Abstract
Purpose
To report a case of Veillonella spondylodiscitis in a healthy 76-year-old lady.
Methods
A previously healthy 76-year-old lady presented with worsening axial back pain at the thoracolumbar junction, fever and loss of weight. Examination revealed deep tenderness over the thoracolumbar junction with painful and restricted spinal movements. The lower limb motor power, sensation and reflexes were normal.
Results
Radiographs of the lumbosacral spine showed evidence of spinal instability with lateral translation and loss of disc space at L1–L2. MRI scans revealed fluid intensity within the L1–L2 disc with infective debris elevating the posterior longitudinal ligament and narrowing the spinal canal. Both tissue and blood cultures were positive for the anaerobic organism, Veillonella. A staged anterior-posterior spinal surgery followed by an extended course of antibiotics resulted in the clinical improvement and normalisation of blood parameters. A review of the literature on Veillonella infections is also presented.
Conclusion
The aim of this report is to bring Veillonella spondylodiscitis to the attention of spinal surgeons and infectious disease specialists and discuss the management options.
Keywords: Veillonella, Infection, Spine, Spondylodiscitis
Introduction
The annual incidence of infective spondylitis reported in two Scandinavian studies was 1 and 2.2 per 100,000 inhabitants per year [1, 2]. While 75% of these infections were pyogenic, the remaining 25% were caused by tuberculosis [2]. In adults, pyogenic infection involves the vertebral body with early destruction of the disc. Staphylococcus aureus, Staphylococcus epidermidis and Streptococcus account for about 68–80% of spondylodiscitis [1, 3, 4]. Anaerobic bacteria account for only 3–5% of spinal infections [3, 4]. Risk factors for spinal infection include increasing age, diabetes, renal failure, intravenous drug abuse, immunodeficiency states and the presence of an extra-spinal source of infection [1–8]. In the pre-antibiotic era, spondylodiscitis carried a mortality rate of between 25 and 100% [5, 9]. Since that time, a majority of spinal infections have been effectively managed with antibiotics alone, and surgery has only been required when there is a failure to respond to antibiotics (with the potential need for an open biopsy) or when there are significant or progressive neurological deficits, spinal instability or deformity [6, 8].
In this report, we present the case of a 76-year-old lady, who was diagnosed with infective spondylodiscitis at the L1–L2 level. Both tissue and blood cultures were positive for the anaerobic organism, Veillonella which is an uncommon cause of spondylodiscitis. A two-staged anterior-posterior surgery followed by an extended course of antibiotics resulted in the clinical improvement and normalisation of blood parameters. The aim of this report is to bring Veillonella spondylodiscitis to the attention of spinal surgeons and infectious disease specialists and discuss the management options.
Case report
A 76-year-old lady presented with a 3-month history of worsening axial pain at the thoracolumbar junction, low-grade fever and weight loss. The pain was constant, moderately severe, non-radiating and aggravated by movement. There was no history of diabetes, immunodeficiency, intravenous drug use, periodontal disease, or previous gastro-intestinal endoscopy. On examination, there was deep tenderness over the thoracolumbar junction. Movements of the spine were painful and restricted in all directions with normal motor power, sensation and reflexes in both lower limbs.
Antero-posterior radiographs of the lumbosacral spine revealed lateral translation of the L1 vertebra over the L2 vertebra with complete loss of the L1–L2 disc space and segmental kyphosis on the lateral view (Figs. 1-2). An MRI scan of the lumbosacral spine revealed fluid signal within the narrowed L1–L2 intervertebral disc space with loss of definition of the adjacent end-plates and infective debris elevating the posterior longitudinal ligament and narrowing the spinal canal (Figs. 3-4, 5-6). Multiple small abscesses were present in the right paraspinal space extending as far laterally as the right diaphragmatic crus and the origin of the right psoas muscle. There was diffuse inflammatory change in the right psoas muscle extending inferiorly to the L3–L4 level.
