Abstract
Background
Nonsurgical management of de Quervain’s tenosynovitis often includes corticosteroid injections. If the injection does not enter the compartment, or all subcompartments, response to the injection is variable. To ensure proper location of injections we evaluated the role of ultrasound.
Questions/Purposes
We determined (1) the incidence of two or more subcompartments, (2) the incidence of anatomic variations during surgical release after failed injections, and (3) the relief of pain after ultrasound-guided injections.
Patients and Methods
A prospective series of 40 consecutive patients (42 wrists) diagnosed with de Quervain’s tenosynovitis by clinical examination were referred to a radiologist for an ultrasound-guided injection. The radiologist injected the first dorsal compartment and noted any septations. Patients returned for followup where outcomes, DASH, and VAS scores were calculated. The treating surgeon was blinded to any anatomic variations. Followup was at 6 weeks and a minimum of 6 months (mean, 6 weeks, range, 3–17 months; mean, 11 months, range, 7–18 months). Four patients were lost to followup.
Results
Multiple subcompartments were noted in 22 of 42 (52%) wrists. At the 6-week followup, 36 of the 37 wrists examined in 36 patients (97%) had at least partial resolution of symptoms. Multiple subcompartments were identified in 52% of cases. At last followup, the mean DASH and VAS scores were 18.4 and 2.2, respectively. However 14% of wrists had recurrence of symptoms, all of which had subcompartments on ultrasound. No adverse effects from the injections were noted.
Conclusion
We found ultrasound-guided injections to be useful for treatment of de Quervain’s tenosynovitis. Our success with ultrasound-guided injections was slightly better than that reported in the literature and without adverse reactions.
Level of Evidence
Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
de Quervain’s tenosynovitis is a stenosing tenosynovitis of the first dorsal compartment of the wrist, affecting the extensor pollicis brevis (EPB) and abductor pollicis longus (APL) tendon sheaths [5, 6]. The diagnosis is clinical and patients are treated nonoperatively with rest, splinting, NSAIDS, and intrasheath corticosteroid injections [12].
Nonsurgical management for de Quervain’s tenosynovitis routinely involves corticosteroid injections into the first dorsal compartment tendon sheath. Injections are safe, cost-effective, and have a favorable adverse effect profile. With as much as 83% achieving pain relief, corticosteroid injection remains the most common and effective treatment option for patients with de Quervain’s tenosynovitis [2, 10, 15, 18, 22, 28, 37]. Treatment failure of injections has been attributed to inaccurate injection technique and anatomic variation in the first dorsal compartment [25, 37]. Some surgeons recommend surgical release when nonoperative treatment fails [12, 24, 36].
The APL and EPB tendons pass through the first dorsal compartment of the wrist, yet in many patients anatomic variations can be seen with a septum often creating two subcompartments [20, 21, 24]. The incidence of septation of the first compartment reportedly varies in cadaveric studies from 24% to 76% [2, 7, 8, 13, 16, 19, 20, 23, 24]. Two studies [9, 37] showed that if the injection does not enter the compartment, or all subcompartments, response to the injection is variable and recurrence of symptoms is common. Furthermore, failure to respond to injections has been attributed to inaccurate technique and these anatomic variations in the first dorsal compartment [25, 37]. Several studies suggested a 40% to 86% incidence of subcompartments identified intraoperatively in patients who had undergone surgical release after the injections failed [9, 13, 24].
The use of ultrasound-guided injections has emerged as another option for nonoperative management of de Quervain’s tenosynovitis [14, 31]. One study of ultrasound-guided injections for de Quervain’s tenosynovitis reported symptomatic relief in 93.8% of patients [14].
To confirm that report we determined (1) the incidence of two or more subcompartments observed with ultrasound, (2) the incidence of anatomic variations identified intraoperatively during surgical release after failed injections, and (3) the relief of pain after ultrasound-guided injections.
