Abstract
Background
Intracardiac myxomas in Carney complex are significant causes of cardiovascular morbidity and mortality through embolic stroke and heart failure. The genetic, clinical and laboratory characteristics of Carney complex-related strokes from atrial myxomas have not been described before. The PRKAR1A gene is mutated in more than 60% of the cases of Carney complex.
Methods and Results
We studied patients with strokes and cardiac myxomas that were hospitalized in our institution and elsewhere: a total of 7 patients with 16 recurrent atrial myxomas and more than 14 episodes of strokes were identified. Neurological deficits were reported; in one patient, an aneurysm developed at the site of a previous stroke. All patients were female, most had presented with Cushing syndrome, and all had additional tumors or other Carney complex manifestations. Other than gender, although there was a trend for patients being overweight and hypertensive, no other risk factors were identified. A total of 5 patients (71%) had a PRKAR1A mutation; all mutations (c418_419delCA, c.340delG/p.Val113fsX15, c.353_365del13/p.Ile118fsX6, c.491_492delTG/p.Val164fsX4, c.177+1G>A) were located in exons 3–5 and introns 2–3, and all led to a non-sense PRKAR1A mRNA.
Conclusions
Female Carney complex patients appear to be at a high risk for recurrent atrial myxomas that lead to multiple strokes. Early identification of a female patient with Carney complex is of paramount importance for the early diagnosis of atrial myxomas and the prevention of strokes.
INTRODUCTION
Carney complex is a rare condition, associated with pigmented skin lesions, multiple endocrine and other tumors, including cardiac myxomas1. It is a genetically heterogeneous disease and linkage analysis has shown that at least two loci are involved: 2p16 and 17q22–24 2. The gene responsible for Carney Complex at locus 2p16 (Carney Complex 2) remains unknown. The gene responsible for Carney Complex type 1, located on 17q22–24, has been identified as the regulatory subunit (R1A) of the protein kinase (PRKAR1A) 2. About 600 patients worldwide have been studied to date, by the National Institute of Health (NIH) – Mayo Clinic (USA) and the Cochin Hospital (France) and more than 120 mutations in PRKAR1A gene have been identified 3.
Cardiac myxomas are rare benign tumors with an estimated incidence of 0.5 – 1 per million population per year; cardiac myxomas account for about half of the primary cardiac tumors in adults and 15% of these lesions in children4. Recurrence is reported in sporadic (4%–7%) and familial cases (10–21%) 5. Although well documented in the adult population, information about cerebral embolism in the pediatric population is still limited.
Due to similar clinical picture, Carney complex should be differentiated from isolated non-syndromic myxoma and other multiple endocrine neoplasias (MEN). Prompted by the study of a recent patient with extensive neurological defects following a stroke, we queried our database of Carney complex patients that have been seen at the NIH Clinical Center over the last 20 years. Although recurrent myxomas overall were relatively frequent, as we have published previously 3, it was striking that only women with Carney complex had their first presentation of the complex as a stroke with extensive neurological damage. These case series indicate that female gender is possibly a risk factor for multiple strokes due to left-sided myxomas in the context of Carney complex.
SUBJECTS & METHODS
Seven patients were studied; one was admitted to the Mother and Child Healthcare Institute (MCHI), Belgrade, Serbia and six were admitted to the NIH Warren Magnuson Clinical Center. These seven patients were selected from a larger group of 258 patients with Carney Complex, because stroke was the first symptom of the disease. All subjects signed on to clinical protocol 95-CH0059 that was approved by the NICHD institutional review board. DNA studies and sequencing for PRKAR1A were completed as previously described 3.
CASE DESCRIPTIONS & RESULTS
Tables 1&2 summarize the main clinical and laboratory data of Carney complex patients with cardiac myxomas complicated by stroke.
Table 1.
