Abstract
Background
Neer Group VI proximal humeral fractures often are related to persistent disability despite surgical treatment. We retrospectively compared the outcome after open reduction and internal fixation with the PHILOS® plate or primary hemiarthroplasty in patients with Neer Group VI fractures focusing on complications, shoulder function, health-related quality of life (SF-36), and potential risk factors for complications.
Questions/purposes
The aim of this study was to compare the PHILOS® plate with primary hemiarthroplasty for treatment of specific Neer Group VI fractures. We asked whether (1) both procedures have comparable clinical and radiologic complication rates; (2) one procedure is superior in terms of revision rate; (3) objective and subjective shoulder function (Constant-Murley score) and health-related quality of life (SF-36) were comparable in both groups at final followup; and (4) there are clinical or radiologic predictors for complications in any group?
Methods
Between 2002 and 2007, 44 consecutive patients (mean, 75.2 years) with a Neer Group VI proximal humeral fracture were included. Twenty-two patients treated with a PHILOS® plate were compared with 22 patients treated by primary hemiarthroplasty. Both groups were similar in all criteria. At minimum followup of 12 months (mean, 30 months; range, 12-83 months), radiographic control, Constant-Murley score, and SF-36 were performed.
Results
Fourteen patients with complications (63.6%) were counted in the PHILOS® plate group, of which 10 (45.4%) needed revision surgery, mostly as a result of avascular necrosis and screw cut-outs. In the primary hemiarthroplasty group, only one patient needed revision surgery (4.5%). Smoking and steroid therapy were substantially associated with complications in the PHILOS® plate group. There were no differences between the two groups regarding Constant-Murley or SF-36 scores.
Conclusions
Angular stable open reduction and internal fixation was associated with high complication and revision rates, especially in patients who smoked and those receiving steroid therapy. Primary hemiarthroplasty provides limited function, which had little influence on the quality of life in this elderly collective. There are predictive factors for complications after the treatment of Neer Group VI proximal humeral fractures with the PHILOS® plate. Primary hemiarthroplasty remains a good option, especially when treating elderly patients.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Fracture of the proximal humerus is a common injury [4], especially in elderly patients with osteoporotic bone. Neer introduced a fracture classification, which is still widely used [24]. In its Group VI, he gathered three- and four-part fracture-dislocations. Displaced fractures of the articular surface (impression or split) also are included in this group, because the noncrushed part of the humeral head is extruded from the joint during impact. These fractures are rare, but they represent a severe injury often leading to permanent functional limitations despite surgical treatment [12]. Neer recommended open reduction and internal fixation (ORIF) for three-part fracture-dislocations and primary hemiarthroplasty (HA) for four-part fracture-dislocations and for fractures with greater than 50% of cartilage-covered articular defect [24, 25]. Angular stable implants have been developed for fractures of the proximal humerus [7, 9, 10, 23, 30]. With these implants, better biomechanical stability could be achieved [16]. Anatomic reconstruction for severely displaced fractures and fractures with glenohumeral dislocations aiming to achieve superior function compared with primary HA has been reported [2, 11, 12, 17, 20, 22, 28].
In 2003, we started to reconstruct proximal humeral fractures with the PHILOS® plate (Synthes GmbH, Solothurn, Switzerland) in our trauma center. Encouraged by good results, we expanded the range of application for ORIF and decided to use this locking plate for treatment of more complex fractures including Neer Group VI fractures. Before then, those fractures were treated by HA only. With further followup, we found a remarkably high complication rate.
The aim of this study was to compare the PHILOS® plate with primary HA for treatment of specific Neer Group VI fractures. The following questions were addressed: (1) Do both procedures have comparable clinical and radiologic complication rates? (2) Is one procedure superior in terms of revision rate? (3) Are objective and subjective shoulder function (Constant-Murley score [6]) and health-related quality of life (SF-36 [31]) comparable in both groups at final followup? (4) Are there clinical or radiologic predictors for complications in any group?
Patients and Methods
Between October 2002 and December 2007, a total of 765 patients with a fracture of the proximal humerus were treated in our trauma unit. In 43 (5.7%) patients, primary HA was performed. Angular stable reconstruction using the PHILOS® plate was performed in 313 (41.0%) patients. In comparison, nonoperative treatment was chosen for the majority of patients (408 patients [53.3%]).
