Abstract
Background
Failure of TKA from aseptic loosening is a growing concern, as TKA is performed with increasing frequency. Loosening is multifactorial and may be associated with elevated inflammatory cytokines in addition to biomechanical failure.
Questions/purposes
We asked whether proinflammatory cytokines and chemokines are elevated in synovial fluid from patients undergoing revision surgery as compared to those with osteoarthritis (OA) or rheumatoid arthritis (RA).
Methods
We obtained synovial fluid samples from 20 patients: six with aseptic loosening of TKA (all with bone loss), 10 with primary OA, and four with RA. A panel of cytokines/chemokines was screened using a SearchLight® Array (Pierce Biotechnology, Rockford, IL, USA) in one revision sample. Using these data, we assayed the synovial fluids for monocyte chemotactic protein 1 (MCP-1) by ELISA.
Results
We observed an increase in synovial MCP-1 levels in samples from patients planned for TKA revision compared to those with OA or RA. In patients undergoing revision arthroplasty, the mean (± SD) MCP-1 concentration was 21,233 ± 18,966 pg/mL (range, 1550–50,657 pg/mL; n = 6). In patients with OA, the mean MCP-1 level was 3012 ± 3321 pg/mL. In patients with RA, the mean MCP-1 concentration was 690 ± 561 pg/mL.
Conclusions
All patients undergoing revision TKA showed elevated concentrations of MCP-1 compared to patients with OA and RA, suggesting MCP-1 may serve as a potential marker or predictor of bone loss in patients undergoing revision surgery.
Clinical Relevance
MCP-1 may be a novel biomarker in patients showing early symptoms of aseptic loosening of TKA.
Introduction
An emerging role for inflammatory cytokines in bone metabolism has recently been recognized [3, 8]. Numerous inflammatory cytokines drive osteoclast maturation, leading to a population of cells with bone-resorbing capabilities. Specifically, tumor necrosis factor α (TNF-α) and interleukin (IL)-1β, IL-6, and IL-17 all have a role in an inflammatory cascade leading to bone loss. Conversely, many cytokines and other factors inhibit bone loss, such as osteoprotegerin (OPG), IL-4, and IL-10 [9, 20, 31, 32, 39]. In the absence of disease, cytokines work in a balanced fashion to oppose each other’s actions, maintaining normal bone mass through continual turnover. However, in pathologic states, this balance is perturbed, leading to net bone loss.
The causative roles of cytokines and chemokines in disorders such as rheumatoid arthritis (RA) and osteoarthritis (OA) are well documented [3, 10, 25]. However, less is known about the relationship between inflammatory cytokines and aseptic loosening of implants in TKA, although studies have detected the presence of numerous cytokines in these patients (Table 1). Patients who undergo TKA are at risk of aseptic loosening, thus needing revision TKA. One long-term study demonstrated 18.3% of all knee revision surgeries failed. Of these, 4.9% failed due to aseptic loosening of the TKA [23]. Aseptic loosening is a chronic inflammatory state secondary to particulate wear debris produced by the implants. This wear debris stimulates an inflammatory cascade that may lead to bone loss and subsequent implant loosening.
Table 1.
