Abstract
AVE0118’s action to prolong effective refractory period (ERP) in atria but not ventricles is thought to be due to its inhibition of IKur. However, in non-remodeled atria, AVE0118 prolongs ERP but not action potential duration (APD70-90), which can be explained with inhibition of sodium, but not potassium channel current. ERP, APD, and the maximum rate of rise of the AP upstroke (Vmax) were measured in canine isolated coronary-perfused right atrial and in superfused ventricular tissue preparations. Whole-cell patch-clamp techniques were used to measure sodium channel current (INa) in HEK293 cells stably expressing SCN5A. AVE0118 (5–10 μM) prolonged ERP (p<0.001), but not APD70 and decreased Vmax (by 15%, 10 μM, p<0.05; n=10 for each). Ventricular ERP, APD90, and Vmax were not changed significantly by 10 μM AVE0118 (all p=ns; n=7). AVE0118 effectively suppressed acetylcholine-mediated persistent atrial fibrillation (AF). AVE0118 (10 μM) reduced peak current amplitude of SCN5A-WT current by 36.5±6.6% (p<0.01; n=7) and shifted half-inactivation voltage (V0.5) of the steady- state inactivation curve from -89.9±0.5 to -96.0±0.9 mV (p<0.01; n=7). Our data suggest that AVE0118-induced prolongation of atrial, but not ventricular ERP, is due largely to atrial- selective depression of INa, which likely contributes to the effectiveness of AVE0118 to suppress AF.
INTRODUCTION
The notion that atrial-selective agents may suppress atrial fibrillation (AF) without the risk of induction of ventricular proarrhythmia has led to the emergence of several investigational atrial-selective pharmacological approaches for rhythm control management of AF. The most investigated and widely promoted as the most promising atrial-specific approach involves inhibition of atrial-specific Kv1.5-pore-forming channels, carrying the ultra-rapid delayed rectified outward potassium current (IKur).1 Numerous agents capable of blocking IKur have been shown to prolong effective refractory period (ERP) specifically in atria.2 ERP can be prolonged due to action potential duration (APD70-90) prolongation and/or development of post- repolarization refractoriness (PRR). The former is commonly due to block of potassium channels and the latter is due to block of sodium channels. Correlation of the ERP and APD70-90 changes induced by IKur blockers has been poorly studied. Of note, INa blockers can produce atrial- selective ERP prolongation due to induction of PRR3 and recent studies have revealed that Ikur blockers such as vernakalant and AZD1305 depress INa – mediated parameters in an atrial- selective manner.4; 5 Vernakalant prolongs atrial ERP largely due to induction of PRR.6
The main purpose of the current study was to test the hypothesis that the effect of AVE0118, a multichannel blocker inhibiting IKur, to produce an atrial selective prolongation of ERP is due largely to its atrial-selective inhibition of the sodium channel. We also used patch- clamp techniques to examine the effects of AVE0118 on INa in HEK293 cells stably expressing SCN5A.
METHODS
This investigation conforms to the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health (The Eighth Edition of the Guide for the Care and Use of Laboratory Animals (NRC 2011)) and was approved by the Institutional Animal Care and Use Committee. Dogs weighing 20–25 kg were anticoagulated with heparin (200 IU/kg) and anesthetized with pentobarbital sodium (35 mg/kg, i.v.). Prior to surgery, the following criteria was met to insure adequate anesthesia: a lack of palpebral reflex, a lack of withdrawal from a noxious stimulus applied to the distal forelimb, a lack of breathing, and an auscultable heart rate that is no greater than 60 bpm. The chest was opened via a left thoracotomy, the heart excised, placed in a cardioplegic solution consisting of cold (4°C) Tyrode's solution containing 8.5 mM [K+]o and transported to a dissection tray.
Experiments were performed using isolated arterially-perfused canine right atrial (RA) preparations and superfused left ventricular endocardial tissue slice preparations ( 1×0.5×0.1 cm). The methods used for isolation and perfusion of these preparations have been described in previous publications.7; 8
Unfolded RA with a rim of the right ventricle was cannulated and perfused through the ostium of the right coronary artery. Unperfused tissue was removed with a razor blade or scissors. The cut ventricular and atrial branches were ligated using silk thread. After these procedures (performed in cold cardioplegic solution, 4–8°C), the preparations were transferred to a temperature-controlled bath and arterially-perfused with Tyrode’s solution by use of a roller pump. The superfused preparations, isolated using a dermatome (Davol Simon Dermatome, Cranston, RI, USA), were placed in a tissue bath (volume 5 ml, flow rate 12 ml/min) and allowed to equilibrate for at least 3 hours while superfused with oxygenated Tyrode’s solution and stimulated at a basic cycle length (BCL) of 500 msec using point stimulation (rectangular stimuli 1 - 3-ms duration, 2–3 times diastolic threshold intensity). The composition of the Tyrode’s solution was (in mM): NaCl 129, KCl 4, NaH2PO4 0.9, NaHCO3 20, CaCl2 1.8, MgSO4 0.5, and D-glucose 5.5, buffered with 95% O2 and 5% CO2 (37±0.5 °C, pH=7.35)
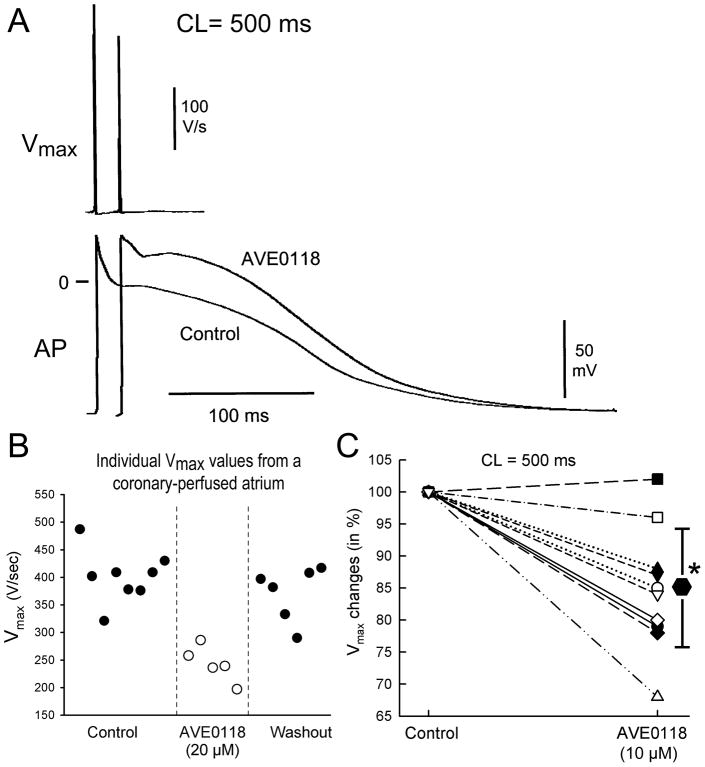
Transmembrane action potential (AP) recordings (sampling rate 41 kHz) were obtained using standard or floating glass microelectrodes (2.7 M KCl, 10–25 MΩ DC resistance). A pseudo-electrocardiogram (ECG) was recorded using two electrodes consisting of Ag/AgCl half cells placed in the Tyrode’s solution bathing the preparation, 1.0 to 1.2 cm from opposite ends of the atrial or ventricular coronary-perfused preparations. Effective refractory period (ERP) was measured by delivering premature stimuli after every 10th regular beat at a pacing cycle length (CL) of 500 (with 10 ms resolution; stimulation with a 2 × diastolic threshold of excitation (DTE) amplitude). Post-repolarization refractoriness (PRR) was recognized when ERP exceeded APD70 in atria and APD90 in ventricles. Note that atrial ERP is generally coincided with APD70-75 in atria and APD90 in ventricles.3; 9 Maximum rate of rise of the AP upstroke (Vmax): Stable AP recordings and Vmax measurements are difficult to obtain in vigorously contracting perfused atrial preparations and there is a significant variability in Vmax values even at the same condition/region (shown in Fig. 1B). In coronary-perfused atria, the effect of AVE01118 on Vmax was first determined in each atrium by averaging the largest three Vmax values in the absence and presence of this agent at a CL of 500 ms. Then, using these average values from each atrium, we compared Vmax data from all atria (n=10). Due to a substantial inter-preparation variability, Vmax values were normalized for each experiment.