Materials and Methods
We prospectively followed a consecutive series of 40 patients presenting with pain, tenderness, and/or swelling over the first dorsal compartment of the wrist diagnosed with de Quervain’s tenosynovitis by clinical examination between 2009 and 2011. All patients were offered and all 40 accepted an ultrasound-guided corticosteroid injection; no patients treated during this time were treated in any other way. A total of 42 wrists were imaged and ultimately 41 wrists were injected under ultrasound-guidance in the 40 patients. Two patients received bilateral injections and one patient had resolution of symptoms on referral to the radiologist and therefore only underwent ultrasound evaluation without injection. Four of the 40 patients were lost to followup before the 6-week visit and two more were lost to followup by the 6-month visit, leaving 36 patients (37 wrists) for evaluation at 6 weeks and 34 patients (34 wrists) for evaluation by 6 months. The average age of the 40 patients was 47 years (range, 16–71 years) and 85% were female. Mean followups were 6 weeks (range, 3–17 weeks) and 11 months (range, 7–18 months). Institutional Review Board approval was obtained for this study, and all patients gave written informed consent.
All injections were performed by one board-certified radiologist (LNN) specializing in musculoskeletal sonography. The ultrasound-guided injection was placed in the following fashion: The skin was prepared with a solution of 2% chlorhexidine gluconate and 70% isopropyl alcohol (ChloraPrep, CareFusion, Leawood, KS, USA). A sterile fenestrated drape then was applied, and a sterile cover (Civ-Flex, Civco, Kalona, IA, USA) was placed over the ultrasound probe. Using 10% povidone-iodine solution (Betadine®, Purdue Products, Stamford, CT, USA) as a scanning medium, the tendons in the first extensor compartment were localized at the level of the distal radius in the short axis plane. (Fig. 1) A dorsal-to-palmar injection path was chosen that was free of overlying veins and of the superficial branch of the radial nerve. Local anesthetic was infiltrated in the skin and subcutaneous tissues using a 25-gauge hypodermic needle with 1% lidocaine. Subsequently, under continuous ultrasound guidance, a 22-gauge hypodermic needle was advanced into the first dorsal extensor compartment, deep to the tendons and along the cortical margin of the radius. A total of 0.5 mL of 1% lidocaine and 0.5 mL (20 mg) of triamcinolone acetonide was injected into the first compartment. If there was only one sheath, the injection was deposited at one location in the sheath (Fig. 2A). However, if there were two sheaths, the septum between the sheaths was pierced with the needle, with half of the injectate given around the APL tendon and, after the needle was drawn back (Fig. 2B), half given around the EPB tendon. The needle then was withdrawn and hemostasis maintained. An iU22 scanner (Philips Medical Systems, Bothell, WA, USA) was used with a compact linear 15–7 MHz multifrequency transducer. The tendons in the first extensor compartment were imaged in the transverse and longitudinal planes, and representative images stored to a picture archiving and communication system (Philips iSite, Philips Medical Systems) for immediate review. If the APL and EPB tendons were completely or partially separated by a hypoechoic septum, they were recorded as being in two sheaths. If the tendons were inseparable, they were recorded as being in one sheath.
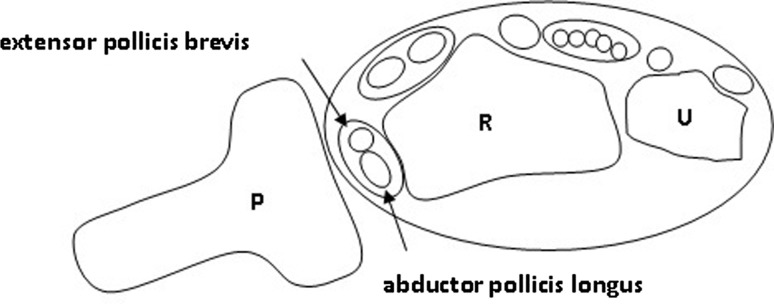
Fig. 1.
The drawing shows the ultrasound probe position over the first dorsal compartment that provides a transverse sonographic image to aid in ultrasound-guided injections for de Quervain’s tenosynovitis. P = probe; R = radius; U = ulna.
Fig. 2A–B.