Fig. 1-2.
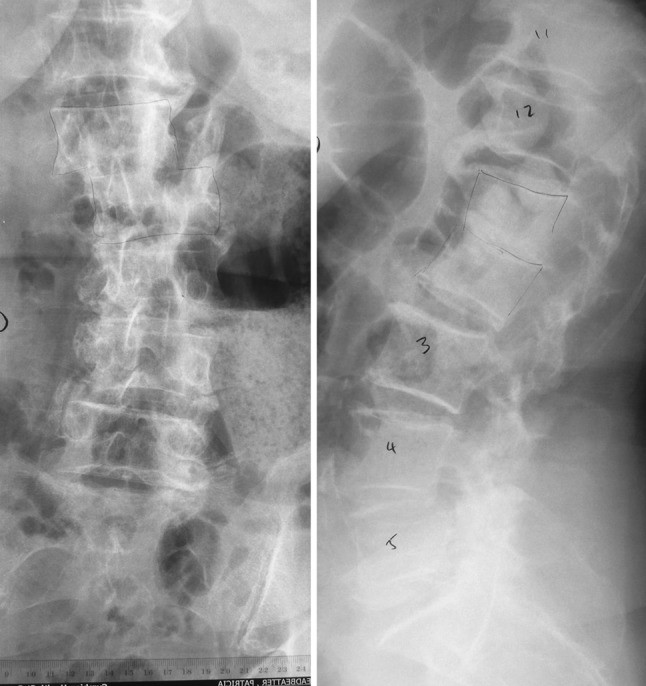
Anteroposterior and lateral radiographs of the lumbosacral spine showing lateral translation and loss of disc space and vertebral destruction at L1–L2 level
Fig. 3-4.
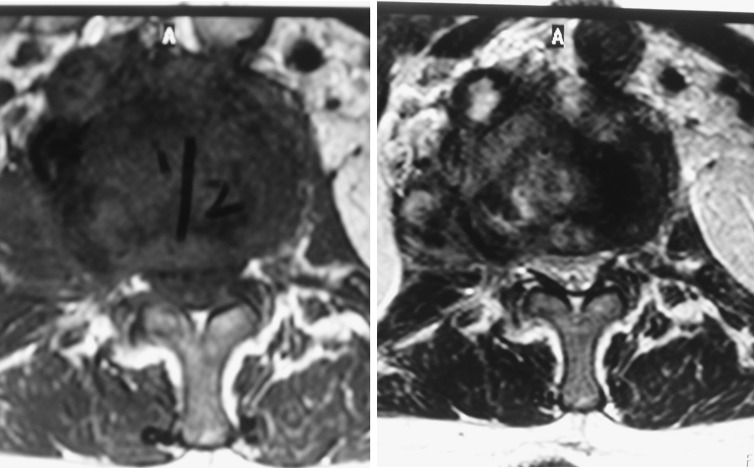
T1 and T2 weighted sagittal MR images of the lumbosacral spine showing fluid in the L1–L2 disc with loss of definition of the adjacent end-plates and infective debris elevating the posterior longitudinal ligament and narrowing the spinal canal
Fig. 5-6.
T1 and T2 weighted axial MR images of the lumbosacral spine showing narrowing of the spinal canal caused by infective debris at the L1–L2 level
On admission, the Haemoglobin was 116 g/L; WBC count = 13.6 × 109/L; ESR = 76 mm/h (normal 1–35) and CRP = 125.2 mg/L (normal 0.0–5.0). A CT guided percutaneous vertebral biopsy was not diagnostic. Aerobic blood culture was negative at 5 days, however Veillonella grew from anaerobic cultures after 7 days of incubation.