Of this cohort, we exclusively concentrated on the patients treated with the PHILOS® plate or primary HA for Neer Group VI fractures. In this single-institution retrospective, comparative study we therefore analyzed all initial AP and transscapular radiographs and available CT scans. This left a total of 54 patients with this specific fracture type. We reviewed these patients’ charts retrospectively. Patients’ preoperative history and medications were documented. To ensure comparability of both groups, comorbidities were measured by the Charlson Index [26]. If patients smoked more than 10 cigarettes per day at the time of injury, they were classified as smokers [1] and any systemic steroid therapy at the time of injury was accounted for. AP and transscapular radiographic examinations at the time of injury, after surgery, and at the time of last followup (mean, 30 months; range, 12–83 months) were available for all patients. Functional and clinical assessments using the age- and gender-specific Constant-Murley [29] and the SF-36 [31] scores were performed at the last followup or at the time of a complication. Inclusion criteria were a traumatic Neer Group VI fracture treated either by the PHILOS® plate or primary HA and a minimum of 12 months followup.
Twenty-six individuals were treated with a PHILOS® plate (PHILOS® group). Three patients died during the 1-year postoperative period independent of shoulder surgery and one patient had incomplete followup. This left a total of 22 (85%) patients finally entering the PHILOS® group. Five patients who needed secondary arthroplasty during followup were not considered for the final comparison of the Constant-Murley and SF-36 scores, but they were included and analyzed for complications and revision surgery.
Twenty-eight patients were treated with primary HA (HA group), of whom six died during the followup unrelated to shoulder surgery. Finally, 22 (78%) of these patients were included in the HA group. One of these patients underwent reverse total shoulder arthroplasty as a result of a complication and therefore that patient’s data were not included for final comparison of the Constant-Murley and SF-36 scores (Table 1).
Table 1.
Comparison of final Constant-Murley and SF-36 scores between groups
| Scoring system | PHILOS® | Hemiarthroplasty | p value |
|---|---|---|---|
| Constant-Murley score (points) | 65.2 (41–100) | 54.4 (38–86) | NS |
| Activities of daily living | 13.4 (8–20) | 12.6 (6–20) | NS |
| Pain | 11.2 (5–15) | 12.1 (10–15) | NS |
| Power | 11.0 (4–22) | 7.9 (2–20) | NS |
| Abduction | 6.5 (4–10) | 3.1 (2–8) | < 0.05 |
| Flexion | 6.2 (2–10) | 3.1 (2–8) | < 0.05 |
| External rotation | 5.7 (0–10) | 4.0 (2–8) | < 0.05 |
| Internal rotation | 5.6 (2–10) | 4.9 (2–8) | NS |
| SF-36 score (points) | 59.4 (30–96) | 56.0 (25–91) | NS |
Data are expressed as mean and minimum to maximum; NS = not significant.
No apparent differences between the two groups regarding group size, age, sex, or comorbidities were seen (Table 2). The minimum followup was 12 months (mean, 21 months; range, 12–60 months) in the PHILOS® group and 12 months (mean, 36 months; range, 12–83 months) in the HA group. The fracture pattern in all of these patients was clearly Neer type VI (Table 2). Almost all patients (40 of 44 [90.1%]; mean age, 77.3 years; range, 55–93 years) had domestic or outdoor falls. Only four younger patients (four of 44 [9.1%]; mean age, 54.5 years; range, 42–66 years) had a high-velocity accident, all treated with a PHILOS® plate; three of them had concomitant injuries. Four patients in the PHILOS® group and three in the HA group received steroid therapy at the time of injury. Seven patients in the PHILOS® group and nine in the HA group were smokers.
Table 2.
Patient characteristics of both groups
| Characteristic | PHILOS® | Hemiarthroplasty |
|---|---|---|
| Patients (number) | 22 | 22 |
| Age* (years) | 75 (42–93) | 76 (55–92) |
| Male/female (number) | 4/18 | 3/19 |
| Charlson Index | 0.4 | 0.9 |
| Smoker (yes/no) | 7/15 | 9/13 |
| Delay to surgery* | 4 (0–8) | 3 (0–16) |
| Three-part dislocation | 3 | 0 |
| Four-part dislocation | 10 | 6 |
| Four-part dislocation with head impression | 1 | 2 |
| Head impression | 7 | 7 |
| Head split | 1 | 7 |
* Data are expressed as mean and minimum to maximum.
