Abstract
Introduction
This study evaluates the short-term clinical outcome, radiological, histological and device retrieval findings of two patients with second generation lumbar total disc replacement (TDR).
Materials and methods
The first patient had a single level L4-L5 Activ-L TDR, the second patient a L4-L5 Mobidisc and L5-S1 Activ-L TDR. The TDRs were implanted elsewhere and had implantation times between 1.3 and 2.8 years.
Results
Plain radiographs and CT-scanning showed slight subsidence of the Activ-L TDR in both patients and facet joint degeneration. The patients underwent revision surgery because of recurrent back and leg pain. After removal of the TDR and posterolateral fusion, the pain improved. Histological examination revealed large ultrahigh molecular weight polyethylene (UHMWPE) particles and giant cells in the retrieved tissue surrounding the Mobidisc. The particles in the tissue samples of the Activ-L TDR were smaller and contained in macrophages. Retrieval analysis of the UHMWPE cores revealed evidence of minor adhesive and abrasive wear with signs of impingement in both TDR designs.
Conclusion
Although wear was unrelated to the reason for revision, this study demonstrates the presence of UHMWPE particles and inflammatory cells in second generation TDR. Long-term follow-up after TDR is indicated for monitoring wear and implant status.
Keywords: Total disc replacement, Disc degeneration, (Un)constrained, Revision surgery, Polyethylene wear
Introduction
Total disc replacement (TDR) has been developed as an alternative to spinal fusion for the treatment of degenerative disc disease [1–3]. Presumed advantages of TDRs are motion preservation, prevention of adjacent level degeneration and restoration of disc height [2–4]. Second generation TDR designs, such as the Mobidisc (LDR Spine, Troyes, France) and Activ-L (Aesculap AG, Tuttlingen, Germany) were developed from the first generation biconvex SB Charité III (Waldemar link, Hamburg, Germany) and Prodisc (Synthes, West Chester, PA, USA) [3, 5–7]. Second generation TDRs have a sliding, convex design with a spherical superior endplate combined with a flat inferior endplate and an ultrahigh molecular weight polyethylene (UHMWPE) core [2, 3, 5, 6]. These new designs were developed because of varying long-term success rates of the Charité TDR [1, 3, 8]. They were supposed to minimize UHMWPE wear debris, allow easier implantation and improve implant stability [7].
A TDR is semi-constrained or unconstrained depending on whether the centre of rotation (COR) is fixed or mobile [5, 6]. Semi-constrained TDRs are divided in designs with and without anterior–posterior (AP) translation [5, 6]. The Mobidisc is an unconstrained TDR and the Activ-L TDR is a semi-constrained design with absence of lateral translation and limited AP translation [7, 9–11]. In a clinical case series on the Mobidisc TDR, the back pain decreased from 6.8 to 2.6 on the visual analog scale (VAS) 2 years after implantation [7, 12]. The back pain of Activ-L patients decreased from 8.2 to 1.5 on the VAS 2 years postoperatively in a prospective clinical trial [13].
There are few retrieval studies available for TDR and little is known about the clinical effects of UHMWPE wear debris in the lumbar spine [14–17]. UHMWPE particles can activate an inflammatory response, which may result in osteolysis and failure of total knee and hip replacements [18, 19]. Punt et al. described the presence of UHMWPE particles and inflammatory cells in periprosthetic tissue after revision of Charité III TDR [14, 15, 17]. It is currently unknown whether UHMWPE wear particles and inflammatory cells are present after second generation TDR. The aim of the present study is to report on the clinical, radiological, histological and device retrieval findings of retrieved Activ-L and Mobidisc TDRs.
Materials and methods
Two patients with a TDR presented with recurrent back and leg pain at our outpatient clinic. The first patient had a single level L4–L5 Activ-L TDR, the second patient a L4–L5 Mobidisc and L5–S1 Activ-L TDR. The TDRs were implanted elsewhere. Radiographs and VAS pain scores were obtained during routine controls at our outpatient clinic. The TDRs were extracted during revision surgery and periprosthetic tissue was randomly collected. Bright field microscopy (Leica DM5000B) was used for examining the presence of inflammatory cells and grading them according to the Mirra classification [20]. Polarized light was used for detection and morphometric analysis (Leica Qwin software V3, Cambridge, UK) of the UHMWPE particles (100×). Damage to the retrieved components was analyzed using light microscopy and scanning electron microscopy (SEM). The UHMWPE cores were inspected for evidence of abrasive, adhesive, third body and fatigue wear mechanisms as well as evidence of impingement.
Case reports
Case 1
A 48-year-old male had experienced low back pain since the age of 20 with irradiation to the right leg and left buttock non-radicular in origin. A herniated disc was removed at L5–S1 when he was 30 years old. After 2 years of pain relief, the pain recurred after an automobile accident. The patient was dissatisfied with the result of corset treatment and a pain management program. In July 2006 at the age of 43, he received an Activ-L TDR at L4–L5, and intercorporal cage fusion with anterior spinal instrumentation at L5–S1 because of two-level degenerative disc disease.