Demographic and clinical characteristics of Carney complex patients with stroke episodes due to cardiac myxomas
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Age (years) | 13 | 52 | 45 | 58 | 29 | 32 | 45 |
| Gender | Female | Female | Female | Female | Female | Female | Female |
| PPNAD | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Multiple freckles/lentigines | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Endocrine disease | No | Cushing’s Acromegaly subclinical hypothyroidism | Cushing’s, Hypothyroidism | Hypothyroidism | Cushing’s | Cushing’s | No |
| Adrenalectomy due to PPNAD | No | Yes | Yes | Yes | Yes | Yes | No |
| Other tumors | None | Pituitary growth hormone secreting tumor, thyroid follicular adenoma, breast myxomas | Myxomas skin, breast | Breast cancer | Schwannoma, breast fibroadenomas right thyroid lobe nodule | Possible pituitary adenoma | Possible pituitary adenoma, thyroid colloid cyst |
| Cardiac Myxoma (number) | 2 | 2 | 2 | 5 | 2 | 2 | 1 |
| Recurrence interval (years) | 0.5 | 23 | 10 | 6 | 0.5 | 3 | NA |
| Stroke episodes (number) | 2 | 1 | 2 | 1 | Multiple bilateral strokes | 4 | 2 |
| Aneurysms | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Neurological deficits | Hemiparesis Impaired speech | anisocoria, Babinski (+) bílaterally | Right upper extremity weakness, impaired speech, memory difficulties | Left sided weakness slurred speech | Ataxia, migraine, visual and memory difficulty | Optic nerve damage | Right-sided weakness. impaired speech, seizures |
| Menstrual periods | Regular | Regular | Regular | Regular (menopausal now) | Regular | Irregular | Regular |
| Weight | Normal | Mildly obese | Overweight | Overweight | Overweight | Slightly overweight | Within normal range |
| Hypertension | No | No | Yes, on ACE inhibitor | No | Resolved | No | No |
| Carney complex family history | Negative | Brother, mother | Brother, daughter | Sister, daughter with atrial myxoma | Negative | Relatives, cousin with pituitary tumor | Son (multiple freckles, testicular enlargement) |
| Family history of pulmonary embolism | Negative | Negative | Negative | Patient’s mother | Negative | 2 family members | Negative |
| Family history of myxomas | Negative | Negative | Mother | Her daughter | Negative | Negative | Negative |
| Family history of strokes | Negative | Negative | Mother | Her sister with Cushing’s | Paternal grandfather | Negative | Negative |
PPNAD: primary pigmented nodular adrenocortical disease
Table 2.
Results of hormonal workup of Carney complex patients with stroke episodes due to cardiac myxomas
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Cortisol, 08.00 am (RR 5–25 mcg/dl) | 3.5 | <1 | 10.5 | 10.2 | 260 | 9.9 | 9.6 |
| ACTH, 08.00 am (RR 9–52 pg/ml) | Not done | 14.1 | 14.9 | 33.4 | 16.3 | 10.2 | 30.8 |
| 24-hour urinary free cortisol (RR 4–50 mcg) | Not done | <1 | 25.4 | 21 | 260 | 4.6 | 31 |
| TSH (RR 0.40 to 4.00 mIU/ml) | 2.09 | 5.03 | 1.2 | 1.82 | 2.29 | 1.05 | 1.39 |
| Prolactin (RR 3–29 mcg/L) | 13.2 | Not done | 1 8.6 | Not done | 22.3 | ||
| FSH (U/L) | 4.9 | Not done | 4 | 87 | 6 | 5 | 39.3 |
| LH (U/L) | 2.8 | Not done | 6 | 43 | 12 | 3 | 12.1 |
| Estradiol (pg/ml) | 45.2 | <10 | 107 | Not done | 37 | 37 | 61 |
| Total testosterone (RR up to 60 ng/dl) | Not done | Not done | 39 | 10 | 14 | 13 | 34 |
Patient 1
A 13-year-old white female presented with respiratory distress, vomiting, abdominal pain, left-sided body weakness and left facial nerve paresis. Multiple hyperpigmented skin spots were also noted. Computed tomography (CT) on admission was normal; a repeated CT scan after 12 hours demonstrated a massive right sided fronto-parietal ischemic zone (Figure 1a). Echocardiogram (ECHO) showed a left atrial myxoma (28×37×57mm) on a short peduncle, arising from the roof of the left atrium protruding through the mitral valve into the left ventricle (Figure 1b). The cardiac mass was completely removed with no evidence of residual tumor 4 months post surgery. However, 6 months after her first ischemic episode, she presented with severe headache, choking and speech difficulties. A new mass (8 × 10mm) was detected with heart ECHO, hanging on a long narrow peduncle from the midportion of the interatrial septum. She underwent a second surgery and the tumor was removed completely. No new cardiac tumors have been detected so far. A PRKAR1A mutation was identified located in exon 4 (Table 3).