Figure 1. AVE0118 decreases the maximum rate of rise of the action potential upstroke (Vmax) in canine coronary perfused right atria.
A: Representative action potentials and corresponding Vmax values recorded under control conditions and following addition of AVE0118 (10 μM). B: Individual Vmax values from an atrial preparation recorded under control conditions, after 20 μM AVE0118 and after washout of the drug. C: AVE0118 (10 μM) - induced reduction of Vmax as a % of baseline values (n=10). * -p<0.05 vs. control.
Experimental Protocols
The equilibration period for the coronary-perfused atrial preparations was 30 min and for superfused ventricular slice preparations 3 hours. The concentration of AVE0118 (5.0 and 10.0 μM) was increased in a step-wise manner, with at least 20 min at each concentration before the start of recording. In an acetylcholine (ACh) -dependent AF model, we tested the effect of AVE0118 to prevent (series 1) the induction of AF as well as, in different series, the ability of these agents to terminate (series 2) persistent AF (lasting > 60 min). In cases in which the drug terminated AF, we tested if the arrhythmia could be re-induced electrically.
Electrophysiology of the Cardiac Sodium Channels in HEK293 Cell Line
The effects of AVE0118 on INa characteristics were evaluated in human embryonic kidney cell line, HEK293, stably expression SCN5A, as previously described.10 Sodium channel characteristic were studied with whole cell patch clamp techniques, as previously described.11 Cells were placed in a chamber for electrophysiological study (EPS; Medical Systems, Greenvale, NY). Macroscopic whole-cell INa was recorded at room temperature (22°C) using an Axopatch 200B amplifier (Molecular Devices, Inc, Sunnyvale, CA). Perfusion bath solution containing (in mmol/L) 140 NaCl, 5 KCl, 1.8 CaCl2, 1 MgCl2, 2.8 Na Acetate, 10 HEPES, and 10 glucose (pH 7.3 with NaOH). Tetraethylammonium chloride (5 mM) was added to the buffer to block TEA-sensitive native currents. Patch clamp pipettes were pulled (1 and 2.5 MΩ) from borosilicate glass (7052; Model PP-89; Narashige, Tokoyo, Japan) and filled with a solution containing (in mM) 5 NaCl, 5 KCl, 130 CsF, 1.0 MgCl2, 5 EGTA and 10 HEPES (pH 7.2 with CsOH). Steady- state availability of the sodium channel was fitted to a Boltzmann equation. Data acquisition and analysis were performed using pCLAMP programs V9.2 (Axon Instruments, Union City, CA) and ORIGIN 6.1 (Microcal Software, Northampton, MA).
Drugs
AVE0118 (a gift from Dr. Gögelein, Sanofi-Aventis , Frankfurt, Germany) was dissolved in 100% DMSO and acetylcholine (SIGMA, MO) was dissolved in distilled water as a stock of 1–10 mM at the start of each experiment.
Statistical Analysis
For data obtained from the multicellular preparations, statistical analysis was performed using paired or unpaired Student’s t-test and one-way repeated measures or multiple comparison analysis of variance (ANOVA) followed by Bonferroni’s test, as appropriate. Statistical differences in voltage clamp analysis were evaluated by Student's unpaired t-test. Data from multicellular preparations and HEK293 Cell Line are expressed as mean ± SD and mean ±SE, respectively. Significance wasassumed for P < 0.05; "NS" indicates non-significant changes.
