Abstract
Purpose
The purpose of this study is to evaluate the effect of intraoperative powdered vancomycin on the rates of postoperative deep spinal wound infection. The use of intraoperative powdered vancomycin as a prophylactic measure in an attempt to reduce the incidence of postoperative spinal wound infection has not been sufficiently evaluated in the existing literature. A retrospective review of a large clinical database was performed to determine the rates of deep wound infection associated with the use of intraoperative operative site powdered vancomycin.
Materials and methods
During the period from 2005 to 2010, 1,512 consecutive spinal surgery cases were performed by the same fellowship-trained spinal surgeon (RWM) at a level 1 trauma-university medical center. One gram of powdered vancomycin was placed in all surgical sites prior to wound closure. Eight hundred forty-nine cases were uninstrumented, 478 cases were instrumented posterior thoracic or lumbar, 12 were instrumented anterior thoracic or lumbar, 126 were instrumented anterior cervical, and 47 were instrumented posterior cervical cases. Fifty-eight cases were combined anterior and posterior surgery and 87 were revision surgeries. A retrospective operative database and medical record review was performed to evaluate for evidence of postoperative deep wound infection.
Results
15 of the 1,512 patients (0.99%) were identified as having evidence of postoperative deep wound infection. At least one pre-existing risk factor for deep infection was present in 8/15 pts (54%). Staphylococcus aureus and methicillin-resistant S. aureus (MRSA) were the most commonly identified organisms (11/15 cases). The rate of deep wound infection was 1.20% (8/663) for instrumented spinal surgeries, and 0.82% (7/849) for uninstrumented surgeries. Deep infection occurred in only 1.23% (4/324) of multilevel instrumented posterior spinal fusions, 1.37% (1/73) of open PLIF procedures, and 1.23% (1/81) of single-level instrumented posterior fusions. Deep infection was not observed in any patient who had uninstrumented spinal fusion (0/64). The deep infection rate for revision surgeries was 1.15% (1/87) and 0.55% (1/183) for trauma surgery. Increased rates of complications related to powdered vancomycin use were not identified in this series. Conclusion
In this series of 1,512 consecutive spinal surgeries, the use of 1 g of powdered intraoperative vancomycin placed in the wound prior to wound closure appears to associated with a low rate deep spinal wound infection for both instrumented and uninstrumented cases. Rates of deep infection for instrumented fusion surgery, trauma, and revision surgery appear to be among the lowest reported in the existing literature. Further investigation of this prophylactic adjunctive measure is warranted.
Keywords: Operative site vancomycin, Intraoperative vancomycin, Postoperative spinal operative site infection, Spinal surgery infection prophylaxis
Introduction
Deep infection after spinal surgery is a potentially devastating complication. Deep spinal infection is associated with higher morbidity, mortality and health care costs [1]. Accurate documentation of rates at which postoperative spine infections occur is important for many reasons to include quality improvement, patient counseling and as a source of information to assist with surgical decision-making. Unfortunately, the literature is confusing with respect to the accurate documentation of the incidence of infection after spinal surgery. Many reports are complicated by the inclusion of broad and inconsistent definitions of superficial spinal infection, including cases of temporary minor wound erythema, minor drainage, or small suture reactions [2–7]. Deep infection appears to be a more accurate parameter for research documentation. Deep infection usually involves reoperation as a standard treatment, and it typically incurs a higher level of health care resources in its management.
Surgeons should make great effort to minimize risk factors for wound infection after spinal surgery. Risks factors for infection after spinal surgery have been well-defined in numerous studies [3, 4, 8–10]. Advanced patient age, obesity, malnutrition, prolonged surgical time, revision surgery, increased blood loss, smoking, use of instrumentation, and revision surgery are among the reported risk factors for increasing rates of deep infection.
The effectiveness of additional intraoperative measures to reduce infection remains unclear. While perioperative antibiotic prophylaxis as a means of lowering infection rates after spinal surgery has been well described, there is a paucity of literature with respect to other adjunct measures to prevent spinal surgery postoperative infection [11]. Diluted povidone–iodine has been demonstrated in two separate case-controlled studies to reduce the rate of postoperative spine infections [12, 13]. There is no currently compelling evidence in the literature to support the role of antibiotic solutions used as wound irrigation substitutes. The use of closed suction wound drainage also has not been demonstrated to decrease the rate of deep spinal wound infection [14]. Additionally, very low level evidence exists to suggest that surgical site shaving may actually increase the incidence of lumbar wound infection after spinal surgery [15]. The same is true for the use of silver-impregnated dressings applied to the wound after spinal surgery [16].
