Abstract
Rationale
Methylphenidate (MPH), the most widely prescribed psychostimulant to treat many neuropsychiatric conditions, is reported to improve attention and speed of processing in survivors of traumatic brain injury (TBI). The neural correlate of this efficacy, however, remains unclear.
Objective
Using perfusion fMRI as a biomarker of regional neural activity, the current study aimed to examine the neural correlates of single-dose (0.3 mg/kg) MPH administration in a randomized double-blind placebo-controlled cross-over study design.
Methods
Twenty-three individuals with moderate to severe TBI were tested on two occasions approximately one week apart. Perfusion fMRI scanning was carried out at rest and while participants performed cognitive tasks requiring sustained attention and working memory.
Results
Behaviorally, MPH significantly improved both accuracy and reaction time (RT) in the sustained attention task, but only RT in the working memory task. A trend of global reduction of cerebral blood flow by MPH was observed in all task conditions including resting. Voxel-wise whole-brain analysis revealed an interaction effect of drug by condition (MPH-placebo X task-rest) for the sustained attention task in the left posterior superior parietal cortex and parieto-occipital junction (BA 7/19). The magnitude of drug-related deactivation of this area during task performance was correlated with improvement in RT.
Conclusion
Suppression of activity in this area during task performance may reflect a compensatory mechanism by which MPH ameliorates attention impairments in TBI.
Keywords: methylphenidate, traumatic brain injury, CBF, fMRI, sustained attention, working memory
INTRODUCTION
Traumatic brain injury (TBI) affects 1.5 million people each year in the United States alone and frequently results in debilitating and persistent cognitive impairment (Rutland-Brown et al. 2006; Whyte et al. 2004a). Many patients live with deficits in higher cognition including attention and executive function for the rest of their lives (Jennett et al. 1981; Millis et al. 2001). Methylphenidate (MPH) is the most widely prescribed psychostimulant and has been demonstrated to enhance cognition in many clinical populations as well as healthy individuals (Aman et al. 1984; Auriel et al. 2006; Rahman et al. 2006; Rapoport and Inoff-Germain 2002; Rhodes et al. 2004; Strauss et al. 1984; Turner et al. 2005). MPH has also been tested in chronic survivors of traumatic brain injury (Siddall 2005; Sivan et al. 2010). Improvements in processing speed and several attention measures have been reported by many researchers, including our group (Gualtieri and Evans 1988; Kaelin et al. 1996; Kim et al. 2006b; Lee et al. 2005; Plenger et al. 1996; Whyte et al. 1997; Whyte et al. 2004b).
Animal studies (for a review, see Challman and Lipsky 2000) have indicated that MPH improves cognition by increasing extracellular dopamine and norepinephrine in cortical and subcortical regions (e.g., Berridge et al. 2006; Tye et al. 2010). Research in healthy humans and individuals with disorders such as ADHD and cocaine addiction has suggested that the neural correlates of MPH efficacy may involve a complex set of mechanisms (for a recent review, see Swanson et al. 2011). Previous neuroimaging studies reported that MPH could induce a reduction (e.g., Mehta et al. 2000; Volkow et al. 2008), an enhancement (e.g., Bullmore et al. 2003; Goldstein and Volkow 2011; Goldstein et al. 2010; Li et al. 2010; Rubia et al. 2011; Shafritz et al. 2004), or both (e.g., Rubia et al. 2009; Tomasi et al. 2011) in task-associated activations/deactivations. In fact, the efficacy of MPH seems to be modulated by many factors including the types of cognitive tasks (e.g., Dodds et al. 2008) and the characteristics of participants tested (Clatworthy et al. 2009; Epstein et al. 2007; Vaidya et al. 1998). Thus, more research with different tasks and populations is warranted to better understand the neural mechanism of MPH efficacy.
The current study is to our knowledge the first attempt to investigate the neural correlates of MPH efficacy in TBI. We aimed to examine the effects of single-dose MPH administration in chronic survivors of TBI with a randomized double-blind placebo-controlled cross-over design. Continuous arterial spin labeled (ASL) perfusion fMRI (Detre et al. 1992; Detre et al. 2009; Williams et al. 1992) was used to quantify CBF non-invasively. The fact that perfusion fMRI reliably measures physiologically meaningful baseline CBF across different time points (Wang et al. 2003b) makes this technique well suited to examining neural responses to pharmacological agents in a cross-over design. We validated the sensitivity of our perfusion fMRI method using sustained attention and working memory tasks in a previous study (Kim et al. 2006a). Based on the results from previous studies of human subjects reviewed above, we initially hypothesized that the mechanism of MPH effects in TBI may involve modulations in regional brain activity associated with task performance. However, we were open to the possibility of an MPH effect outside the normal task-related areas because there can be brain reorganization after TBI.
METHODS
Participants
Thirty three participants with TBI were recruited from clinical databases of current and former patients at MossRehab (Schwartz et al. 2005), as well as through community advertisement. To be included, participants had to be between the ages of 16 and 60, and to have a history of non-penetrating traumatic brain injury of at least moderate severity at least 3 months prior to enrollment. Severity level was defined by significant and well-documented loss or alteration of consciousness following injury (i.e., lowest Glasgow Coma Scale (GCS) score of less than 12, or prospectively documented post-traumatic amnesia (PTA) of greater than 1 day), or focal abnormality on a neuroimaging study that was attributable to traumatic injury. Self- or clinician-reported attention complaints were also required. Potential participants were excluded if they had a history of premorbid neurologic disease, psychosis, major affective disorder, developmental disability, Attention Deficit Hyperactivity Disorder, or if they were currently abusing alcohol or recreational drugs. Persons who were taking psychoactive medications other than anticonvulsants were excluded. During the study period, only psychoactive medications were monitored. Individuals who had a remote substance abuse with likely permanent organic sequelae judged by the study physician were also excluded. Individuals were excluded who had extensive focal lesions in the middle and inferior frontal cortices, which are frequently implicated in higher cognition including sustained attention or working memory.
Procedure
Participants and/or their involved caregivers (depending on the participant’s cognitive capacity) provided informed consent. The study protocol was approved by the Albert Einstein Healthcare Network and the University of Pennsylvania IRBs. Participants with TBI were tested on 2 occasions approximately 1 week apart at the same time of day. All participants were interviewed regarding their usual pattern of caffeine and nicotine intake. From this interview, a participant-specific agreement was reached to consume similar quantities of these substances on the two testing days. Participants consumed a capsule on each testing day that contained either placebo or MPH in a dose of 0.3 mg/kg rounded to the nearest 2.5 mg (as in Whyte et al. 2004b), approximately an hour prior to testing, based on pharmacokinetic data on the peak drug effect. The order of drug vs. placebo was randomized and both participants and investigators were blinded to drug condition. Thirteen participants received the placebo condition first and then MPH. Blood pressure and pulse were assessed prior to drug administration and immediately following the scanning session to screen for adverse drug effects.