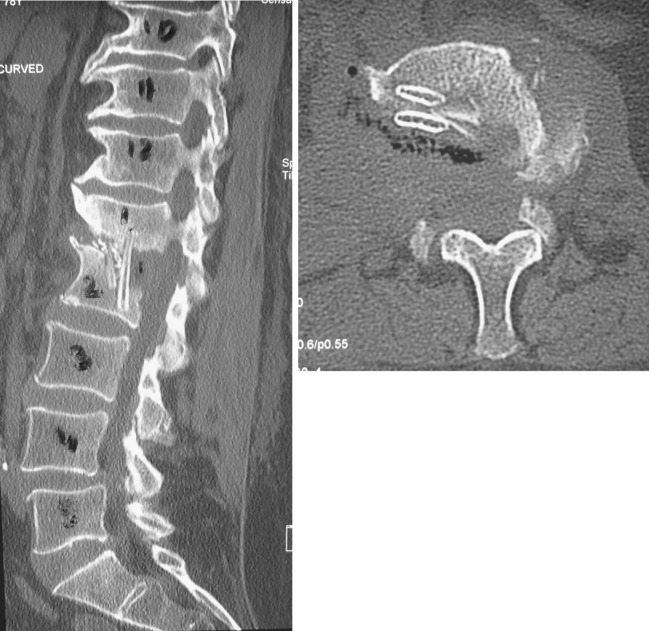
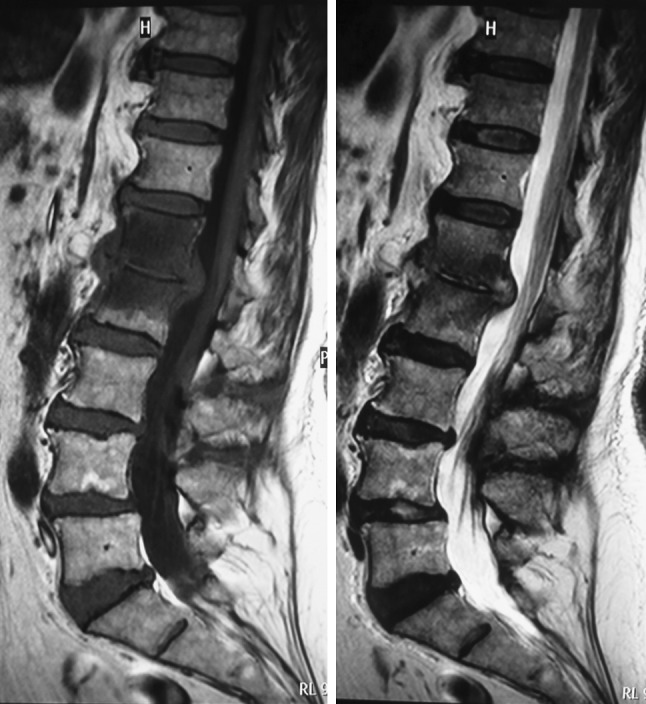
In view of the instability caused by the lateral translation of the L1 vertebra over the L2 vertebra, a spinal stabilization was planned. In the first stage, an anterior L1–L2 debridement and autologous rib grafting was performed through a retroperitoneal approach (Figs. 7-8). Intravenous antibiotics were commenced following the anterior surgery. Culture of the debris and excised tissue was positive for Veillonella at 7 days on enrichment medium. In the second stage (3 days later), a posterior pedicle screw instrumented fusion was performed extending from T11–L4 (Figs. 9-10). In addition, the patient received a combination of Cefotaxime (1 g, twice a day) and Metronidazole (500 mg, thrice a day) intravenously for 4 weeks followed by a combination of oral Augmentin Duo Forte (twice a day) and oral Metronidazole (400 mg, thrice a day) for a further 6 weeks. Serial blood tests confirmed a fall in the ESR and CRP with both parameters attaining normal levels 3 weeks after commencement of antibiotics.
Fig. 7-8.
Sagittal and axial CT scan sections performed after the first stage of surgery showing the extent of the surgical decompression and the rib grafts in situ
Fig. 9-10.