RESULTS
Electrophysiologic effects of AVE0118 atrial and ventricular preparations
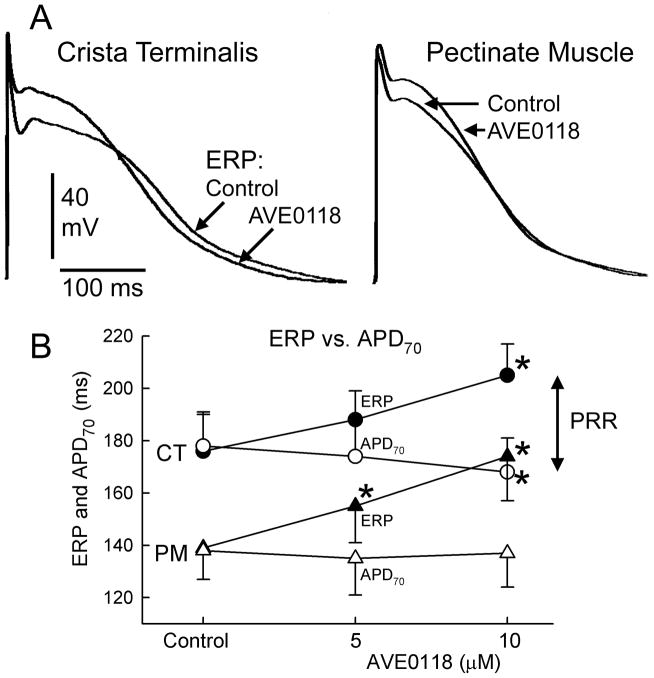
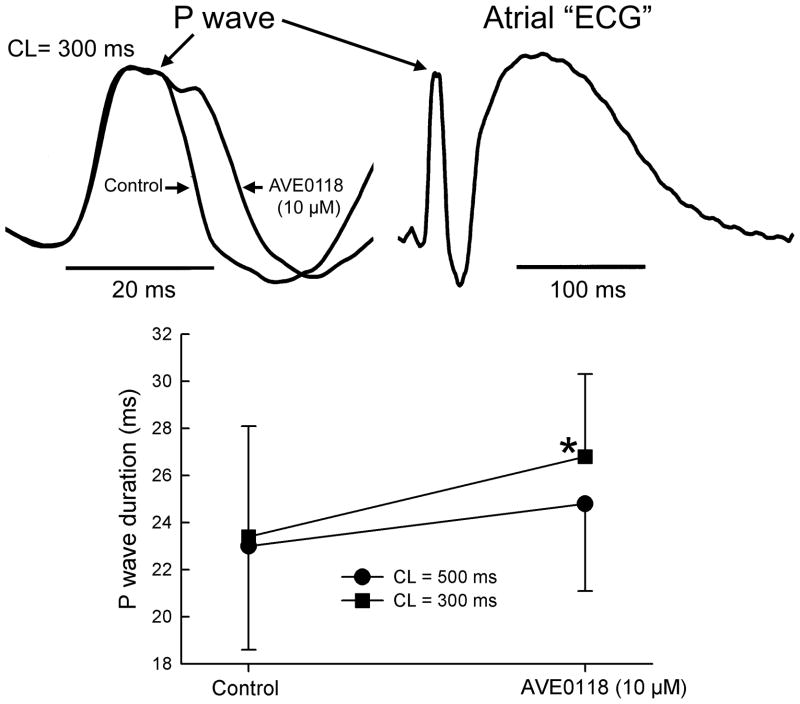
In atria, AVE0118 reduced Vmax by an average of 15% at a concentration of 10 μM (CL = 500 ms, Fig. 1). AVE0118 (5–10 μM) significantly prolonged ERP in both pectinate muscle (PM) and crista terminalis (CT) regions, but abbreviated APD70 in CT and caused no change in APD70 in PM, revealing the development of PRR (Fig. 2). The early repolarization phase in atria was significantly prolonged by AVE0118; APD20 in CT increased from 5±3 to 51±12 ms at a concentration of 10 μM (p<0.001; CL = 500 ms; n=8) (Fig. 2). AVE0118 did not significantly alter DTE (0.22±0.04 mA in control vs. 0.23±0.03 mA at 10 μM AVE0118; CL = 500 ms; n=10). The duration of P wave was significantly increased at a CL of 300 ms, but not 500 ms by 10 μM AVE0118 (Fig. 3).
Figure 2.
AVE0118 causes little change in APD70 but causes a significant prolongation of ERP in canine atria, leading to development of post-repolarization refractoriness (PRR). In atria, ERP corresponds to APD at 70–75% of repolarization. Shown are: superimposed action potential tracings recorded before and after AVE0118 in crista terminalis (CT) and pectinate muscle (PM) regions (A) and summary data of APD70 and ERP (B). * - p<0.05 vs. control. CL = 500 ms. n=10
Figure 3.
AVE0118 significantly increased the duration of the atrial P wave complex (a measure of atrial conduction) at a pacing CL of 300 ms in coronary-perfused atrial preparations.
* - p<0.05 vs. control. n=5 for each.
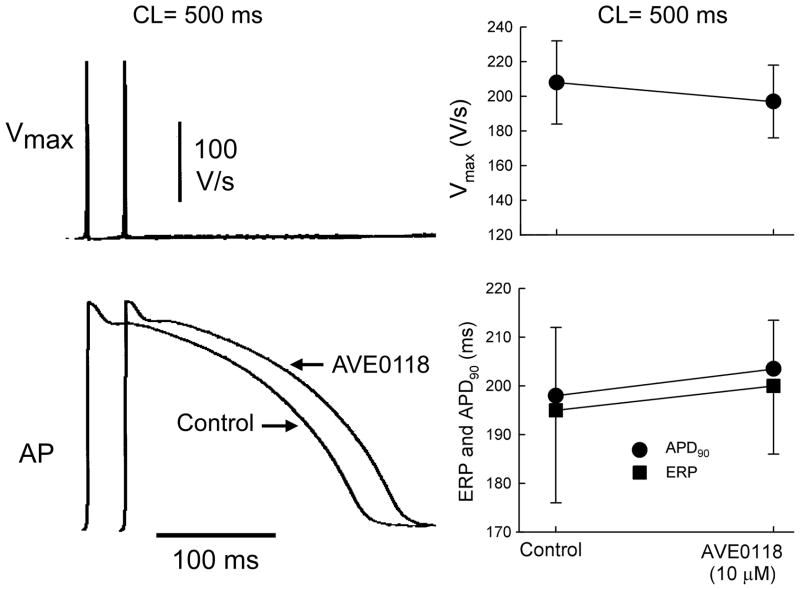
In contrast to atrial preparations, ERP, APD90, Vmax, and action potential morphology were not altered significantly by AVE0118 in ventricular preparations (Fig. 4). DTE was also unaffected by AVE0118 in ventricular preparations (0.29±0.05 mA in control vs. 0.28±0.06 mA in presence of 10 μM of AVE0118; n=7; CL = 500 ms).
Figure 4. AVE0118 fails to significantly alter Vmax, APD, and ERP in canine left ventricular superfused endocardial tissue slices.
Left panels: action potentials recordings and corresponding Vmax values. Right panels: Summary data for Vmax, ERP and APD90. n=7. CL = 500 ms.