Figure 1.
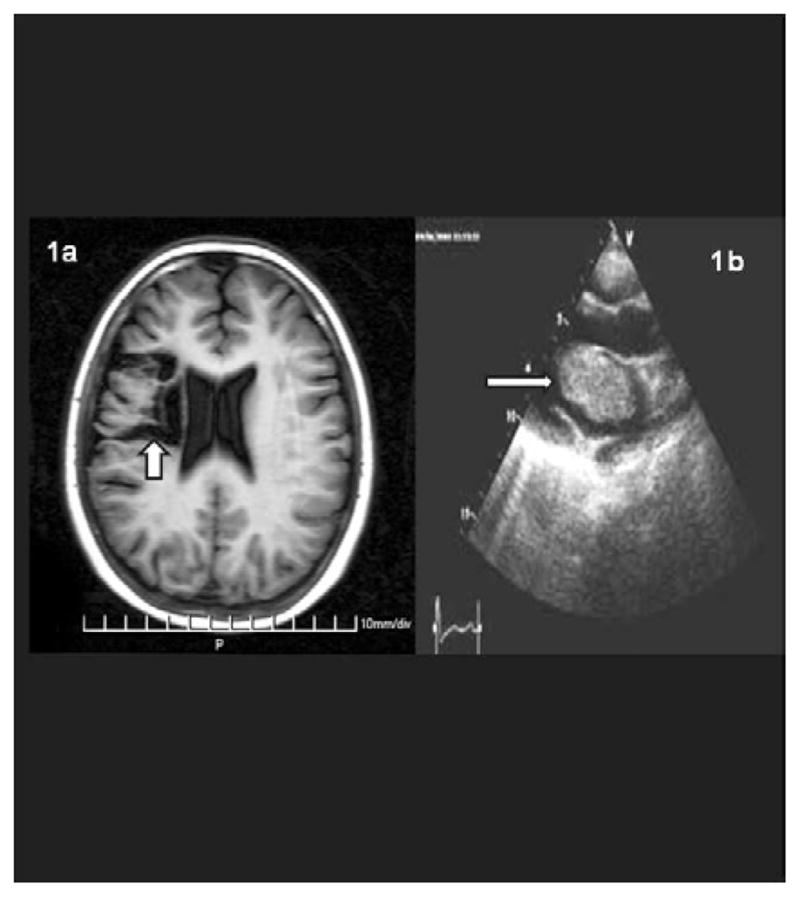
a. Axial T1-weighted MRI scan of the brain reveals an abnormal area of decreased signal intensity (arrow). This is due to encephalomacia, the end result of an old ischemic infarction; b. Heart echocardiogram showing a left atrial mass (arrow).
Table 3.