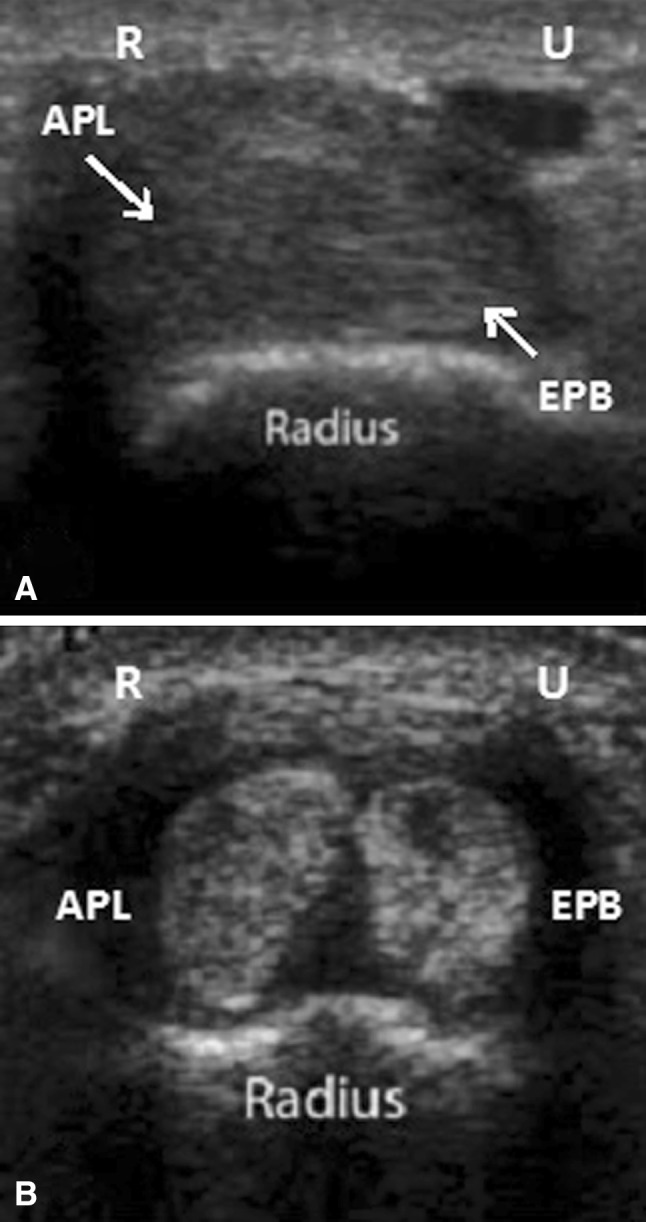
Transverse axis sonogram of the first dorsal compartment show (A) a single compartment versus (B) two subcompartments. APL = abductor pollicis longus; EPB = extensor pollicis brevis; R = radial; U = ulnar. The presence of hypoechoic or anechoic material between the two tendons as seen in B also can be seen with a tendon sheath effusion and is not necessarily diagnostic of a septum. However, on injection there was no communication seen between two tendons.
All patients underwent the same protocol after the procedure, including unsupervised nighttime splint use with a custom-made splint for 6 weeks and told to resume normal activities without restrictions.
All patients were examined at 6 weeks and 6 months after the injection by the hand surgeon (CHL) who enrolled and treated the patients in the study. The resolution or return of symptoms was noted at the 6-week visit using a scoring system between 0% to 100% improvement. DASH scores [11], VAS scores [4], and resolution or return of symptoms were noted at the 6-month visit.
Patients who did not achieve adequate relief from the cortisone injection at either the 6-week or 6-month followup were offered surgical release. Surgery was performed with the patient under local anesthesia with sedation, through a transverse incision over the first dorsal compartment. The extensor retinaculum was incised, and the APL and EPB tendon sheaths were identified and released. At the time of surgery, the surgeon noted any anatomic variations including the presence of separate subcompartments. The surgeon was blinded to the anatomic results found during the ultrasound-guided injection. These results were later compared with the findings of the radiologist.