The patient reported some pain relief for several weeks, however, the pain exacerbated to the pre-operative level. At presentation in February 2007 at our outpatient clinic, the pain was localized in the lower back with irradiation and numbness in the buttocks and both legs. Walking was limited to 10 min, standing to 10 min and sitting to 20 min. He woke up approximately four times a night due to pain. The VAS was 8 for the back, 4 for the right leg and 2 for the left leg.
Plain radiographs demonstrated that the TDR was slightly undersized, eccentrically positioned (Fig. 1). Limited motion of the Activ-L TDR was visible on flexion–extension radiographs [range of motion (ROM): 3°]. CT- and MRI-scanning revealed facet joint degeneration at L4–L5, but no signs of disc herniation, spinal stenosis or post-operative scarring. Therefore, the recurrent back pain was assumed to be due to facet joint degeneration. It was decided to remove the TDR by a left sided lumbotomy in October 2007, 1 year and 4 months after initial surgery because the patient strongly posed for TDR removal. Despite good fixation of the TDR to the bone, there was no osseous integration of the bone into the coating. The disc space was filled with a strut allograft. A small peritoneal lesion was sutured without remaining complaints. Instrumented posterolateral fusion of L4–L5 was performed during the same session. Intra-operative cultures were sterile.
Fig. 1.
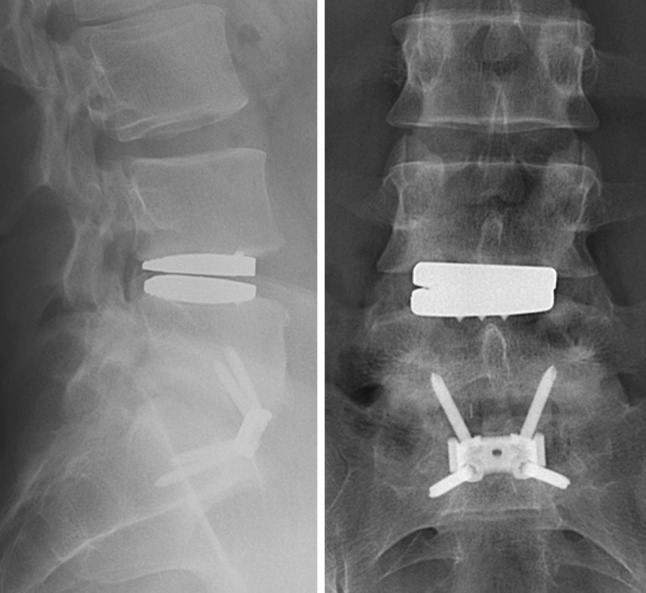
Lateral (left) and AP (right) radiographs showing a slightly undersized L4–L5 Activ-L TDR that slightly subsided
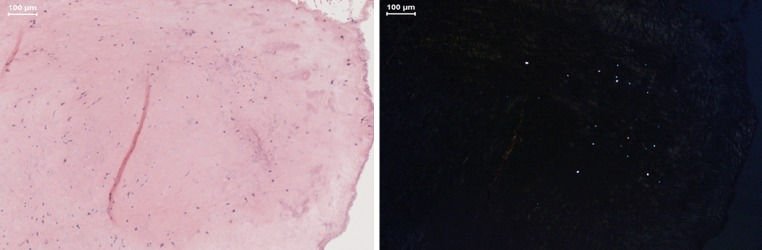
The mean number of UHMWPE particles in the periprosthetic tissue was 3 particles/mm2 with a mean curve length of 3.89 μm (range 2.05–9.59 μm). The particles had a round to oval shaped morphology. Histological examination showed a mild inflammatory reaction with mainly macrophages and an incidental giant cell (Fig. 2). An overview of the size and morphology of the UHMWPE particles and the amount of inflammatory cells is summarized in Table 1. There was no evidence of metallosis in the periprosthetic tissue.
Fig. 2.
Hematoxylin and eosin stained sections of retrieved periprosthetic fibrous tissue surrounding the Activ-L TDR, with light microscopy (left) and polarized light (right), containing small round polyethylene particles in macrophages
Table 1.