Cytokines and chemokines in aseptic loosening of total joint arthroplasty
| Cytokine | Site | Cytokine source | Study |
|---|---|---|---|
| IL-8 | Hip | Pseudocapsular tissue Synoviallike interface membrane |
Lassus et al. [21] |
| IL-6 | Hip | Pseudosynovial tissue Synoviallike interface membrane |
Konttinen et al. [18] |
| IL-1β | Hip | Synovium | Clarke et al. [4] |
| IL-6 | Knee | ||
| IL-8 | |||
| IL-10 | |||
| IL-6 | Hip | Synovium | Beraudi et al. [2] |
| IL-8 | Sabokbar and Rushton [29] | ||
| IL-8 | Hip | Serum and synovium | Tanaka et al. [35] |
| IL-1β | Hip | Serum | Granchi et al. [12] |
| IL-6 | |||
| GM-CSF | |||
| M-CSF | Hip | Synoviumlike interface tissue and synovium | Takei et al. [34] |
| M-CSF | Hip | Synoviallike membrane | Xu et al. [41] |
| IL-1α | Hip | Interfacial membrane | Shanbhag et al. [33] |
| IL-1α | Hip | Pseudosynovial membrane | Westacott et al. [38] |
| IL-1β | |||
| IL-1β | Hip | Membranous tissue | Jiranek et al. [15] |
| PDGF | |||
| IL-12 | Hip | Pseudosynovial fluid | Inomoto et al. [13] |
| IL-11 | Hip | Pseudocapsular tissue | Xu et al. [42] |
| IL-15 | Hip | Interface membrane | Revell and Jellie [27] |
| Saeed and Revell [30] | |||
| TGF-β1 and 2 | Hip | Pseudocapsular tissue | Konttinen et al. [17] |
| TNF-α | Hip | Synoviallike membrane | Xu et al. [40] |
| MCP-1 | Hip | Periprosthetic tissue | Nakashima et al. [24] |
| MIP-1α | |||
| MCP-1 | Knee | Synovial fluid | Current study |
IL = interleukin; GM-CSF = granulocyte-macrophage colony-stimulating factor; M-CSF = macrophage colony-stimulating factor; PDGF = platelet-derived growth factor; TGF = transforming growth factor; TNF = tumor necrosis factor; MCP = monocyte chemotactic protein; MIP = macrophage inflammatory protein.
While several in vitro studies have examined the role of monocyte chemotactic protein 1 (MCP-1) in the synovial tissues of patients with chronic wear debris-induced inflammation [6, 7, 11, 19, 24, 36], none assessed MCP-1 in synovial fluid of patients undergoing TKA revision surgery for aseptic loosening. Collectively, these in vitro studies demonstrate increased MCP-1 gene expression and protein associated with chronic wear debris. These studies focused on titanium [6, 7] and polymethylmethacrylate particles [24]. Fritz et al. [6, 7] performed analyses with human bone marrow-derived and osteosarcoma cells. Their studies determined that titanium particles caused increased IL-8 expression and activation of the NF-κB transcription factor, giving further insight into the osteoblast role in the pathogenesis of osteolysis. By studying granulomatous tissue retrieved during revision, Nakashima et al. [24] found MCP-1 expressed within this tissue. Moreover, antibodies to MCP-1 inhibited expression of this chemokine in cell culture medium [24]. Identification of inflammatory mediators in patients undergoing aseptic loosening of TKA is important for developing rational therapeutics aimed at mitigating wear debris-induced inflammation and subsequent bone loss. Currently, anticytokine therapies are used successfully to limit inflammation and bone loss in patients with RA [25]. Therefore, MCP-1 may be a therapeutic target for mitigating aseptic loosening of prosthetic implants or a predictive marker of osteolysis in these individuals.
We therefore asked whether proinflammatory cytokines and chemokines are elevated in (1) synovial fluid from patients undergoing revision surgery (2) known inflammatory states such as RA and OA, and (3) patients undergoing revision surgery compared to patients with OA or RA.
Patients and Methods
We obtained synovial fluid samples from 20 patients: six undergoing revision arthroplasties with radiographic evidence of aseptic loosening, 10 with primary OA undergoing primary TKA, and four with self-reported RA. For patients undergoing revision arthroplasty, the primary diagnosis before the initial TKA was OA in all patients. We used the following criteria to identify absence of sepsis in the six patients: normal inflammatory markers (erythrocyte sedimentation rate, C-reactive protein) and intraoperative frozen section demonstrating fewer than five neutrophils per high-power field. Intraoperatively, we inserted an 18-gauge needle into the knee before incision of the joint capsule to prevent blood and debris contamination. We centrifuged samples for 10 minutes at 2500 rpm to remove cells and other debris as previously described [1]. All patients undergoing revision TKA had cobalt-chromium components. One patient had a cobalt-chromium femoral component and titanium tibial base plate. The mean age of these six patients was 63 years (range, 37–78 years). There were four women and two men. Three patients were on NSAID treatment for at least 2 years before revision surgery. The average duration to revision surgery was 8.5 years (range, 2–12 years); one patient with aseptic loosening was less than 6 years from primary arthroplasty, and in that patient, we identified no factors for early failure. The mean age of the 10 patients undergoing primary TKA for OA was 72 years (range, 56–83 years). There were five women and five men. Nine patients were on NSAID treatment for at least 1 month before surgery. The four patients with self-reported RA all had radiographic evidence of RA. Their mean age was 59 years (range, 46–75 years). There were three women and one man. One patient was treated with steroids, and all patients reported taking NSAIDs. All patients in this study were ambulatory. This study was approved by the institutional review board with informed consent obtained from all patients.
