Abstract
Background
Coexistence of degenerative arthritis and calcium pyrophosphate dihydrate (CPPD) crystals (or radiological chondrocalcinosis) with osteoarthritis (OA) of the knees is frequent at the time of arthroplasty. Several studies suggest more rapid clinical and radiographic progression with CPPD than with OA alone. However, it is unclear whether chondrocalcinosis predisposes to higher risks of progression of arthritis in other compartments.
Question/purposes
We questioned whether chondrocalcinosis influences clinical scores, degeneration of other compartments, rupture of the ACL, survivorship, reason for revision, or timing of failures in case of UKA.
Methods
We retrospectively reviewed 206 patients (234 knees) who had UKAs between 1990 and 2000. Of these 234 knees, 85 had chondrocalcinosis at the time of surgery and 63 of the knees subsequently had radiographic evidence of chondrocalcinosis observed during followup. We evaluated patients with The Knee Society rating system and compared function and radiographic progression in the other compartments of patients without and with chondrocalcinosis.
Results
The use of conventional NSAIDs, radiographic progression of OA in the opposite femorotibial compartment of the knee, failure of the ACL, and aseptic loosening did not occur more frequently among patients with chondrocalcinosis. The 15-year cumulative survival rates were 90% and 87% for the knees without and with chondrocalcinosis, respectively, using revision to TKA as the end point.
Conclusion
Our findings show chondrocalcinosis does not influence progression and therefore is not a contraindication to UKA.
Level of Evidence
Level II, prognostic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Proposed advantages of UKA over TKA for patients include less blood loss, easier and shorter rehabilitation, improved knee function, and shorter inpatient stay [7, 8]. However, coexistence of degenerative arthritis and calcium pyrophosphate dihydrate (CPPD) crystals with osteoarthritis (OA) of the knees is common in these patients. The term pseudogout also is used to describe CPPD arthropathy because it is clinically similar to gout (monosodium urate crystal arthropathy). McCarty et al. [22] first used the term in 1966. The deposition of CPPD crystals in articular tissue is common in the elderly, affecting more than 60% of those older than 85 years [12, 13, 33]. CPPD is the most common cause of chondrocalcinosis in the knee. Several studies suggest chondrocalcinosis predisposes to the development of rapidly destructive arthropathy, loosening of arthroplasty, alteration of the ACL, and inflammatory reactions [3, 18, 20, 23, 24]. Some studies show more clinical and radiographic progression with CPPD than with OA [25, 27]. Several authors [5, 32] believe chondrocalcinosis is a contraindication to UKA because it may lead to progression of the OA into the opposite femorotibial compartment. Thus, the potential advantages of UKA could be offset by an increased risk of complications if the patient has chondrocalcinosis at the time of surgery. Furthermore, when the patient is operated on at a younger age, the risk of complications related to chondrocalcinosis could appear later during the followup because the prevalence of chondrocalcinosis increases with age [12].
Woods et al. [34] suggested chondrocalcinosis is not a contraindication for UKA. However, the authors studied only knees with mobile-bearing medial UKA and chondrocalcinosis at the time of surgery and the mean followup was only 4 years. Thus, it is unclear whether chondrocalcinosis predisposes to higher risks in patients with UKA as has been suggested.
We therefore determined (1) the frequency of radiographic chondrocalcinosis during followup and whether medial or lateral fixed-bearing UKA in the presence or absence of chondrocalcinosis differed in (2) clinical outcome, (3) use of NSAIDs, (4) joint space narrowing in the opposite femorotibial compartment or the patellofemoral joint, (5) ACL rupture during followup, (6) loosening of components, and (7) long-term (10–15 years) survivorship.
Patients and Methods
We retrospectively reviewed prospectively collected data on all 206 patients (234 knees) who underwent fixed-bearing UKAs between the 1990 and 2000. There were 64 lateral and 170 medial UKAs. The indications for UKA were (1) the presence of a functioning ACL, (2) a fixed flexion deformity less than 10º, a correctable varus or valgus deformity less than 10º, and (3) intact, healthy, articular cartilage in the opposite compartment (with stress radiograph) and in the patellofemoral joint (with patellofemoral view). This patient cohort of 206 patients with 234 unicompartmental arthroplasties represented 7% of all 3340 primary knee arthroplasties performed during this period. The median age of the patients at surgery was 70 years (range, 60–89 years). There were 91 men and 115 women. Twelve patients were lost to followup between 3 and 9 years, and 82 died at a mean of 6 years (range, 2–10 years) after the arthroplasty. These losses left 112 of the 206 patients (31 with lateral and 81 with medial UKAs). The minimum followup of these 112 patients was 10 years (mean, 15 years; range, 10–20 years). No patients were recalled specifically for this study; all data were obtained from medical records and radiographs.
At the time of surgery (Table 1) demographics for the patients with OA and with chondrocalcinosis were similar for the proportion of medial and lateral UKAs and sex. Patient demographics were not similar regarding age, with an increased age for patients with chondrocalcinosis.
