Abstract
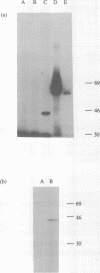
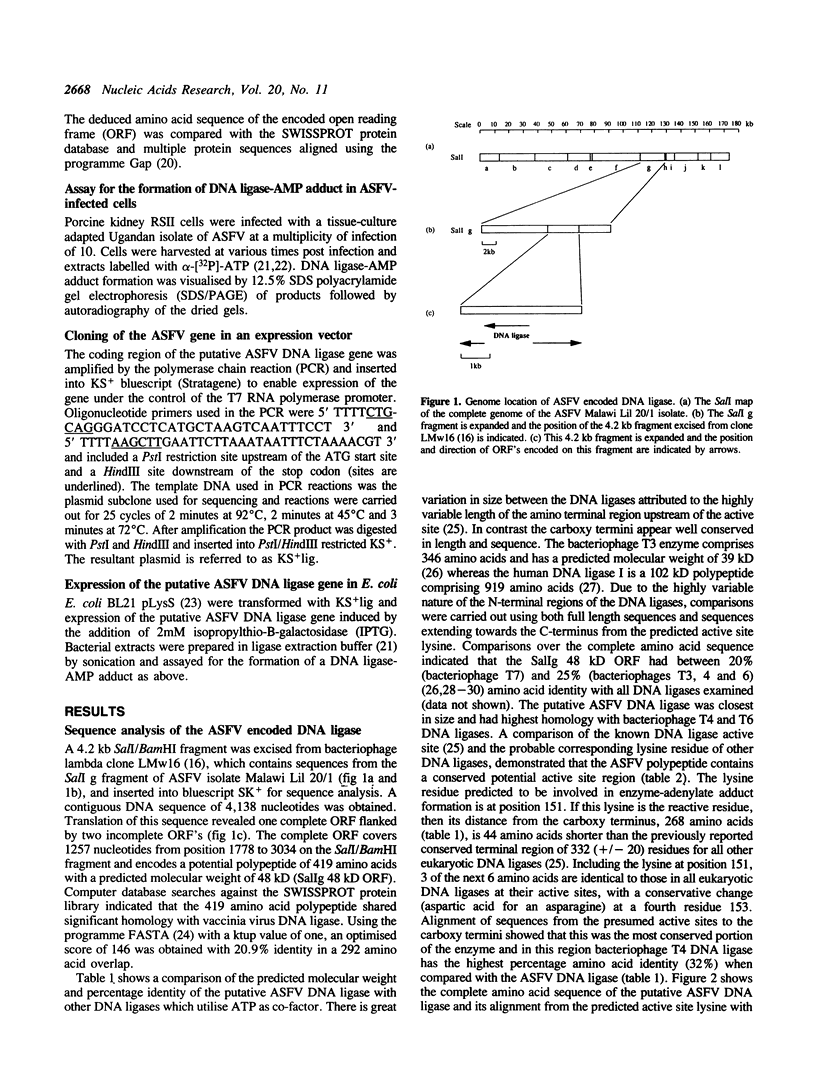
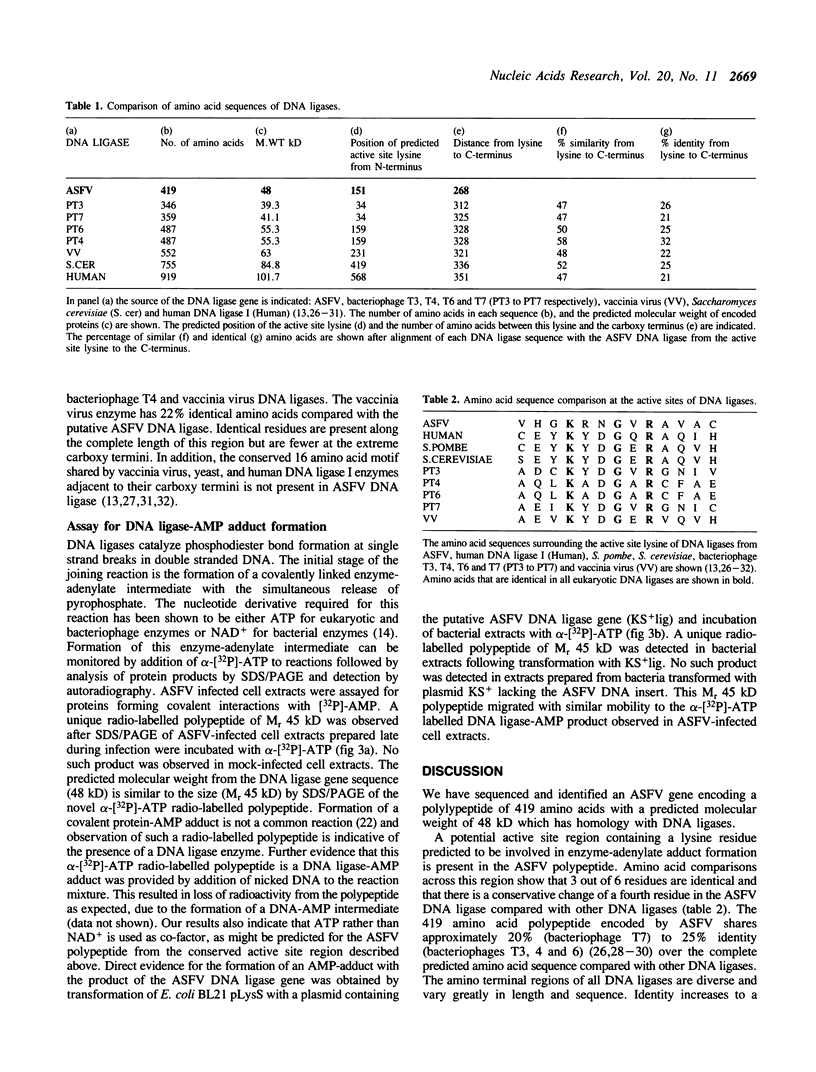
Sequence analysis of the SalI g region of the genome of a virulent isolate of ASFV (Malawi Lil 20/1) has revealed an open reading frame with the potential to encode a 48 kilodalton (kD) polypeptide which has significant homology with eukaryotic and prokaryotic DNA ligases. This ASFV encoded gene also contains the putative active site region of DNA ligases including the lysine residue which is necessary for enzyme-adenylate adduct formation, but lacks the C-terminal basic region conserved in other eukaryotic DNA ligases. A novel [32P]-labelled potential DNA ligase-adenylate adduct of M(r) 45 kD was observed upon incubation of ASFV infected cell cytoplasmic extracts with alpha-[32P]-ATP and subsequent analysis of products by SDS/PAGE. These data together suggest that ASFV encodes its own DNA ligase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J., Brown R. S., Tsugita A. Primary structure and genetic organization of phage T4 DNA ligase. Nucleic Acids Res. 1983 Oct 25;11(20):7145–7156. doi: 10.1093/nar/11.20.7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEDSON H. S., DUMBELL K. R. HYBRIDS DERIVED FROM THE VIRUSES OF ALASTRIM AND RABBIT POX. J Hyg (Lond) 1964 Jun;62:141–146. doi: 10.1017/s0022172400039875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEDSON H. S., DUMBELL K. R. HYBRIDS DERIVED FROM THE VIRUSES OF VARIOLA MAJOR AND COWPOX. J Hyg (Lond) 1964 Jun;62:147–158. doi: 10.1017/s0022172400039887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball L. A. High-frequency homologous recombination in vaccinia virus DNA. J Virol. 1987 Jun;61(6):1788–1795. doi: 10.1128/jvi.61.6.1788-1795.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankier A. T., Weston K. M., Barrell B. G. Random cloning and sequencing by the M13/dideoxynucleotide chain termination method. Methods Enzymol. 1987;155:51–93. doi: 10.1016/0076-6879(87)55009-1. [DOI] [PubMed] [Google Scholar]
- Banks G. R., Barker D. G. DNA ligase-AMP adducts: identification of yeast DNA ligase polypeptides. Biochim Biophys Acta. 1985 Dec 18;826(4):180–185. doi: 10.1016/0167-4781(85)90004-1. [DOI] [PubMed] [Google Scholar]
- Barker D. G., White J. H., Johnston L. H. Molecular characterisation of the DNA ligase gene, CDC17, from the fission yeast Schizosaccharomyces pombe. Eur J Biochem. 1987 Feb 2;162(3):659–667. doi: 10.1111/j.1432-1033.1987.tb10688.x. [DOI] [PubMed] [Google Scholar]