The method of surgical treatment was either chosen preoperatively or intraoperatively. The fracture could not be reduced anatomically during the operation or the head fragment showed signs of avascularity (not quantified by laser Doppler flowmetry or borehole bleeding) in nine patients, therefore primary HA was performed. For three patients with severe displacement of the head fragment observed on the radiograph and 10 patients with more than one cartilage-covered articular fragment of the humeral head observed on the preoperative CT scan, no attempt was made to perform ORIF.
Three different attending trauma surgeons were involved (AP, UC, MD) in all of the operations. Surgery was performed with the patient under general anesthesia and in a beach chair position. A deltopectoral approach was used in all patients. Instruments were available for PHILOS® and the shoulder HA using a Howmedica prosthesis (Stryker Howmedica Osteonics, Mahwah, NJ, USA).
If, after the mentioned considerations, the decision was made to use a PHILOS® plate (short, three holes), open reduction was performed. The humeral head fragment always was stabilized with at least seven locking head screws. Tuberosities additionally were secured to the plate with nonabsorbable sutures (3-Ethicon®-Mersilene, polyester; Johnson & Johnson®, Dublin, Ireland). For HAs, the prosthesis was cemented in all cases aiming for 25° retroversion. The tuberosities were fixed to each other and to the fin system of the implant by nonabsorbable sutures (3-Ethicon®-Mersilene, polyester; Johnson & Johnson®). In all cases, cancellous bone graft from the humeral head was applied between the tuberosities and the humeral diaphysis to facilitate bony union.
The postoperative rehabilitation program was the same for both groups: immobilization for 7 days, then pendulum exercises of the shoulder for another 2 weeks. After 3 weeks postoperatively, passive ROM up to 90° elevation was initiated. Free active ROM with strengthening was started 6 weeks after surgery.
Preoperative AP and transscapular radiographs were analyzed retrospectively by two of the authors (CS, MD), accounting for exact fracture pattern and predictive factors of humeral head ischemia according to Hertel et al. [14]. In case of no accordance, operation protocols were consulted. This procedure was blinded for further followup. In the PHILOS® group, the postoperative reduction result was assessed by the head-shaft angulation on the AP view [15]. Secondary displacement was measured by the degree of varus collapse on the immediate postoperative radiograph compared with the last radiograph. Complications assessed radiographically were screw perforations into the glenohumeral joint and partial or total avascular necrosis (AVN) of the humeral head in the PHILOS® group. In the HA group, the initial and final greater tuberosity malposition in the vertical plane were assessed according to Boileau et al. [5]. In the horizontal plane, it was considered malposition when it was not seen on the AP radiograph but was observed on the transscapular radiograph. The greater tuberosity was considered resorbed when it was not seen in any plane of the last radiographs obtained. Proximal migration of the prosthesis was assessed on the postoperative and last AP radiographs [5]. The last radiograph was examined for prosthetic loosening if radiolucent lines around the prosthetic stem were seen and periarticular ectopic bone formations were observed [18].
Statistical analysis was performed using SPSS 17.0 software (Chicago, IL, USA). For group comparisons, we used Student’s t-test and for nonparametric comparisons, Fisher’s exact test. Correlations were calculated using Spearman’s rho, and for dichotomous data, we calculated the phi coefficient. Probability values less than 0.05 were considered significant.
Results
Clinical and radiographic complication rates were comparable in both groups at the last followup. The complication rates were 63.6% (14 of 22) for the PHILOS® group (Table 3) and 77.2% (17 of 22) (p = 0.2) for the HA group. The most frequent complication in the PHILOS® group was AVN of the humeral head (12 of 22; 54.5%) (Fig. 1) followed by secondary perforation of the head screws into the glenohumeral joint (eight of 12; 66.6%) (Fig. 2). Screw cut-out attributable to secondary collapse of the fracture was seen twice (two of 22; 9.1%). In the HA group, posterior malreduction of the greater tuberosity was seen in six patients (six of 22; 27.3%); four healed in this position (Fig. 3). Twelve patients (12 of 22; 54.5%) had an acromiohumeral distance less than 7 mm at the last radiographic followup. Of those, a total of seven patients had complete resorption of the greater tuberosity (two with primary posterior malposition and five with primary correct reduction). In one patient ectopic bone formation was found (final Constant-Murley score, 86 points). No sign of prosthetic loosening was seen in any patient at the time of the last radiographic followup.
Table 3.
