL-51952); NIH to S.H. Lindsey (HL-103974); NIH to A.F. Chen (R01GM077352); AHA: to M.C. Chappell (AHA-151521); L.M. Yamaleyeva (AHA-525586U); ADA to A.F. Chen (7-08-RA-23).
Conflicts of interest: None
Conflict of Interest: No relationships to disclose
Literature Cited
- 1.Alderman MH. Salt, blood pressure, and human health. Hypertension. 2000;36:890–893. doi: 10.1161/01.hyp.36.5.890. [DOI] [PubMed] [Google Scholar]
- 2.Cubeddu LX, Alfieri AB, Hoffmann IS, Jimenez E, Roa CM, Cubeddu R, Palermo C, Baldonedo RM. Nitric oxide and salt sensitivity. Am J Hypertens. 2000;13:973–979. doi: 10.1016/s0895-7061(00)00283-1. [DOI] [PubMed] [Google Scholar]
- 3.Singh I, Grams M, Wang WH, Yang T, Killen P, Smart A, Schnermann J, Briggs JP. Coordinate regulation of renal expression of nitric oxide synthase, renin, and angiotensinogen mRNA by dietary salt. Am J Physiol. 1996;270:F1027–F1037. doi: 10.1152/ajprenal.1996.270.6.F1027. [DOI] [PubMed] [Google Scholar]
- 4.Ponnuchamy B, Khalil RA. Cellular mediators of renal vascular dysfunction in hypertension. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1001–R1018. doi: 10.1152/ajpregu.90960.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Persson AE, Gutierrez A, Pittner J, Ring A, Ollerstam A, Brown R, Liu R, Thorup C. Renal NO production and the development of hypertension. Acta Physiol Scand. 2000;168:169–174. doi: 10.1046/j.1365-201x.2000.00669.x. [DOI] [PubMed] [Google Scholar]
- 6.Ni Z, Vaziri ND. Effect of salt loading on nitric oxide synthase expression in normotensive rats. Am J Hypertens. 2001;14:155–163. doi: 10.1016/s0895-7061(00)01234-6. [DOI] [PubMed] [Google Scholar]
- 7.Scuteri A, Stuehlinger MC, Cooke JP, Wright JG, Lakatta EG, Anderson DE, et al. Nitric oxide inhibition as a mechanism for blood pressure increase during salt loading in normotensive postmenopausal women. J Hypertens. 2003;21:1339–1346. doi: 10.1097/00004872-200307000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Bloch J, Qiu C, Erdely A, Baylis C. Inhibition of inducible nitric oxide synthase during high dietary salt intake. Am J Hypertens. 2002;15:230–235. doi: 10.1016/s0895-7061(01)02321-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattson DL, Bellehumeur TG. Neural nitric oxide synthase in the renal medulla and blood pressure regulation. Hypertension. 1996;28:297–303. doi: 10.1161/01.hyp.28.2.297. [DOI] [PubMed] [Google Scholar]
- 10.Welch WJ, Wilcox CS. What is brain nitric oxide synthase doing in the kidney? Curr Opin Nephrol Hypertens. 2002;11:109–115. doi: 10.1097/00041552-200201000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Huang PL. Neuronal and endothelial nitric oxide synthase gene knockout mice. Braz J Med Biol Res. 1999;32:1353–1359. doi: 10.1590/s0100-879x1999001100005. [DOI] [PubMed] [Google Scholar]
- 12.Ollerstam A, Persson AE. Macula densa neuronal nitric oxide synthase. Cardiovasc Res. 2002;56:189–196. doi: 10.1016/s0008-6363(02)00536-9. [DOI] [PubMed] [Google Scholar]
- 13.Taylor NE, Maier KG, Roman RJ, Cowley AW., Jr NO synthase uncoupling in the kidney of Dahl S rats: role of dihydrobiopterin. Hypertension. 2006;48:1066–1071. doi: 10.1161/01.HYP.0000248751.11383.7c. [DOI] [PubMed] [Google Scholar]
- 14.Yamaleyeva LM, Gallagher PE, Vinsant S, Chappell MC. Discoordinate regulation of renal nitric oxide synthase isoforms in ovariectomized mRen2. Lewis rats Am J Physiol Regul Integr Comp Physiol. 2007;292:R819–R826. doi: 10.1152/ajpregu.00389.2006. [DOI] [PubMed] [Google Scholar]