Block of INa by AVE-0118
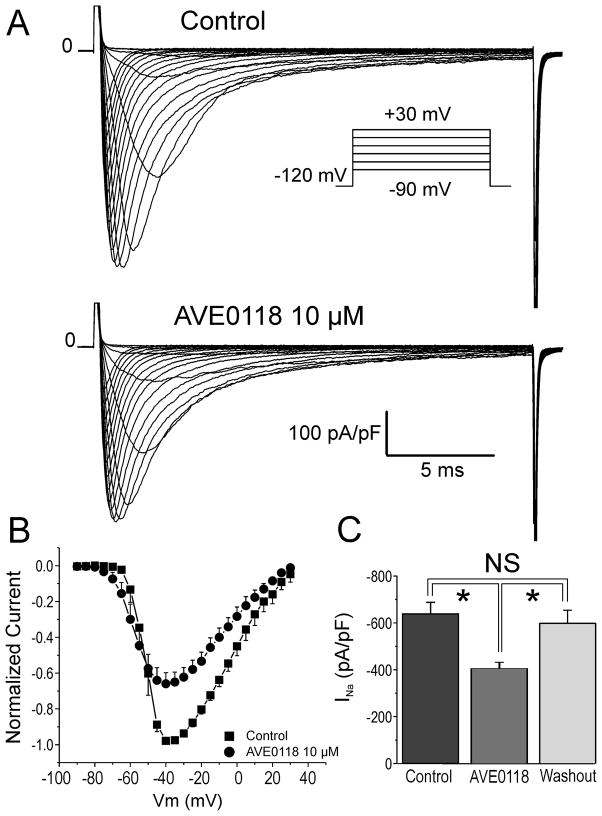
Whole cell sodium current was recorded at room temperature in HEK293 cells stably expressing SCN5A (Nav1.5). Macroscopic sodium currents (INa) elicited by 20 ms test pulses from a holding potential of −120 mV to potentials between −90 mV and +30 mV in increments of +5 mV are shown in Fig. 5A. Figure 5B shows the effect of 10 μM AVE0118 on the current- voltage (I-V) relationship for INa. AVE0118 (10 μM) caused a significant reduction of peak INa (Fig. 5C). INa elicited with pulses to −30 mV was reduced by 36.5±6.6% (p<0.01; n=7). Washout of AVE0118 was associated with restoration of the current.
Figure 5. Effect of AVE0118 on sodium channel current (INa) in HEK293 cells stably expressing SCN5A.
A: INa elicited by 20 ms test pulses from a holding potential of -120 mV to potentials between -90 mV and +30 mV, in increments of 5 mV (inset). B: Current voltage (I-V) relationship for INa in the absence and presence of 10 μM AVE0118. Peak currents were normalized to the maximum current recorded under control conditions and following application of AVE0118. C: Effect of 10 μM AVE0118 to decrease INa. INa density was reduced by 36.5±6.6% (*- p<0.01; n=7) at -30 mV. Washout of AVE0118 is associated with restoration of the current. Mean ± SEM (n=7).
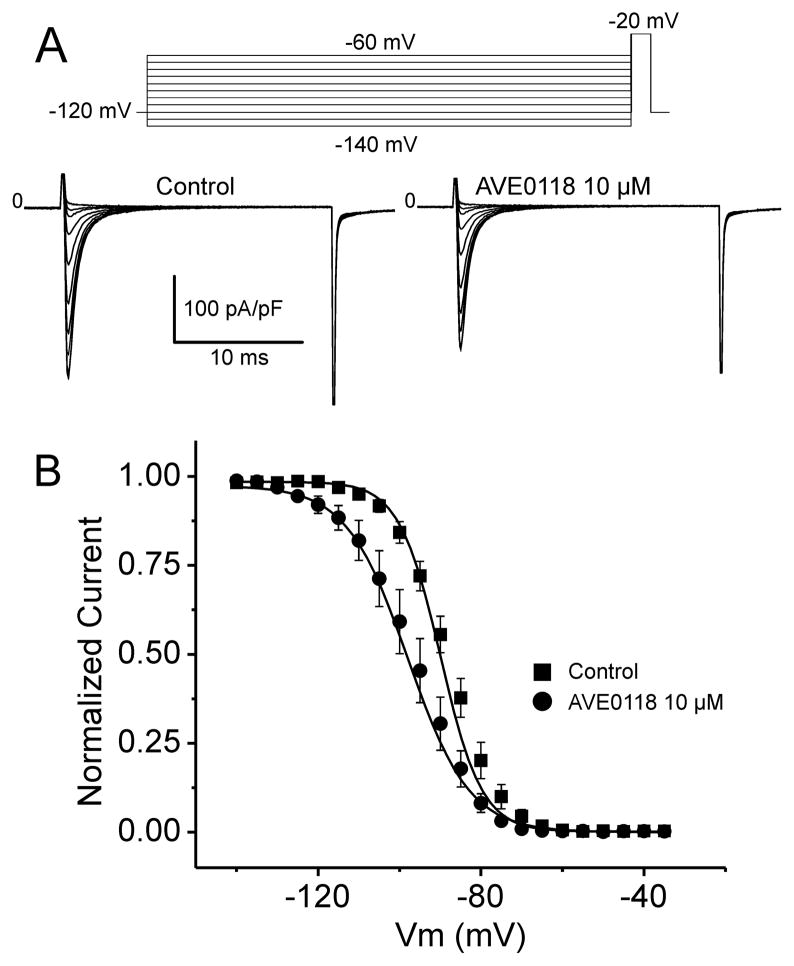
The steady-state inactivation relationship was obtained using a 500 ms prepulse to different voltages followed by a step to −20 mV (20 ms duration). AVE0118 shifted half inactivation voltage (V0.5) to more negative potentials (from −89.9±0.5 to -96.0±0.9 mV; p<0.01; n=7; Fig. 6), thus further reducing the availability of sodium channels.
Figure 6. Effect of AVE0118 on Steady-state Inactivation of Cardiac Sodium Channels in HEK293 cells.
A: Representative Nav1.5 current traces recorded before and after 10 μM AVE0118. Currents were recorded using the protocol pictured in the inset. B: Peak Nav1.5 current was normalized to the maximum current recorded under control conditions or following 10 μM AVE0118 (-89.0 ± 0.55 mV vs. -95.96 ± 0.55 mV, respectively). Steady-state inactivation is plotted as a function of conditioning potential and fitted to a Boltzmann distribution. AVE0118 induces a significant shift in mid-inactivation voltage (V1/2; p<0.01). Mean ± SEM (n=7).