Powdered forms of antibiotics which are deposited directly into the spinal surgical wound prior to closure may be successful means to reduce postoperative deep spinal wound infection. Directly depositing the powdered form of the antibiotic into the operative site theoretically achieves the highest levels of antibiotic concentration in the spinal wound. Techniques involving the placement of powdered antibiotic into presumably clean spinal surgical sites prior to wound closure have not been addressed to date in the existing literature. Such techniques theoretically provide high concentrations of prophylactic antibiotic directly in the operative area. Placement of the powdered form of an antibiotic into the wound immediately prior closure may provide the highest intrawound antibiotic levels for a prolonged period of time after the operation.
The purpose of this manuscript is to report the rates of deep infection among a large series of consecutive patients who were treated with this technique. Outcomes regarding rate of deep infection in this study may be compared to previously reported rates of deep spinal wound infections per case subcategory to determine if further study with regard to this prophylactic measure is warranted.
Methods
This study included 1,512 consecutive patients who had spinal surgery performed by the same surgeon at the same university medical center during the years 2005–2010. There were 1,481 adult and only 31 pediatric patients. One gram of powdered vancomycin antibiotic was placed into each wound at the conclusion of each procedure and prior to closure of the deep lumbar fascia (Fig. 1a, b). Surgical procedures were separated into appropriate subcategories based on the spinal region, the presence or absence of instrumentation, and whether or not a fusion was performed. Operative subcategories were the following: (1) multilevel posterior thoracic or lumbar instrumented fusions, (2) single-level instrumented posterior fusion, (3) instrumented lumbar single-level PLIF, (4) uninstrumented lumbar fusion, (5) multilevel lumbar decompression, (6) single-level lumbar decompression, (7) instrumented anterior cervical, and (8) instrumented posterior cervical. Revision, trauma, deformity, tumor, and combined anterior–posterior procedures were also identified.
Fig. 1.
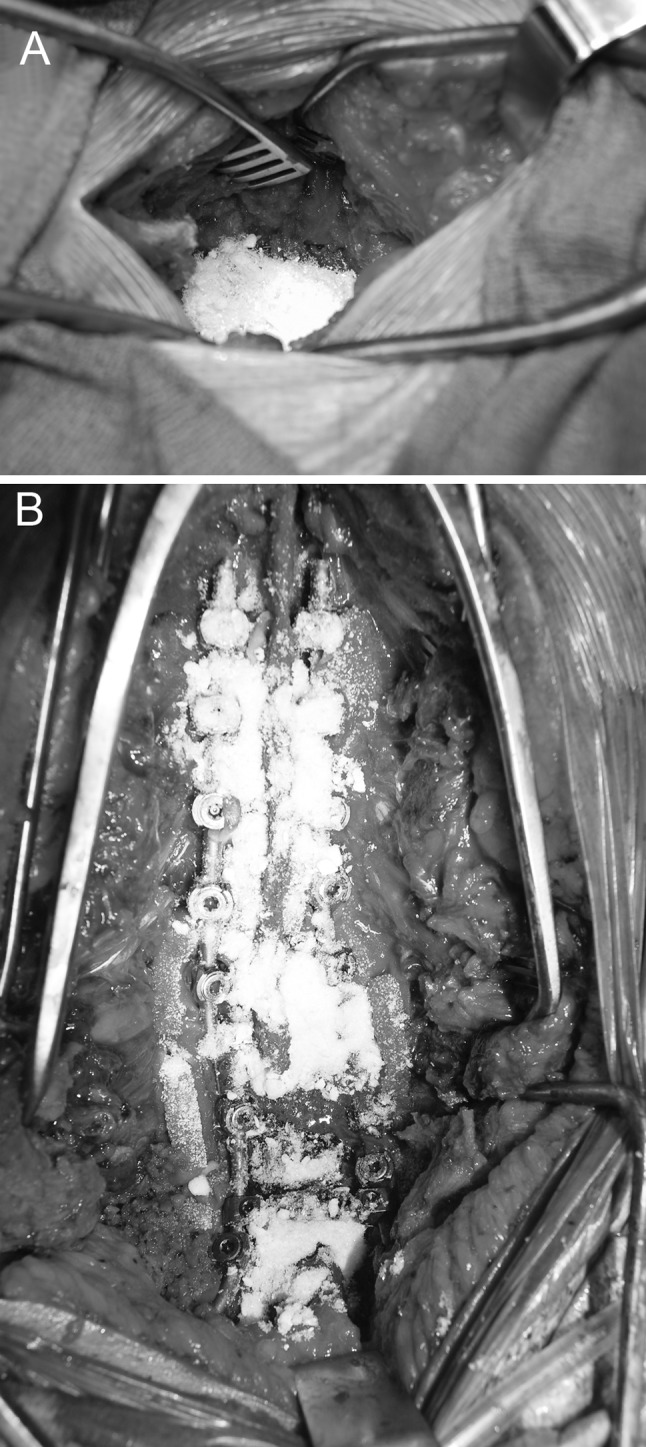
Intraoperative images demon stating the coverage area of 1 g of powdered vancomycin placed in the deep wound region prior to wound closure in a uninstrumented lumbar surgery and b instrumented thoracolumbar surgery
All patients received the usual in-place hospital-mandated surgical infection prophylactic measures required for all surgeries performed in the university facility. Existing hospital policy required all patients to receive a perioperative dose of IV antibiotic within 60 min of the surgical incision for routine infection prophylaxis. One gram of cefazolin was administered to all patients who did not have evidence of a preexisting penicillin allergy. Clindamycin 900 mg was administered intravenously to those patients who had been identified as having preexisting penicillin allergy. Operative drainage tubes were typically used for all surgeries with the exception of single-level lumbar decompressive procedures. Operative procedure subcategories were recorded in a surgical database and patient clinical information was obtained as part of the routine surgical follow-up for each procedure performed. All patient clinical information was stored in electronic medical record format.