- 15.Groban l, Yamaleyeva LM, Westwood BM, Houle TT, Lin M, Kitzman DW, Chappell MC. Progressive diastolic dysfunction in the female mRen(2). Lewis rat: influence of salt and ovarian hormones. J Gerontol A Biol Sci Med Sci. 2008;63:3–11. doi: 10.1093/gerona/63.1.3. [DOI] [PubMed] [Google Scholar]
- 16.Jessup JA, Zhang L, Chen AF, Presley TD, Kim-Shapiro DB, Chappell MC, Groban L. Neuronal nitric oxide synthase inhibition improves diastolic function and reduces oxidative stress in ovariectomized mRen2.Lewis rats. Menopause. 2011;18:698–708. doi: 10.1097/gme.0b013e31820390a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jessup JA, Zhang L, Presley TD, Kim-Shapiro DB, Wang H, Chen AF, Chappell MC, Groaban L. Tetrahydrobiopterin restores diastolic function and attenuates superoxide production in ovariectomized mRen2. Lewis rats Endocrinology. 2011;152:2428–2436. doi: 10.1210/en.2011-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chappell MC, Yamaleyeva LM, Westwood BM. Estrogen and salt sensitivity in the female mRen(2) Lewis rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1557–R1563. doi: 10.1152/ajpregu.00051.2006. [DOI] [PubMed] [Google Scholar]
- 19.Pendergrass KD, Pirro NT, Westwood BM, Ferrario CM, Brosnihan KB, Chappell MC. Sex differences in circulating and renal angiotensins of hypertensive mRen(2). Lewis but not normotensive Lewis rats. Am J Physiol Heart Circ Physiol. 2008;295:H10–H20. doi: 10.1152/ajpheart.01277.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen JA, Lindsey SH, Pirro NT, Brosnihan KB, Gallagher PE, Chappell MC. Influence of estrogen depletion and salt loading on renal angiotensinogen expression in the mRen(2). Lewis strain. Am J Physiol Renal Physiol. 2010;299:F35–F42. doi: 10.1152/ajprenal.00138.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stallings SP, Mertz HL, Liu J, Valego NK, Figueroa JP, Rose JC. Selective inhibition of nos 1 and effects on renin production in late-gestation fetal lambs. J Soc Gynecol Invest. 2003;10(2):347A. [Google Scholar]
- 22.Babu BR, Griffith OW. N5-(1-Imino-3-butenyl)-L-ornithine. A neuronal isoform selective mechanism-based inactivator of nitric oxide synthase. J Biol Chem. 1998;273:8882–8889. doi: 10.1074/jbc.273.15.8882. [DOI] [PubMed] [Google Scholar]
- 23.Yamaleyeva LM, Pendergrass KD, Pirro NT, Gallagher PE, Groban l, Chappell MC. Ovariectomy is protective against renal injury in the high-salt-fed older mRen2.Lewis rat. Am J Physiol Heart Circ Physiol. 2007;293 doi: 10.1152/ajpheart.00427.2007. [DOI] [PubMed] [Google Scholar]
- 24.Zheng JS, Yang XQ, Lookingland KJ, Fink GD, Hesslinger C, Kapatos G, Kovesdi I, Chen AF. Gene transfer of human guanosine 5′-triphosphate cyclohydrolase I restores vascular tetrahydrobiopterin level and endothelial function in low renin hypertension. Circulation. 2003;108:1238–1245. doi: 10.1161/01.CIR.0000089082.40285.C3. [DOI] [PubMed] [Google Scholar]
- 25.Stegbauer J, Vonend O, Oberhauser V, Rump LC. Effects of angiotensin-(1-7) and other bioactive components of the renin-angiotensin system on vascular resistance and noradrenaline release in rat kidney. J Hypertens. 2003;21:1391–1399. doi: 10.1097/00004872-200307000-00030. [DOI] [PubMed] [Google Scholar]