Cognitive tasks
Visual sustained attention task (VSAT)
A simple go/no-go visual reaction time task was used to examine the neural network involved in maintaining visual sustained attention (Whyte et al. 2004b; Whyte et al. 1995). Stimuli consisted of pairs of vertical lines presented for a brief period in the center of the screen. The central area of the screen was covered by a random pattern mask with a fixation cross except when a stimulus was presented. The mask subtended approximately 1 and 4 of horizontal and vertical visual angle, respectively. Subjects were taught that a pair of identical lines constituted a target, whereas a pair of grossly unequal lines constituted a foil (one line was the same length as the target and the other was 50% shorter), and to press the button with their dominant hand as quickly and accurately as possible in response to targets only. They were also explicitly told that only 20% of the stimuli were targets. A total of 60 stimuli were presented during an uninterrupted 6 minute task block with an average inter-stimulus interval of 6 seconds (range: 4 to 8 seconds).
Two-back task
A letter version of 2-back task (Awh et al. 1996; Cohen et al. 1997) was employed to examine the neural network involved in continuous performance of a working memory task. In this task, subjects were presented with a series of letters in the center of the screen. The letters subtended approximately 1.5 × 1.5 of visual angle. Subjects were required to press the button whenever the letter presented was identical to the one presented two items previously in the sequence. A total of 180 letters were presented with an exposure duration of 1 second and an inter-stimulus interval (ISI) of 2 seconds. The target rate for this task was 12%.
Data acquisition
All participants were trained on the study tasks outside the scanner prior to data collection to ensure comprehension of the instructions, adequate performance, and, in the case of the VSAT task, to select a participant-specific stimulus duration that resulted in approximately 75% accuracy. Details of the calibration procedure are available elsewhere (Whyte et al. 1995). The order of task blocks was always resting first, the sustained attention task second, and the 2-back task last. Each task block was approximately 6 minutes and the intervals between task blocks were approximately 30 seconds. During the resting condition, which was used as the baseline control, participants were instructed to close their eyes but stay awake. For both tasks, responses and reaction times (RTs) were recorded for further analysis.
Imaging was conducted on a Siemens 3.0 T Trio whole-body scanner (Siemens AG, Erlangen, Germany), using a standard Transmit/Receive head coil. An amplitude-modulated CASL technique was used for perfusion fMRI scans (Wang et al. 2005). Interleaved images with and without labeling were acquired using a gradient echo echo-planar imaging sequence with the following acquisition parameters: FOV = 22cm, matrix = 64×64, TR = 4sec, TE = 17ms, flip angle = 90°. Fourteen slices (6mm thickness with 1.5mm gap) were acquired from inferior to superior in a sequential order to cover the whole brain supratentorially. A delay time of 1 second was inserted between the end of labeling pulses and image acquisition to reduce transit related effects. Each subject performed three CASL scans each with 92 acquisitions (approximately 6 minutes). Before the functional scans, high resolution T1-weighted anatomic images were obtained using 3D MPRAGE: TR = 1620ms, TI = 950ms, TE = 3ms, flip angle = 15°, 160 contiguous slices of 1.0 mm thickness, FOV = 192×256mm2, matrix = 192×256, 1NEX with a scan time of 6 minutes.
Data Analysis
Behavioral data
Performance of the participants was characterized with respect to two dimensions: discrimination and speed. Discrimination was measured with d’. Speed was operationalized as median RT on hits (correct button presses to targets). In order to ensure that the scanning results reflected performance of the cognitive tasks as instructed, accuracy thresholds were set for inclusion in the final analysis. For both tasks, we required that accuracy was significantly above chance across the two sessions as measured by the binomial test.
Behavioral performance between drug conditions was compared with the Wilcoxon Signed Ranks Test for each task. Statistical analyses were performed using PASW Statistics software version 18 (SPSS Inc., Chicago, IL, USA).
Imaging data
The location and extent of focal lesions was quantified by a trained observer under supervision of a neurologist (H.B.C.) with extensive experience in lesion assessment. Focal lesions included any cystic cavities and other focal regions of abnormal signal in the white or gray matter. For more technical details, see our previous study (Kim et al. 2008).
Functional image pre-processing and individual-level analysis were carried out using VoxBo software (Center for Functional Neuroimaging, Philadelphia, PA, http://www.voxbo.org). The group-level analysis was performed with Statistical Parametric Mapping software (SPM5, Wellcome Department of Cognitive Neurology, London, UK, http://www.fil.ion.ucl.ac.uk/spm). For each participant, functional images were realigned to correct the head motion using a 6-parameter rigid-body least squares realignment routine (Friston et al. 1995). If the average of maximal translational displacements along three axes (x, y, and z) during a session exceeded the average of voxel dimensions along three axes, it was regarded as excessive motion. Perfusion weighted image series were generated by pair-wise subtraction of the label and control images, followed by conversion to absolute CBF image series based on a single compartment CASL perfusion model (Wang et al. 2005). The resulting CBF data sets contained 46 images for each 6 minute task block with an effective TR of 8s. The CBF images were then normalized to a custom template using symmetric normalization (Avants et al. 2008; Avants et al. 2006) as implemented in the Advanced Normalization Tools (ANTS;http://sourceforge.net/projects/advants). Normalized images were resampled to a 3 mm isotropic cubic voxels. For detailed procedures for template building and spatial normalization, see our previous studies (Kim et al. 2008; Kim et al. 2010).
For each cognitive task, the following voxel-based statistical analyses were first conducted. For each subject, voxel-wise individual GLMs were built to quantify CBF values for each task condition. The global signal covariate was included in the GLM to reduce spatially coherent noise in the data (Aguirre et al. 1998). Perfusion MRI data are known to be free from any substantial temporal autocorrelation (Aguirre et al. 2002; Wang et al. 2003a). Therefore, no filtering, autocorrelation modeling, or smoothing was done for the time series. The resulting parameter estimates were then fed into a random effects model to allow population-level inferences (Holmes and Friston 1998). For multiple comparison correction for our whole brain voxel-wise analysis, we adopted a family-wise error (FWE) corrected cluster-level threshold of p < 0.01 after applying a peak height threshold of uncorrected p < 0.001 for the voxel-level. The resulting peak coordinates were mapped to MNI space by registering the custom template to the Colin brain (Van Essen et al. 2001) using ANTS. The anatomical labels of the peaks were manually obtained using the AAL atlas (Tzourio-Mazoyer et al. 2002) and an atlas by Mai and colleagues (2004).