Mutational data of Carney complex patients with stroke episodes due to cardiac myxomas
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| PRKAR1A Mutation | c418_419delCA | c.340delG/p.Val113 fsX15 | c.353_365del13/p.Ile118fs X6 | None | c.491_492delTG/p.Val164f sX4 | c.177+1G >A | None |
| Gene location of the mutation | Exon 4 | Exon 3 | Exon 4 | NA | Exon 5 | Intron 2–3 | NA |
Patient 2
A 32-year-old white female presented with a recurrent left ventricular myxoma; in the course of her investigation she was found to also have Cushing syndrome due to Primary Pigmented Nodular Adrenocortical Disease (PPNAD) and was diagnosed with Carney complex. A magnetic resonance imaging (MRI) of the brain showed a region of cortical encephalomalacia with surrounding gliosis in the right parietal lobe; she indicated that she had experienced “some visual changes” and headaches over a period of 2 years. Although the patients’ parents were not known to suffer from any disease, the patient was found to have at least 3 siblings affected with Carney complex.
Patient 3
A 38-year-old white female presented with inability to speak and right upper extremity weakness. ECHO showed a cardiac myxoma that measured 40×45×30 mm. A year later she presented again with speaking difficulty and increased right arm clumsiness; another heart myxoma was identified and resected from the same location as previously. The patient recovered her speech ability but there was residual right upper extremity weakness, numbness and memory difficulties. MRI showed an old infarction within the left frontal and frontal parietal lobe along the left middle-cerebral artery (MCA) distribution. The patient’s mother had a history of myxomas. The patient’s brother was also diagnosed with Carney complex.
Patient 4
A 58-year-old white female presented with an 8-month-long history of headaches, blurred vision, numbness in her distal upper extremities, hearing loss, and intermittent chest pain. A left atrial myxoma had been excised in the past; nultiple (up to four) left atrial myxomas were detected at presentation, as well as pigmented skin lesions typical of Carney complex. During her follow-up she also developed Cushing’s syndrome due to PPNAD and underwent bilateral adrenalectomy. She remains well to date and has recovered from most of her neurological deficits.
Patient 5
A 29-year-old white female with a history of a fifth cranial nerve schwannoma and left atrial myxoma, which removed at the age of 19 years, presented with blurred vision, frequent headaches and tingling of her left lip, that often extended to the rest of the left side of the face. The symptoms were attributed to the schwannoma which was excised. The clinical findings were suggestive of Carney Complex. PPNAD was also diagnosed and bilateral adrenalectomy was performed. However, four months following adrenalectomy, she developed acute ataxia; MRI showed multiple bilateral strokes and an echocardiogram showed a left ventricular cardiac myxoma. At present, the patient has almost completely recovered from the neurologic deficits of these events, but scaring remains evident on brain MRI.
Patient 6
A 32-year-old white female was diagnosed with Carney Complex after a history of recurrent left cardiac atrial myxomas for which she was operated twice within three years. In both cases, surgery had been preceded by a presentation with a stroke, resulting in permanent optic nerve damage. MRI showed diffuse atrophy of the left cerebral hemisphere with focal encephalomalacia adjacent to the left lateral ventricle.
Patient 7
A 45 year-old white female was diagnosed with Carney complex after her 7 year-old son was found to have the disease. There was a history of multiple transient ischemic attacks; after a hemorrhagic stroke she was found to have an atrial myxoma that was surgically resected. A brain MRI showed areas of encephalomalacia (Figure 2a, b), right caudate head and adjacent corona radiate as well as dilatation of lateral ventricle and an aneurysm in the left sylvian fissure (Figure 2c). Residual symptoms of stroke involved right sided weakness, impaired speech and seizures.
Figure 2.


Two axial T2-weighted images of the brain show prominent abnormal signal changes (arrows) in the left basal ganglia (a) and in the white matter of the left cerebral hemisphere (b). Both abnormalities are due to gliosis secondary to old ischemic infarctions. A small lacunar infarction is also noted in the right basal ganglia (a, small arrow). Coronal T1-weighted scan of the brain (c) demonstrates an aneurysm at the bifurcation of the left middle cerebral artery (arrow)
Summary of clinical findings and DNA studies
All patients were females and four of seven patients were also diagnosed with Cushing syndrome due to PPNAD. Six of the seven patients presented with a recurrent cardiac myxoma, two of them in less than a year after the first one. Strokes recurred in more than half of the patients leaving them with significant neurological deficits, whereas one of the patients had already developed an aneurysm. Family history for Carney complex was mostly positive, whereas on two occasions there was also a positive family history for either pulmonary embolism or cardiac myxomas.