Because the vancomycin powder was placed under the deep fascia, only the rate of deep spinal surgical wound infection was determined to be the most appropriate study parameter. Both the surgical database and the patient medical records were searched to identify evidence of post surgical deep wound infection. Additionally, all patients in this series with clinical evidence of deep wound infection after surgery were treated with an additional surgical procedure for wound irrigation and debridement—thus making this parameter easily identifiable in the database and electronic medical records. The authors specifically chose not to include rates of superficial infection because of the high level of variability of superficial infection clinical presentation.
The operative procedure breakdown in this spinal surgery case series was as follows: 849 cases were uninstrumented, 478 cases were instrumented posterior thoracic or lumbar, 12 instrumented anterior thoracic or lumbar, 126 instrumented anterior cervical, 47 instrumented posterior cervical (Table 1). Eighty-seven patients had revision spinal surgery and 58 patients underwent same-day anterior-posterior procedures.
Table 1.
Rates of deep wound infection
| Uninstrumented spinal surgeries | 0% (0/64) |
| Instrumented spinal surgeries | 1.20% (8/663 |
| Uninstrumented spinal fusion | 0% (0/64) |
| Multilevel instrumented posterior spinal fusions | 1.23% (4/324) |
| Open PLIF procedures | 1.37% (1/73) |
| Single-level instrumented posterior fusions | 1.23% (1/81) |
| Multiple-level posterior decompression | 1.00% (6/600) |
| Single-level posterior decompression/discectomy | 0.40% (1/249) |
| Anterior cervical fusion | 0.00% (0/146) |
| Posterior cervical fusion | 2.13% (1/47) |
| Posterior decompressive laminectomy | 0.82% (7/849) |
The rate of total deep wound infection in the consecutive case series of 1,512 patients was determined as well as the rates of deep infection per surgical procedure subcategory.
Results
A retrospective review of the surgical database and the patients’ medical records revealed 15 of the 1,512 patients (0.99%) as having evidence of postoperative deep wound infection. Eight of the 15 patients (54%) who developed deep infection had at least one significant risk factor for infection (obesity, revision surgery, metastatic cancer, and radiation). All 15 patients required reoperation for wound irrigation, debridement, and reclosure as well as at least a 6-week course of intravenous antibiotic therapy. Staphylococcus aureus was the most commonly identified organism and appeared in 11 of the 15 cases—6 of these patients had a methicillin-resistant strain of S. aureus (MRSA). Enterococcus was the next most common infecting organism and was identified in two patients.