In addition, a region-of-interest (ROI) analysis approach was used to examine the MPH effects within the task-related brain networks for each task. This analysis strategy was chosen to increase the statistical power and to reduce Type I error (Poldrack 2007). A priori ROIs were selected based on previous neuroimaging studies using visual sustained attention and N-back working memory tasks. For the visual sustained attention task, the following four ROIs in the AAL atlas (Tzourio-Mazoyer et al. 2002) were constructed based on previous studies of visual sustained attention (Coull et al. 1998; Kinomura et al. 1996; Lawrence et al. 2003; Lim et al. 2010): bilateral anterior cingulate gyri, right lateral frontal cortex, right inferior parietal cortex including angular and supramarginal gyri, and bilateral thalami. For the 2-back task, the following ROIs were constructed based on a meta-analysis by Owen and colleagues (2005): bilateral premotor areas including precentral gyri, bilateral lateral frontal cortices, bilateral thalami, and bilateral inferior parietal lobes including angular and supramarginal gyri. Because the ROIs were in the MNI space, ROIs were first warped to our custom template space using ANTS. Individual CBF time-series were extracted from these ROIs for each subject and then averaged. Similarly, global CBF values were calculated from the CBF time series from a whole-brain gray matter mask. The mask was obtained by segmenting the custom template using SPM5. Global CBF values were compared across drug conditions with the Wilcoxon Signed Ranks Test.
RESULTS
Participant Characteristics
Thirty-three individuals with TBI were originally enrolled in this study. Two individuals could not participate in the second session due to personal reasons that were not related to the study. Data from two participants were excluded due to data corruption. Two participants were excluded due to brain coverage issues after spatial normalization. Two participants had extensive focal lesions (greater than 50 cm3) in the areas known to be involved in the tasks to be performed (middle and inferior prefrontal cortices). Motion during one or more task sessions resulted in the exclusion of an additional two participants from the VSAT task and four participants from the 2-back task. In addition, five participants were excluded from the analysis of the VSAT task because their behavioral performance did not meet the necessary cutoff (see the data analysis section). No one was excluded from the 2-back task due to low performance. Because the participants with accuracy or motion exclusions differed between the 2 tasks, we chose to analyze slightly different participant samples for the 2 tasks. Consequently, the final analysis was conducted on 18 participants with TBI for the VSAT task and 21 participants for the 2-back task. Tables 1 and 2 summarize the demographic and lesion characteristics of the TBI survivors who enter into either or both analyses.
Table 1.
Selected Demographic and Clinical Characteristics of TBI Survivors
| All | Subgroup | ||
|---|---|---|---|
| 23 | VSAT 18 | Two-back | |
| N | 23 | 18 | 21 |
| Male/Female | 18/5 | 14/4 | 17/4 |
| Age | 34.2 (11.5) | 34.2 (10.1) | 34.3 (11.7) |
| Ethnicity (C/AA/H/A) |
11/9/2/1 | 8/7/2/1 | 10/8/2/1 |
| Handedness (Right/Left) |
19/4 | 15/3 | 17/4 |
| Education | 13.3 (2.7) | 13.5 (2.9) | 13.3 (2.9) |
| Months Post Injury | 51.1 (63.3) | 55.5 (67.3) | 44.0 (53.1) |
Numbers in parentheses are standard deviations. C=Caucasian. AA=African American. H=Hispanic. A=Asian. VSAT=Visual Sustained Attention Task.
Table 2.
Lesion characteristics of TBI participants with focal lesions
| Patient ID | Lesion location at the time of testing | Total lesion volume (cm3) | Included in |
|---|---|---|---|
| 3 | L temporal pole; R occipital | 43.8 | VSAT and 2-back |
| 5 | R superior frontal | 0.7 | VSAT and 2-back |
| 7 | L superior temporal and orbitofrontal; L superior frontal | 9.9 | 2-back |
| 9 | L frontal pole and orbitofrontal lesion extending to superior frontal | 64.5 | VSAT and 2-back |
| 10 | R temporal pole; R orbitofrontal; L orbitofrontal | 87.7 | 2-back |
| 12 | R temporal pole; L superior frontal | 23.7 | 2-back |
| 15 | R thalamus | 0.2 | VSAT and 2-back |
| 19 | L subcortical lesion involving thalamus, basal ganglia, and internal/external capsule extending into centrum semiovale |
17.1 | VSAT and 2-back |
| 22 | Bilateral orbitofrontal extending into frontal pole superiorly | 113.3 | 2-back |
| 28 | L superior frontal; L temporal; R internal capsule; R putamen; L thalamus | 2.3 | VSAT and 2-back |
| 31 | L temporal and bilateral superior frontal; R putamen | 43.6 | VSAT and 2-back |
R=Right. L=Left. VSAT=Visual Sustained Attention Task.
MPH Effects on Task Performance
For the VSAT, accuracy as measured by d’ was significantly better, and median RT was significantly faster on MPH than on placebo. For the two-back task, MPH resulted in significantly faster response time and a non-significant trend toward greater accuracy on MPH than on placebo (see Table 3).
Table 3.
Effects of Methylphenidate on Performance during the Sustained Attention and 2-Back Tasks
| Placebo | MPH | Effect Size | p-value | |||
|---|---|---|---|---|---|---|
| VSAT | ||||||
| Accuracy (d’) | 1.62 | (1.03) | 2.23 | (1.07) | 0.90 | < .005 |
| Median RT | 827.47 | (291.17) | 752.03 | (256.87) | 0.69 | < .05 |
| 2-Back | ||||||
| Accuracy (d’) | 2.39 | (0.78) | 2.65 | (0.82) | 0.36 | = .14 |
| Median RT | 929.31 | (192.92) | 835.02 | (136.12) | 0.63 | < .05 |
Mean and standard deviation, in parenthesis, of behavioral measures are reported with corresponding within-subject effect sizes defined by Morris and DeShon (2002). VSAT=Visual Sustained Attention Task.
MPH Effects on CBF
Global resting perfusion
Table 4 presents global gray matter CBF values for each condition. For all task conditions, global CBF values on MPH were consistently lower than those on placebo. However, these differences did not reach statistical significance (see Table 4). This tendency of global reduction in CBF is consistent with results from previous studies that demonstrated vaso-constrictive properties of MPH (Wang et al. 1994).
Table 4.