An overview of the characteristics of the UHMWPE particles and the amount of inflammatory cells graded according to the Mirra classification [18]
| Case number | Type TDR | Level | Implantation time (years) | Macrophagesa | Giant cellsb | Mean particles/mm2 | Mean curve length particles (μm) | Range curve length particles (μm) | Mean curve width particles (μm) | Mean roundnessc |
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | Activ-L | L4–L5 | 1.3 | 2+ | 1+ | 3 | 3.89 | 2.05–9.59 | 1.74 | 1.45 |
| Case 2 | Activ-L | L5–S1 | 1.6 | 2+ | 1+ | 1 | 3.07 | 2.05–4.10 | 1.02 | 1.61 |
| Mobidisc | L4–L5 | 2.8 | 0 | 1+ | 2 | 39.48 | 2.05–73.56 | 7.96 | 2.12 |
a1+: 1–9 macrophages/high power field (500×), 2+: 10–49 macrophages/high power field (500×), 3+: ≥50 macrophages/high power field (500×), five microscopic fields were scored
b1+: 1–2 cells/medium power field (250×), 2+: 3–8 cells/medium power field (250×), 3+: ≥9 cells/medium power field (250×), five microscopic fields were scored
cA mean roundness of 1.00 indicates a perfectly round shaped UHMWPE particle
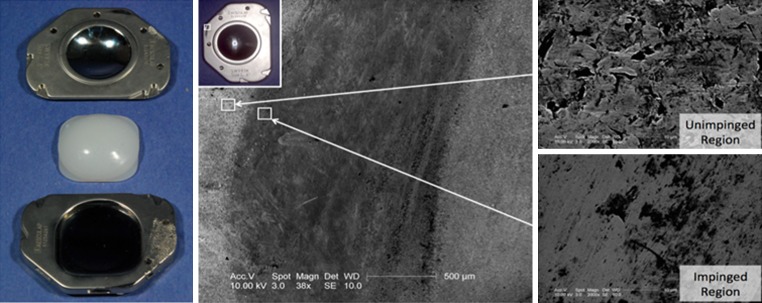
Overall, this retrieved UHMWPE core exhibited minimal wear. Burnishing and multi-directional scratching was observed on the backside and the articulating dome of the core. However, the original machining marks were still present, indicative of material removal on the order of micrometers. There was also evidence of the metallic endplates contacting one another at the anterior portion of the device. Impingement was confirmed using SEM. At high resolutions (38–2,000×), the areas where the endplates impinged were burnished and very smooth as compared to the unimpinged areas (Fig. 3).
Fig. 3.
Retrieved Activ-L TDR, 1.3 years after insertion. Note the impingement in the anterior portion of the superior endplate (center). The impinged region has a smoother appearance (lower right) than the unimpinged region (upper right)
Three years and 5 months after removal of the TDR, the patient experienced relief of the back pain (VAS 3.5) and no leg pain (VAS 0). The back pain consisted of a cramping sensation in the lower back at night which did not disturb sleeping. Radiographs showed fusion of the operated segment with slight anterior collapse into the allograft (Fig. 4).
Fig. 4.
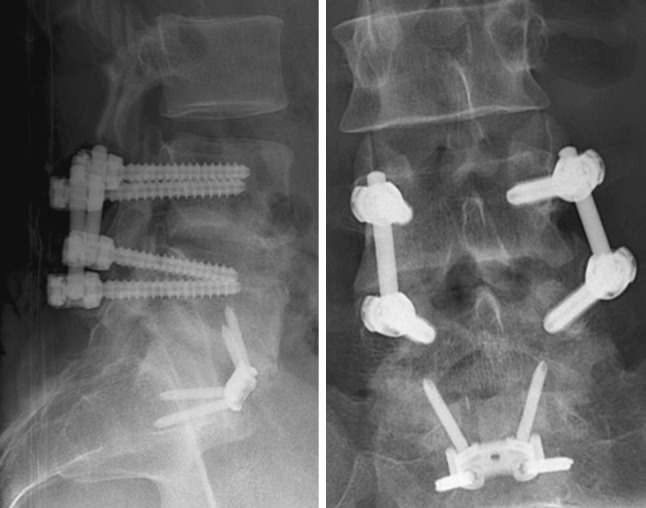
Lateral (left) and AP (right) postoperative radiographs after removal of the Activ-L TDR and posterolateral fusion of L4–L5
Case 2
A 49-year-old female patient had low back complaints with non-radicular irradiation to the right buttock since 2001. Discography revealed painful disc degeneration at L4–L5 and L5–S1. She received a Mobidisc TDR at level L4–L5 in August 2004 at age 42 and an Activ-L TDR at L5–S1 in September 2005.
The back and buttock complaints were relieved after insertion of the Activ-L TDR but recurred in 2006. When the patient presented in August 2006 at our outpatient clinic, walking was limited to 30 min, standing to 10 min and sitting to 60 min. At night she woke up approximately three times and had to get out of bed to relieve the pain. The pain did not improve with pain medication, physical therapy and nerve blockage.
Plain radiographs showed slight subsidence of the Activ-L TDR and a slight eccentric position of the Mobidisc TDR (Fig. 5). Flexion–extension radiographs showed a ROM of 7° for the Mobidisc and 4° for the Activ-L TDR. CT-scanning revealed facet joint degeneration at L4–L5 and L5–S1, with no signs of spinal stenosis or compression. The facet joint degeneration could be a feasible explanation for the recurrent back pain. It was the patient’s preference to have both TDRs removed. In June 2007, the Mobidisc and Activ-L TDR were retrieved using a left sided lumbotomy extended distally, 2 years and 10 months and 1 year and 5 months after implantation, respectively [21]. The TDRs were well fixed to the bone, but there was no osseous integration. The disc spaces were filled with a strut allograft and bone chips. Two days after the operation the patient developed respiratory complaints due to acute respiratory distress syndrome most likely caused by an allergic reaction to medication. She recovered without remaining complaints. Six weeks later, after a delay of 5 weeks, posterior instrumentation and fusion at L4–S1 was performed. All intra-operative cultures were sterile.