Results
Separate subcompartments were noted in 52% of wrists. Three patients had partial septums, two of whom had two separate subcompartments.
At the 6-week followup, 97% (36 of 37) of the wrists in 36 patients had partial symptomatic relief of symptoms (60% or better improvement after one injection) and 92% of patients had near complete resolution of symptoms after one injection (80% or better improvement). At the last followup, the mean DASH and VAS scores were 18.39 and 2.2, respectively. However, 14% (five of 34) of patients had recurrence of symptoms.
Three patients subsequently underwent surgery, all three of whom had two separate subcompartments, as observed by ultrasound and confirmed at surgery. Two patients refused surgery and received a second injection without ultrasound, both of whom also had subcompartments identified by ultrasound. Of the patients who had subcompartments on ultrasound, 85% (17 of 20) had long-term relief of symptoms with one injection. No adverse effects from the injections were noted in any case.
Discussion
de Quervain’s disease is a stenosing tenosynovitis that is commonly treated nonsurgically with corticosteroid injections into the tendon sheath (Table 1). Given that response to treatment is related to injection accuracy and anatomic variation [9, 25, 29, 37], ultrasound-guided injections have emerged as an option in the nonoperative management of de Quervain’s disease [14, 17, 26]. However, to our knowledge, only one study has assessed ultrasound and ultrasound-guided injections in de Quervain’s tenosynovitis [14]. We therefore determined (1) the incidence of two or more subcompartments determined ultrasonically, (2) the incidence of anatomic variations during surgical release after failed injections, and (3) the relief of pain after ultrasound-guided injections.
Table 1.
Selected characteristics of several studies in the treatment of de Quervain’s tenosynovitis
| Parameter | Weiss et al. [32] | Harvey et al. [10] | Anderson et al. [2] | Witt et al. [33] | McKenzie [23] | Zingas et al. [37] | Current study |
|---|---|---|---|---|---|---|---|
| Female patients’ wrists | 80 | 70 | 50 | 64 | 25 | 12 | 34 |
| Male patients’ wrists | 13 | 12 | 5 | 19 | 5 | 7 | 6 |
| Bilateral | 0 | 0 | 1 | 4 | 0 | 0 | 2 |
| Age of males, years (range) | 38 (17–72) | 45 (16–83) | 47 (21–80) | 44 (16–75) | 49.5 (25–74) | 39 (24–63) | 47 (16–71) |
| Criteria for diagnosis | Pain at radial wrist, tender over 1st dorsal compartment, Finkelstein’s test | Pain at radial wrist, tender over 1st dorsal compartment, Finkelstein’s test | Pain at radial wrist, tender over 1st dorsal compartment, Finkelstein’s test | Pain at radial wrist, tender over 1st dorsal compartment, Finkelstein’s test | Pain at radial wrist, tender over 1st dorsal compartment, Finkelstein’s test | Pain at radial wrist, tender over 1st dorsal compartment, Finkelstein’s test | Pain at radial wrist, tender over 1st dorsal compartment, Finkelstein’s test |
| Injection medication used | 1 mL betamethasone, 1 mL 1% lidocaine | Hydrocortisone acetate or methyl prednisolone acetate, local anesthetic | 80 mg methyl prednisolone, 1.5 mL 1% xylocaine | 40 mg methyl prednisolone, 1 mL 1% lidocaine | 25 mg hydrocortisone, 1 mL 2% lidocaine | 6 mg celestone, 3 mL 1% lidocaine | 0.5 mL (20 mg) triamcinolone acetonide, 0.5 mL 1% lidocaine |
| One injection | 42 | 45 | 33 | 87 | 24 | 19 | 41 |
| Repeat injection | 0 | 7 | 14 | 0 | 5 | 0 | 2 |
| Percentage of success | 67 | 82 | 90 | 62 | 93 | 58 | 93 |
| Failure | 45 (all underwent surgery) | 11 (6 had 2 injections, 5 had 1 injection) | 5 | 33 (30 underwent surgery) | 1 | 8 (3 had surgery, 5 had return of criteria) | 3 (3 had surgery) |
| Followup, months (range) | 4, 13 (6–14) | 0.5, 20 (0.25–84) | 1.5, 48 (6–71) | 18 (12–30) | 0.5, 1, 3, 18 | 1, 3 | 1.5, 6 (0.5–17.5) |
We recognize limitations to our study. First, we had no control group without ultrasound-guided injections with which we could compare our findings. Although potentially improving our study, comparing our data with that in the literature can provide meaningful conclusions. Second, we lacked comparison of our DASH and VAS scores at three separate times: preinjection, 6 weeks, and 6 months. These comparisons could have provided more standardized outcome measures, however given the lack of DASH and VAS scores used in the current literature to assess the injection efficacy of de Quervain’s tenosynovitis, these data would have been difficult to correlate without the use of a control group. Third, the observations at followup could be operator dependent, as with all ultrasound-related studies. Correctly identifying subcompartments and partial septa in the first dorsal compartment of the wrist could be more challenging for physicians with varying experience.