Global Gray Matter CBF Values on MPH and Placebo
| Condition | Placebo | MPH | Effect Size | p-value | ||
|---|---|---|---|---|---|---|
| Rest | 49.68 | (9.59) | 46.81 | (7.52) | 0.37 | = .12 |
| VSAT | 49.91 | (12.17) | 46.60 | (6.84) | 0.39 | = .13 |
| Two-back | 50.08 | (9.82) | 46.30 | (7.14) | 0.38 | = .10 |
Mean and standard deviation, in parenthesis, of CBF values are reported with corresponding within-subject effect sizes defined by Morris and DeShon (2002). VSAT=Visual Sustained Attention Task.
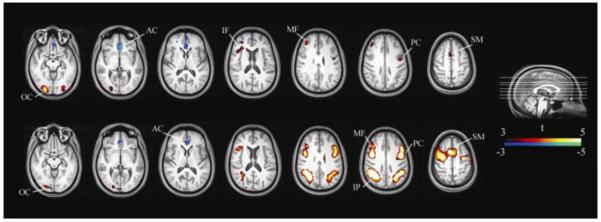
Whole brain voxel-based analysis
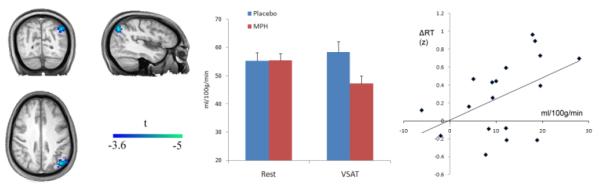
Table 5 and Fig. 1 show the loci of activation and deactivation associated with the main effect of each task, which are generally in agreement with previous studies using similar tasks in healthy controls (Coull et al. 1998; Coull et al. 1996; Kim et al. 2006a; Lawrence et al. 2003; Owen et al. 2005; Pardo et al. 1991). The MPH main effect did not reveal any significant voxels. However, a drug by condition interaction effect (defined by a paired t-test between [MPH task – MPH rest] and [placebo task – placebo rest] subtraction images) identified a single region in the left posterior superior parietal lobule near the parieto-occipital junction (BA 7/19; cluster size 113; MNI coordinate [−42, −70, 43]; Z = 3.90; FWE-corrected cluster-level threshold p = 0.001) in the sustained attention task (see Fig. 2). Subsequent analysis of mean CBF values from this area revealed that the interaction was mainly driven by a deactivation of this area during VSAT task performance on MPH. Furthermore, the magnitude of the parameter estimate for the interaction effect—i.e., (task-rest on MPH) – (task-rest on placebo)—was correlated with MPH-induced reduction of RT (Pearson’s r = −.48, p < .05).
Table 5.
Regions of Significant CBF Changes during Task Performance Compared to Rest
| Size (voxels) |
Anatomical Label | BA | MNI Coordinates |
Z Score | Δ CBF (ml/100g/min) |
% CBF change |
|||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Activation | |||||||||
| VSAT | |||||||||
| 135 | R occipital | 18 | 23 | −94 | 4 | 4.47 | 7.3 ± 7.8 | 17.5 | |
| 68 | L occipital | 18 | −34 | −97 | 0 | 3.84 | 7.1 ± 9.1 | 17.9 | |
| 86 | R middle frontal | 46 | 39 | 36 | 12 | 3.80 | 4.4 ± 5.6 | 10.0 | |
| 39 | R superior frontal/SMA | 6 | 11 | 9 | 45 | 3.64 | 3.6 ± 5.4 | 7.5 | |
| 44 | R inferior frontal/insula | 48 | 33 | 23 | 4 | 3.61 | 3.1 ± 4.5 | 6.7 | |
| 47 | L precentral | 6 | −40 | −5 | 33 | 3.58 | 3.4 ± 4.5 | 7.2 | |
| Deactivation | |||||||||
| 173 | L SC/medial orbitofrontal | 25 | −6 | 21 | −11 | 4.29 | −6.7 ± 10.9 | −13.5 | |
| Activation | |||||||||
| Two-back | |||||||||
| 842 | R inferior parietal | 39/40/7 | 32 | −56 | 40 | 6.24 | 5.4 ± 4.9 | 12.1 | |
| 1229 | R precentral/middle frontal/SMA | 6/44 | 40 | −3 | 34 | 6.12 | 5.5 ± 5.3 | 12.4 | |
| 632 | L precentral/middle frontal/SMA | 6/44 | −35 | 0 | 33 | 5.78 | 5.3 ± 5.3 | 11.1 | |
| 511 | L inferior parietal | 39/40/7 | −32 | −49 | 42 | 5.15 | 4.6 ± 4.6 | 9.7 | |
| 94 | R occipital | 18 | 21 | −94 | 7 | 3.67 | 5.9 ± 9.9 | 16.1 | |
| Deactivation | |||||||||
| 77 | L posterior cingulate | 11 | −3 | 26 | −11 | 3.99 | −6.1 ± 10.4 | −11.8 | |
Cluster sizes are in voxels. A CBF and % CBF values (mean ± standard deviation) are changes from the resting baseline. R=Right. L=Left. BA=Brodmann area. SC=subgenual cingulate. SMA=Supplementary motor area. VSAT=Visual Sustained Attention Task.
Fig. 1.
Topography of Task Effects. Top: Areas associated with the VSAT task. Bottom: Areas associated with the two-back task. AC: anterior cingulate, IF: inferior frontal, IP: inferior parietal, MF: middle frontal, OC: occipital, PC: precentral, SM: supplementary motor. VSAT=Visual Sustained Attention Task.
Fig. 2.
The Drug by Condition Interaction for the Sustained Attention Task and Relationship with Behavior. Left: An interaction effect of drug by condition (MPH-placebo X VSAT-rest) was located in the left posterior superior parietal lobule. Middle: An ROI analysis indicated that the interaction was due to a deactivation of the area during task performance on MPH. Right: Relationship between the magnitude of the deactivation and MPH-induced RT reduction. Each individual’s median RT was transformed to a z-score based on the group mean before ΔRT (drug-induced RT change) was calculated. ΔRT’s were flipped along y-axis so that positive numbers represent improvement in speed.
A priori anatomical ROI analysis
As the method section describes, four anatomical ROIs were identified as a priori regions of interest. Two analyses were conducted using these ROIs. First, we re-ran our voxel-based GLM analysis for the drug-related main and interaction effects within those ROIs. However, no voxel clusters survived after a multiple comparison correction for both tasks. Second, the average CBF values from each ROI were fed into a repeated measures ANOVA with three within-subject factors: task (rest, task), drug (placebo, drug), and region (four regions for each task). For the VSAT task, only the main effect of region was significant (F[3,51]=30.07, p < .001). For the 2-back task, the mains effects of region (F[3,60]=66.19, p < .001) and task (F[1,20]=21.67, p < .001) were significant. No drug-related main and interaction effects were significant in either task (all p > .2).