Patients with complications in the PHILOS® group
| Patient number | Sex | Age (years) | Steroid use | Smoker | Fracture type | Head extension > 8 mm | Complications | Days to complication | Constant-Murley score | Revision 1 | Revision 2 | Final followup (months) | Final Constant-Murley score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 9 | F | 86 | Yes | No | Four-part with head impression | No | Partial AVN | 240 | 56 | – | – | 12 | 56 |
| 11 | F | 92 | Yes | No | Three-part dislocation | No | AVN, screw cut-out | 181 | 45 | Removal of hardware | – | 28 | 50 |
| 12 | F | 83 | No | Yes | Four-part dislocation | Yes | Secondary dislocation and screw cut-out | 220 | 45 | – | – | 12 | 49 |
| 14 | F | 66 | Yes | No | Head split | No | Partial AVN | 180 | 40 | – | – | 13 | 41 |
| 16 | F | 84 | No | No | Four-part dislocation | Yes | Secondary dislocation and screw cut-out | 101 | 40 | Partial removal of hardware | – | 12 | 78 |
| 17 | M | 66 | No | Yes | Head impression | No | AVN, screw cut-out | 261 | 37 | Removal of hardware | – | 24 | 38 |
| 21 | F | 72 | No | Yes | Head impression | No | AVN, screw cut-out and rotator cuff tear | 358 | 40 | Reverse arthroplasty | – | 42 | 54 |
| 22 | F | 88 | No | No | Four-part dislocation with head split | No | Partial AVN | 200 | 50 | – | – | 42 | 74 |
| 23 | M | 49 | Yes | Yes | Four-part dislocation | Yes | AVN, screw cut-out and rotator cuff tear | 116 | 32 | Change of screw | Reverse arthroplasty | 40 | 23 |
| 24 | F | 84 | No | Yes | Four-part dislocation | No | AVN, screw cut-out | 240 | 50 | Partial removal of hardware | – | 26 | 62 |
| 25 | F | 62 | No | Yes | Four-part dislocation | No | AVN, screw cut-out | 272 | 27 | Reverse arthroplasty | – | 39 | 61 |
| 30 | F | 78 | No | No | Three-part dislocation | No | AVN, screw cut-out | 355 | 44 | Hemiarthroplasty | – | 28 | 65 |
| 31 | F | 71 | No | Yes | Head impression | No | AVN, screw cut-out | 343 | 44 | Hemiarthroplasty | – | 36 | 32 |
| 33 | M | 56 | No | No | Four-part dislocation | No | Early wound infection, rotator cuff tear, and partial AVN | 13 | – | Wound revision | – | 12 | 47 |
AVN = avascular necrosis.
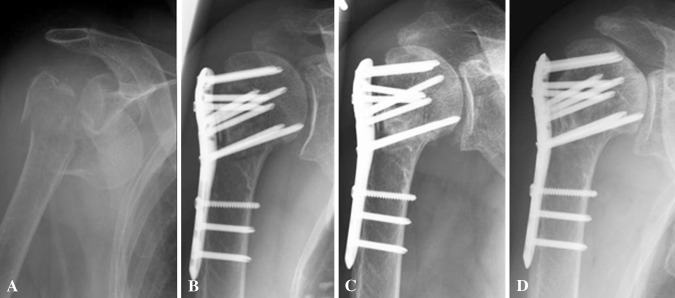
Fig. 1A–D.
Radiographs obtained at (A) the time of injury, (B) postoperatively, (C) after 200 days followup with beginning necrosis of the humeral head and (D) last followup after 4 years are shown. The 88-year-old patient had no pain and did not want additional surgery.
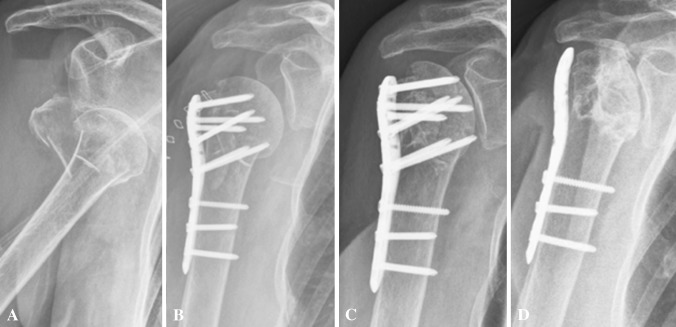
Fig. 2A–D.
Radiographs obtained at (A) the time of injury, (B) postoperatively, (C) after 240 days with AVN and consecutive screw cut-out, and (D) last followup 26 months after removal of the head screws are shown for an 84-year-old patient.