The APL and EPB tendons have been described as encompassing one compartment. It now is understood that separate compartments for the APL and EPB tendons may exist. Although slip multiplicity may affect de Quervain’s tenosynovitis, current studies reflect a more prominent role of compartment septation [3, 6, 13, 26, 28, 32–34]. The reported average incidence of a division into two compartments in cadaveric studies is 43% (Table 2) [1, 7, 8, 13, 16, 19, 20, 22–25, 27]. In our study population, 52% of patients had multiple subcompartments, showing a lower percentage of divided septums in patients with de Quervain’s tenosynovitis than has been reported [1, 3, 13, 26, 33, 34, 36]. Other authors using ultrasound reported lower percentages of septums, 44% and 56% respectively [14, 17]. Some agree the higher correlation of a septum in patients with de Quervain’s tenosynovitis is a large predisposing factor in the development of the disease [12, 33].
Table 2.
Incidence of septation in studies of de Quervain’s tenosynovitis
| Study | Type of study | Septum/wrist* | Percentage |
|---|---|---|---|
| Alemohammad et al. [1] | CD/CS | 18/19:102/143 | 20:71 |
| Bahm et al. [3] | CS | 42/70 | 60 |
| Giles [7] | CD | 27/50 | 54 |
| Gonzalaez et al. [8] | CD | 31/66 | 45 |
| Jackson et al. [13] | CD/CS | 120/300:27/40 | 40:67 |
| Jeyapalan and Choudhary [14] | US | 10/17 | 56 |
| Kwon et al. [17] | US | 19/43 | 44 |
| Leslie et al. [20] | CD | 34/100 | 34 |
| Mahakkanukrauh and Mahakkanukrauh [22] | CD | 155/200 | 77.5 |
| Minamikawa et al. [24] | CD | 53/71 | 75 |
| Mirzanli et al. [25] | CD | 42/150 | 28 |
| Visuthikosol and Chanyasawat [34] | CS | 38/42 | 90 |
| Yuasa and Kiyoshige [36] | CS | 16/22 | 73 |
| Witt et al. [37] | CS | 22/30 | 73 |
| Current study | US/CS | 22/42:3/3 | 52:100 |
* Number of wrists with septums identified in the first dorsal compartment versus total number of wrists studied; CD = cadaveric series; CS = clinical series; US = ultrasound series.
The prevalence of a dividing septum in clinical studies including surgically treated patients has been reported more frequently than in cadaveric studies, averaging 72% (Table 2) [1, 3, 13, 26, 33, 34, 36]. It is important to recognize the possible selection bias inherent in some of these clinical studies that included patients who underwent surgery owing to failure of nonoperative treatment [17]. Therefore, they are more likely to have a septum present leading to surgery and their inclusion in a study documenting anatomic variation in the first dorsal compartment. In our study population, of the three patients for whom nonoperative treatment failed and who underwent surgery, all had two separate subcompartments, as observed on ultrasound and at surgery. Albeit small, our findings and those of others indicate that a selection bias exists in other studies in which surgical patients for whom nonoperative management failed, most likely owing to separate subcompartments, were included secondary to that failure [17]. This could have led to an artificially high prevalence of septums currently reported clinical studies of patients with de Quervain’s tenosynovitis [1, 3, 13, 26, 33, 34, 36]. Ultrasound has proven to be a useful nonoperative method to document septation in patients with de Quervain’s tenosynovitis (Table 3) [17, 35].