The rate of deep wound infection was 1.20% (8/663) for instrumented spinal surgeries, and 0.82% (7/849) for posterior decompressive laminectomy. Five patients required multiple operative debridement procedures for recurrent deep wound infection.
Deep infection occurred in only 1.23% (4/324) of multilevel instrumented posterior spinal fusions, 1.37% (1/73) of open PLIF procedures, and 1.23% (1/81) of single-level instrumented posterior fusions. Deep infection was not observed in any patient who had uninstrumented spinal fusion (0/64) (Table 1). The deep infection rate for revision surgeries was 1.15% (1/87) and 1.72% (1/58) for same-day anterior and posterior surgery.
The majority of the deep infections involving uninstrumented spinal surgery occurred in patients who were noted to have multiple-level decompressive procedures. Six of the seven infections that occurred in the 849 uninstrumented spinal surgeries involved two or more levels of thoracic or lumbar laminectomy decompression. Only one infection occurred in the portion of the group that had single-level decompressive surgery. The overall infection rate for the entire group of uninstrumented spinal surgeries was low (0.82%).
The occurrence of deep infection in this series of patients appeared to be relatively evenly distributed throughout the 6-year time period of the study. At least one deep infection was noted to occur in each of the 6 years of the study’s time period. The highest number of deep infections occurring in a single year was five (2007). Years 2006 and 2009 each had three deep infections. Two infections occurred in 2005 and one in the year 2010 (Table 2).
Table 2.
Deep infection rates by study year
| 2005 | 1.14% (2/175) |
| 2006 | 1.50% (3/200) |
| 2007 | 2.0% (5/254) |
| 2008 | 0.36% (1/275) |
| 2009 | 0.98% (3/306) |
| 2010 | 0.33% (1/302) |
Rates of deep infection per case category were as follows: trauma: 0.55% (1/183), revision surgery: 1.15% (1/87), deformity: 1.61% (1/62), anterior/posterior: 1.72% (91/58), and tumor: 5.4% (2/37) (Table 3).
Table 3.
Institutional deep infection rates per case category
| Trauma | 0.55% (1/183) |
| Revision surgery | 1.15% (1/87) |
| Deformity | 1.61% (1/62) |
| Anterior/posterior | 1.72% (91/58) |
| Tumor | 5.4% (2/37) |
Increased rates of complications related to powdered vancomycin use were not identified in this series. A search of the medical records and surgical database did not reveal evidence of increased rates of postoperative renal insufficiency or renal failure among the 1,512 consecutive spinal surgical cases, and only one patient (0.07%) was identified as having unexplained renal failure or insufficiency after elective spinal surgery. Two additional patients (0.13%) experienced transient hearing loss that resolved in the early postoperative period. Other complications with respect to the use of operative site vancomycin were not readily identifiable in this series.
Discussion
Infection after spinal surgery is a dreaded complication that can greatly increase patient morbidity and mortality. Surgical site infection after spinal surgery has been reported to occur in rates ranging from 0.3 to 20% of patients [2, 6, 7, 12, 16–23]. Calderone et al. [1] estimated that health care costs can increase up to fourfold once an infection is encountered after spinal surgery. Successful adjunctive perioperative measures to reduce the incidence of deep spinal wound infection can have a profoundly positive effect on both patient outcomes and health care costs.
Risk factors for deep spinal wound infection have been widely reported in the literature, and surgeons should make great efforts to minimize those risk factors prior to performing invasive spinal procedures [3–5, 8–10, 24]. Among the reported patient risk factors for infection are advanced patient age, obesity, diabetes, urinary incontinence, smoking, and malnutrition. Reported surgical risk factors include revision surgery, increased surgical blood loss, prolonged surgical time, use of instrumentation, and multilevel posterior fusions. While many of these studies were limited by small sample sizes and other confounding variables, they do present some degree of evidence to associate the aforementioned risk factors with increased rates of spinal infection after surgery.
Intravenous antibiotics administered perioperatively are standard measures used in an attempt to reduce the incidence of postoperative infection. The North American Spine Society has published evidence-based guidelines evaluating the prophylactic use of antibiotics in spinal surgery [4]. The guidelines indicate that all patients who undergo spinal surgery should receive perioperative prophylactic antibiotic. The superiority of one agent over another could not be recommended in this guideline publication. Specific changes to antibiotic protocols for higher infection risk patients also could not be recommended based on the available evidence.