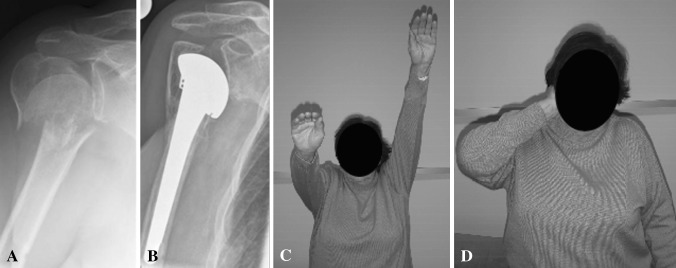
Fig. 3A–D.
(A) This radiograph was obtained at the time this 82-year-old patient sustained injury. (B) Three years postoperative, her radiograph shows dorsal malunion of the greater tuberosity, which also appears to be positioned high, but still in the normal range of 5 mm in relation to the head of the prosthesis. (C) The patient has limited function but no pain. (D) The surgically treated shoulder did not influence her quality of life.
We counted more revision surgeries in the PHILOS® group, which is significant (10 of 22 versus one of 22, or 45.5% versus 4.5%; p = 0.002). Five patients (22.7%) in the PHILOS® group needed conversion to secondary arthroplasty. Of those, a reverse shoulder arthroplasty was performed three times and HA was performed twice (Table 3). Owing to persistent pain caused by a dorsally displaced greater tuberosity, a reverse shoulder arthroplasty was performed 210 days after HA. We did not include patients with a secondary arthroplasty in the final comparison of Constant-Murley and SF-36 scores because the treatment modality changed, and reverse shoulder arthroplasty was not performed in our institution.
There was no difference in the Constant-Murley (p = 0.4) or SF-36 (p = 0.6) scores between the HA- and PHILOS®-treated fractures. For the different fracture subtypes (dislocation, head impression, head split), the Constant-Murley score did not differ in the treatment groups. In the HA group neither posterior malpositioning of the greater tuberosity (p = 0.6) (Fig. 3) nor an acromiohumeral distance less than 7 mm (p = 0.2) had significant influence on the Constant-Murley score. The mean final Constant-Murley scores were 65.2 (range, 41–100) in the PHILOS® group and 54.4 (range, 38–86) for the HA group. The final SF-36 score was 59.4 (range, 30–96) in the PHILOS® group and 56 (range, 25–91) in the HA group. Patients without complications (eight of 22; 36.4%) had a mean final Constant-Murley score of 72.8 in the PHILOS® group (range, 46–100), which was significantly higher compared with the HA group (p = 0.001). The mean SF-36 score (67.3; range 39–93) for these patients also was higher, but not statistically significantly higher (p = 0.3).
Smoking was a significant predictor of complications in the PHILOS® group (p = 0.02) (Table 3). Steroid therapy also was associated with complications in that group as all patients (four of four) (Table 3) were concerned, however, there was no statistical significance (p = 0.1). In the HA group, there was no association between smoking or steroid therapy and complications. In the PHILOS® group, if the metaphyseal head extension measured greater than 8 mm, it was less likely (p = 0.005) (Table 3) that AVN would develop. The medial hinge was greater than 2 mm in all fractures and therefore was not a predictor for complications. Neither the mean postoperative head-shaft angle (130°, range, 125°–140° versus 129°, range, 115°–145°) nor varus collapse (5.2°, range, 0°–20° versus 7.9°, range, 0°–15°of 130°) differed significantly for patients without complications compared with patients with complications in the PHILOS® group.
Discussion
Since Neer’s work [25], there has been a lack of comparative studies concerning different treatment options for specific fracture types. Looking for functional outcome or quality of life after the treatment of fracture-dislocations with angular stable implants or primary HA in the literature, only sparse information exists integrated in several studies.
We compared two similar groups of patients with Neer Group VI proximal humeral fractures in this series. The group treated with the PHILOS® plate was at significantly higher risk for revision surgery. Smoking was the main risk factor for complications in this group. The Constant-Murley and SF-36 scores were comparable in both groups. Respecting the relatively high age of the patients in this study, we conclude that primary HA remains a valuable option in the treatment of Neer Group VI fractures. It is a reliable treatment of pain with a small number of complications needing revision (Fig. 3). Angular stable ORIF of Neer Group VI fractures can be reserved for younger patients trying to reach better functionality accepting the high risk of avascular necrosis and revision surgery while paying attention to secondary screw perforations.