Preoperatively we obtained AP and lateral knee radiographs in all patients. We recorded the Anderson Orthopaedic Research Institute (AORI) classification for osteolytic bone lesions [5]. The AORI classification is commonly used in revision knee arthroplasty to assess bone loss and its impact on implant stability. The classification progresses from minimal bone loss (Type 1) to substantial bone loss requiring a hinged prosthesis in certain circumstances (Type 3). All six patients undergoing revision had bone loss: two had femurs with F1 bone loss, and four F2 bone loss; five of the tibias had T2 bone loss, and one T1 bone loss.
We analyzed all 20 synovial fluid samples using LINCOplex® technology for simultaneous, multianalyte detection of human IL-1β, TNF-α, and IL-6 (LINCO Research Inc, St Charles, MO, USA). IL-17 and MCP-1 levels were determined by ELISA (Biosource, Camarillo, CA, USA; and BD Pharmingen, San Diego, CA, USA). All MCP-1 and IL-17 samples were analyzed in triplicate at 450 nm using a microplate reader (Bio-Rad Laboratories, Inc, Hercules, CA, USA). We confirmed the abilities of representative ELISA kits to detect cytokines in synovial fluid using positive controls included in each of the kits.
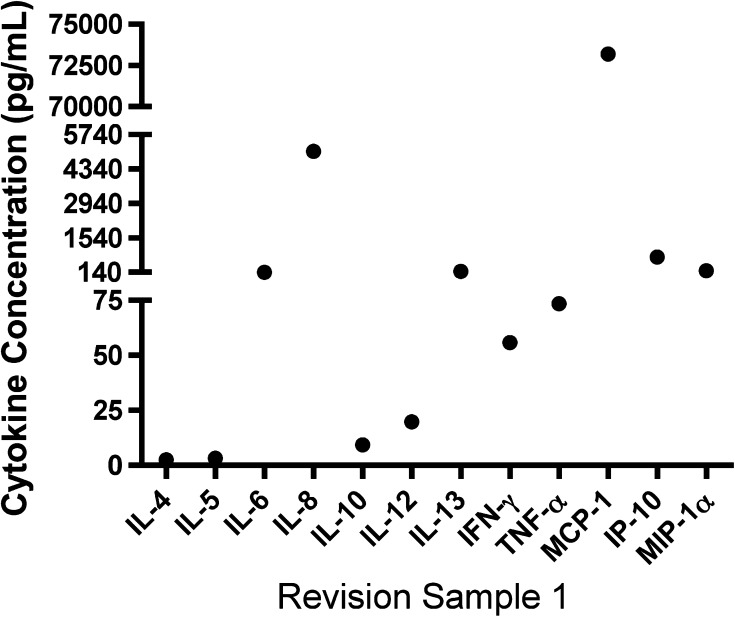
A SearchLight® Array (Pierce Biotechnology, Rockford, IL, USA) was performed using 50 μL of one of the samples of synovial fluid from the six patients undergoing revision TKA. Performing a SearchLight® Array on all six patients was cost prohibitive; thus, we chose one sample to assess whether there were any significant cytokine differences. The SearchLight® Array was a multiplexed sandwich ELISA for the quantitative measurement of up to 16 proteins per well. We visualized cytokines and chemokines using a chemiluminescent signal imaged with a charge-coupled device (CCD) camera. Using the SearchLight® CCD Imaging and Analysis System software, we calculated the values (in picograms per milliliter) for unknowns from standard curves based on the position of the spots and comparison of density values for unknown samples to density values for known standards.
Since the data were not normally distributed, we used the Kruskal-Wallis test to determine differences in the MCP-1 levels (from the ELISA) in the synovial fluid samples from patients with RA, OA, and revisions. On finding a significant difference across the three groups, we performed Bonferroni-adjusted pairwise comparisons (p < 0.017) using the Mann-Whitney test.