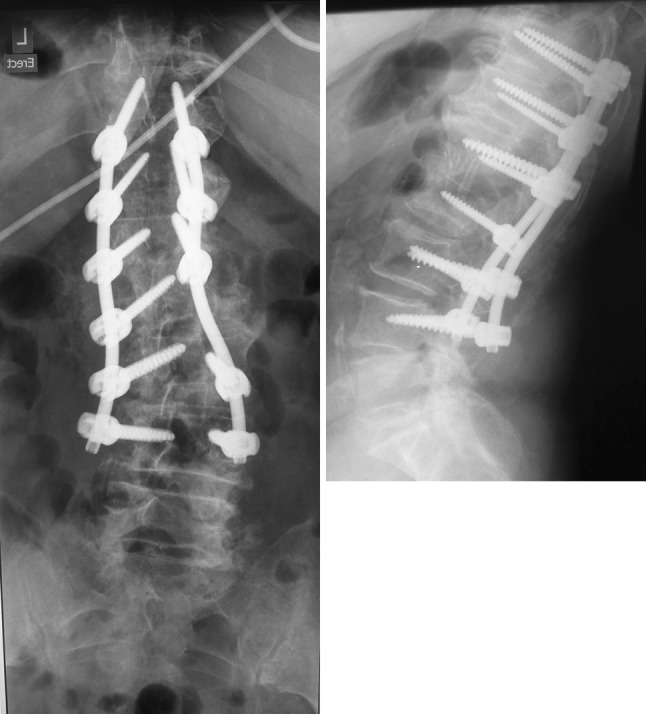
Post-operative radiographs (anteroposterior and lateral) showing a T11–L4 posterior instrumented spinal fusion with spinal realignment
Discussion
In the study performed by Hall et al. [10], 78% of 182 cases of osteomyelitis (including spine) were caused by aerobic organisms and of the remainder, a mixture of aerobic and anaerobic organisms was isolated in 17% and a pure growth of anaerobic organisms was isolated in 5% of cases. Peptococcus magnus, P. prevotii, P. asaccharalyticus and P. variabilis were the most common isolates with one instance of Veillonella alcalescens. In view of the reported incidence of anaerobic infections, we would like to emphasise the necessity for the routine use of anaerobic culture.
Veillonella is an anaerobic, non-motile, non-sporulating Gram-negative coccus that forms part of the normal microflora in the oral cavity, gastrointestinal tract, and vagina. Veillonella has been implicated in dental infections, pulmonary infections, cardiac prosthetic valve infections, endocarditis [11], osteomyelitis [10], prosthetic joint infections [12, 13] and fatal sepsis [14]. Differentiation of the various species of Veillonella (Veillonella dispar, Veillonella atypica, and Veillonella parvula) requires restricted fragment-length polymorphism (RFLP) analysis of polymerase chain reaction (PCR) amplified 16s rDNA [15]. Veillonella parvula is the most frequently isolated pathogenic subtype. Sutter [16] found that 100% of the strains of Veillonella were sensitive to cephalothin, cefoxitin, clindamycin and metronidazole. In a recent study, Veillonella species isolated from the oral cavity of humans demonstrated a high level of resistance to penicillin G [17].
Marriott [18] reported on a 55-year-old gentleman who presented with back pain, night sweats and rigors 2 months after a colonoscopy and rectal biopsy. Veillonella was isolated from the blood and from tissue obtained by a percutaneous biopsy taken from the L2–L3 disc (the latter grown from horse blood agar and chocolate agar incubated for 7 days at 35°C under 5% CO2 atmospheric conditions). The patient recovered after treatment with intravenous ceftriaxone for 6 weeks followed by oral amoxicillin–clavulanic acid for a further 6 weeks. The authors believe that the rectal biopsy site was the portal of entry of the organism. Isner-Horobeti [19] reported on a 27-year-old man with unknown risk factors for infection, who presented with an L4–L5 spondylodiscitis. A fine needle biopsy from the L4–L5 disc revealed Veillonella after delayed cultures. The infection responded to a 3-week course of intravenous Amoxicillin (12 g/day) followed by 8 weeks of oral Amoxicillin (6 g/day). Barnhart [20] reported on a 31-year-old male who presented with a post-operative Veillonella infection in the cervical spine. The portal of entry was probably the post-operative oesophageal perforation that had occurred. The patient recovered completely after drainage of the retropharyngeal abscess and treatment with intravenous Penicillin for 6 weeks. Finally, Bongaerts [21] reported on a 74-year-old gentleman with Veillonella parvula infection at T12–L1 that was treated with 6 weeks of intravenous Penicillin.