- Barker D. G., White J. H., Johnston L. H. The nucleotide sequence of the DNA ligase gene (CDC9) from Saccharomyces cerevisiae: a gene which is cell-cycle regulated and induced in response to DNA damage. Nucleic Acids Res. 1985 Dec 9;13(23):8323–8337. doi: 10.1093/nar/13.23.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D. E., Johnston L. H., Kodama K., Tomkinson A. E., Lasko D. D., Lindahl T. Human DNA ligase I cDNA: cloning and functional expression in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6679–6683. doi: 10.1073/pnas.87.17.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroudy B. M., Venkatesan S., Moss B. Incompletely base-paired flip-flop terminal loops link the two DNA strands of the vaccinia virus genome into one uninterrupted polynucleotide chain. Cell. 1982 Feb;28(2):315–324. doi: 10.1016/0092-8674(82)90349-x. [DOI] [PubMed] [Google Scholar]
- Blasco R., López-Otín C., Muñz M., Bockamp E. O., Simón-Mateo C., Viñuela E. Sequence and evolutionary relationships of African swine fever virus thymidine kinase. Virology. 1990 Sep;178(1):301–304. doi: 10.1016/0042-6822(90)90409-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursnell M., Shaw K., Yáez R. J., Viñuela E., Dixon L. The sequences of the ribonucleotide reductase genes from African swine fever virus show considerable homology with those of the orthopoxvirus, vaccinia virus. Virology. 1991 Sep;184(1):411–416. doi: 10.1016/0042-6822(91)90860-e. [DOI] [PubMed] [Google Scholar]
- Chernos V. I., Antonova T. P., Senkevich T. G. Recombinants between vaccinia and ectromelia viruses bearing the specific pathogenicity markers of both parents. J Gen Virol. 1985 Mar;66(Pt 3):621–626. doi: 10.1099/0022-1317-66-3-621. [DOI] [PubMed] [Google Scholar]
- Colinas R. J., Goebel S. J., Davis S. W., Johnson G. P., Norton E. K., Paoletti E. A DNA ligase gene in the Copenhagen strain of vaccinia virus is nonessential for viral replication and recombination. Virology. 1990 Nov;179(1):267–275. doi: 10.1016/0042-6822(90)90295-3. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Moss B. Structure of vaccinia virus early promoters. J Mol Biol. 1989 Dec 20;210(4):749–769. doi: 10.1016/0022-2836(89)90107-1. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Moss B. Structure of vaccinia virus late promoters. J Mol Biol. 1989 Dec 20;210(4):771–784. doi: 10.1016/0022-2836(89)90108-3. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon L. K. Molecular cloning and restriction enzyme mapping of an African swine fever virus isolate from Malawi. J Gen Virol. 1988 Jul;69(Pt 7):1683–1694. doi: 10.1099/0022-1317-69-7-1683. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Nucleotide sequence from the genetic left end of bacteriophage T7 DNA to the beginning of gene 4. J Mol Biol. 1981 Jun 5;148(4):303–330. doi: 10.1016/0022-2836(81)90178-9. [DOI] [PubMed] [Google Scholar]