Table 3.
Findings on ultrasound before surgery for de Quervain’s tenosynovitis.
A couple studies have assessed the efficacy of corticosteroid injections [10, 33]. In a pooled quantitative literature evaluation, an overall success rate of 83% was identified using one to two injections [30]. In a dye-injection accuracy study, the authors reported that 84% of injections were placed accurately in the first dorsal compartment; however, only 26% (five of 19) of injections were deposited successfully in the APL and EPB compartments, correlating to greater symptom relief [37]. Another study testing injection technique with cadaveric models showed accurate placement of 72% of all injections in the APL and EPB compartments; the authors concluded that to improve outcomes, two separate injections should be made to successfully inject both compartments given the high incidence of a septum [25]. A less invasive option for accurate identification and injection into both potential compartments would be to use ultrasound guidance. In the only published study assessing ultrasound-guided injections in de Quervain’s tenosynovitis, the authors found 93.8% of their patients (15 of 16) had symptomatic relief at a mean followup of 6.75 weeks with no adverse reactions [14]. Three of their patients (20%) had recurrence of symptoms [14]. In our study, at 6 weeks followup, 97% of patients (36 of 37 wrists) reported partial resolution of symptoms and 92% reported near complete resolution (34 of 37), which is greater than the 83% in the pooled quantitative literature evaluation of blind injections (Table 1) [30]. Moreover, at last followup (mean, 11 months), we identified continued symptom improvement with a recurrence rate of 14%. Of the five patients in whom ultrasound-guided injection failed, all five had two compartments observed on ultrasound, correlating the findings from the radiologist and surgeon.
We found that ultrasound-guided injections may be useful in the treatment of de Quervain’s tenosynovitis. We found the incidence of multiple subcompartments to be within the range of the general population, but less common than reported in prior studies. All symptomatic wrists that required surgery in our study had multiple subcompartments. We identified better success with ultrasound-guided injections than previously reported. We identified no adverse effects from ultrasound-guided injections.
Footnotes
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that he, or a member of their immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
References
- 1.Alemohammad AM, Yazaki N, Morris RP, Buford WL, Viegas SF. Thumb interphalangeal joint extension by the extensor pollicis brevis: association with a subcompartment and de Quervain’s disease. J Hand Surg Am. 2009;34:719–723. doi: 10.1016/j.jhsa.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 2.Anderson BC, Manthey R, Brouns MC. Treatment of de Quervain’s tenosynovitis with corticosteroids: a prospective study of the response to local injection. Arthritis Rheum. 1991;34:793–798. doi: 10.1002/art.1780340703. [DOI] [PubMed] [Google Scholar]
- 3.Bahm J, Szabo Z, Foucher G. The anatomy of De Quervain’s disease: a study of operative findings. Int Orthop. 1995;19:209–211. doi: 10.1007/BF00185223. [DOI] [PubMed] [Google Scholar]
- 4.Chaffee A, Yakuboff M, Tanabe T. Responsiveness of the VAS and McGill pain questionnaire in measuring changes in musculoskeletal pain. J Sport Rehabil. 2011;20:250–255. doi: 10.1123/jsr.20.2.250. [DOI] [PubMed] [Google Scholar]