Results
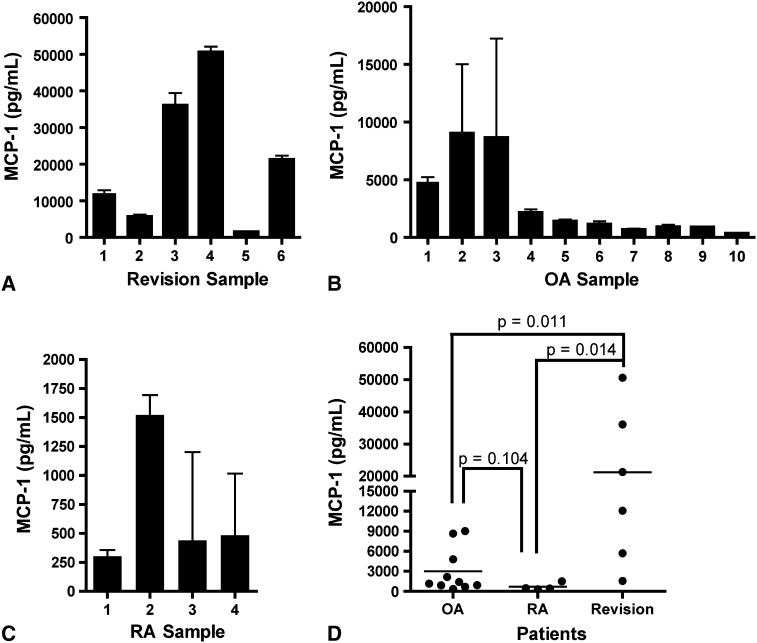
In patients with revision TKA, the mean ± SD MCP-1 concentration was 21,233 ± 18,966 pg/mL (range, 1550–50,657 pg/mL) (Fig. 1A). Patients 2 and 5 had lower levels of MCP-1 relative to other patients. When we examined cytokine profiles in patients undergoing revision TKA, there was no detectable IL-1β (< 0.6 pg/mL) or IL-17 (< 2.0 pg/mL). However, TNF-α concentrations varied among patients with revisions, with a mean of 208 ± 506 pg/mL. Since none of these cytokines were consistently elevated in these patients, we performed a broad screen of inflammatory factors, using SearchLight® Array (Fig. 2). Expression of the chemokine MCP-1 was high in this representative patient. In subjects without inflammation, MCP-1 expression is ordinarily undetectable, and thus its high expression is indicative of inflammatory activity [28].
Fig. 1A–D.
Elevated levels of MCP-1 occurred in patients undergoing revision surgery and some patients with OA. Graphs show the MCP-1 concentrations in synovial fluid of (A) patients undergoing revision surgery for aseptic loosening of TKA (n = 6), (B) patients with OA undergoing primary TKA (n = 10), and (C) patients with RA undergoing primary TKA (n = 4). All samples were analyzed in triplicate, and SDs are shown. (D) A compilation of MCP-1 data shows elevated MCP-1 in patients undergoing revision TKA for aseptic loosening (n = 6) as compared to patients with OA (n = 10) or RA (n = 4) undergoing primary TKA.
Fig. 2.
Screening of a large panel of cytokines and chemokines identifies high levels of MCP-1 in synovial fluid from a patient undergoing revision surgery for aseptic loosening of TKA. A SearchLight® Array (Pierce Biotechnology) was performed using 50 μL of synovial fluid from one patient undergoing revision (Patient 1). Concentrations of the indicated cytokines or chemokines are indicated. IFN-γ = interferon γ; IP-10 = 10-kDa IFN-γ-induced protein; MIP-1α = macrophage inflammatory protein 1α.
The mean MCP-1 concentration was 3012 ± 3321 pg/mL (range, 365–9032 pg/mL) for OA samples (Fig. 1B) and 690 ± 561 pg/mL (range, 303–1522 pg/mL) for RA samples (Fig. 1C). Patients with OA fell into two distinct subsets based on MCP-1 concentration: greater than 4000 pg/mL and less than 2500 pg/mL (Fig. 1B).