- Esteves A., Marques M. I., Costa J. V. Two-dimensional analysis of African swine fever virus proteins and proteins induced in infected cells. Virology. 1986 Jul 15;152(1):192–206. doi: 10.1016/0042-6822(86)90384-3. [DOI] [PubMed] [Google Scholar]
- Gershon P. D., Kitching R. P., Hammond J. M., Black D. N. Poxvirus genetic recombination during natural virus transmission. J Gen Virol. 1989 Feb;70(Pt 2):485–489. doi: 10.1099/0022-1317-70-2-485. [DOI] [PubMed] [Google Scholar]
- González A., Talavera A., Almendral J. M., Viñuela E. Hairpin loop structure of African swine fever virus DNA. Nucleic Acids Res. 1986 Sep 11;14(17):6835–6844. doi: 10.1093/nar/14.17.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond J. M., Dixon L. K. Vaccinia virus-mediated expression of African swine fever virus genes. Virology. 1991 Apr;181(2):778–782. doi: 10.1016/0042-6822(91)90917-z. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hingamp P. M., Arnold J. E., Mayer R. J., Dixon L. K. A ubiquitin conjugating enzyme encoded by African swine fever virus. EMBO J. 1992 Jan;11(1):361–366. doi: 10.1002/j.1460-2075.1992.tb05058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliman A. V., Zimin A. A., Nazipova N. N., Kriukov V. M., Taniashin V. I. Sravnitel'nyi analiz genov DNK-ligaz fagov T6 i T4. Dokl Akad Nauk SSSR. 1988;299(3):737–742. [PubMed] [Google Scholar]
- Kerr S. M., Johnston L. H., Odell M., Duncan S. A., Law K. M., Smith G. L. Vaccinia DNA ligase complements Saccharomyces cerevisiae cdc9, localizes in cytoplasmic factories and affects virulence and virus sensitivity to DNA damaging agents. EMBO J. 1991 Dec;10(13):4343–4350. doi: 10.1002/j.1460-2075.1991.tb05012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr S. M., Smith G. L. Vaccinia virus DNA ligase is nonessential for virus replication: recovery of plasmids from virus-infected cells. Virology. 1991 Feb;180(2):625–632. doi: 10.1016/0042-6822(91)90076-n. [DOI] [PubMed] [Google Scholar]
- Kerr S. M., Smith G. L. Vaccinia virus encodes a polypeptide with DNA ligase activity. Nucleic Acids Res. 1989 Nov 25;17(22):9039–9050. doi: 10.1093/nar/17.22.9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznar J., Salas M. L., Viñuela E. DNA-dependent RNA polymerase in African swine fever virus. Virology. 1980 Feb;101(1):169–175. doi: 10.1016/0042-6822(80)90493-6. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Reddy M. K., Bauer W. R. Activation of the vaccinia virus nicking-joining enzyme by trypsinization. J Biol Chem. 1989 Jan 5;264(1):443–449. [PubMed] [Google Scholar]