Fig. 5.
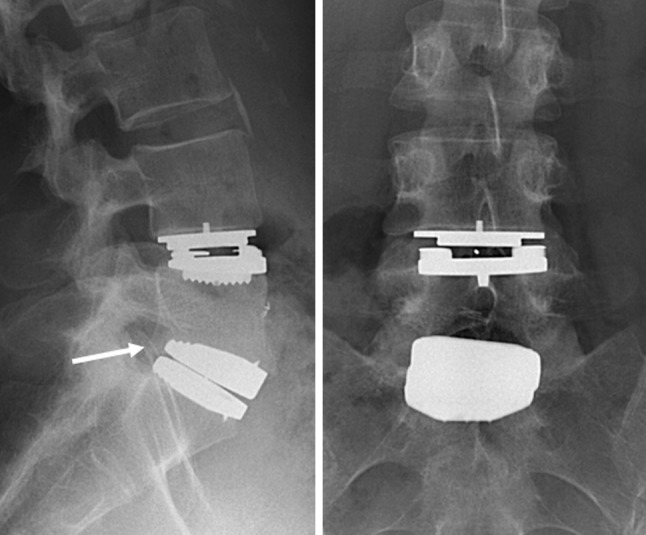
Lateral (left) and AP (right) radiographs showing a Mobidisc TDR at L4–L5 and an Activ-L TDR at L5–S1. The Mobidisc is correctly sized and correctly positioned, the Activ-L TDR is adequately sized but subsided (arrow)
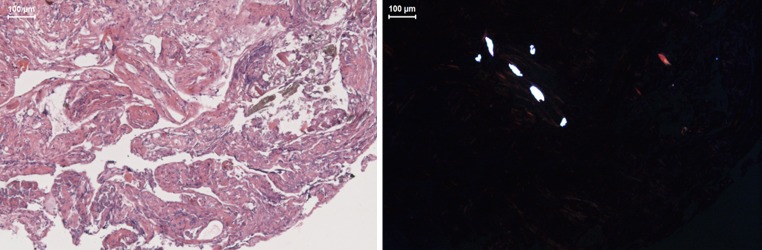
The mean number of UHMWPE particles in the retrieved periprosthetic tissue was 1 and 2 particles/mm2 for the Activ-L and Mobidisc, respectively (Table 1). The particles retrieved from the Activ-L TDR had a round to oval shaped morphology, the particles from the Mobidisc were more flake shaped. The mean curve length was 3.07 μm (range 2.05–4.10 μm) for the Activ-L and 39.48 μm (range 2.05–73.56 μm) for the Mobidisc. Surrounding the Activ-L TDR we observed macrophages and on both levels a small amount of giant cells (Fig. 6). No metal particles were found in the tissue surrounding both TDRs.
Fig. 6.
Hematoxylin and eosin stained sections of retrieved periprosthetic fibrous tissue surrounding the Mobidisc TDR, with light microscopy (left) and polarized light (right), containing large flakes of polyethylene in giant cells
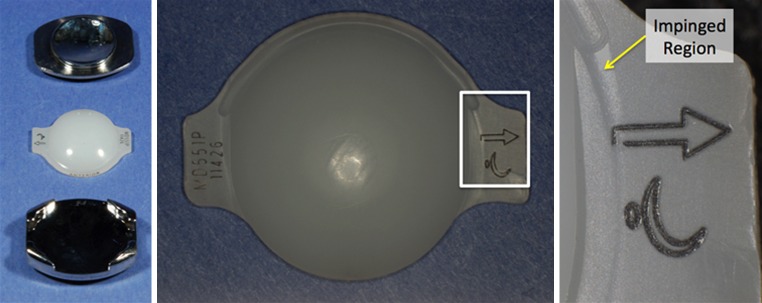
The UHMWPE cores in both retrievals exhibited minimal wear. Although machining marks were still present on the superior and inferior articulating surfaces of the Activ-L core, they were eroded at locations on the Mobidisc core. Nevertheless, we were unable to measure any deviation in total height of the core from the manufacturer’s specifications (accuracy 0.001 mm). Multi-directional scratching and burnishing was observed for both the Activ-L and the Mobidisc TDR on both the dome and the backside of the cores. There was evidence of metal-on-metal impingement on the left side of the superior endplate of the Activ-L TDR. In the case of the Mobidisc TDR, the right lateral wing of the core had impinged with the superior endplate (Fig. 7).
Fig. 7.
Retrieved Mobidisc TDR (left), 2.8 years after implantation, with impingement on right lateral wing (center and right)
Three years and 9 months after removal, the back complaints were relieved (VAS 3) and there was no buttock pain (VAS 0). Initially, the back pain varied in severity but gradually decreased after a revalidation program and epidural injections. She resumed sports activities and was able to refrain from pain medication. Radiographs showed a solid fusion with unaltered position of the instrumentation (Fig. 8).