Anti-AF effect of AVE0118
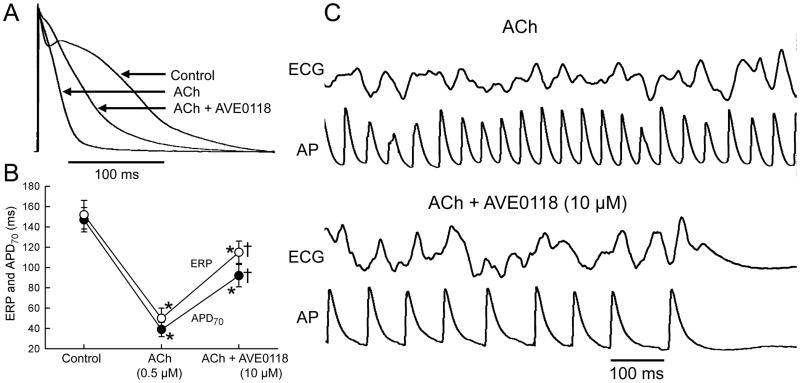
In the presence of ACh (0.5 μM), the atrial action potential was markedly abbreviated (Fig. 7). Under these conditions, addition of AVE0118 (10 μM) significantly prolonged both APD70 and ERP. ERP prolonged more than APD70, showing the development of PRR. With ACh alone (0.5 μM), persistent AF was inducible in 100% of coronary-perfused atria (10/10). AVE0118 (10 μM) prevented the induction of persistent ACh-mediated AF in 100% of atria (4/4). Non-sustained AF or tachycardia (<30 sec) were induced in 2 of the 4 atrial preparations. In another series of experiments, AVE0118 (10 μM) was found to terminate persistent acetylcholine-mediated AF in 6/7 atria (Fig. 7).
Figure 7. AVE0118 prolongs atrial APD and ERP in the presence of acetylcholine (ACh) and effectively terminates ACh-mediated AF.
A: Superimposed action potentials (AP) recorded in control, in the presence of ACh, and ACh plus AVE0118. B: Plot depicts average changes in APD70 and ERP. All data are from pectinate muscle stimulated at a CL of 500 ms. * - p<0.05 vs. control. † p<0.05 vs. ACh. n=5–10. C: Shown are ECG and AP tracings recorded during persistent AF in the presence of ACh alone and following the addition of AVE0118, which terminated the arrhythmia on the 12th minute.
DISCUSSION
Our main finding is that the IKur blocker AVE0118 also importantly inhibits sodium channel activity in an atrial-selective manner, which largely accounts for the atrial selective prolongation of ERP and may contribute to anti-AF properties of AVE0118.
Atrial-selective agents for treatment of AF: IKur blockers
A major limitation of many effective anti-AF agents is the risk of induction of ventricular arrhythmias. This risk can be eliminated or diminished with the use of agents that selectively alter atrial electrophysiological parameters. Block of IKur, an atrial specific target,1 has long been considered to be a promising atrial-specific approach for effective and safe AF therapy.2; 12 However, enthusiasm for selective IKur blockers for AF management has diminished in recent years.13–17
AVE0118 is known to inhibit multiple ion channels including IKur, Ito, IK-ACh, and constitutively active IK-ACh and to prolong atrial but not ventricular ERP.18; 19 The atrial-selective 20; 21 prolongation of ERP by AVE0118 has previously been attributed to inhibition of IKur. However, APD70-90 is abbreviated or not affected by AVE0118 in “healthy” atria and only slightly prolonged in remodeled atria.22; 23 Cardiac ERP corresponds to APD70-90. In the present study, we compared AVE-0118-induced APD and ERP changes and demonstrated that in healthy atria AVE0118 causes little change in APD70-90 but significantly prolongs ERP (Fig. 2). Prolongation of ERP without lengthening of APD70-90 is due to induction of PRR, which is due to the inhibition of peak INa, but not IKur. The ability of AVE0118 to block INa is supported by the fact that this agent reduces Vmax in atrial preparations and reduces peak INa in heterologously- expressed sodium channels.
Atrial-selective prolongation of ERP: IKur or INa inhibition?
Many agents capable of inhibiting IKur have been shown to selectively prolong ERP in atria.2 More recent studies have shown that block of INa can also prolong atrial ERP selectively, without altering ventricular ERP.3; 15 Interestingly, recent studies indicate that agents with IKur blocking activity capable of producing atrial-specific/predominant ERP prolongation (such as vernakalant, AZD7009, and AZD1305) also potently inhibit peak INa.5; 24; 25 Atrial-selective ERP prolongation with vernakalant is due to inhibition of peak INa6 and with AZD1305 is due to block of both peak INa and IKr.5; 26
Several IKur blockers (vernakalant, ISQ-1 and TAEA) slow conduction in atria, but not in ventricles, 4; 27 pointing to atrial-selective INa inhibition. “Pure” inhibition of IKur with low concentrations of 4-AP, cause no or only minor changes in APD70-90 and as expected the changes in ERP parallel the changes in APD.13 Of note, the potential INa blocking ability of most of the IKur blockers either has not been studied or studied under conditions that may not unmask this effect of the drug (i.e., studied in ventricular myocytes/preparations at slow pacing rates).28 The atrial selective INa blocking effect is observed at normal and rapid, but not slow, activation rates owing to the fact that atrial-selective INa blockers possess relatively rapid unbinding kinetics from the sodium channel.6; 15
We determined the effect of AVE0118 on APD, Vmax, PRR, DTE only at one pacing CL in the current study (CL = 500 ms). It is expected that at faster activation rates, sodium channel-mediated parameters (Vmax, PRR, DTE, etc) would be altered by AVE0118 to a greater degree, as previously demonstrated for a number of atrial-selective INa blockers, including ranolazine, vernakalant, amiodarone and AZD1305.3; 5; 6; 29 Atrial conduction time was increased by AVE0118 in a rate-dependent manner (Fig. 3) Note that at rapid activation rates (CL = 200 ms), AVE0118 statistically significantly slowed conduction velocity in atria of goat in vivo.20; 30
While AVE0118 (3–10 μM) prolongs ERP in atria of goats20; 31 pigs32 and dogs33 in vivo as well as in coronary-perfused atria of rabbits34 and dogs (current study), this drug (at 6 μM) produces no change in ERP in human superfused atrial preparations isolated from patients in sinus rhythm patients.19 The difference may be species-dependent, but it can be also explained by a relatively poor pharmacological sensitivity of atrial superfused vs. perfused (or in vivo) preparations. A statistically significant ERP prolongation with vernakalant, ranolazine, and dl-sotalol was obtained at 3 μM in canine coronary-perfused left atrial appendage preparations,6 but only at 30 μM in canine superfused pulmonary vein preparations.35 The extent of prolongation of atrial ERP by the atrial-selective INa blockers ranolazine and vernakalant is strongly rate-dependent, being small or absent at 1000 ms CL and significant at ≤500 ms CL in canine atrial preparations.6; 35 Considering that effect of AVE0118 on ERP in the human atrial preparations was recorded at a CL of 1000 ms19 and in goat, pig, rabbit, and dog atria at much faster rates (CL = ≤400, ≤400, 200, and 500 ms, respectively), 20; 31–34 the failure of AVE0118 to prolong ERP in human atrial preparations may be attributed to the relatively slow pacing rate.