- Salas M. L., Kuznar J., Viñuela E. Effect of rifamycin derivatives and coumermycin A1 on in vitro RNA synthesis by African swine fever virus. Brief report. Arch Virol. 1983;77(1):77–80. doi: 10.1007/BF01314866. [DOI] [PubMed] [Google Scholar]
- Salas M. L., Kuznar J., Viñuela E. Polyadenylation, methylation, and capping of the RNA synthesized in vitro by African swine fever virus. Virology. 1981 Sep;113(2):484–491. doi: 10.1016/0042-6822(81)90176-8. [DOI] [PubMed] [Google Scholar]
- Santarén J. F., Viñuela E. African swine fever virus-induced polypeptides in Vero cells. Virus Res. 1986 Sep;5(4):391–405. doi: 10.1016/0168-1702(86)90031-6. [DOI] [PubMed] [Google Scholar]
- Schmitt M. P., Beck P. J., Kearney C. A., Spence J. L., DiGiovanni D., Condreay J. P., Molineux I. J. Sequence of a conditionally essential region of bacteriophage T3, including the primary origin of DNA replication. J Mol Biol. 1987 Feb 5;193(3):479–495. doi: 10.1016/0022-2836(87)90261-0. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Chan Y. S., Kerr S. M. Transcriptional mapping and nucleotide sequence of a vaccinia virus gene encoding a polypeptide with extensive homology to DNA ligases. Nucleic Acids Res. 1989 Nov 25;17(22):9051–9062. doi: 10.1093/nar/17.22.9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Söderhäll S., Lindahl T. DNA ligases of eukaryotes. FEBS Lett. 1976 Aug 1;67(1):1–8. doi: 10.1016/0014-5793(76)80858-7. [DOI] [PubMed] [Google Scholar]
- Tomkinson A. E., Roberts E., Daly G., Totty N. F., Lindahl T. Three distinct DNA ligases in mammalian cells. J Biol Chem. 1991 Nov 15;266(32):21728–21735. [PubMed] [Google Scholar]
- Tomkinson A. E., Totty N. F., Ginsburg M., Lindahl T. Location of the active site for enzyme-adenylate formation in DNA ligases. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):400–404. doi: 10.1073/pnas.88.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traktman P. The enzymology of poxvirus DNA replication. Curr Top Microbiol Immunol. 1990;163:93–123. doi: 10.1007/978-3-642-75605-4_4. [DOI] [PubMed] [Google Scholar]
- Viñuela E. African swine fever virus. Curr Top Microbiol Immunol. 1985;116:151–170. doi: 10.1007/978-3-642-70280-8_8. [DOI] [PubMed] [Google Scholar]
- Yuen L., Moss B. Oligonucleotide sequence signaling transcriptional termination of vaccinia virus early genes. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6417–6421. doi: 10.1073/pnas.84.18.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]