Fig. 8.
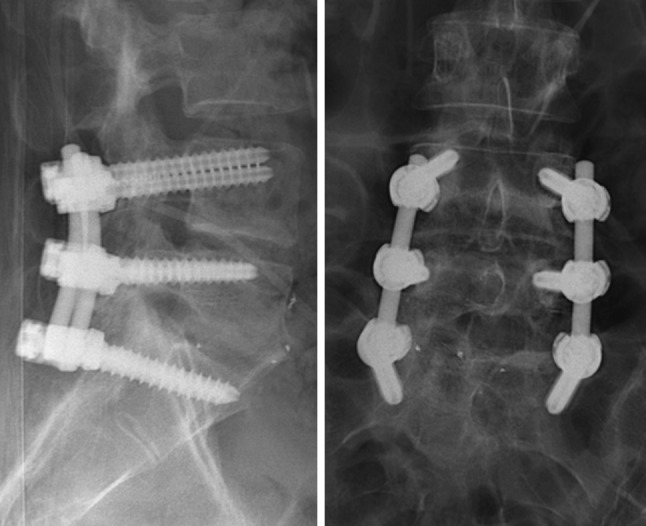
Lateral (left) and AP (right) postoperative radiographs after removal of the Mobidisc and Activ-L TDR and posterolateral fusion of L4–S1
Discussion
Two patients with second generation Activ-L and Mobidisc TDRs had recurrent back and leg pain, which improved after TDR removal and subsequent posterolateral fusion. Plain radiographs and CT-scanning showed slight subsidence of both Activ-L TDRs and facet joint degeneration. UHMWPE particles and inflammatory cells were present in the tissue surrounding the TDRs. The UHMWPE cores exhibited minimal abrasive and adhesive wear, but showed evidence of impingement.
The mean number of UHMWPE particles was two orders of magnitude lower in the Activ-L (1–3 particles/mm2) and Mobidisc (2 particles/mm2) compared to a previous study on the SB Charité III TDR (231 particles/mm2) [14, 17]. The lower wear in the present study can be explained by a combination of factors, including implantation time, TDR design and material factors. The higher number of UHMWPE particles in the Charité TDR study can result from differences in mean implantation time (1.6 vs. 10.0 years) [14, 17]. Further, the Charité retrievals were gamma sterilized in air, in a first generation oxygen permeable package [14, 17, 22–24]. The cores of the Mobidisc and Activ-L TDR were gamma irradiation sterilized in a second generation barrier package, designed to preserve the mechanical properties of UHMWPE [3, 7, 22].
The difference in UHMWPE particle size between the Activ-L and Mobidisc TDR could be explained by having an unconstrained and semi-constrained TDR design. In total knee replacement (TKR), the same phenomenon is described [25, 26]. Larger UHMWPE particles are found in tissue surrounding failed mobile bearing TKR than in tissue surrounding failed fixed bearing TKR [25, 26]. Design-dependant differences in loading and wear mechanisms can be an explanation for these size differences [3, 7]. Large flake shaped UHMWPE particles, present in the tissue surrounding the Mobidisc TDR, were associated with fewer inflammatory cells. It could be possible that the particles of the Mobidisc TDR have a tendency to be less bioactive.
Faint signs of impingement were observed in all three TDRs, which is perhaps not surprising since the devices had subsided into biomechanically unfavorable positions. Unnatural motion patterns also could have contributed to the impingement. Impingement has been observed in many types of joint replacement, including the hip and shoulder, as well as previous TDR designs [27–30]. It may lead to UHMWPE degradation and increased amounts of wear [27–30]. Because of the short implantation times, the long-term implications of impingement of the two designs in this study are unknown. However, the metal-on-metal wear observed in the Activ-L TDR is of particular concern, as it may produce metal wear debris.
The presence of UHMWPE wear particles results in the activation of an inflammatory response, associated with osteolysis and aseptic loosening of joint replacements [18, 19]. Macrophages and giant cells release various cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1) and interleukin-6 (IL-6). These cytokines are considered to be potent inducers of osteoclasts [18, 19]. They also play a role in the development of neuro-inflammatory pain [31, 32]. Since osteolysis after TDR is rarely observed, it could be possible that the UHMWPE particle concentration after TDR is too low for causing osteolysis in most cases, but is potent enough to initiate neuro-inflammation and recurrent back pain. Therefore, the presence of wear after TDR continues to be of clinical concern. This is especially true for the younger and active patients who frequently receive TDR.
Facet joint degeneration can contribute to recurrent back pain after TDR [10, 33–35]. The patient in case 1 had an anterior spinal fusion at L5–S1, which can accelerate facet joint degeneration, as well as unnatural motions in the L4–L5 Activ-L TDR [36–38]. In case 2, the patient received a two-level TDR which can lead to hypermobility and accelerates facet joint degeneration [39–41]. It is stated that a fixed COR should prevent overloading of the facets [6, 10]. While, others state that a mobile COR leads to a lower facet contact force by an equilibrium between facet loading and ligament tension [5, 9]. Also malpositioning of the TDR causes higher stresses on the facets [11, 42]. Nevertheless, facet joint degeneration was present at L4–L5 and L5–S1 in both cases with both TDR designs.