A number of factors are likely to contribute to the atrial selectivity of INa blockers, including a more depolarized resting membrane potential (RMP), more negative half-inactivation voltage (V0.5), and more gradual phase 3 of the action potential in atrial cells as compared with ventricular cells (for detailed discussion see3; 10; 36). Rate of recovery from sodium channel block is thought to contribute to the atrial selectivity of sodium-channel blockers. Drugs, such as propafenone, which exhibit slow dissociation from the sodium channel, show little to no atrial selectivity,37 whereas agents that dissociate rapidly, such as ranolazine, amiodarone, and vernakalant, tend to be highly atrial-selective in their inhibition of sodium channel-dependent parameters.3; 29; 37 The unbinding kinetics of AVE0118 the sodium channel remains unknown.
Anti – AF effectiveness of AVE0118
Our demonstration of good anti-AF efficacy of AVE-0118 in coronary-perfused canine atrial preparation is consistent with the report of Blaauw et al20 demonstrating good efficacy AVE0118 in remodeled atria of the goat in vivo. When atrial APD was significantly abbreviated (as after ACh), AVE-0118 prolonged both APD and ERP, the former less than the latter (Fig. 6). Prolongation of APD and ERP is likely due to block of IK-ACh, IKur, Ito, and INa. The ERP prolonging effect of AVE0118 appears to be responsible for its anti-AF efficacy.
“Pure” IKur block for AF?
At concentrations that are able to effectively suppress AF, IKur blockers such as vernakalant, AVE0118, AZD1305, AZD7009, potently inhibit other currents, particularly INa, Ito, IK-ACh, CA-IK-ACh, and IKr.5; 19; 20; 24; 26; 38 It is therefore difficult to dissect out the degree to which IKur inhibition contributes to the anti-AF effects of an agent. It is noteworthy that specific block 13; of IKur using low concentrations of 4-AP neither prevent the induction of AF nor terminate AF. 17 Indeed, selective IKur block has been shown to abbreviate atrial APD90/ERP and promote the induction of AF in atria displaying a plateau-shaped action potential morphology.13 It is also noteworthy that loss of function mutations in KCNA5, the gene that encodes for the α subunit of the IKur channel, have been associated with inherited AF.39; 40
Ito inhibition is likely to contribute to the atrial-selective and anti-AF effects of AVE0118. Ito is larger in atrial vs. ventricular cell.41 The predominant α-subunit of the Ito channel, Kv4.3, is expressed significantly more strongly in human atria vs. ventricles,42 and 4-AP block of Ito is much more effective in atrial vs. ventricular myocytes.43; 44
Because the density of IKur is reduced with acceleration of pacing rate,45 the relative contribution of IKur to atrial repolarization may be relatively small in the setting of AF. IKur density has been reported to be decreased in cells isolated from chronic AF hearts.19; 46 While block of IKur alone may not be sufficient to suppress AF,13; 15–17 the contribution of IKur block may 30; 36 be important in combination with inhibition of other currents (e.g., Ito, IKr, INa).
Study limitations
Our data were obtained from “healthy” cardiac preparations that did not manifest the structural and electrical cardiac changes commonly encountered in patients with AF. Pharmacological responses of remodeled atria may differ from those of healthy atria. Our experiments were performed in isolated atria coronary-perfused with Tyrode’s solution. The presence of autonomic influences and other factors present in vivo may modulate the effect of IKur inhibition, resulting in outcomes different from those observed in the present study.
While the effect of AVE0118 on INa – dependent parameters (Vmax, DTE and ERP) was measure in intact canine coronary-perfused atrial and ventricular preparations, the effect of AVE0118 on INa was measured in a heterologous expression system, HEK293 cells stably expressing SCN5A. The sodium channel in heart is known to co-associate with many auxiliary subunits, which may not be present in HEK293 cells. Consequently, our quantification of the effects of AVE0018 on INa may less than accurately reflect the responses of native atrial and ventricular myocytes.
Conclusions
Our data indicate that AVE0118-induced atrial-selective prolongation of ERP is due largely to the effect of the drug to produce an atrial-selective depression of INa which likely contributes to the effectiveness of AVE0118 to suppress AF. Our results provide support for the hypothesis that the atrial selectivity of multichannel blockers inhibiting IKur to prolong ERP is due largely to atrial-selective depression of INa6; 14; 15; 36.
Acknowledgments
We gratefully acknowledge the expert technical assistance of Judy Hefferon and Robert Goodrow.
Footnotes
Conflict of Interest Disclosure: Dr. Antzelevitch is a consultant to Gilead Sciences and AstraZeneca and received research grant funds from Gilead Sciences, AstraZeneca, Merck, Cardiome and Buchang Group
Reference List
- 1.Wang ZG, Fermini B, Nattel S. Sustained depolarization-induced outward current in human atrial myocytes: Evidence for a novel delayed rectifier K+ current similar to Kv1. 5 cloned channel currents. Circ Res. 1993;73:1061–76. doi: 10.1161/01.res.73.6.1061. [DOI] [PubMed] [Google Scholar]
- 2.Ford JW, Milnes JT. New drugs targeting the cardiac ultra-rapid delayed-rectifier current (IKur): rationale, pharmacology and evidence for potential therapeutic value. J Cardiovasc Pharmacol. 2008;52(2):105–20. doi: 10.1097/FJC.0b013e3181719b0c. [DOI] [PubMed] [Google Scholar]
- 3.Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116(13):1449–57. doi: 10.1161/CIRCULATIONAHA.107.704890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bechard J, Pourrier M. Atrial selective effects of intravenously administrated vernakalant in conscious beagle dog. J Cardiovasc Pharmacol. 2011;58(1):49–55. doi: 10.1097/FJC.0b013e31821b8608. [DOI] [PubMed] [Google Scholar]