- 5.Quervain F. On a form of chronic tendovaginitis by Dr. Fritz de Quervain in la Chaux-de-Fonds. 1895. Am J Orthop (Belle Mead NJ). 1997;26:641–644. [PubMed] [Google Scholar]
- 6.Finkelstein H. Stenosing tendovaginitis at the radial styloid process. J Bone Joint Surg Am. 1930;12:509–540. [Google Scholar]
- 7.Giles KW. Anatomical variations affecting the surgery of de Quervain’s disease. J Bone Joint Surg Br. 1960;42:352–355. doi: 10.1302/0301-620X.42B2.352. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez MH, Sohlberg R, Brown A, Weinzweig N. The first dorsal extensor compartment: an anatomic study. J Hand Surg Am. 1995;20:657–660. doi: 10.1016/S0363-5023(05)80286-2. [DOI] [PubMed] [Google Scholar]
- 9.Gousheh J, Yavari M, Arasteh E. Division of the first dorsal compartment of the hand into two separate canals: rule or exception? Arch Iran Med. 2009;12:52–54. [PubMed] [Google Scholar]
- 10.Harvey FJ, Harvey PM, Horsley MW. De Quervain’s disease: surgical or nonsurgical treatment. J Hand Surg Am. 1990;15:83–87. doi: 10.1016/S0363-5023(09)91110-8. [DOI] [PubMed] [Google Scholar]
- 11.Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG) Am J Ind Med. 1996;29:602–608. doi: 10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 12.Ilyas AM, Ast M, Schaffer AA, Thoder J. De quervain tenosynovitis of the wrist. J Am Acad Orthop Surg. 2007;15:757–764. doi: 10.5435/00124635-200712000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Jackson WT, Viegas SF, Coon TM, Stimpson KD, Frogameni AD, Simpson JM. Anatomical variations in the first extensor compartment of the wrist: a clinical and anatomical study. J Bone Joint Surg Am. 1986;68:923–926. [PubMed] [Google Scholar]
- 14.Jeyapalan K, Choudhary S. Ultrasound-guided injection of triamcinolone and bupivacaine in the management of De Quervain’s disease. Skeletal Radiol. 2009;38:1099–1103. doi: 10.1007/s00256-009-0721-y. [DOI] [PubMed] [Google Scholar]
- 15.Jirarattanaphochai K, Saengnipanthkul S, Vipulakorn K, Jianmongkol S, Chatuparisute P, Jung S. Treatment of de Quervain disease with triamcinolone injection with or without nimesulide: a randomized, double-blind, placebo controlled trial. J Bone Joint Surg Am. 2004;86:2700–2706. doi: 10.2106/00004623-200412000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Keon-Cohen B. De Quervain’s disease. J Bone Joint Surg Br. 1951;33:96–99. doi: 10.1302/0301-620X.33B1.96. [DOI] [PubMed] [Google Scholar]
- 17.Kwon BC, Choi SJ, Koh SH, Shin DJ, Baek GH. Sonographic identification of the intracompartmental septum in de Quervain’s disease. Clin Orthop Relat Res. 2010;468:2129–2134. doi: 10.1007/s11999-009-1199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lane LB, Boretz RS, Stuchin SA. Treatment of de Quervain’s disease: role of conservative management. J Hand Surg Br. 2001;26:258–260. doi: 10.1054/jhsb.2001.0568. [DOI] [PubMed] [Google Scholar]
- 19.De Leao L. Quervain’s disease: a clinical and anatomical study. J Bone Joint Surg Am. 1958;40:1063–1070. [PubMed] [Google Scholar]
- 20.Leslie BM, Ericson WB, Jr, Morehead JR. Incidence of a septum within the first dorsal compartment of the wrist. J Hand Surg Am. 1990;15:88–91. doi: 10.1016/S0363-5023(09)91111-X. [DOI] [PubMed] [Google Scholar]
- 21.Loomis LK. Variations of stenosing tenosynovitis at the radial styloid process. J Bone Joint Surg Am. 1951;33:340–346. [PubMed] [Google Scholar]