In five of the seven patients (71%), a PRKAR1A mutation was identified. All mutations (c418_419delCA, c.340delG/p.Val113fsX15, c.353_365del13/p.Ile118fsX6, c.491_492delTG/p.Val164fsX4, c.177+1G>A) were located in exons 3–5 and introns 2–3, and all led to a non-sense PRKAR1A mRNA (Table 3).
DISCUSSION
As it has been previously reported in Carney complex, most of our patients presented with cardiac myxomas in the second or third decade of their life 7. Intracardiac tumors in the pediatric age group are rare with an overall incidence of 0.17%. Myxomas account for 6% of all pediatric cardiac tumors and they are much less frequent than rhabdomyomas which account for 63% of cardiac lesions in childhood; most of the myxomas in childhood occur within the context of Carney complex. Our study highlights the variability in age-of-onset for Carney complex-related myxomas from age 4 to 61 years 8. Patients usually present with symptoms of left heart outflow obstruction (heart failure, dyspnoea, syncope and sudden death), constitutional symptoms (autoimmune/vasculitis symptoms, weight loss, fatigue or fever) or embolic disease 9. Although most of our patients had the first two of the classic triad symptoms, none of them had the third. Importantly, one of our patients developed an aneurysm at the bifurcation of the left middle cerebral artery. Although unusual, scarce fusiform and saccular cerebral aneurysms after multiple cerebral embolisms as a result of atrial myxoma have been reported 10–11. Accordingly, myxoma cells in the walls of such aneurysms have been previously determined by histopathological studies 12.
In a previous study of 112 patients with cardiac myxomas, only 6 had recurrence of their myxoma (5%), 3 of whom (2.5%) were patients with Carney complex; thus, although the reported recurrence rate for sporadic tumors ranges from 1%–3% 13, the overall risk of recurrence for familial and Carney complex-related myxomas maybe as high as 22% 14. In our series of myxomas complicated by stroke, 6 of the 7 patients (86%) had 2–5 recurrences of their tumor. Carney complex might be one of the most significant risk factors for recurrence of a myxoma and its associated complications. Other risk factors include incomplete resection, intracardiac implantation, embolization, multicentricity of the tumor, and/or retention of tumor precursor cells in the subendocardium. In the series of 112 consecutive cases of left atrial myxoma mentioned before, 72 were women and 40 men 15. However, among patients with sporadic cardiac myxomas, males were reported to be statistically at greater risk than females of developing embolic complications. It is interesting that in contrast to the male predominance (70.8%) in septic cardioembolic strokes after infective endocarditis 16, we found that strokes complicated the course of recurrent myxomas only in female patients with Carney complex. Hormonal and clinical characteristics of our patients (tables 1–2), however, could not offer a clear explanation for this prevalence, so that further studies are needed to adequately address this association.
Five of our patients (71%) were found to carry five different inactivating mutations of the PRKAR1A gene (Carney complex type 1) (Table 3). In this genetically heterogeneous entity, however, we did not find any case with myxoma-related stroke caused by mutations on chromosome 2p16 (Carney complex type 2). The only other gene that has been involved in myxomas is the G-alpha stimulatory subunit of the G-protein coupled receptor (GNAS); these mutations were detected in the myocardium of patients with McCune-Albright syndrome 17. It is noteworthy that these two genes are among the most essential players of the cyclic AMP signaling pathway.
Other Mendelian disorders that have been associated with strokes are presented in Table 4. Clearly, patients with Carney complex are readily distinguishable from the other conditions presented in Table 4, due to their unique presentation with skin lesions and other, primarily endocrine manifestations, such as Cushing syndrome due to PPNAD.