DISCUSSION
The current study aimed to investigate the neural correlates of the efficacy of single-dose administration of MPH in chronic survivors of TBI. MPH enhanced behavioral performance in both tasks used, but the effect was more prominent in the sustained attention task compared with the working memory task. A trend of global CBF reduction found in our ROI analysis together with the lack of any drug-induced regional resting CBF modulations in our voxel-wise analysis corroborates previous [15O]H2O PET results that reported similar CBF decreases in healthy volunteers (Wang et al. 1994). It was interpreted as vasoactive effects of the drug because CBF reduction was homogenous across regions. In line with the vasoactivity interpretation, a recent [18F]FDG PET study (Volkow et al. 2008) reported that there were no regional MPH-induced metabolic changes at rest.
Against our initial hypothesis, we could not identify MPH-induced modulations of brain activity within the task-associated regions of interest, indicating that the drug’s effects on perfusion were not task-dependent in these regions. However, a voxel-wise exploratory analysis identified a locus of the drug by condition interaction in the left posterior superior parietal lobule and parieto-occipital junction (BA 7/19) in the visual sustained attention task. Subsequent analysis of mean CBF values from this area revealed that the interaction was mainly driven by a deactivation of this area during task performance on MPH. Furthermore, the magnitude of the interaction was correlated with behavioral improvement on MPH, providing further evidence of its functional significance.
What might the significance of this MPH-induced reduction of left posterior parietal CBF be? If healthy subjects deactivate this area while performing the task, it could be concluded that MPH restores the normal suppression of activity in this area. If controls do not modulate this region during task performance, TBI survivors’ MPH-induced deactivation might reflect a compensatory reaction to improve task performance. To answer this question, we looked at our previously published data on healthy controls (Kim et al. 2006a). We noted that healthy control subjects showed neither activation nor deactivation in the superior parietal lobule with task performance. The same pattern is observed in this area of TBI patients: we could not detect a task-related CBF increase in patients even with a lowered threshold. These results suggest that suppression of activity in this area during task performance may instead be a compensatory mechanism by which MPH ameliorates attention impairments in TBI. This conclusion is in contrast with the majority of previous neuroimaging studies in ADHD and cocaine addiction that reported MPH-induced ‘normalization’ of altered task-related regional activity (Goldstein and Volkow 2011; Goldstein et al. 2010; Rubia et al. 2009; Rubia et al. 2011; Shafritz et al. 2004). One potential explanation is that TBI is associated with a larger degree of brain reorganization, mandating a compensatory mechanism of recovery.
Why is suppression of the posterior superior parietal cortex associated with improved performance? One explanation is based on the animal (e.g., Colby et al. 1988) and human (e.g., Portin and Hari 1999) studies reporting that the parieto-occipital area preferentially represents the peripheral visual field (cf. Palmer and Rosa 2006). Thus, suppression in this area might reflect increased focused attention on the stimulus at the center of the visual field. We further speculate that control subjects might not have deactivated this region during the task because their attentional capacity was not depleted to the level where deactivation of this region is required. Another explanation is that the superior parietal cortex is a part of the resting state network the activity of which may be suppressed when the brain is engaged in an active cognitive task. In fact, this area has been identified as part of “left parietal-frontal resting-state network” (Beckmann et al. 2005; Damoiseaux et al. 2006; van den Heuvel et al. 2008). However, the exact behavior and the functional meaning of this network remain unclear (van den Heuvel and Hulshoff Pol 2010). Still another interpretation is based on the studies proposing posterior superior parietal cortex as part of the dorsal attention system that helps select features of interest (Behrmann et al. 2004; Corbetta et al. 2008; Corbetta and Shulman 2002). Thus, decreased CBF during task performance in this area on MPH might reflect increased efficiency of the dorsal attention system. This explanation is in line with some previous studies of MPH efficacy in healthy subjects (Mehta et al. 2000; Volkow et al. 2008). However, this interpretation cannot explain why the CBF level in this area during the task dropped further below the level of resting after MPH administration. A fourth potential interpretation is based on the studies showing that deactivation in the superior parietal area has been associated with the state of meditation (Newberg et al. 2001; Newberg and Iversen 2003; Wang et al. 2011). Similar to the beneficial effect of meditation, an increased ability to focus during sustained attention performance on MPH might have prevented ‘mind wandering,’ consequently facilitating task performance. This interpretation might also explain the fact that this area was not deactivated on MPH during the two-back task, since the fast-paced nature of the working memory task, itself, might have prevented mind wandering. Unfortunately, our current limited knowledge does not allow us to preferentially choose one explanation over the others. We expect that further research on the role of this area will shed light on our empirical finding in the future.
Several potential limitations of the current study should be noted. First, the order of task blocks was not counterbalanced. The sustained attention task was always administered before the 2-back task. For this reason, task order effects cannot be ruled out when comparing the results from the two tasks. This time confound could be an alternative explanation why we observed stronger behavioral enhancement in the sustained attention task compared to the two-back task. Another limitation of the study is the relatively noisy nature of ASL time series. Despite using lenient thresholding for examining perfusion fMRI results, a low signal-to-noise ratio could have prevented identification of other brain regions showing MPH effects. Third, the group analysis approach we took might have overlooked individual-specific patterns of reorganization in brain activation. Fourth, one should be reminded that the present study used a blocked design with a very long task period, so that brain activation likely reflected primarily enduring aspects of “task set,” rather than transient neural events associated with stimulus processing and response. While this design allows us to detect tonic cognitive components with increased sensitivity, effects of MPH on these more transient cognitive processes may have been missed. Finally, a relatively small sample size could have reduced the statistical power to detect the effects of interest.
In conclusion, our perfusion fMRI study confirmed the effect of MPH on performance of higher cognitive tasks in chronic TBI and identified a locus of the MPH effect in the left posterior superior parietal area. The magnitude of task-related tonic CBF reduction in this area was correlated with improvement in performance. The fact that healthy controls did not activate this region during task performance suggests that suppression of activity in this area may reflect a TBI-specific mechanism by which MPH ameliorates attention impairments. In future studies, the significance of the left posterior parietal area for modulating sustained attention in TBI could be confirmed by using transcranial magnetic stimulation to suppress its activity. A study implementing both BOLD and perfusion fMRI would also be useful in investigating both tonic and transient components of higher cognition in TBI.