There is an ongoing discussion about the optimal revision strategy for failed TDRs [21, 43–45]. With acceptable implant status and position, posterior fusion can be addressed for treatment of recurrent back pain due to facet joint degeneration [44, 45]. When the TDR has subsided, migrated or mechanically failed, the pain can be addressed by TDR removal [45]. The outcome of secondary fusion with the TDR in situ has not been well reported. In our case load of failed TDRs we started with posterolateral fusion without removal of the TDR. Unfortunately, the results were disappointing in most patients [21, 46]. Thereafter, we combined fusion with TDR removal if the patient accepts the risks of retrieval surgery.
Acknowledgments
Institutional support for two of the authors (SMK and DWM) has been received from Medtronic, LDR Spine, National Institutes of Health (NIH) R01 AR56264 and the Food and Drug Administration (FDA) Critical Path initiative (HHSF223200930112G).
Conflict of interest
None of the authors has any potential conflict of interest.
References
- 1.Yajun W, Yue Z, Xiuxin H, Cui C. A meta-analysis of artificial total disc replacement versus fusion for lumbar degenerative disc disease. Eur Spine J. 2010;19:1250–1261. doi: 10.1007/s00586-010-1394-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bono CM, Garfin SR. History and evolution of disc replacement. Spine J. 2004;4:145S–150S. doi: 10.1016/j.spinee.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Kurtz SM (2009) Ultra high molecular weight polyethylene in total joint replacement and medical devices. In: UHMWPE Biomaterials Handbook, 2nd edn. Academic Press, New York
- 4.Harrop JS, Youssef JA, Maltenfort M, Vorwald P, Jabbour P, Bono CM, Goldfarb N, Vaccaro AR, Hilibrand AS. Lumbar adjacent segment degeneration and disease after arthrodesis and total disc arthroplasty. Spine (Phila Pa 1976) 2008;33:1701–1707. doi: 10.1097/BRS.0b013e31817bb956. [DOI] [PubMed] [Google Scholar]
- 5.Chung SK, Kim YE, Wang KC. Biomechanical effect of constraint in lumbar total disc replacement: a study with finite element analysis. Spine (Phila Pa 1976) 2009;34:1281–1286. doi: 10.1097/BRS.0b013e3181a4ec2d. [DOI] [PubMed] [Google Scholar]
- 6.Huang RC, Girardi FP, Cammisa FP, Jr, Wright TM. The implications of constraint in lumbar total disc replacement. J Spinal Disord Tech. 2003;16:412–417. doi: 10.1097/00024720-200308000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Steib J, Aubourg L, Beaurain J. Mobidisc prosthesis. In: Yue JJ, editor. Motion preservation surgery of the spine. Philadelphia: Saunders; 2008. pp. 326–329. [Google Scholar]
- 8.Eerenbeemt KD, Ostelo RW, Royen BJ, Peul WC, Tulder MW. Total disc replacement surgery for symptomatic degenerative lumbar disc disease: a systematic review of the literature. Eur Spine J. 2010;19:1262–1280. doi: 10.1007/s00586-010-1445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ha SK, Kim SH, Kim DH, Park JY, Lim DJ, Lee SK. Biomechanical study of lumbar spinal arthroplasty with a semi-constrained artificial disc (activ L) in the human cadaveric spine. J Korean Neurosurg Soc. 2009;45:169–175. doi: 10.3340/jkns.2009.45.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zander T, Rohlmann A, Bergmann G. Influence of different artificial disc kinematics on spine biomechanics. Clin Biomech (Bristol, Avon) 2009;24:135–142. doi: 10.1016/j.clinbiomech.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Moumene M, Geisler FH. Comparison of biomechanical function at ideal and varied surgical placement for two lumbar artificial disc implant designs: mobile-core versus fixed-core. Spine (Phila Pa 1976) 2007;32:1840–1851. doi: 10.1097/BRS.0b013e31811ec29c. [DOI] [PubMed] [Google Scholar]
- 12.Delécrin J, Allain J, Steib J, Beucrain J, Dufour T, Chataigner H, Gau M, Huppert J, Onimus M, Zeegers W (2008) Mobidisc lumbar arthroplasty: Multi-center study with 2 years follow-up. In: 9th Congress of the European Federation of National Associations of Orthopaedics and Traumatology Nice, France
- 13.Yue JJ (2010) A prospective clinical comparison of 3 biomechanical types of lumbar total disc replacements: A semi-constrained device, a controlled translation device, and an unconstrained device, minimum 2 year follow-up. In: Annual Conference of the International Society for the Advancement of Spine Surgery. Perpignan, France