Table 4.
Monogenic disorders Associated with Stroke
| Disorder | Gene/locus | Inheritance pattern | Clinical picture |
|---|---|---|---|
| CADASIL | Notch 3/19p13.2-p13.1 | AD | Adult-onset dementia and stroke |
| CARASIL | HTRA1/10q26.3 | AR | subcortical infarcts, leukoencephalopathy, alopecia, spondylosis, dementia |
| HERNS | TREX1/3p21.31 | AD | 1. Hereditary endotheliopathy, retinopathy, nephropathy, and stroke |
| CRV | ≫ | AD | 2. Cerebroretinal vasculopathy |
| HVR | ≫ | AD | 3. Hereditary vascular ritonapathy |
| COL4A1-related disorder (Stroke syndrome) | COL4A1/13q34 | AD | Infantile hemiparesis, migraine, intracerebral hemorrhage, seizures, Raynaud phenomenon, dementia |
| Fabry disease | Alpha-galactosidase (GLA)/Xq22 | XL-R | premature stroke, dolichoectasia, white matter hyperintensities |
| Homocystinuria | Cystathionine beta synthase (CBS)/21q22.3 | AR | multisystemic disorder of the connective tissue, muscles, CNS, and cardiovascular system: extensive atheroma formation at young age, Intra vascular thrombosis |
| Ehlers-Danlos syndrome type IV | COL3A1/2q32.2 | AD | Characteristic facial appearance, small stature, thin, pale, translucent skin, arterial, intestinal, uterine fragility |
| Marfan syndrome | FBN1/15q21.1 | AD | Musculoskeletal disorder, neurovascular complications (including TIA, cerebral infarction) |
| MoyaMoya disease | Linkage to 3p24.2–26, and 17q | Polygenic or AR | cerebral ischemia, TIAs, sensorimotor paralysis, convulsions and/or migraine-like headaches |
| Pseudoxanthoma Elasticum | ABCC6/16p13.1 | AR or AD | Connective tissue, cardiovascular complications including intracranial aneurysms and ischaemic stroke |
| HHT – Hereditary hemorrhagic telangiectasia (Osler-Weber-Randu disease) | Endoglin (ENG)/9q33–q34.1 and ALK1or ACVRL1/12q11–q14 | AD | Telangiectasias of skin and mucosal membranes, arteriovenous malformations in internal organs, embolic stroke due to pulmonary arteriovenous malformations |
| Sickle Cell Disease | HBB/11p15.5 | AR | Chronic anemia, periodic episodes of pain, ischemic/hemorrhagic strokes, TIA |
| Carney complex | PRKAR1A/2p16 and 17q22–24 (unknown gene) | AD | Spotty pigmentation of the skin, heart myxomas, extracardiac myxomas ( breast, testis, thyroid, brain, adrenal gland), pituitary adenoma, psammomatous melanotic schwannoma, Sertoli cell tumors of the testis, PPNAD |
AD: Autosomal dominant, AR: Autosomal recessive, XL-R: X-linked recessive, TIA: Transient ischemic attack, CNS: Central nervous system, PPNAD: primary pigmented nodular adrenocortical disease
In conclusion these case series indicate that female patients with Carney complex in particular, may be at risk for multiple strokes, a manifestation of the disease that can be prevented with frequent cardiac imaging and early detection and excision of the causative myxomas.