Table 1.
Patient demographics
| Demographics | OA | Chondrocalcinosis | p value |
|---|---|---|---|
| Total number of UKAs (medial/lateral) | 149 (99/50) | 85 (54/29) | 0.24 |
| Surgical date | 1990–2000 | 1990–2000 | |
| Gender (men/women) | 69/77 | 31/29 | 0.12 |
| Median age (years) ± standard deviation | 67 ± 5.2 | 74 ± 6.3 | 0.02 |
| Age range (years) | 60–85 | 65–89 | |
| Followup in years, mean (range) ± standard deviation | 12 (2–20) ± 6.8 | 10 (3–18) ± 3.4 | 0.04 |
| Lost to followup | 7 | 5 | 0.08 |
| Deaths before 10 years followup | 46 | 36 | 0.04 |
UKA = unicompartmental arthroplasty; OA = osteoarthritis without chondrocalcinosis.
All patients had cemented fixation for the femoral and tibial components. All patients received the Ceraver uni Knee (Ceraver Osteal, Roissy, France). The prosthesis (Fig. 1) had a cobalt-chrome femoral component implanted with instrumentation that ensured three minimal cuts (distal, posterior, and chamfer cut) and reproducible position of the implant. The tibial component had a titanium alloy tibial metal back into which a flat polyethylene tibial implant could be inserted with a snap fit. The surgical instrumentation included an intramedullary cutting jig for the femur and an extramedullary cutting attachment for the tibial component. We aligned the femoral component to be perpendicular to the tibial component and parallel to the long axis of the tibia. We sized the component to extend to the anterior femur without overhanging anteriorly.
Fig. 1.

The Ceraver unicompartmental system is shown.
We determined the diagnosis of chondrocalcinosis at the time of surgery using synovial fluid collection, radiographs, compensated polarized light microscopy, and histology. We collected synovial fluid of all the knees in syringes without anticoagulant and examined them within 24 hours using compensated polarized light microscopy for crystal analysis. Chondrocalcinosis observed before or during surgery was not a contraindication for UKA. Then, we analyzed one drop of each uncentrifuged fluid for CPPD crystals using a compensated polarized light microscope. CPPD crystals appeared as weakly birefringent or nonbirefringent rod- or rhomboid-shaped crystals. We used conventional radiographs to determine chondrocalcinosis without grading the radiographic appearance of crystal disease. Weightbearing extended AP, lateral, and supine skyline films were obtained during the year before surgery. At the time of surgery, we found CPPD crystals in synovial fluids and on histologic examination from 85 knees and chondrocalcinosis were present on radiographs from 68 knees. At the time of the operation, inspection also showed extensive precipitate deposition of the CPPD in the synovial lining of the ACL for 50 knees. In some of these knees, we observed partial loss of the synovial covering of the ACL and longitudinal splitting of the ligament into separate fiber bundles. These bundles were discolored and unnaturally friable, suggesting that the ligament may slightly deteriorate with chondrocalcinosis. However, this ACL was without notable degeneration or other abnormality, and we considered it to be normal in these 50 knees. The 17 knees that had synovial fluid crystals without radiographic chondrocalcinosis at the time of surgery had chondrocalcinosis visible on radiographs at the most recent followup. Therefore, we considered these 85 knees to have chondrocalcinosis at the time of surgery. Sixty-three of the149 knees without chondrocalcinosis subsequently had radiographic evidence of chondrocalcinosis. Therefore, during followup, chondrocalcinosis was detected on radiographs of 148 of the original 234 knees. Among the 112 knees that could be studied after 10 years followup (mean, 15 years; range, 10–20 years), chondrocalcinosis was present in 43 knees at the time of surgery and in 71 knees at the most recent evaluation (Fig. 2). We evaluated the occurrence of chondrocalcinosis in both knees when it occurred on the surgically treated knee.
Fig. 2.
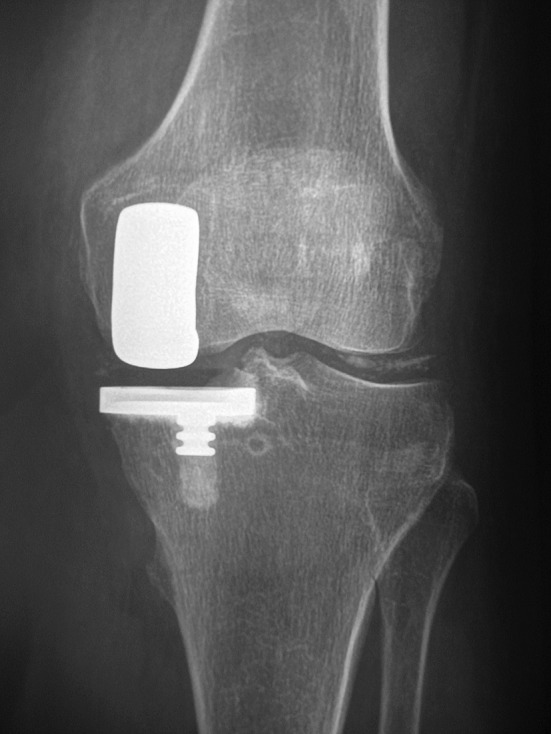
A UKA was performed in a patient who had chondrocalcinosis at the time of surgery. The radiograph obtained 10 years postoperative is shown.