Our patient presented with a 3-month history of worsening back pain and constitutional symptoms. The reported duration between onset of symptoms and diagnosis can vary between 2 weeks and 9 months [2, 4, 6]. In addition, a causative organism is not found in 8–15% of the cases [1, 4]. Recovery of Veillonella requires adequate samples collected under strictly anaerobic conditions and the organism grows slowly, taking an average of 4–5 days [19]. In addition to prolonged administration of the appropriate antibiotic, our patient underwent a surgical debridement and stabilization because of the spinal instability caused by the infection. In a review of 15 studies comprising 312 procedures relating to the surgical treatment of pyogenic spondylodiscitis [6], Chen et al. reported that 66% of the patients had undergone a combined anterior-posterior surgery. A decreased incidence of recurrence (6.3 vs. 10.4%) and revision surgery (2.9 vs. 6.3%) was noted following the combined approach compared to the posterior and anterior approaches [6]. Although we chose to perform an anterior debridement and posterior stabilization in two stages, a single-stage anterior-posterior surgery, a posterior alone debridement and stabilization and an anterior alone debridement and stabilization are equally efficacious options. Furthermore, spinal instrumentation and anterior bone grafting have been found to be safe in the presence of infection [6].
Conclusion
Veillonella forms part of the normal flora in the oral cavity and gastro-intestinal tract and rarely causes infection. However, both surgeons and infectious disease specialists should be aware of the potential of Veillonella to cause spondylodiscitis. The routine use of anaerobic culture is necessary to isolate these organisms. Veillonella grows slowly, hence allowing adequate time for culture under strictly anaerobic conditions is important. Whenever possible, tissue in addition to blood should be submitted for culture. The outcome in our case was favourable using appropriate antibiotics and following a two-stage surgical approach which was required in view of the spinal instability at presentation.
Conflict of interest
None of the authors has any potential conflict of interest.
References
- 1.Chelsom J, Solberg CO. Vertebral osteomyelitis at a Norwegian university hospital 1987–97: clinical features, laboratory findings and outcome. Scand J Infect Dis. 1998;30(2):147–151. doi: 10.1080/003655498750003537. [DOI] [PubMed] [Google Scholar]
- 2.Beronius M, Bergman B, Andersson R. Vertebral osteomyelitis in Goteborg, Sweden: a retrospective study of patients during 1990–95. Scand J Infect Dis. 2001;33(7):527–532. doi: 10.1080/00365540110026566. [DOI] [PubMed] [Google Scholar]
- 3.Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ. Hematogenous pyogenic spinal infections and their surgical management. Spine. 2000;25(13):1668–1679. doi: 10.1097/00007632-200007010-00010. [DOI] [PubMed] [Google Scholar]
- 4.Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1997;79(6):874–880. doi: 10.2106/00004623-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Kulowski J. Pyogenic osteomyelitis of the spine. An analysis and discussion of 102 cases. J Bone Joint Surg Am. 1936;18:343–364. [Google Scholar]