Acknowledgments
We thank all patients for their participation in this study. This work was supported, in part, by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), intramural National Institutes of Health (NIH) project Z01-HD-000642-04 to Dr. C. A. Stratakis, and by the Intensive Care Unit, Department of Pediatrics, Regional University Hospital, University of Crete, Heraklion, Greece (Dr. Briassoulis).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carney JA, Hruska LS, Beauchamp GD, et al. Dominant inheritance of the complex of myxomas, spotty pigmentation, and endocrine over activity. Mayo Clin Proc. 1986;61:165–172.2. doi: 10.1016/s0025-6196(12)61843-6. [DOI] [PubMed] [Google Scholar]
- 2.Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000 Sep;26(1):89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 3.Horvath A, Bertherat J, Groussin L, et al. Mutations and polymorphisms in the gene encoding regulatory subunit type 1-alpha of protein kinase A (PRKAR1A): an update. Hum Mutat. 2010;31:369–379. doi: 10.1002/humu.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacGowan SW, Sidhu P, Aherne T, et al. Atrial myxoma: National incidence, diagnosis and surgical management. Ir J Med Sci. 1993;162:223–226. doi: 10.1007/BF02945200. [DOI] [PubMed] [Google Scholar]
- 5.Turhan S, Tulunay C, Altin T, et al. Second recurrence of familial cardiac myxomas in atypical locations. Can J Cardiol. 2008;24:715–716. doi: 10.1016/s0828-282x(08)70671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Mateen M, Hood M, Trippel D, et al. Cerebral Embolism From Atrial Myxoma in Pediatric Patients. Pediatrics. 2003;112:162–167. doi: 10.1542/peds.112.2.e162. [DOI] [PubMed] [Google Scholar]
- 7.Stratakis CA, Kirschner LS, Carney JA. Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab. 2001;86:4041–4046. doi: 10.1210/jcem.86.9.7903. [DOI] [PubMed] [Google Scholar]
- 8.Zahedi RG, Wald DS, Ohri S. Carney complex. Ann Thorac Surg. 2006;82:320–322. doi: 10.1016/j.athoracsur.2005.07.099. [DOI] [PubMed] [Google Scholar]
- 9.Vandersteen A, Turnbull J, Jan W, et al. Cutaneous signs are important in the diagnosis of the rare neoplasia syndrome Carney complex. Eur J Pediatr. 2009;168:1401–1404. doi: 10.1007/s00431-009-0935-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Q, Shang H, Zhou D, et al. Repeated embolism and multiple aneurysms: central nervous system manifestations of cardiac myxoma. Eur J Neurol. 2008;15:e112–113. doi: 10.1111/j.1468-1331.2008.02295.x. [DOI] [PubMed] [Google Scholar]
- 11.Ryou KS, Lee SH, Park SH. Multiple fusiform myxomatous cerebral aneurysms in a patient with Carney complex. J Neurosurg. 2008;109:318–320. doi: 10.3171/JNS/2008/109/8/0318. [DOI] [PubMed] [Google Scholar]
- 12.Jean WC, Walski-Easton SM, Nussbaum ES. Multiple intracranial aneurysms as delayed complications of an atrial myxoma: case report. Neurosurgery. 2001;49:200–202. doi: 10.1097/00006123-200107000-00031. [DOI] [PubMed] [Google Scholar]
- 13.Reynen K. Cardiac myxomas. N Engl J Med. 1995;333:1610–1617. doi: 10.1056/NEJM199512143332407. [DOI] [PubMed] [Google Scholar]
- 14.Stratakis CA, Carney JA, Lin JP, et al. Carney complex, a familial multiple neoplasia and lentiginosis syndrome. Analysis of 11 kindreds and linkage to the short arm of chromosome 2. J Clin Invest. 1996;97:699–705. doi: 10.1172/JCI118467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Baltimore) 2001;80:159–172. doi: 10.1097/00005792-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Ruttmann E, Willeit J, Ulmer H, et al. Neurological outcome of septic cardioembolic stroke after infective endocarditis. Stroke. 2006;37:2094–2099. doi: 10.1161/01.STR.0000229894.28591.3f. [DOI] [PubMed] [Google Scholar]
- 17.Mantovani G, Bondioni S, Corbetta S, et al. Analysis of GNAS1 and PRKAR1A gene mutations in human cardiac myxomas not associated with multiple endocrine disorders. J Endocrinol Invest. 2009;32:501–504. doi: 10.1007/BF03346496. [DOI] [PubMed] [Google Scholar]