Lonstein et al. [17] identified intraoperative contamination of wounds as an important cause for postoperative spinal infection. Antibiotics and antiseptic irrigation of surgical wounds are commonly used in an attempt to decrease postoperative spinal infection rates. However, little clinical literature exists to support these techniques. Haines et al. [25] reported the effectiveness of topical antibiotics used intraoperatively may be beneficial for wound with higher infection risk (>15%). The author did not demonstrate any benefit from topical antibiotic solution applied to spinal wounds for lesser-risk cases.
There are conflicting reports in the orthopaedic literature with regard to the effectiveness of prophylactic topical wound antibiotic instillation in addition to systemic antibiotic therapy. Maguire [26] investigated the use of bacitracin and neomycin powder and found reduced infection rates. Nachamie et al. [27] investigated the use of dilute neomycin and found the technique to be ineffective. To date, the literature remains unclear on the effectiveness of these techniques.
Closed wound suction drainage has not been demonstrated to be an effective means of reducing the incidence of spinal infection after surgery. Two separate reports exist demonstrating no significant differences between groups of spinal surgical patients with and without drainage tubes [14, 19]. Also, drains were not recommended as a means to reduce infection in The North American Spine Society’s published guidelines on spinal surgery and postoperative infection [4].
There is low-level evidence to support the use of wound irrigation with 0.35% povidone–iodine solution (3.5% betadine). Chang et al. and Cheng et al. compared wounds irrigated with 3.5% povidone–iodine to wounds irrigated with normal saline solution in patients with diverse spinal disorders. Both studies reported a 0% infection rate in the povidone–iodine group [12, 13].
In another recent randomized trial, Celik et al. [15] compared a small group or patients undergoing predominately uninstrumented spinal surgery who had shaving versus no shaving of the surgical site. Patients who were shaved demonstrated a higher infection rate (1.07 vs. 0.23%). This study was limited by its relatively small sample size and the fact that only four patients who were shaved developed infection versus only one patient who was not shaved and developed infection.
Epstein compared the use of silver-impregnated dressings with iodine or alcohol-based swab and dry gauze dressings. The silver-impregnated dressing group demonstrated a 0% overall infection rate, and the dry gauze group had a 2.5% deep infection rate. This study was limited by the lack of statistical analysis comparing the two groups [16].
Schuster et al. [28] performed an evidenced-based review involving the influence of therapeutic interventions on infection rates after spine surgery. Eight studies were identified by the authors evaluating wound care methods other than antibiotics for preventing postoperative surgical site infection after spinal surgery including the aforementioned studies in this manuscript. The authors concluded that there is some moderate evidence to support the use of betadine solution in preventing postoperative spinal infection. All other measures to include suction drainage, shaving, and silver dressings were deemed to have extremely low levels of supporting evidence in the literature [28].
A recent report was published from the Scoliosis Research Society (SRS) Morbidity and Mortality Committee on rates of infection following spine surgery [6]. This study evaluated 108,419 reported surgical procedures from the society’s members with emphasis on the overall rate of infection as well as the rates for various surgical procedure subcategories. The overall deep infection rate identified the group of 108,419 database of surgical cases was 1.3%. Cases that included spinal fusion had a 33% higher rate of infection (2.4 vs. 1.8%). The overall infection rate for cases with implants was 28% higher than the rate of infection for cases without implants (2.3 vs. 1.8%). Revision spinal surgery in the SRS Database review had a 2.2% infection rate and surgery for spinal tumors was noted to have a 3.7% infection rate. The SRS study did not comment on the adjunctive measures for infection prophylaxis used by the contributing surgeons. The data from this study suggest that even among skilled spine surgeons postsurgical infection remains an inherent complication.