This study has certain limitations: First, we presume there might be bias to treat more severe fracture types with HA, because there were more head split fractures in the HA group and some indications were made intraoperatively. However, fracture subtypes had no influence on the final outcome. Second, we excluded five patients with complications and secondary arthroplasty for the final comparison of the Constant-Murley and SF-36 scores in the PHILOS® group. Third, the involved surgeons had more experience with the PHILOS® plate than with HA. Finally, there was an older type of HA used in this study; newer implants may provide better functional results.
In the PHILOS® group, 54.5% of the patients had AVN of the humeral head. The rate of AVN is reported to be as much as 80% for fracture dislocations [12]. The vascularization of the humeral head is known to be fragile [8], especially in four-part fracture dislocations [25] (Fig. 1). Perforation of head screws often was associated with AVN; this is a specific complication duo after angular stable ORIF [28]. Furthermore, this study showed that Neer Group VI fractures are difficult to stabilize with a PHILOS® plate. The implant could not provide stability with time, leading to varus collapse during followup. Hertel’s eggshell model provides a possible explanation for this observation [13]. Concerning final malunion, absorption, and nonunion of the greater tuberosity the literature varies from 21% to 53% for primary HA of displaced three- and four-part fractures [5, 19, 21, 22]. We explain our comparatively high incidence of mainly radiographic complications with strict analysis of the greater tuberosity, the acromiohumeral distance, and the complexity of the Neer group VI fractures, which are only partially included in other studies [3, 5, 19, 21, 22, 27].
In the PHILOS® group, 71% of the complications needed revision surgery, whereas only 6% of the complications in the HA group needed additional surgical treatment. The revisions in the PHILOS® group were attributable mainly to partial or total AVN with subsequent secondary screw cut-outs, which needed surgical intervention to avoid further damage to the joint. The low revision rate in the HA group may be explained by the high number of radiographic complications, which had no influence on the Constant-Murley score. The only patient needing revision had persistent pain and limited function after HA. Specific statements regarding revision rate of Neer Group VI fractures are sparse in literature, which makes a comparison to our data difficult. Kettler et al. reported a rate of 42% [17], which is comparable to our results. We did not find any specification regarding revision rate for primary HA in Neer Group VI fractures in the literature [3, 5, 19, 21, 22, 27].
Comparing the age- and gender-adapted Constant-Murley score of our two treatment groups, there was no significant difference. The treatment of pain was even better in the HA group. In contrast, the objective shoulder function was better in the PHILOS® group (Table 1). Patients without complications in the PHILOS® group had significantly better shoulder function. However, the SF-36 score did not differ significantly between our two groups, not even in patients without complications after angular stable ORIF. Therefore, limited shoulder functionality did not have an influence on the quality of life in our collective. We explain this with the high mean age in both groups with the assumption that shoulder function has limited influence on the quality of life of the elderly patient (Fig. 3). Kettler et al. reported a Constant-Murley score between 52 to 72 points after ORIF with the PHILOS® plate [17]. Hente et al. reached a mean Constant-Murley score of 55 points in these specific fracture types, which was lower than for fractures without dislocation [12]. These results match ours, knowing that the Constant-Murley score of different studies are difficult to compare. For the outcome after HA, we found studies that incorporated 21% to 50% of fractures with glenohumeral displacement, or head split pattern [3, 5, 19, 21, 22, 27]. These studies showed a final Constant-Murley score that ranged from 40 to 83 points, while many other studies mention no fracture-specific outcome. The functional outcome in our HA group is in the same range. Concerning the SF-36, we found no comparable studies in the literature.
In the PHILOS® group, smoking was a significant predictor of complications, and a metaphyseal head extension greater than 8 mm [14] was a reliable predictor for sufficient vascularization without AVN. In the HA group, no predictor was found. Smoking is known to have a bad influence on fracture healing [1]. Steroid therapy was not significantly related to complications in the PHILOS® group, but our results show a clear trend toward it, as all concerned patients had complications (Table 3). When looking for possible reasons for the limited functionality after HA, we found no association between a malpositioned greater tuberosity or reduced acromiohumeral distance and the Constant-Murley score [5, 19]. The relatively small number of patients, which allows only limited comparison, might be a possible explanation.
Footnotes
Each author certifies that he or she, or a member of their immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Hospital Zurich-Triemli, Zurich, Switzerland.