- 6.Chen WH, Jiang LS, Dai LY. Surgical treatment of pyogenic vertebral osteomyelitis with spinal instrumentation. Eur Spine J. 2007;16(9):1307–1316. doi: 10.1007/s00586-006-0251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia A, Jr, Grantham SA. Hematogenous pyogenic vertebral osteomyelitis. J Bone Joint Surg Am A. 1960;42:429–436. [PubMed] [Google Scholar]
- 8.Rezai AR, Woo HH, Errico TJ, Cooper PR. Contemporary management of spinal osteomyelitis. Neurosurgery. 1999;44(5):1018–1025. doi: 10.1097/00006123-199905000-00047. [DOI] [PubMed] [Google Scholar]
- 9.Butler E, Blusger I, Perry K (1941) Staphylococcal osteomyelitis of the spine. Lancet 480–481
- 10.Hall BB, Fitzgerald RH, Jr, Rosenblatt JE. Anaerobic osteomyelitis. J Bone Joint Surg Am. 1983;65(1):30–35. [PubMed] [Google Scholar]
- 11.Rovery C, Etienne A, Foucault C, Berger P, Brouqui P. Veillonella montpellierensis endocarditis. Emerg Infect Dis. 2005;11(7):1112–1114. doi: 10.3201/eid1107.041361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaninetti-Schaerer A, Delden C, Genevay S, Gabay C. Total hip prosthetic joint infection due to Veillonella species. Joint Bone Spine. 2004;71(2):161–163. doi: 10.1016/j.jbspin.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Marchandin H, Jean-Pierre H, Carriere C, Canovas F, Darbas H, Jumas-Bilak E. Prosthetic joint infection due to Veillonella dispar. Eur J Clin Microbiol Infect Dis. 2001;20(5):340–342. doi: 10.1007/s100960100493. [DOI] [PubMed] [Google Scholar]
- 14.Liu JW, Wu JJ, Wang LR, Teng LJ, Huang TC. Two fatal cases of Veillonella bacteremia. Eur J Clin Microbiol Infect Dis. 1998;17(1):62–64. doi: 10.1007/BF01584370. [DOI] [PubMed] [Google Scholar]
- 15.Sato T, Matsuyama J, Sato M, Hoshino E. Differentiation of Veillonella atypica, Veillonella dispar and Veillonella parvula using restricted fragment-length polymorphism analysis of 16S rDNA amplified by polymerase chain reaction. Oral Microbiol Immunol. 1997;12(6):350–353. doi: 10.1111/j.1399-302X.1997.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 16.Sutter VL, Finegold SM. Susceptibility of anaerobic bacteria to 23 antimicrobial agents. Antimicrob Agents Chemother. 1976;10(4):736–752. doi: 10.1128/aac.10.4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nyfors S, Kononen E, Bryk A, Syrjanen R, Jousimies-Somer H. Age-related frequency of penicillin resistance of oral Veillonella. Diagn Microbiol Infect Dis. 2003;46(4):279–283. doi: 10.1016/S0732-8893(03)00082-8. [DOI] [PubMed] [Google Scholar]
- 18.Marriott D, Stark D, Harkness J. Veillonella parvula discitis and secondary bacteremia: a rare infection complicating endoscopy and colonoscopy? J Clin Microbiol. 2007;45(2):672–674. doi: 10.1128/JCM.01633-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isner-Horobeti ME, Lecocq J, Dupeyron A, Martino SJ, Froehlig P, Vautravers P. Veillonella discitis. A case report. Joint Bone Spine. 2006;73(1):113–115. doi: 10.1016/j.jbspin.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Barnhart RA, Weitekamp MR, Aber RC. Osteomyelitis caused by Veillonella. Am J Med. 1983;74(5):902–904. doi: 10.1016/0002-9343(83)91083-5. [DOI] [PubMed] [Google Scholar]
- 21.Bongaerts GP, Schreurs BW, Lunel FV, Lemmens JA, Pruszczynski M, Merkx MA. Was isolation of Veillonella from spinal osteomyelitis possible due to poor tissue perfusion? Med Hypotheses. 2004;63(4):659–661. doi: 10.1016/j.mehy.2004.02.052. [DOI] [PubMed] [Google Scholar]