Acknowledgements
The authors wish to thank Kathy Z. Tang, BA, John Slattery, BA, Geoffrey K. Aguirre, MD, PhD, Daniel Kimberg, PhD, John Pluta, BS, and Allen Osman, PhD for their help with subject recruitment, data analysis, and manuscript review. The assistance of MRI technicians Doris Cain, Patricia O’Donnell, and Norman Butler is also gratefully acknowledged. This study was supported by grant R24HD39621 (to J.W.), R24HD050836 (to J.W.; www.ncrrn.org), and P30NS045839 (to J.A.D.) from the NIH. Dr. Detre is an inventor on the University of Pennsylvania’s patent for ASL MRI and is entitled to institutional royalty sharing for its licensure.
Footnotes
Disclosure and Conflict of Interest
The authors declare that there are no other potential conflicts of interest related to this study.
Dr. Detre is an inventor on the University of Pennsylvania’s patent for ASL MRI and is entitled to institutional royalty sharing for its licensure.
Contributor Information
Junghoon Kim, Moss Rehabilitation Research Institute, Albert Einstein Healthcare Network, 50 Township Line Rd., Elkins Park, PA 19027.
John Whyte, Moss Rehabilitation Research Institute.
Sunil Patel, Moss Rehabilitation Research Institute.
Eduardo Europa, Moss Rehabilitation Research Institute.
Jiongjiong Wang, Ahmanson-Lovelace Brain Mapping Center, University of California at Los Angeles, Los Angeles, CA 90095.
H. Branch Coslett, Department of Neurology, University of Pennsylvania, Philadelphia, PA 19104.
John A. Detre, Department of Neurology and Center for Functional Neuroimaging, University of Pennsylvania.
REFERENCES
- Aguirre GK, Detre JA, Zarahn E, Alsop DC. Experimental design and the relative sensitivity of BOLD and perfusion fMRI. Neuroimage. 2002;15:488–500. doi: 10.1006/nimg.2001.0990. [DOI] [PubMed] [Google Scholar]
- Aguirre GK, Zarahn E, D’Esposito M. The inferential impact of global signal covariates in functional neuroimaging analyses. Neuroimage. 1998;8:302–6. doi: 10.1006/nimg.1998.0367. [DOI] [PubMed] [Google Scholar]
- Aman MG, Vamos M, Werry JS. Effects of methylphenidate in normal adults with reference to drug action in hyperactivity. Aust N Z J Psychiatry. 1984;18:86–8. doi: 10.3109/00048678409161040. [DOI] [PubMed] [Google Scholar]
- Auriel E, Hausdorff JM, Herman T, Simon ES, Giladi N. Effects of methylphenidate on cognitive function and gait in patients with Parkinson’s disease: a pilot study. Clin Neuropharmacol. 2006;29:15–7. doi: 10.1097/00002826-200601000-00005. [DOI] [PubMed] [Google Scholar]
- Avants B, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B, Schoenemann PT, Gee JC. Lagrangian frame diffeomorphic image registration: Morphometric comparison of human and chimpanzee cortex. Med Image Anal. 2006;10:397–412. doi: 10.1016/j.media.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Awh E, Jonides J, Smith EE, Schumacher EH, Koeppe RA, Katz S. Dissociation of storage and rehearsal in verbal working memory: Evidence from positron emission tomography. Psychological Science. 1996;7:25–31. [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2005;360:1001–13. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann M, Geng JJ, Shomstein S. Parietal cortex and attention. Curr Opin Neurobiol. 2004;14:212–7. doi: 10.1016/j.conb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–20. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Suckling J, Zelaya F, Long C, Honey G, Reed L, Routledge C, Ng V, Fletcher P, Brown J, Williams SC. Practice and difficulty evoke anatomically and pharmacologically dissociable brain activation dynamics. Cereb Cortex. 2003;13:144–54. doi: 10.1093/cercor/13.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challman TD, Lipsky JJ. Methylphenidate: its pharmacology and uses. Mayo Clin Proc. 2000;75:711–21. doi: 10.4065/75.7.711. [DOI] [PubMed] [Google Scholar]
- Clatworthy PL, Lewis SJ, Brichard L, Hong YT, Izquierdo D, Clark L, Cools R, Aigbirhio FI, Baron JC, Fryer TD, Robbins TW. Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J Neurosci. 2009;29:4690–6. doi: 10.1523/JNEUROSCI.3266-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–8. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Colby CL, Gattass R, Olson CR, Gross CG. Topographical organization of cortical afferents to extrastriate visual area PO in the macaque: a dual tracer study. J Comp Neurol. 1988;269:392–413. doi: 10.1002/cne.902690307. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frackowiak RS, Frith CD. Monitoring for target objects: activation of right frontal and parietal cortices with increasing time on task. Neuropsychologia. 1998;36:1325–34. doi: 10.1016/s0028-3932(98)00035-9. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Frackowiak RS, Grasby PM. A fronto-parietal network for rapid visual information processing: a PET study of sustained attention and working memory. Neuropsychologia. 1996;34:1085–95. doi: 10.1016/0028-3932(96)00029-2. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–53. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23:37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- Detre JA, Wang J, Wang Z, Rao H. Arterial spin-labeled perfusion MRI in basic and clinical neuroscience. Curr Opin Neurol. 2009;22:348–55. doi: 10.1097/WCO.0b013e32832d9505. [DOI] [PubMed] [Google Scholar]
- Dodds CM, Muller U, Clark L, van Loon A, Cools R, Robbins TW. Methylphenidate has differential effects on blood oxygenation level-dependent signal related to cognitive subprocesses of reversal learning. J Neurosci. 2008;28:5976–82. doi: 10.1523/JNEUROSCI.1153-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JN, Casey BJ, Tonev ST, Davidson MC, Reiss AL, Garrett A, Hinshaw SP, Greenhill LL, Glover G, Shafritz KM, Vitolo A, Kotler LA, Jarrett MA, Spicer J. ADHD- and medication-related brain activation effects in concordantly affected parent-child dyads with ADHD. J Child Psychol Psychiatry. 2007;48:899–913. doi: 10.1111/j.1469-7610.2007.01761.x. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline J, Heather JD, Frackowiak R. Spatial registration and normalization of images. Human Brain Mapping. 1995;3:165–189. [Google Scholar]