- 26.Forbes MS, Thornhill BA, Park MH, Chevalier RL. Lack of Endothelial Nitric-Oxide Synthase Leads to Progressive Focal Renal Injury. Am J Pathol. 2007;170:87–99. doi: 10.2353/ajpath.2007.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayakawa H, Raij L. Nitric Oxide Synthase Activity and Renal Injury in Genetic Hypertension. Hypertension. 1998;31:266–270. doi: 10.1161/01.hyp.31.1.266. [DOI] [PubMed] [Google Scholar]
- 28.Pollock JS, Carmines PK. NOS3 Regulation: Renal Tubular Epithelial Cells Are Not Simply Large Endothelial Cells. Hypertension. 2006;47:19–21. doi: 10.1161/01.HYP.0000196276.29211.6f. [DOI] [PubMed] [Google Scholar]
- 29.Tojo A, Kimoto M, Wilcox CS. Renal expression of constitutive NOS and DDAH: Separate effects of salt intake and angiotensin. Kidney Int. 2000;58:2075–2083. doi: 10.1111/j.1523-1755.2000.00380.x. [DOI] [PubMed] [Google Scholar]
- 30.Cai H, Dikalov S, Griendling KK, Harrison DG. Detection of reactive oxygen species and nitric oxide in vascular cells and tissues: comparison of sensitivity and specificity. Methods Mol Med. 2007;139:293–312. doi: 10.1007/978-1-59745-571-8_20. [DOI] [PubMed] [Google Scholar]
- 31.Vasquez-Vivar J, Hogg N, Martasek P, Karoui H, Pritchard KA, jr, Kalyanaraman B. Tetrahydrobiopterin-dependent inhibition of superoxide generation from neuronal nitric oxide synthase. J Biol Chem. 1999;274:26736–26742. doi: 10.1074/jbc.274.38.26736. [DOI] [PubMed] [Google Scholar]
- 32.Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol. 2009;302:148–158. doi: 10.1016/j.mce.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li JM, Shah AM. Mechanism of endothelial cell NADPH oxidase activation by angiotensin II. Role of the p47phox subunit. J Biol Chem. 2003;278:12094–12100. doi: 10.1074/jbc.M209793200. [DOI] [PubMed] [Google Scholar]
- 34.Kakoki M, Zou AP, Mattson DL. The influence of nitric oxide synthase 1 on blood flow and interstitial nitric oxide in the kidney. Am J Physiol Regul Integr Comp Physiol. 2001;281:R91–R97. doi: 10.1152/ajpregu.2001.281.1.R91. [DOI] [PubMed] [Google Scholar]
- 35.Sears CE, Bryant SM, Ashley EA, Lygate CA, Rakovic S, Wallis HL, Neubauer S, Terrar DA, Casadei B. Cardiac neuronal nitric oxide synthase isoform regulates myocardial contraction and calcium handling. Circ Res. 2003;92:e52–e59. doi: 10.1161/01.RES.0000064585.95749.6D. [DOI] [PubMed] [Google Scholar]
- 36.Babu BR, Griffith OW. Design of isoform-selective inhibitors of nitric oxide synthase. Current Opinion in Chemical Biology. 1998;2:491–500. doi: 10.1016/s1367-5931(98)80125-7. [DOI] [PubMed] [Google Scholar]
- 37.Tinel H. Beneficial Effects of the combination of nifedipine and losartan in hypertensive Dahl salt-sensitive rats. J Cardiovasc Pharmacol. 2007;50:75–82. doi: 10.1097/FJC.0b013e318058820c. [DOI] [PubMed] [Google Scholar]
- 38.Nishiyama A, Yoshizumi M, Rahman M, Kobori H, Seth DM, Miyatake A, Zhang GX, Yao L, Hitomi H, Shokoji T, Kiyomoto H, Kimura S, Tamaki T, Kohno M, Abe Y. Effects of AT1 receptor blockade on renal injury and mitogen-activated protein activity in Dahl salt-sensitive rats. Kidney Int. 2004;65:972–981. doi: 10.1111/j.1523-1755.2004.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sartori-Valinotti JC, Iliescu R, Fortepiani LA, Yanes LL, Reckelhoff JF. Sex differences in oxidative stress and the impact on blood pressure control and cardiovascular disease. Clin and Exp Pharmacol and Physiol. 2006;34:938–945. doi: 10.1111/j.1440-1681.2007.04643.x. [DOI] [PubMed] [Google Scholar]