References
- 1.Adams CI, Keating JF, Court-Brown CM. Cigarette smoking and open tibial fractures. Injury. 2001;32:61–65. doi: 10.1016/S0020-1383(00)00121-2. [DOI] [PubMed] [Google Scholar]
- 2.Agudelo J, Schurmann M, Stahel P, Helwig P, Morgan SJ, Zechel W, Bahrs C, Parekh A, Ziran B, Williams A, Smith W. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21:676–681. doi: 10.1097/BOT.0b013e31815bb09d. [DOI] [PubMed] [Google Scholar]
- 3.Antuna SA, Sperling JW, Cofield RH. Shoulder hemiarthroplasty for acute fractures of the proximal humerus: a minimum five-year follow-up. J Shoulder Elbow Surg. 2008;17:202–209. doi: 10.1016/j.jse.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 4.Bengner U, Johnell O, Redlund-Johnell I. Changes in the incidence of fracture of the upper end of the humerus during a 30-year period: a study of 2125 fractures. Clin Orthop Relat Res. 1988;231:179–182. [PubMed] [Google Scholar]
- 5.Boileau P, Krishnan SG, Tinsi L, Walch G, Coste JS, Mole D. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11:401–412. doi: 10.1067/mse.2002.124527. [DOI] [PubMed] [Google Scholar]
- 6.Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987 Jan;(214):160–164. [PubMed]
- 7.Dietrich M, Meier C, Lattmann T, Zingg U, Gruninger P, Platz A. Complex fracture of the proximal humerus in the elderly: locking plate osteosynthesis vs hemiarthroplasty][in German. Chirurg. 2008;79:231–240. doi: 10.1007/s00104-007-1436-z. [DOI] [PubMed] [Google Scholar]
- 8.Gerber C, Schneeberger AG, Vinh TS. The arterial vascularization of the humeral head: an anatomical study. J Bone Joint Surg Am. 1990;72:1486–1494. [PubMed] [Google Scholar]
- 9.Handschin AE, Cardell M, Contaldo C, Trentz O, Wanner GA. Functional results of angular-stable plate fixation in displaced proximal humeral fractures. Injury. 2008;39:306–313. doi: 10.1016/j.injury.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Helmy N, Hintermann B. New trends in the treatment of proximal humerus fractures. Clin Orthop Relat Res. 2006;442:100–108. doi: 10.1097/01.blo.0000194674.56764.c0. [DOI] [PubMed] [Google Scholar]
- 11.Helwig P, Bahrs C, Epple B, Oehm J, Eingartner C, Weise K. Does fixed-angle plate osteosynthesis solve the problems of a fractured proximal humerus? A prospective series of 87 patients. Acta Orthop. 2009;80:92–96. doi: 10.1080/17453670902807417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hente R, Kampshoff J, Kinner B, Fuchtmeier B, Nerlich M. Treatment of dislocated 3- and 4-part fractures of the proximal humerus with an angle-stabilizing fixation plate][in German. Unfallchirurg. 2004;107:769–782. doi: 10.1007/s00113-004-0818-7. [DOI] [PubMed] [Google Scholar]
- 13.Hertel R. Fractures of the proximal humerus in osteoporotic bone. Osteoporos Int. 2005;16(suppl 2):S65–S72. doi: 10.1007/s00198-004-1714-2. [DOI] [PubMed] [Google Scholar]
- 14.Hertel R, Hempfing A, Stiehler M, Leunig M. Predictors of humeral head ischemia after intracapsular fracture of the proximal humerus. J Shoulder Elbow Surg. 2004;13:427–433. doi: 10.1016/j.jse.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 15.Hertel R, Knothe U, Ballmer FT. Geometry of the proximal humerus and implications for prosthetic design. J Shoulder Elbow Surg. 2002;11:331–338. doi: 10.1067/mse.2002.124429. [DOI] [PubMed] [Google Scholar]
- 16.Hessmann MH, Sternstein W, Krummenauer F, Hofmann A, Rommens PM. Internal fixation of proximal humerus fractures][in German. Chirurg. 2005;76:167–174. doi: 10.1007/s00104-004-0927-4. [DOI] [PubMed] [Google Scholar]