- Goldstein RZ, Volkow ND. Oral methylphenidate normalizes cingulate activity and decreases impulsivity in cocaine addiction during an emotionally salient cognitive task. Neuropsychopharmacology. 2011;36:366–7. doi: 10.1038/npp.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Woicik PA, Maloney T, Tomasi D, Alia-Klein N, Shan J, Honorio J, Samaras D, Wang R, Telang F, Wang GJ, Volkow ND. Oral methylphenidate normalizes cingulate activity in cocaine addiction during a salient cognitive task. Proc Natl Acad Sci U S A. 2010;107:16667–72. doi: 10.1073/pnas.1011455107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualtieri CT, Evans RW. Stimulant treatment for the neurobehavioural sequelae of traumatic brain injury. Brain Inj. 1988;2:273–90. doi: 10.3109/02699058809150898. [DOI] [PubMed] [Google Scholar]
- Holmes A, Friston KJ. Generalizability, random effects, and population inference. Neuroimage. 1998;7:S754. [Google Scholar]
- Jennett B, Snoek J, Bond MR, Brooks N. Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry. 1981;44:285–93. doi: 10.1136/jnnp.44.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin DL, Cifu DX, Matthies B. Methylphenidate effect on attention deficit in the acutely brain-injured adult. Arch Phys Med Rehabil. 1996;77:6–9. doi: 10.1016/s0003-9993(96)90211-7. [DOI] [PubMed] [Google Scholar]
- Kim J, Avants B, Patel S, Whyte J, Coslett BH, Pluta J, Detre JA, Gee JC. Structural consequences of diffuse traumatic brain injury: A large deformation tensor-based morphometry study. Neuroimage. 2008;39:1014–26. doi: 10.1016/j.neuroimage.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Whyte J, Patel S, Avants B, Europa E, Wang J, Slattery J, Gee J, Coslett BH, Detre JA. Resting Cerebral Blood Flow Alterations in Chronic Traumatic Brain Injury: An Arterial Spin Labeling Perfusion fMRI Study. Journal of Neurotrauma. 2010;27:1399–1411. doi: 10.1089/neu.2009.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Whyte J, Wang J, Rao H, Tang KZ, Detre JA. Continuous ASL perfusion fMRI investigation of higher cognition: quantification of tonic CBF changes during sustained attention and working memory tasks. Neuroimage. 2006a;31:376–85. doi: 10.1016/j.neuroimage.2005.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Ko MH, Na SY, Park SH, Kim KW. Effects of single-dose methylphenidate on cognitive performance in patients with traumatic brain injury: a double-blind placebo-controlled study. Clin Rehabil. 2006b;20:24–30. doi: 10.1191/0269215506cr927oa. [DOI] [PubMed] [Google Scholar]
- Kinomura S, Larsson J, Gulyas B, Roland PE. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science. 1996;271:512–5. doi: 10.1126/science.271.5248.512. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA. Multiple neuronal networks mediate sustained attention. J Cogn Neurosci. 2003;15:1028–38. doi: 10.1162/089892903770007416. [DOI] [PubMed] [Google Scholar]
- Lee H, Kim SW, Kim JM, Shin IS, Yang SJ, Yoon JS. Comparing effects of methylphenidate, sertraline and placebo on neuropsychiatric sequelae in patients with traumatic brain injury. Hum Psychopharmacol. 2005;20:97–104. doi: 10.1002/hup.668. [DOI] [PubMed] [Google Scholar]
- Li CS, Morgan PT, Matuskey D, Abdelghany O, Luo X, Chang JL, Rounsaville BJ, Ding YS, Malison RT. Biological markers of the effects of intravenous methylphenidate on improving inhibitory control in cocaine-dependent patients. Proc Natl Acad Sci U S A. 2010;107:14455–9. doi: 10.1073/pnas.1002467107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Wu WC, Wang J, Detre JA, Dinges DF, Rao H. Imaging brain fatigue from sustained mental workload: an ASL perfusion study of the time-on-task effect. Neuroimage. 2010;49:3426–35. doi: 10.1016/j.neuroimage.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai JK, Assheuer JK, Paxinos G. Atlas of the human brain. 2nd edn Academic Press; San Diego: 2004. [Google Scholar]
- Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000;20:RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millis SR, Rosenthal M, Novack TA, Sherer M, Nick TG, Kreutzer JS, High WM, Jr., Ricker JH. Long-term neuropsychological outcome after traumatic brain injury. J Head Trauma Rehabil. 2001;16:343–55. doi: 10.1097/00001199-200108000-00005. [DOI] [PubMed] [Google Scholar]
- Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods. 2002;7:105–25. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- Newberg AB, Alavi A, Baime M, Pourdehnad M, Santanna J, d’Aquili E. The measurement of regional cerebral blood flow during the complex cognitive task of meditation: a preliminary SPECT study. Psychiatry Res. 2001;106:113–22. doi: 10.1016/s0925-4927(01)00074-9. [DOI] [PubMed] [Google Scholar]
- Newberg AB, Iversen J. The neural basis of the complex mental task of meditation: neurotransmitter and neurochemical considerations. Med Hypotheses. 2003;61:282–91. doi: 10.1016/s0306-9877(03)00175-0. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer SM, Rosa MG. A distinct anatomical network of cortical areas for analysis of motion in far peripheral vision. The European journal of neuroscience. 2006;24:2389–405. doi: 10.1111/j.1460-9568.2006.05113.x. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME. Localization of a human system for sustained attention by positron emission tomography. Nature. 1991;349:61–4. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- Plenger PM, Dixon CE, Castillo RM, Frankowski RF, Yablon SA, Levin HS. Subacute methylphenidate treatment for moderate to moderately severe traumatic brain injury: a preliminary double-blind placebo-controlled study. Arch Phys Med Rehabil. 1996;77:536–40. doi: 10.1016/s0003-9993(96)90291-9. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Region of interest analysis for fMRI. Soc Cogn Affect Neurosci. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portin K, Hari R. Human parieto-occipital visual cortex: lack of retinotopy and foveal magnification. Proceedings Biological sciences / The Royal Society. 1999;266:981–5. doi: 10.1098/rspb.1999.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Robbins TW, Hodges JR, Mehta MA, Nestor PJ, Clark L, Sahakian BJ. Methylphenidate (‘Ritalin’) can ameliorate abnormal risk-taking behavior in the frontal variant of frontotemporal dementia. Neuropsychopharmacology. 2006;31:651–8. doi: 10.1038/sj.npp.1300886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport JL, Inoff-Germain G. Responses to methylphenidate in Attention-Deficit/Hyperactivity Disorder and normal children: update 2002. J Atten Disord. 2002;6(Suppl 1):S57–60. doi: 10.1177/070674370200601s07. [DOI] [PubMed] [Google Scholar]