Powdered forms of antibiotics which are deposited directly into the spinal surgical wound prior to closure may be successful means to reduce postoperative deep spinal wound infection. Directly depositing the powdered form of the antibiotic into the operative site theoretically achieves the highest levels of antibiotic concentration in the spinal wound. There is evidence in the general orthopaedic literature to support the concept on placing antibiotics directly into wounds via orthopaedic implants or cement beads. Schmidmaier et al. [29] concluded that the local application of gentamycin in the form of coated implants might support systemic antibiotic prophylaxis in preventing implant-associated osteomyelitis. This concept has not been adequately explored in the existing spine literature. In our consecutive series of spinal surgery, patients who had 1 g of prophylactic powdered vancomycin placed in the wound prior to closure of the seep fascia, the overall rate of deep wound infection was 1.20% (8/663) for instrumented spinal surgeries, and 0.82% (7/849) for uninstrumented surgeries.
Vancomycin was chosen for use because of its relatively low hospital cost, its ease of use in the powdered form, and its very broad and effective coverage against the organisms that typically infect deep spinal wounds such as MRSA. These reported figures in our study for the occurrence of deep wound infection appear to be among the lowest reported for deep spinal wound infection in the existing literature.
With respect to reported deep infection rates for surgical procedure subcategories in our series, deep infection occurred in only 1.23% (4/324) of multilevel instrumented posterior spinal fusions. This figure appears to compare favorably to the rates of deep infection for similar procedures reported in the literature [2, 6, 14, 18, 20, 21, 30]. Deep infection after open PLIF procedures occurred in 1.37% (1/73) of cases and in only 1.23% (1/81) of single-level instrumented posterior fusions. The finding of the Scoliosis Research Society Database postoperative infection study demonstrated a higher deep infection rate for open TLIF and for TLIF performed through a minimally invasive approach (1.9 vs. 0.4%). Deep infection rates reported from SRS Database study, which the largest series of spinal surgical patients in the existing literature, reveal the following rates of deep infection per subcategory: kyphosis 2.8%, fracture 1.5%, spondylolisthesis 1.2%, scoliosis 2.5%, lumbar spondolytic radiculopathy 1.5%, lumbar spinal stenosis 1.2%, and lumbar disc herniation 0.45% [6]. Deep infection reported in our series of patients who had powdered vancomycin placed as an adjunctive prophylactic measure appears to occur at a lower rate for most comparable surgical subcategory. Deep infection was not observed in any patient who had uninstrumented spinal fusion (0/64) in this study.
Additionally, our reported deep infection rates for the case categories of trauma and revision spinal surgery are among the lowest to be reported in the existing literature. Interestingly, we observed a higher rate of deep infection for cases involving tumor excision and reconstruction. This may reflect the inherent higher risk of patient population undergoing tumor surgery-many of whom are immunocompromised at presentation or have had concomitant chemo or radiotherapy in the perioperative period.
The limitations of this study include the lack of a control group and the heterogeneous group of spinal surgical procedures—some with relatively small sample size. While many other studies investigating the occurrence of infection after spinal surgery include the reported rates of both superficial and deep spinal infection, the authors of this manuscript purposely chose to omit superficial infection rates from this study. Previous studies have reported superficial infection rates ranging from 0 to 20% after spinal surgery [2, 6, 7, 20]. The wide variation in reported superficial infection rates may be directly related to the unclear and poorly established definition of superficial infection. Some studies include a broad definition with the occurrence of wound erythema, minor superficial stitch pustule, and minor temporary wound drainage included among cases of superficial infection, while others do not include similar patients. Deep infection after spinal surgery, on the other hand, is typically documented by the patient’s return to the operative suite for an incision and debridement procedure as well as the presence of positive deep wound cultures. In the senior author’s opinion, deep infection, as a primary study parameter, may be potentially more accurate in the documentation or true-positive cases of infection occurring after spinal surgery. Furthermore, the powdered vancomycin used in this study was only placed in the deep tissue level below the wound’s fascia. One would expect the highest concentrations of antibiotic and thus the greatest potential effect of the technique to occur in the deep tissue level of the wound.
Conclusion
In this series of 1,512 consecutive spinal surgeries, the use of 1 g of powdered intraoperative vancomycin placed in the wound prior to wound closure appears to be associated with a low rate deep spinal wound infection in both instrumented and uninstrumented cases. Rates of deep infection for instrumented fusion surgery, trauma and revision surgery appear to be among the lowest reported in the existing literature. Further investigation of this technique using the case-controlled methodology with larger surgical subpopulations is warranted.
Acknowledgments
The authors wish to thank Kimberly A. Napoli for assistance preparing this manuscript.
Conflict of interest
None of the authors has any potential conflict of interest.