We found a difference (p = 0.004) in MCP-1 in revision, OA, and RA synovial fluid (Fig. 1D). Post hoc analysis with a Mann-Whitney test showed the mean concentration of MCP-1 in revision samples was larger (p = 0.011 and p = 0.014, respectively) than OA and RA samples. However, when comparing OA and RA, there was no difference (p = 0.104) between mean MCP-1 concentrations. The mean concentration of the high MCP-1 for the OA group (> 4000 pg/mL) was lower than that for patients with revisions: 7502 pg/mL versus 21,233 pg/mL, respectively. Cytokines typically associated with bone loss did not distinguish patients undergoing revision from patients with OA and RA.
Discussion
Inflammatory cytokines play well-established roles in the development and progression of chronic inflammation in diseases such as RA and to a lesser extent OA [10, 16]. A possible consequence of advanced arthritis is the need for TKA, which has the risk of developing the need for revision surgery from aseptic loosening. The relationship between specific cytokine expression and aseptic loosening is not well understood, and how these cytokines relate to pathology seen in OA and RA patients has not been examined. We therefore asked whether proinflammatory cytokines and chemokines are elevated in (1) synovial fluid from patients undergoing revision surgery, (2) known inflammatory states such as RA and OA, and (3) patients undergoing revision surgery compared to patients with OA or RA.
We acknowledge several limitations to our study. First, with such a small sample size, it was not possible to detect small but potentially important differences in inflammatory markers. However, given the large differences in MCP-1 values among groups, MCP-1 appears to be associated with osteolysis and bone loss. Second, this study lacks confirmatory pathology for patients with inflammatory arthropathy such as RA, although these patients did have radiographic evidence consistent with their reported disease state. Third, we observed a difference between the values found using SearchLight® Array and those found using ELISA. The sensitivities of both platforms differ, which explains the difference in values between the two assays. However, the absolute findings of elevated MCP-1 were consistent in both assays. Finally, it is difficult to obtain adequate controls to establish normality. To obtain a true control, synovial fluid from a pain-free, well-functioning arthroplasty would be needed. This would create ethical concerns owing to the small but real risk of infection. Further studies to correlate MCP-1 activity with degree of osteolysis would be valuable based on these preliminary findings.
Our findings suggest a role for MCP-1 in patients with failed TKA (Fig. 1). When compared to patients with OA and RA, those patients undergoing revision TKA had elevated synovial fluid MCP-1 levels. This is consistent with previous reports connecting bone loss and inflammation with elevated MCP-1 levels. Several in vitro studies have shown an association between wear debris and MCP-1 production [6, 7, 11, 19, 24, 36], but few in vivo studies have examined MCP-1 levels in patients undergoing revision TKA. Ishiguro et al. [14] demonstrated the MCP-1 transcript is expressed at the bone-cement interface tissue in patients with failed TKAs. Transient MCP-1 expression is also observed in a murine model of titanium particle-induced inflammation [37]. MCP-1 is also expressed in inflamed bone. Specifically, one study examined the role of MCP-1 in an inflammatory lesion in a murine mandible, where the number of MCP-1-expressing cells correlated with the number of monocytes, suggesting MCP-1 is an important mediator in the recruitment of these cells to inflamed bone [26].
The OA group fell into two distinct categories with respect to MCP-1 levels. While the majority of patients with OA had low levels of MCP-1, a distinct group demonstrated high MCP-1 levels (Fig. 1B). Importantly, one study [22] reported a negative correlation between MCP-1 and TNF-α levels and muscle strength in patients with OA, suggesting inflammatory cytokines, and MCP-1 in particular, have relevance to clinical disease parameters. These findings may indicate an important role for MCP-1 in certain patients with OA. Thus, it may be prudent to direct future investigation into MCP-1 profiles in primary TKA and OA to determine whether the higher-MCP-1 group correlates with earlier revision and a shorter survivorship of the primary surgery.
Acknowledgments
We thank Ms. Cathy Buyea of the State University of New York at Buffalo and Dr. Cristina Sison of the Feinstein Institute for Medical Research for assistance with statistical analyses.
Footnotes
The institution of one or more of the authors (SLG) has received funding from the NIH (AR054389); one author (JMK) was supported by an individual F30 grant from the NIH (DE014831); one author (KLK) was supported by the NIH (DE14460, DE18290, and RR017696). Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the State University of New York at Buffalo (Buffalo, NY, USA).