- 5.Burashnikov A, Zygmunt AC, Di Diego JM, Linhardt G, Carlsson L, Antzelevitch C. AZD1305 exerts atrial-predominant electrophysiological actions and is effective in suppressing atrial fibrillation and preventing its re-induction in the dog. J Cardiovasc Pharmacol. 2010;56(1):80–90. doi: 10.1097/FJC.0b013e3181e0bc6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burashnikov A, Pourrier M, Gibson JK, Lynch JJ, Antzelevitch C. Rate-dependent effects of vernakalant in the isolated non-remodeled canine left atria are primarily due to block of the sodium channel. Comparison with ranolazine and dl-sotaol. Circ Arrhythm Electrophysiol. 2012 doi: 10.1161/CIRCEP.111.968305. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, Cordeiro JM, Thomas GP. Electrophysiologic effects of ranolazine: a novel anti-anginal agent with antiarrhythmic properties. Circulation. 2004;110(8):904–10. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burashnikov A, Mannava S, Antzelevitch C. Transmembrane action potential heterogeneity in the canine isolated arterially-perfused atrium: effect of IKr and Ito/IKur block. Am J Physiol. 2004;286:H2393–H2400. doi: 10.1152/ajpheart.01242.2003. [DOI] [PubMed] [Google Scholar]
- 9.Bode F, Kilborn M, Karasik P, Franz MR. The repolarization-excitability relationship in the human right atrium is unaffected by cycle length, recording site and prior arrhythmias. J Am Coll Cardiol. 2001;37(3):920–5. doi: 10.1016/s0735-1097(00)01189-x. [DOI] [PubMed] [Google Scholar]
- 10.Zygmunt AC, Nesterenko VV, Rajamani S, Hu D, Barajas-Martinez H, Belardinelli L, Antzelevitch C. Mechanisms of atrial-selective block of sodium channel by ranolazine I. Experimental analysis of the use-dependent block. Am J Physiol Heart Circ Physiol. 2011;301(4):H1606–H1614. doi: 10.1152/ajpheart.00242.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barajas-Martínez HM, Hu D, Cordeiro JM, Wu Y, Kovacs RJ, Meltser H, Kui H, Burashnikov E, Brugada R, Antzelevitch C, Dumaine R. Lidocaine-induced Brugada syndrome phenotype linked to a novel double mutation in the cardiac sodium channel. Circ Res. 2008;103(4):396–404. doi: 10.1161/CIRCRESAHA.108.172619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nattel S, Carlsson L. Innovative approaches to anti-arrhythmic drug therapy. Nat Rev Drug Discov. 2006;5(12):1034–49. doi: 10.1038/nrd2112. [DOI] [PubMed] [Google Scholar]
- 13.Burashnikov A, Antzelevitch C. Can inhibition of IKur promote atrial fibrillation? Heart Rhythm. 2008;5(5):1304–9. doi: 10.1016/j.hrthm.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burashnikov A, Antzelevitch C. How do atrial-selective drugs differ from antiarrhythmic drugs currently used in the treatment of atrial fibrillation? J Atrial Fibrillation. 2008;1(2):98–107. doi: 10.4022/jafib.v1i1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burashnikov A, Antzelevitch C. Atrial-selective sodium channel block for the treatment of atrial fibrillation. Expert Opin Emerg Drugs. 2009;14(2):233–49. doi: 10.1517/14728210902997939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravens U, Wettwer E. Ultra-rapid delayed rectifier channels: molecular basis and therapeutic implications. Cardiovasc Res. 2011;89:843–51. doi: 10.1093/cvr/cvq398. [DOI] [PubMed] [Google Scholar]
- 17.Pandit SV, Zlochiver S, Filgueiras-Rama D, Mironov S, Yamazaki M, Ennis SR, Noujaim SF, Workman AJ, Berenfeld O, Kalifa J, Jalife J. Targeting atrio-ventricular differences in ion channel properties for terminating acute atrial fibrillation in pigs. Cardiovasc Res. 2011;89:843–51. doi: 10.1093/cvr/cvq359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gogelein H, Brendel J, Steinmeyer K, Strubing C, Picard N, Rampe D, Kopp K, Busch AE, Bleich M. Effects of the atrial antiarrhythmic drug AVE0118 on cardiac ion channels. Naunyn Schmiedebergs Arch Pharmacol. 2004;370(3):183–92. doi: 10.1007/s00210-004-0957-y. [DOI] [PubMed] [Google Scholar]
- 19.Christ T, Wettwer E, Voigt N, Hala O, Radicke S, Matschke K, Varro A, Dobrev D, Ravens U. Pathology-specific effects of the IKur/Ito/IK,ACh blocker AVE0118 on ion channels in human chronic atrial fibrillation. Br J Pharmacol. 2008;154(8):1619–30. doi: 10.1038/bjp.2008.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blaauw Y, Gogelein H, Tieleman RG, van HA, Schotten U, Allessie MA. "Early" class III drugs for the treatment of atrial fibrillation: efficacy and atrial selectivity of AVE0118 in remodeled atria of the goat. Circulation. 2004;110(13):1717–24. doi: 10.1161/01.CIR.0000143050.22291.2E. [DOI] [PubMed] [Google Scholar]
- 21.Knobloch K, Brendel J, Rosenstein B, Bleich M, Busch AE, Wirth KJ. Atrial-selective antiarrhythmic actions of novel Ikur vs. Ikr, Iks, and IKAch class Ic drugs and beta blockers in pigs. Med Sci Monit. 2004;10(7):BR221–BR228. [PubMed] [Google Scholar]
- 22.Wettwer E, Hala O, Christ T, Heubach JF, Dobrev D, Knaut M, Varro A, Ravens U. Role of IKur in controlling action potential shape and contractility in the human atrium: influence of chronic atrial fibrillation. Circulation. 2004;110(16):2299–306. doi: 10.1161/01.CIR.0000145155.60288.71. [DOI] [PubMed] [Google Scholar]
- 23.Schotten U, de Haan S, Verheule S, Harks EG, Frechen D, Bodewig E, Greiser M, Ram R, Maessen J, Kelm M, Allessie M, Van Wagoner DR. Blockade of atrial-specific K+-currents increases atrial but not ventricular contractility by enhancing reverse mode Na+/Ca2+-exchange. Cardiovasc Res. 2007;73(1):37–47. doi: 10.1016/j.cardiores.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 24.Fedida D. Vernakalant (RSD1235): a novel, atrial-selective antifibrillatory agent. Expert Opin Investig Drugs. 2007;16(4):519–32. doi: 10.1517/13543784.16.4.519. [DOI] [PubMed] [Google Scholar]
- 25.Carlsson L, Chartier D, Nattel S. Characterization of the in vivo and in vitro electrophysiological effects of the novel antiarrhythmic agent AZD7009 in atrial and ventricular tissue of the dog. J Cardiovasc Pharmacol. 2006;47(1):123–32. doi: 10.1097/01.fjc.0000196242.04384.c3. [DOI] [PubMed] [Google Scholar]