- 14.Punt IM, Cleutjens JP, Bruin T, Willems PC, Kurtz SM, Rhijn LW, Schurink GW, Ooij A. Periprosthetic tissue reactions observed at revision of total intervertebral disc arthroplasty. Biomaterials. 2009;30:2079–2084. doi: 10.1016/j.biomaterials.2008.12.071. [DOI] [PubMed] [Google Scholar]
- 15.Kurtz SM, Ooij A, Ross R, Waal Malefijt J, Peloza J, Ciccarelli L, Villarraga ML. Polyethylene wear and rim fracture in total disc arthroplasty. Spine J. 2007;7:12–21. doi: 10.1016/j.spinee.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Punt I, Baxter R, Ooij A, Willems P, Rhijn L, Kurtz S, Steinbeck M. Submicron sized ultra-high molecular weight polyethylene wear particle analysis from revised SB Charite III total disc replacements. Acta Biomater. 2011;7:3404–3411. doi: 10.1016/j.actbio.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Punt IM, Austen S, Cleutjens JP, Kurtz SM, Ten Broeke RH, van Rhijn LW, Willems PC, Ooij AV (2011) Are periprosthetic tissue reactions observed after revision of total disc replacement comparable to the reactions observed after total hip or knee revision surgery? Spine (Phila Pa 1976) (in press) [DOI] [PMC free article] [PubMed]
- 18.Gallo J, Raska M, Mrazek F, Petrek M. Bone remodeling, particle disease and individual susceptibility to periprosthetic osteolysis. Physiol Res. 2008;57:339–349. doi: 10.33549/physiolres.931140. [DOI] [PubMed] [Google Scholar]
- 19.Dumbleton JH, Manley MT, Edidin AA. A literature review of the association between wear rate and osteolysis in total hip arthroplasty. J Arthroplast. 2002;17:649–661. doi: 10.1054/arth.2002.33664. [DOI] [PubMed] [Google Scholar]
- 20.Mirra JM, Amstutz HC, Matos M, Gold R. The pathology of the joint tissues and its clinical relevance in prosthesis failure. Clin Orthop Relat Res. 1976;117:221–240. [PubMed] [Google Scholar]
- 21.Maat GH, Punt IM, Rhijn LW, Schurink GW, Ooij A. Removal of the Charite lumbar artificial disc prosthesis: surgical technique. J Spinal Disord Tech. 2009;22:334–339. doi: 10.1097/BSD.0b013e3181761d0c. [DOI] [PubMed] [Google Scholar]
- 22.Kurtz SM, MacDonald D, Ianuzzi A, Ooij A, Isaza J, Ross ER, Regan J. The natural history of polyethylene oxidation in total disc replacement. Spine (Phila Pa 1976) 2009;34:2369–2377. doi: 10.1097/BRS.0b013e3181b20230. [DOI] [PubMed] [Google Scholar]
- 23.Costa L, Bracco P, Brach del Prever EM, Kurtz SM, Gallinaro P. Oxidation and oxidation potential in contemporary packaging for polyethylene total joint replacement components. J Biomed Mater Res B Appl Biomater. 2006;78:20–26. doi: 10.1002/jbm.b.30454. [DOI] [PubMed] [Google Scholar]
- 24.Kurtz SM. Wear in highly crosslinked polyethylenes. Curr Orthop. 2008;22:392–399. doi: 10.1016/j.cuor.2008.10.011. [DOI] [Google Scholar]
- 25.Minoda Y, Kobayashi A, Iwaki H, Miyaguchi M, Kadoya Y, Ohashi H, Takaoka K. Characteristics of polyethylene wear particles isolated from synovial fluid after mobile-bearing and posterior-stabilized total knee arthroplasties. J Biomed Mater Res B Appl Biomater. 2004;71:1–6. doi: 10.1002/jbm.b.30005. [DOI] [PubMed] [Google Scholar]
- 26.Hirakawa K, Bauer TW, Stulberg BN, Wilde AH, Borden LS. Characterization of debris adjacent to failed knee implants of 3 different designs. Clin Orthop Relat Res. 1996;331:151–158. doi: 10.1097/00003086-199610000-00021. [DOI] [PubMed] [Google Scholar]
- 27.Kurtz SM, Austin MS, Azzam K, Sharkey PF, MacDonald DW, Medel FJ, Hozack WJ. Mechanical properties, oxidation, and clinical performance of retrieved highly cross-linked Crossfire liners after intermediate-term implantation. J Arthroplast. 2010;25(614–623):e611–e612. doi: 10.1016/j.arth.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Currier BH, Currier JH, Mayor MB, Lyford KA, Collier JP, Citters DW. Evaluation of oxidation and fatigue damage of retrieved crossfire polyethylene acetabular cups. J Bone Joint Surg Am. 2007;89:2023–2029. doi: 10.2106/JBJS.F.00336. [DOI] [PubMed] [Google Scholar]
- 29.Nam D, Kepler CK, Nho SJ, Craig EV, Warren RF, Wright TM. Observations on retrieved humeral polyethylene components from reverse total shoulder arthroplasty. J Shoulder Elb Surg. 2010;19:1003–1012. doi: 10.1016/j.jse.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Day JS, Macdonald DW, Olsen M, Getz C, Williams GR, Kurtz SM (2011) Polyethylene wear in retrieved reverse total shoulder components. J Shoulder Elbow Surg (in press) [DOI] [PMC free article] [PubMed]