- Rhodes SM, Coghill DR, Matthews K. Methylphenidate restores visual memory, but not working memory function in attention deficit-hyperkinetic disorder. Psychopharmacology (Berl) 2004;175:319–30. doi: 10.1007/s00213-004-1833-7. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Mohammad AM, Brammer M, Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naive children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57:640–52. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Mohammad AM, Taylor E, Brammer M. Methylphenidate normalizes frontocingulate underactivation during error processing in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;70:255–62. doi: 10.1016/j.biopsych.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil. 2006;21:544–8. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Brecher AR, Whyte J, Klein MG. A patient registry for cognitive rehabilitation research: a strategy for balancing patients’ privacy rights with researchers’ need for access. Arch Phys Med Rehabil. 2005;86:1807–14. doi: 10.1016/j.apmr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Marchione KE, Gore JC, Shaywitz SE, Shaywitz BA. The effects of methylphenidate on neural systems of attention in attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161:1990–7. doi: 10.1176/appi.ajp.161.11.1990. [DOI] [PubMed] [Google Scholar]
- Siddall OM. Use of methylphenidate in traumatic brain injury. Ann Pharmacother. 2005;39:1309–13. doi: 10.1345/aph.1E637. [DOI] [PubMed] [Google Scholar]
- Sivan M, Neumann V, Kent R, Stroud A, Bhakta BB. Pharmacotherapy for treatment of attention deficits after non-progressive acquired brain injury. A systematic review. Clin Rehabil. 2010;24:110–21. doi: 10.1177/0269215509343234. [DOI] [PubMed] [Google Scholar]
- Strauss J, Lewis JL, Klorman R, Peloquin LJ, Perlmutter RA, Salzman LF. Effects of methylphenidate on young adults’ performance and event-related potentials in a vigilance and a paired-associates learning test. Psychophysiology. 1984;21:609–21. doi: 10.1111/j.1469-8986.1984.tb00247.x. [DOI] [PubMed] [Google Scholar]
- Swanson J, Baler RD, Volkow ND. Understanding the effects of stimulant medications on cognition in individuals with attention-deficit hyperactivity disorder: a decade of progress. Neuropsychopharmacology. 2011;36:207–26. doi: 10.1038/npp.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang GJ, Wang R, Telang F, Caparelli EC, Wong C, Jayne M, Fowler JS. Methylphenidate enhances brain activation and deactivation responses to visual attention and working memory tasks in healthy controls. Neuroimage. 2011;54:3101–10. doi: 10.1016/j.neuroimage.2010.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DC, Blackwell AD, Dowson JH, McLean A, Sahakian BJ. Neurocognitive effects of methylphenidate in adult attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) 2005;178:286–95. doi: 10.1007/s00213-004-1993-5. [DOI] [PubMed] [Google Scholar]
- Tye KM, Tye LD, Cone JJ, Hekkelman EF, Janak PH, Bonci A. Methylphenidate facilitates learning-induced amygdala plasticity. Nat Neurosci. 2010;13:475–81. doi: 10.1038/nn.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JD. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci U S A. 1998;95:14494–9. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M, Mandl R, Hulshoff Pol H. Normalized cut group clustering of resting-state FMRI data. PLoS One. 2008;3:e2001. doi: 10.1371/journal.pone.0002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2010;20:519–34. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Lewis JW, Drury HA, Hadjikhani N, Tootell RB, Bakircioglu M, Miller MI. Mapping visual cortex in monkeys and humans using surface-based atlases. Vision Res. 2001;41:1359–78. doi: 10.1016/s0042-6989(01)00045-1. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Wong C, Ma J, Pradhan K, Benveniste H, Swanson JM. Methylphenidate decreased the amount of glucose needed by the brain to perform a cognitive task. PLoS One. 2008;3:e2017. doi: 10.1371/journal.pone.0002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Ferrieri R, Schlyer DJ, Alexoff D, Pappas N, Lieberman J, King P, Warner D, et al. Methylphenidate decreases regional cerebral blood flow in normal human subjects. Life Sci. 1994;54:PL143–6. doi: 10.1016/0024-3205(94)00873-6. [DOI] [PubMed] [Google Scholar]
- Wang J, Aguirre GK, Kimberg DY, Detre JA. Empirical analyses of null-hypothesis perfusion FMRI data at 1.5 and 4 T. Neuroimage. 2003a;19:1449–62. doi: 10.1016/s1053-8119(03)00255-6. [DOI] [PubMed] [Google Scholar]
- Wang J, Aguirre GK, Kimberg DY, Roc AC, Li L, Detre JA. Arterial spin labeling perfusion fMRI with very low task frequency. Magn Reson Med. 2003b;49:796–802. doi: 10.1002/mrm.10437. [DOI] [PubMed] [Google Scholar]
- Wang J, Rao H, Korczykowski M, Wintering N, Pluta J, Khalsa DS, Newberg AB. Cerebral blood flow changes associated with different meditation practices and perceived depth of meditation. Psychiatry Research: Neuroimaging. 2011;191:60–67. doi: 10.1016/j.pscychresns.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang Y, Wolf RL, Roc AC, Alsop DC, Detre JA. Amplitude-modulated continuous arterial spin-labeling 3.0-T perfusion MR imaging with a single coil: feasibility study. Radiology. 2005;235:218–28. doi: 10.1148/radiol.2351031663. [DOI] [PubMed] [Google Scholar]
- Whyte J, Hart T, Laborde A, Rosenthal M. Rehabilitation issues in traumatic brain injury. In: DeLisa JA, Gans BM, Walsh NE, editors. Physical medicine and rehabilitation: principles and practice. Lippincott Williams & Wilkins; Philadelphia: 2004a. pp. 1677–1714. [Google Scholar]
- Whyte J, Hart T, Schuster K, Fleming M, Polansky M, Coslett HB. Effects of methylphenidate on attentional function after traumatic brain injury. A randomized, placebo-controlled trial. Am J Phys Med Rehabil. 1997;76:440–50. doi: 10.1097/00002060-199711000-00002. [DOI] [PubMed] [Google Scholar]
- Whyte J, Hart T, Vaccaro M, Grieb-Neff P, Risser A, Polansky M, Coslett HB. Effects of methylphenidate on attention deficits after traumatic brain injury: a multidimensional, randomized, controlled trial. Am J Phys Med Rehabil. 2004b;83:401–20. doi: 10.1097/01.phm.0000128789.75375.d3. [DOI] [PubMed] [Google Scholar]
- Whyte J, Polansky M, Fleming M, Coslett HB, Cavallucci C. Sustained arousal and attention after traumatic brain injury. Neuropsychologia. 1995;33:797–813. doi: 10.1016/0028-3932(95)00029-3. [DOI] [PubMed] [Google Scholar]
- Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A. 1992;89:212–6. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]