Vinod Dasa and Jill M. Kramer equally contributed to this work.
Contributor Information
Vinod Dasa, Email: vdasa@lsuhsc.edu.
William M. Mihalko, Email: wmihalko@campbellclinic.com.
References
- 1.Andersson MK, Anissian L, Stark A, Bucht E, Felländer-Tsai L, Tsai JA. Synovial fluid from loose hip arthroplasties inhibits human osteoblasts. Clin Orthop Relat Res. 2000;378:148–154. doi: 10.1097/00003086-200009000-00024. [DOI] [PubMed] [Google Scholar]
- 2.Beraudi A, Stea S, Cremonini S, Visentin M, Toni A. Assessment of five interleukins in human synovial fluid as possible markers for aseptic loosening of hip arthroplasty. Artif Organs. 2009;33:538–543. doi: 10.1111/j.1525-1594.2009.00736.x. [DOI] [PubMed] [Google Scholar]
- 3.Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 4.Clarke SA, Brooks RA, Hobby JL, Wimhurst JA, Myer BJ, Rushton N. Correlation of synovial fluid cytokine levels with histological and clinical parameters of primary and revision total hip and total knee replacements. Acta Orthop Scand. 2001;72:491–498. doi: 10.1080/000164701753532835. [DOI] [PubMed] [Google Scholar]
- 5.Engh GA, Ammeen DJ. Bone loss with revision total knee arthroplasty: defect classification and alternatives for reconstruction. Instr Course Lect. 1999;48:167–175. [PubMed] [Google Scholar]
- 6.Fritz EA, Glant TT, Vermes C, Jacobs JJ, Roebuck KA. Titanium particles induce the immediate early stress responsive chemokines IL-8 and MCP-1 in osteoblasts. J Orthop Res. 2002;20:490–498. doi: 10.1016/S0736-0266(01)00154-1. [DOI] [PubMed] [Google Scholar]
- 7.Fritz EA, Glant TT, Vermes C, Jacobs JJ, Roebuck KA. Chemokine gene activation in human bone marrow-derived osteoblasts following exposure to particulate wear debris. J Biomed Mater Res A. 2006;77:192–201. doi: 10.1002/jbm.a.30609. [DOI] [PubMed] [Google Scholar]
- 8.Goldring MB. Osteoarthritis and cartilage: the role of cytokines. Curr Rheumatol Rep. 2000;2:459–465. doi: 10.1007/s11926-000-0021-y. [DOI] [PubMed] [Google Scholar]
- 9.Goldring MB. Anticytokine therapy for osteoarthritis. Expert Opin Biol Ther. 2001;1:817–829. doi: 10.1517/14712598.1.5.817. [DOI] [PubMed] [Google Scholar]
- 10.Goldring SR. Pathogenesis of bone and cartilage destruction in rheumatoid arthritis. Rheumatology (Oxford). 2003;42(suppl 2):ii11–ii16. [DOI] [PubMed]
- 11.Goodman SB, Ma T. Cellular chemotaxis induced by wear particles from joint replacements. Biomaterials. 2010;31:5045–5050. doi: 10.1016/j.biomaterials.2010.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Granchi D, Verri E, Ciapetti G, Stea S, Savarino L, Sudanese A, Mieti M, Rotini R, Dallari D, Zinghi G, Montanaro L. Bone-resorbing cytokines in serum of patients with aseptic loosening of hip prostheses. J Bone Joint Surg Br. 1998;80:912–917. doi: 10.1302/0301-620X.80B5.8513. [DOI] [PubMed] [Google Scholar]
- 13.Inomoto M, Miyakawa S, Mishima H, Ochiai N. Elevated interleukin-12 in pseudosynovial fluid in patients with aseptic loosening of hip prosthesis. J Orthop Sci. 2000;5:369–373. doi: 10.1007/s007760070045. [DOI] [PubMed] [Google Scholar]