References
- 1.Calderone RR, Garland DE, Capen DA, Oster H. Cost of medical care for postoperative spinal infections. Orthop Clin North Am. 1996;27:171–182. [PubMed] [Google Scholar]
- 2.Banco SP, Vaccaro AR, Blam O, Eck JC, Cotler JM, Hilibrand AS, Albert TJ, Murphey S. Spine infections: variations in incidence during the academic year. Spine (Phila Pa 1976) 2002;27:962–965. doi: 10.1097/00007632-200205010-00016. [DOI] [PubMed] [Google Scholar]
- 3.Blam OG, Vaccaro AR, Vanichkachorn JS, Albert TJ, Hilibrand AS, Minnich JM, Murphey SA. Risk factors for surgical site infection in the patient with spinal injury. Spine (Phila Pa 1976) 2003;28:1475–1480. doi: 10.1097/01.BRS.0000067109.23914.0A. [DOI] [PubMed] [Google Scholar]
- 4.Olsen MA, Mayfield J, Lauryssen C, Polish LB, Jones M, Vest J, Fraser VJ. Risk factors for surgical site infection in spinal surgery. J Neurosurg. 2003;98:149–155. [PubMed] [Google Scholar]
- 5.Wimmer C, Gluch H, Franzreb M, Ogon M. Predisposing factors for infection in spine surgery: a survey of 850 spinal procedures. J Spinal Disord. 1998;11:124–128. [PubMed] [Google Scholar]
- 6.Smith JS, Shaffrey CI, Sansur CA, Berven SH, Fu KM, Broadstone PA, Choma TJ, Goytan MJ, Noordeen HH, Knapp DR, Hart RA, Donaldson WF, 3rd, Polly DW, Jr, Perra JH, Boachie-Adjei O. Rates of infection following spine surgery based on 108, 419 procedures: a REPORT from the Scoliosis Research Society Morbidity and Mortality Committee. Spine (Phila Pa 1976) 2010;35:2140–2149. doi: 10.1097/BRS.0b013e3181cbc8e7. [DOI] [PubMed] [Google Scholar]
- 7.Pull ter Gunne AF, Cohen DB. Incidence, prevalence, and analysis of risk factors for surgical site infection following adult spinal surgery. Spine (Phila Pa 1976) 2009;34:1422–1428. doi: 10.1097/BRS.0b013e3181a03013. [DOI] [PubMed] [Google Scholar]
- 8.Fang A, Hu SS, Endres N, Bradford DS. Risk factors for infection after spinal surgery. Spine (Phila Pa 1976) 2005;30:1460–1465. doi: 10.1097/01.brs.0000166532.58227.4f. [DOI] [PubMed] [Google Scholar]
- 9.Ho C, Sucato DJ, Richards BS. Risk factors for the development of delayed infections following posterior spinal fusion and instrumentation in adolescent idiopathic scoliosis patients. Spine (Phila Pa 1976) 2007;32:2272–2277. doi: 10.1097/BRS.0b013e31814b1c0b. [DOI] [PubMed] [Google Scholar]
- 10.Klekamp J, Spengler DM, McNamara MJ, Haas DW. Risk factors associated with methicillin-resistant staphylococcal wound infection after spinal surgery. J Spinal Disord. 1999;12:187–191. [PubMed] [Google Scholar]
- 11.Mastronardi L, Tatta C (2004) Intraoperative antibiotic prophylaxis in clean spinal surgery: a retrospective analysis in a consecutive series of 973 cases. Surg Neurol 61:129–135. pii:S0090301903006566 (discussion 135) [DOI] [PubMed]
- 12.Cheng MT, Chang MC, Wang ST, Yu WK, Liu CL, Chen TH (2005) Efficacy of dilute betadine solution irrigation in the prevention of postoperative infection of spinal surgery. Spine (Phila Pa 1976) 30:1689–1693. pii:00007632-200508010-00002 [DOI] [PubMed]
- 13.Chang FY, Chang MC, Wang ST, Yu WK, Liu CL, Chen TH. Can povidone–iodine solution be used safely in a spinal surgery? Eur Spine J. 2006;15:1005–1014. doi: 10.1007/s00586-005-0975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown MD, Brookfield KF. A randomized study of closed wound suction drainage for extensive lumbar spine surgery. Spine (Phila Pa 1976) 2004;29:1066–1068. doi: 10.1097/00007632-200405150-00003. [DOI] [PubMed] [Google Scholar]