- 31.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 32.Thacker MA, Clark AK, Marchand F, McMahon SB. Pathophysiology of peripheral neuropathic pain: immune cells and molecules. Anesth Analg. 2007;105:838–847. doi: 10.1213/01.ane.0000275190.42912.37. [DOI] [PubMed] [Google Scholar]
- 33.Siepe CJ, Zelenkov P, Sauri-Barraza JC, Szeimies U, Grubinger T, Tepass A, Stabler A, Mayer MH. The fate of facet joint and adjacent level disc degeneration following total lumbar disc replacement: a prospective clinical, X-ray, and magnetic resonance imaging investigation. Spine (Phila Pa 1976) 2010;35:1991–2003. doi: 10.1097/BRS.0b013e3181d6f878. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt H, Midderhoff S, Adkins K, Wilke HJ. The effect of different design concepts in lumbar total disc arthroplasty on the range of motion, facet joint forces and instantaneous center of rotation of a L4–5 segment. Eur Spine J. 2009;18:1695–1705. doi: 10.1007/s00586-009-1146-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siepe CJ, Korge A, Grochulla F, Mehren C, Mayer HM. Analysis of post-operative pain patterns following total lumbar disc replacement: results from fluoroscopically guided spine infiltrations. Eur Spine J. 2008;17:44–56. doi: 10.1007/s00586-007-0519-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cunningham BW, Gordon JD, Dmitriev AE, Hu N, McAfee PC. Biomechanical evaluation of total disc replacement arthroplasty: an in vitro human cadaveric model. Spine (Phila Pa 1976) 2003;28:S110–S117. doi: 10.1097/01.BRS.0000092209.27573.90. [DOI] [PubMed] [Google Scholar]
- 37.Ahn DK, Park HS, Choi DJ, Kim KS, Yang SJ. Survival and prognostic analysis of adjacent segments after spinal fusion. Clin Orthop Surg. 2010;2:140–147. doi: 10.4055/cios.2010.2.3.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee CS, Hwang CJ, Lee SW, Ahn YJ, Kim YT, Lee DH, Lee MY. Risk factors for adjacent segment disease after lumbar fusion. Eur Spine J. 2009;18:1637–1643. doi: 10.1007/s00586-009-1060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ching AC, Birkenmaier C, Hart RA. Short segment coronal plane deformity after two-level lumbar total disc replacement. Spine (Phila Pa 1976) 2010;35:44–50. doi: 10.1097/BRS.0b013e3181b9d556. [DOI] [PubMed] [Google Scholar]
- 40.Siepe CJ, Mayer HM, Heinz-Leisenheimer M, Korge A. Total lumbar disc replacement: different results for different levels. Spine (Phila Pa 1976) 2007;32:782–790. doi: 10.1097/01.brs.0000259071.64027.04. [DOI] [PubMed] [Google Scholar]
- 41.Huppert J, Beaurain J, Steib JP, Bernard P, Dufour T, Hovorka I, Stecken J, Dam-Hieu P, Fuentes JM, Vital JM, Vila T, Aubourg L. Comparison between single- and multi-level patients: clinical and radiological outcomes 2 years after cervical disc replacement. Eur Spine J. 2011;20:1417–1426. doi: 10.1007/s00586-011-1722-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gstoettner M, Heider D, Liebensteiner M, Bach CM. Footprint mismatch in lumbar total disc arthroplasty. Eur Spine J. 2008;17:1470–1475. doi: 10.1007/s00586-008-0780-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spivak JM, Petrizzo AM. Revision of a lumbar disc arthroplasty following late infection. Eur Spine J. 2010;19:677–681. doi: 10.1007/s00586-009-1226-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tropiano P. Expert’s comment concerning Grand Rounds case entitled “Revision of a lumbar disc arthroplasty following late infection” (by Jeffrey M. Spivak and Anthony M. Petrizzo) Eur Spine J. 2010;19:682–684. doi: 10.1007/s00586-009-1225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel AA, Brodke DS, Pimenta L, Bono CM, Hilibrand AS, Harrop JS, Riew KD, Youssef JA, Vaccaro AR. Revision strategies in lumbar total disc arthroplasty. Spine (Phila Pa 1976) 2008;33:1276–1283. doi: 10.1097/BRS.0b013e3181714a1d. [DOI] [PubMed] [Google Scholar]
- 46.Punt IM, Visser VM, Rhijn LW, Kurtz SM, Antonis J, Schurink GW, Ooij A. Complications and reoperations of the SB Charite lumbar disc prosthesis: experience in 75 patients. Eur Spine J. 2008;17:36–43. doi: 10.1007/s00586-007-0506-8. [DOI] [PMC free article] [PubMed] [Google Scholar]