- 26.Persson F, Andersson B, Duker G, Jacobson I, Carlsson L. Functional effects of the late sodium current inhibition by AZD7009 and lidocaine in rabbit isolated atrial and ventricular tissue and Purkinje fibre. Eur J Pharmacol. 2007;558(1–3):133–43. doi: 10.1016/j.ejphar.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 27.Regan CP, Kiss L, Stump GL, McIntyre CJ, Beshore DC, Liverton NJ, Dinsmore CJ, Lynch JJ., Jr Atrial antifibrillatory effects of structurally distinct IKur blockers 3- [(dimethylamino)methyl]-6-methoxy-2-methyl-4-phenylisoquinolin-1(2H)-one and 2-phenyl-1,1-dipyridin-3-yl-2-pyrrolidin-1-yl-ethanol in dogs with underlying heart failure. J Pharmacol Exp Ther. 2008;324(1):322–30. doi: 10.1124/jpet.107.127654. [DOI] [PubMed] [Google Scholar]
- 28.Wirth KJ, Brendel J, Steinmeyer K, Linz DK, Rutten H, Gogelein H. In vitro and in vivo effects of the atrial selective antiarrhythmic compound AVE1231. J Cardiovasc Pharmacol. 2007;49(4):197–206. doi: 10.1097/FJC.0b013e318032002f. [DOI] [PubMed] [Google Scholar]
- 29.Burashnikov A, Di Diego JM, Sicouri S, Ferreiro M, Carlsson L, Antzelevitch C. Atrial-selective effects of chronic amiodarone in the management of atrial fibrillation. Heart Rhythm. 2008;5(12):1735–42. doi: 10.1016/j.hrthm.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blaauw Y, Schotten U, van HA, Neuberger HR, Allessie MA. Cardioversion of persistent atrial fibrillation by a combination of atrial specific and non-specific class III drugs in the goat. Cardiovasc Res. 2007;75(1):89–98. doi: 10.1016/j.cardiores.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 31.Linz DK, Afkham F, Itter G, Rutten H, Wirth KJ. Effect of atrial electrical remodeling on the efficacy of antiarrhythmic drugs: comparison of amiodarone with IKr- and Ito/IKur-blockade in vivo strial electrical remodeling and antiarrhythmic drugs. J Cardiovasc Electrophysiol. 2007;18(12):1313–20. doi: 10.1111/j.1540-8167.2007.00962.x. [DOI] [PubMed] [Google Scholar]
- 32.Wirth KJ, Paehler T, Rosenstein B, Knobloch K, Maier T, Frenzel J, Brendel J, Busch AE, Bleich M. Atrial effects of the novel K+-channel-blocker AVE0118 in anesthetized pigs. Cardiovasc Res. 2003;60(2):298–306. doi: 10.1016/s0008-6363(03)00543-1. [DOI] [PubMed] [Google Scholar]
- 33.Oros A, Volders PG, Beekman JD, van der NT, Vos MA. Atrial-specific drug AVE0118 is free of torsades de pointes in anesthetized dogs with chronic complete atrioventricular block. Heart Rhythm. 2006;3(11):1339–45. doi: 10.1016/j.hrthm.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 34.Lofberg L, Jacobson I, Carlsson L. Electrophysiological and antiarrhythmic effects of the novel antiarrhythmic agent AZD7009: a comparison with azimilide and AVE0118 in the acutely dilated right atrium of the rabbit in vitro. Europace. 2006;8(7):549–57. doi: 10.1093/europace/eul061. [DOI] [PubMed] [Google Scholar]
- 35.Sicouri S, Pourrier M, Gibson JK, Lynch JJ, Antzelevitch C. Comparison of electrophysiological and antiarrhythmic effects of vernakalant, ranolazine, and sotalol in canine pulmonary vein sleeve preparations. Heart Rhythm. 2012 doi: 10.1016/j.hrthm.2011.10.021. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burashnikov A, Antzelevitch C. New development in atrial antiarrhythmic drug therapy. Nat Rev Cardiol. 2010;7(3):139–48. doi: 10.1038/nrcardio.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burashnikov A, Belardinelli L, Antzelevitch C. Atrial-selective sodium channel block strategy to suppress atrial fibrillation. Ranolazine versus propafenone. J Pharmacol Exp Ther. 2012;340(1):161–8. doi: 10.1124/jpet.111.186395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldstein RN, Khrestian C, Carlsson L, Waldo AL. Azd7009: a new antiarrhythmic drug with predominant effects on the atria effectively terminates and prevents reinduction of atrial fibrillation and flutter in the sterile pericarditis model. J Cardiovasc Electrophysiol. 2004;15(12):1444–50. doi: 10.1046/j.1540-8167.2004.04354.x. [DOI] [PubMed] [Google Scholar]
- 39.Olson TM, Alekseev AE, Liu XK, Park SJ, Zingman LV, Bienengraeber M, Sattiraju S, Ballew JD, Jahangir A, Terzic A. Kv1. 5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum Mol Genet. 2006;15:2185–91. doi: 10.1093/hmg/ddl143. [DOI] [PubMed] [Google Scholar]
- 40.Yang T, Yang P, Roden DM, Darbar D. Novel KCNA5 mutation implicates tyrosine kinase signaling in human atrial fibrillation. Heart Rhythm. 2010;7(9):1246–52. doi: 10.1016/j.hrthm.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giles WR, Imaizumi Y. Comparison of potassium currents in rabbit atrial and ventricular cells. J Physiol (Lond ) 1988;405:123–45. doi: 10.1113/jphysiol.1988.sp017325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaborit N, Le BS, Szuts V, Varro A, Escande D, Nattel S, Demolombe S. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J Physiol. 2007;582(Pt 2):675–93. doi: 10.1113/jphysiol.2006.126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amos GJ, Wettwer E, Metzger F, Li Q, Himmel HM, Ravens U. Differences between outward currents of human atrial and subepicardial ventricular myocytes. J Physiol. 1996;491 ( Pt 1):31–50. doi: 10.1113/jphysiol.1996.sp021194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nattel S, Matthews C, De Blasio E, Han W, Li D, Yue L. Dose-dependence of 4-aminopyridine plasma concentrations and electrophysiological effects in dogs : potential relevance to ionic mechanisms in vivo. Circulation. 2000;101(10):1179–84. doi: 10.1161/01.cir.101.10.1179. [DOI] [PubMed] [Google Scholar]
- 45.Feng J, Xu D, Wang Z, Nattel S. Ultrarapid delayed rectifier current inactivation in human atrial myocytes: properties and consequences. Am J Physiol. 1998;275(5 Pt 2):H1717–H1725. doi: 10.1152/ajpheart.1998.275.5.H1717. [DOI] [PubMed] [Google Scholar]
- 46.Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward K+ current densities and Kv1. 5 expression are reduced in chronic human atrial fibrillation. Circ Res. 1997;80(6):772–81. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]