- 17.Kettler M, Biberthaler P, Braunstein V, Zeiler C, Kroetz M, Mutschler W. Treatment of proximal humeral fractures with the PHILOS angular stable plate: presentation of 225 cases of dislocated fractures][in German. Unfallchirurg. 2006;109:1032–1040. doi: 10.1007/s00113-006-1165-7. [DOI] [PubMed] [Google Scholar]
- 18.Kjaersgaard-Andersen P, Frich LH, Sojbjerg JO, Sneppen O. Heterotopic bone formation following total shoulder arthroplasty. J Arthroplasty. 1989;4:99–104. doi: 10.1016/S0883-5403(89)80061-0. [DOI] [PubMed] [Google Scholar]
- 19.Kontakis G, Koutras C, Tosounidis T, Giannoudis P. Early management of proximal humeral fractures with hemiarthroplasty: a systematic review. J Bone Joint Surg Br. 2008;90:1407–1413. doi: 10.1302/0301-620X.90B11.21070. [DOI] [PubMed] [Google Scholar]
- 20.Koukakis A, Apostolou CD, Taneja T, Korres DS, Amini A. Fixation of proximal humerus fractures using the PHILOS plate: early experience. Clin Orthop Relat Res. 2006;442:115–120. doi: 10.1097/01.blo.0000194678.87258.6e. [DOI] [PubMed] [Google Scholar]
- 21.Kralinger F, Schwaiger R, Wambacher M, Farrell E, Menth-Chiari W, Lajtai G, Hubner C, Resch H. Outcome after primary hemiarthroplasty for fracture of the head of the humerus: a retrospective multicentre study of 167 patients. J Bone Joint Surg Br. 2004;86:217–219. doi: 10.1302/0301-620X.86B2.14553. [DOI] [PubMed] [Google Scholar]
- 22.Mighell MA, Kolm GP, Collinge CA, Frankle MA. Outcomes of hemiarthroplasty for fractures of the proximal humerus. J Shoulder Elbow Surg. 2003;12:569–577. doi: 10.1016/S1058-2746(03)00213-1. [DOI] [PubMed] [Google Scholar]
- 23.Moonot P, Ashwood N, Hamlet M. Early results for treatment of three- and four-part fractures of the proximal humerus using the PHILOS plate system. J Bone Joint Surg Br. 2007;89:1206–1209. doi: 10.1302/0301-620X.89B9.18528. [DOI] [PubMed] [Google Scholar]
- 24.Neer CS., 2nd Displaced proximal humeral fractures: I. Classification and evaluation . J Bone Joint Surg Am. 1970;52:1077–1089. [PubMed] [Google Scholar]
- 25.Neer CS., 2nd Displaced proximal humeral fractures: II. Treatment of three-part and four-part displacement. J Bone Joint Surg Am. 1970;52:1090–1103. [PubMed] [Google Scholar]
- 26.Roos LL, Stranc L, James RC, Li J. Complications, comorbidities, and mortality: improving classification and prediction. Health Serv Res. 1997;32:229–238; discussion 239–242. [PMC free article] [PubMed]
- 27.Solberg BD, Moon CN, Franco DP, Paiement GD. Surgical treatment of three and four-part proximal humeral fractures. J Bone Joint Surg Am. 2009;91:1689–1697. doi: 10.2106/JBJS.H.00133. [DOI] [PubMed] [Google Scholar]
- 28.Sudkamp N, Bayer J, Hepp P, Voigt C, Oestern H, Kaab M, Luo C, Plecko M, Wendt K, Kostler W, Konrad G. Open reduction and internal fixation of proximal humeral fractures with use of the locking proximal humerus plate: results of a prospective, multicenter, observational study. J Bone Joint Surg Am. 2009;91:1320–1328. doi: 10.2106/JBJS.H.00006. [DOI] [PubMed] [Google Scholar]
- 29.Tavakkolizadeh A, Ghassemi A, Colegate-Stone T, Latif A, Sinha J. Gender-specific Constant score correction for age. Knee Surg Sports Traumatol Arthrosc. 2009;17:529–533. doi: 10.1007/s00167-009-0744-x. [DOI] [PubMed] [Google Scholar]
- 30.Wanner GA, Wanner-Schmid E, Romero J, Hersche O, Smekal A, Trentz O, Ertel W. Internal fixation of displaced proximal humeral fractures with two one-third tubular plates. J Trauma. 2003;54:536–544. doi: 10.1097/01.TA.0000052365.96538.42. [DOI] [PubMed] [Google Scholar]
- 31.Ware JE Jr. SF-36 Health Survey Manual and Interpretation Guide. Boston: The Health Institute, New England Medical Centre; 1993.