- 14.Ishiguro N, Kojima T, Ito T, Saga S, Anma H, Kurokouchi K, Iwahori Y, Iwase T, Iwata H. Macrophage activation and migration in interface tissue around loosening total hip arthroplasty components. J Biomed Mater Res. 1997;35:399–406. doi: 10.1002/(SICI)1097-4636(19970605)35:3<399::AID-JBM14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 15.Jiranek WA, Machado M, Jasty M, Jevsevar D, Wolfe HJ, Goldring SR, Goldberg MJ, Harris WH. Production of cytokines around loosened cemented acetabular components: analysis with immunohistochemical techniques and in situ hybridization. J Bone Joint Surg Am. 1993;75:863–879. doi: 10.2106/00004623-199306000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 17.Konttinen YT, Waris V, Xu JW, Jiranek WA, Sorsa T, Virtanen I, Santavirta S. Transforming growth factor-beta 1 and 2 in the synovial-like interface membrane between implant and bone in loosening of total hip arthroplasty. J Rheumatol. 1997;24:694–701. [PubMed] [Google Scholar]
- 18.Konttinen YT, Xu JW, Waris E, Li TF, Gómez-Barrena E, Nordsletten L, Santavirta S. Interleukin-6 in aseptic loosening of total hip replacement prostheses. Clin Exp Rheumatol. 2002;20:485–490. [PubMed] [Google Scholar]
- 19.Koreny T, Tunyogi-Csapó M, Gál I, Vermes C, Jacobs JJ, Glant TT. The role of fibroblasts and fibroblast-derived factors in periprosthetic osteolysis. Arthritis Rheum. 2006;54:3221–3232. doi: 10.1002/art.22134. [DOI] [PubMed] [Google Scholar]
- 20.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, Martin TJ, Suda T. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lassus J, Salo J, Jiranek WA, Santavirta S, Nevalainen J, Matucci-Cerinic M, Horák P, Konttinen Y. Macrophage activation results in bone resorption. Clin Orthop Relat Res. 1998;352:7–15. doi: 10.1097/00003086-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Levinger I, Levinger P, Trenerry MK, Feller JA, Bartlett JR, Bergman N, McKenna MJ, Cameron-Smith D. Increased inflammatory cytokine expression in the vastus lateralis of patients with knee osteoarthritis. Arthritis Rheum. 2011;63:1343–1348. doi: 10.1002/art.30287. [DOI] [PubMed] [Google Scholar]
- 23.Mortazavi SM, Molligan J, Austin MS, Purtill JJ, Hozack WJ, Parvizi J. Failure following revision total knee arthroplasty: infection is the major cause. Int Orthop. 2011;35:1157–1164. doi: 10.1007/s00264-010-1134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakashima Y, Sun DH, Trindade MC, Chun LE, Song Y, Goodman SB, Schurman DJ, Maloney WJ, Smith RL. Induction of macrophage C-C chemokine expression by titanium alloy and bone cement particles. J Bone Joint Surg Br. 1999;81:155–162. doi: 10.1302/0301-620X.81B1.8884. [DOI] [PubMed] [Google Scholar]
- 25.O’Dell JR. Anticytokine therapy—a new era in the treatment of rheumatoid arthritis? N Engl J Med. 1999;340:310–312. doi: 10.1056/NEJM199901283400411. [DOI] [PubMed] [Google Scholar]
- 26.Rahimi P, Wang CY, Stashenko P, Lee SK, Lorenzo JA, Graves DT. Monocyte chemoattractant protein-1 expression and monocyte recruitment in osseous inflammation in the mouse. Endocrinology. 1995;136:2752–2759. doi: 10.1210/en.136.6.2752. [DOI] [PubMed] [Google Scholar]
- 27.Revell PA, Jellie SE. Interleukin 15 production by macrophages in the implant interface membrane of aseptically loosened joint replacements. J Mater Sci Mater Med. 1998;9:727–730. doi: 10.1023/A:1008903018885. [DOI] [PubMed] [Google Scholar]
- 28.Rollins B. MCP-1, MCP-2, MCP-3, MCP-4 and MCP-5. In: Oppenheim JJ, Feldmann M, Durum SK, Hirano T, Vilcek J, Nicos NA, editors. Cytokine Reference. New York, NY: Academic Press; 2000. pp. 1145–1160. [Google Scholar]
- 29.Sabokbar A, Rushton N. Role of inflammatory mediators and adhesion molecules in the pathogenesis of aseptic loosening in total hip arthroplasties. J Arthroplasty. 1995;10:810–816. doi: 10.1016/S0883-5403(05)80080-4. [DOI] [PubMed] [Google Scholar]