- 22.Mahakkanukrauh P, Mahakkanukrauh C. Incidence of a septum in the first dorsal compartment and its effects on therapy of de Quervain’s disease. Clin Anat. 2000;13:195–198. doi: 10.1002/(SICI)1098-2353(2000)13:3<195::AID-CA6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 23.McKenzie JM. Conservative treatment of de Quervain’s disease. Br Med J. 1972;4:659–660. doi: 10.1136/bmj.4.5841.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minamikawa Y, Peimer CA, Cox WL, Sherwin FS. De Quervain’s syndrome: surgical and anatomical studies of the fibroosseous canal. Orthopedics. 1991;14:545–549. doi: 10.3928/0147-7447-19910501-07. [DOI] [PubMed] [Google Scholar]
- 25.Mirzanli C, Ozturk K, Esenyel CZ, Ayanoglu S, Imren Y, Aliustaoglu S. Accuracy of intrasheath injection techniques for de Quervain’s disease: a cadaveric study. J Hand Surg Eur Vol. 2012;37:155–160. doi: 10.1177/1753193411409126. [DOI] [PubMed] [Google Scholar]
- 26.Nagaoka M, Matsuzaki H, Suzuki T. Ultrasonographic examination of de Quervain’s disease. J Orthop Sci. 2000;5:96–99. doi: 10.1007/s007760050134. [DOI] [PubMed] [Google Scholar]
- 27.Nayak RS, Hussein M, Krishnamurthy A, Mansur DI, Prabhu LV, D’Souza P, Potu BK, Chettiar GK. Variation and clinical significance of extensor pollicis brevis: a study in South Indian cadavers. Chang Gung Med J. 2009;32:600–604. [PubMed] [Google Scholar]
- 28.Opreanu RC, Wechter J, Tabbaa H, Kepros JP, Baulch M, Xie Y, Lackey W, Katranji A. Anatomic variations of the first extensor compartment and abductor pollicis longus tendon in trapeziometacarpal arthritis. Hand (NY). 2010;5:184–189. doi: 10.1007/s11552-009-9234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters-Veluthamaningal C, Winters JC, Groenier KH, Meyboom-DeJong B. Randomised controlled trial of local corticosteroid injections for de Quervain’s tenosynovitis in general practice. BMC Musculoskelet Disord. 2009;10:131. doi: 10.1186/1471-2474-10-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richie CA, 3rd, Briner WW., Jr Corticosteroid injection for treatment of de Quervain’s tenosynovitis: a pooled quantitative literature evaluation. J Am Board Fam Pract. 2003;16:102–106. doi: 10.3122/jabfm.16.2.102. [DOI] [PubMed] [Google Scholar]
- 31.Sawaizumi T, Nanno M, Ito H. De Quervain’s disease: efficacy of intra-sheath triamcinolone injection. Int Orthop. 2007;31:265–268. doi: 10.1007/s00264-006-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiss AP, Akelman E, Tabatabai M. Treatment of de Quervain’s disease. J Hand Surg Am. 1994;19:595–598. doi: 10.1016/0363-5023(94)90262-3. [DOI] [PubMed] [Google Scholar]
- 33.Witt J, Pess G, Gelberman RH. Treatment of de Quervain tenosynovitis: a prospective study of the results of injection of steroids and immobilization in a splint. J Bone Joint Surg Am. 1991;73:219–222. [PubMed] [Google Scholar]
- 34.Visuthikosol V, Chanyasawat S. Surgical treatment of de Quervain’s diseases: a clinical review of 42 cases. J Med Assoc Thai. 1988;71:637–639. [PubMed] [Google Scholar]
- 35.Volpe A, Pavoni M, Marchetta A, Caramaschi P, Biasi D, Zorzi C, Arcaro G, Grassi W. Ultrasound differentiation of two types of de Quervain’s disease: the role of retinaculum. Ann Rheum Dis. 2010;69:938–939. doi: 10.1136/ard.2009.123026. [DOI] [PubMed] [Google Scholar]
- 36.Yuasa K, Kiyoshige Y. Limited surgical treatment of de Quervain’s disease: decompression of only the extensor pollicis brevis subcompartment. J Hand Surg Am. 1998;23:840–843. doi: 10.1016/S0363-5023(98)80160-3. [DOI] [PubMed] [Google Scholar]
- 37.Zingas C, Failla JM, Holsbeeck M. Injection accuracy and clinical relief of de Quervain’s tendinitis. J Hand Surg Am. 1998;23:89–96. doi: 10.1016/S0363-5023(98)80095-6. [DOI] [PubMed] [Google Scholar]