- 15.Celik SE, Kara A. Does shaving the incision site increase the infection rate after spinal surgery? Spine (Phila Pa 1976) 2007;32:1575–1577. doi: 10.1097/BRS.0b013e318074c39f. [DOI] [PubMed] [Google Scholar]
- 16.Epstein NE (2007) Do silver-impregnated dressings limit infections after lumbar laminectomy with instrumented fusion? Surg Neurol 68:483–485. doi:10.1016/j.surneu.2007.05.045 (discussion 485) [DOI] [PubMed]
- 17.Lonstein J, Winter R, Moe J, Gaines D (1973) Wound infection with Harrington instrumentation and spine fusion for scoliosis. Clin Orthop Relat Res 96:222–233 [PubMed]
- 18.Massie JB, Heller JG, Abitbol JJ, McPherson D, Garfin SR (1992) Postoperative posterior spinal wound infections. Clin Orthop Relat Res 284:99–108 [PubMed]
- 19.Payne DH, Fischgrund JS, Herkowitz HN, Barry RL, Kurz LT, Montgomery DM. Efficacy of closed wound suction drainage after single-level lumbar laminectomy. J Spinal Disord. 1996;9:401–403. doi: 10.1097/00002517-199610000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Omeis IA, Dhir M, Sciubba DM, Gottfried ON, McGirt MJ, Attenello FJ, Wolinsky JP, Gokaslan ZL. Postoperative surgical site infections in patients undergoing spinal tumor surgery: incidence and risk factors. Spine (Phila Pa 1976) 2011;36:1410–1419. doi: 10.1097/BRS.0b013e3181f48fa9. [DOI] [PubMed] [Google Scholar]
- 21.Rechtine GR, Bono PL, Cahill D, Bolesta MJ, Chrin AM. Postoperative wound infection after instrumentation of thoracic and lumbar fractures. J Orthop Trauma. 2001;15:566–569. doi: 10.1097/00005131-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Collins I, Wilson-MacDonald J, Chami G, Burgoyne W, Vineyakam P, Berendt T, Fairbank J. The diagnosis and management of infection following instrumented spinal fusion. Eur Spine J. 2008;17:445–450. doi: 10.1007/s00586-007-0559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schimmel JJ, Horsting PP, Kleuver M, Wonders G, Limbeek J. Risk factors for deep surgical site infections after spinal fusion. Eur Spine J. 2010;19:1711–1719. doi: 10.1007/s00586-010-1421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capen DA, Calderone RR, Green A. Perioperative risk factors for wound infections after lower back fusions. Orthop Clin North Am. 1996;27:83–86. [PubMed] [Google Scholar]
- 25.Haines SJ. Topical antibiotic prophylaxis in neurosurgery. Neurosurgery. 1982;11:250–253. doi: 10.1227/00006123-198208000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Maguire WB. The use of antibiotics, locally and systemically, in orthopedic surgery. Med J Aust. 1964;2:412–414. [PubMed] [Google Scholar]
- 27.Nachmie B, Siffert RS. A study of neomycin instillation into orthopaedic wounds. JAMA. 1968;204:687–689. doi: 10.1001/jama.1968.03140210041010. [DOI] [Google Scholar]
- 28.Schuster JM, Rechtine G, Norvell DC, Dettori JR. The influence of perioperative risk factors and therapeutic interventions on infection rates after spine surgery: a systematic review. Spine(Phila Pa 1976) 2010;35:S125–S137. doi: 10.1097/BRS.0b013e3181d8342c. [DOI] [PubMed] [Google Scholar]
- 29.Schmidmaier G, Lucke M, Wildemann B, Haas NP, Raschke M. Prophylaxis and treatment of implant-related infections by antibiotic-coated implants: a review. Injury. 2006;37(Suppl 2):S105–S112. doi: 10.1016/j.injury.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Brown EM, Pople IK, Louvois J, Hedges A, Bayston R, Eisenstein SM, Lees P. Spine update: prevention of postoperative infection in patients undergoing spinal surgery. Spine (Phila Pa 1976) 2004;29:938–945. doi: 10.1097/00007632-200404150-00023. [DOI] [PubMed] [Google Scholar]