- 30.Saeed S, Revell PA. Production and distribution of interleukin 15 and its receptors (IL-15Ralpha and IL-R2beta) in the implant interface tissues obtained during revision of failed total joint replacement. Int J Exp Pathol. 2001;82:201–209. doi: 10.1111/j.1365-2613.2001.iep185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saidenberg-Kermanac’h N, Cohen-Solal M, Bessis N, Vernejoul MC, Boissier MC. Role for osteoprotegerin in rheumatoid inflammation. Joint Bone Spine. 2004;71:9–13. doi: 10.1016/S1297-319X(03)00131-3. [DOI] [PubMed] [Google Scholar]
- 32.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shanbhag AS, Jacobs JJ, Black J, Galante JO, Glant TT. Cellular mediators secreted by interfacial membranes obtained at revision total hip arthroplasty. J Arthroplasty. 1995;10:498–506. doi: 10.1016/S0883-5403(05)80152-4. [DOI] [PubMed] [Google Scholar]
- 34.Takei I, Takagi M, Ida H, Ogino T, Santavirta S, Konttinen YT. High macrophage-colony stimulating factor levels in synovial fluid of loose artificial hip joints. J Rheumatol. 2000;27:894–899. [PubMed] [Google Scholar]
- 35.Tanaka R, Yasunaga Y, Hisatome T, Yamasaki T, Iwamori H, Ochi M. Serum interleukin 8 levels correlate with synovial fluid levels in patients with aseptic loosening of hip prosthesis. J Arthroplasty. 2005;20:1049–1054. doi: 10.1016/j.arth.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 36.Trindade MC, Schurman DJ, Maloney WJ, Goodman SB, Smith RL. G-protein activity requirement for polymethylmethacrylate and titanium particle-induced fibroblast interleukin-6 and monocyte chemoattractant protein-1 release in vitro. J Biomed Mater Res. 2000;51:360–368. doi: 10.1002/1097-4636(20000905)51:3<360::AID-JBM9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 37.Warme BA, Epstein NJ, Trindade MC, Miyanishi K, Ma T, Saket RR, Regula D, Goodman SB, Smith RL. Proinflammatory mediator expression in a novel murine model of titanium-particle-induced intramedullary inflammation. J Biomed Mater Res B Appl Biomater. 2004;71:360–366. doi: 10.1002/jbm.b.30120. [DOI] [PubMed] [Google Scholar]
- 38.Westacott CI, Taylor G, Atkins R, Elson C. Interleukin 1 alpha and beta production by cells isolated from membranes around aseptically loose total joint replacements. Ann Rheum Dis. 1992;51:638–642. doi: 10.1136/ard.51.5.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong PK, Campbell IK, Egan PJ, Ernst M, Wicks IP. The role of the interleukin-6 family of cytokines in inflammatory arthritis and bone turnover. Arthritis Rheum. 2003;48:1177–1189. doi: 10.1002/art.10943. [DOI] [PubMed] [Google Scholar]
- 40.Xu JW, Konttinen YT, Lassus J, Natah S, Ceponis A, Solovieva S, Aspenberg P, Santavirta S. Tumor necrosis factor-alpha (TNF-alpha) in loosening of total hip replacement (THR) Clin Exp Rheumatol. 1996;14:643–648. [PubMed] [Google Scholar]
- 41.Xu JW, Konttinen YT, Waris V, Pätiälä H, Sorsa T, Santavirta S. Macrophage-colony stimulating factor (M-CSF) is increased in the synovial-like membrane of the periprosthetic tissues in the aseptic loosening of total hip replacement (THR) Clin Rheumatol. 1997;16:243–248. doi: 10.1007/BF02238958. [DOI] [PubMed] [Google Scholar]
- 42.Xu JW, Li TF, Partsch G, Ceponis A, Santavirta S, Konttinen YT. Interleukin-11 (IL-11) in aseptic loosening of total hip replacement (THR) Scand J Rheumatol. 1998;27:363–367. doi: 10.1080/03009749850154393. [DOI] [PubMed] [Google Scholar]