We followed patients annually after the first year and recorded the Knee Society scores every year after surgery and at the most recent followup. We recorded the types of medical treatment received after surgery and difficulty level in performing activities of daily living (including using the restroom [6], cooking, standing up from a chair, going shopping, and going upstairs). These were rated from 0 (no difficulty) to 5 (almost impossible to perform). At each visit we obtained AP, lateral, and patella view radiographs of the knees.
Various observers, including consultants, fellows, and residents evaluated and recorded radiographic progression of arthritis in the contralateral tibiofemoral compartment and patellofemoral joint by measuring the width of the joint space and the presence of osteophytes, using Ahlback’s criteria [1]. We measured joint space in millimeters on standard radiographs, which we obtained before surgery, early during the postoperative period, and usually annually thereafter until the last or the most recent followup. We defined radiographic progression of OA as 25% joint space loss. We evaluated the status of the ACL at the time of the most recent followup on standing lateral radiographs with tibial translation, as previously reported [17]. Therefore, we used the displacement of the nonimplanted femoral condyle with the posterior edge of the nonresurfaced tibial plateau as the reference line, and measured the posterior of the tibial plateau on the lateral radiograph. We measured postoperative alignment (hip-knee-ankle angle) on radiographs of the entire limb taken with the patient weightbearing [16]. We defined the hip-knee-ankle angle as the angle between the line joining the center of the femoral head to the center of the knee and the line joining the center of the knee to the center of the ankle. These axes usually formed a straight line (180°). According to these measurements, only eight knees had postoperative overcorrection of the deformity, ie, they were in valgus deformity after medial UKA. The other knees had undercorrection of the deformity, ie, they were in varus with medial UKA and in valgus with lateral UKA. We did not perform additional analysis of the radiographs with blinded multiple observers except for the diagnosis of chondrocalcinosis during followup.
We expressed qualitative data (ie, sex) as counts and percentages within groups and quantitative data by mean standard deviation or range. We compared these qualitative data between the two groups using chi-square or Fisher’s exact test. We determined any differences in the outcome scores (The Knee Society clinical rating score) between preoperative and latest followup using a Student’s t-test or a nonparametric Mann-Whitney U test. Some data were nonnormal using a one-sample Kolmogorov-Smirnov Z test. We compared difficulty levels in performing activities and satisfaction with preoperative treatment using Mann-Whitney U test. The cumulative survival rates for implants (end point as revision surgery) were determined using a Kaplan-Meier analysis [19] with 95% confidence intervals (to deal with missing data). Differences between the timing of failures in knees with and without chondrocalcinosis were determined with the Gehan-Breslow test, using a statistical software package (Prophet, version 5.0; BBN Technologies; Cambridge, MA, USA).
Results
Eighty-five of the original 234 knees (36%) had primary chondrocalcinosis at the time of surgery whereas 63 (27%) had subsequent chondrocalcinosis that appeared on the radiographs of both knees during followup. Therefore chondrocalcinosis ultimately occurred in 148 of the 234 knees (63%) of the knees by the last followup.
At last followup, for the 112 knees (112 patients) followed at least 10 years we observed no differences in mean Knee Society clinical scores between the 71 knees with chondrocalcinosis and the 41 without chondrocalcinosis at the most recent evaluation. The mean postoperative Knee Society scores at the latest followup were similar (p = 0.3) for the knees with chondrocalcinosis (174; range, 85–196), and for knees without chondrocalcinosis (178; range, 110–200). Self-reported difficulty in performing various activities, namely cooking (p = 0.25), standing from a sitting position (p = 0.77), using restrooms (p = 0.88), going upstairs (p = 0.41), and going shopping (p = 0.36), were similar between the two groups with and without chondrocalcinosis.
The use of conventional NSAIDs, coxibs, glucosamine and chondroitin sulfate, topical agents, and intraarticular injection of steroid, and hyaluronan was similar after surgery in patients with and without chondrocalcinosis at the time of surgery (Table 2). Occurrence of chondrocalcinosis on radiographs during the evolution did not change treatments during the evolution.
Table 2.
Treatment of patients at most recent followup according to the status at the time of surgery
| Treatment | Osteoarthritis (n = 149) | Chondrocalcinosis (n = 85) | p value |
|---|---|---|---|
| Paracetamol | 79 (53%) | 52 (61%) | 0.6 |
| Tramadol | 11 (7%) | 14 (16%) | 0.1 |
| NSAIDs | 35 (20%) | 26 (31%) | 0.4 |
| Cox-2 inhibitors | 76 (51%) | 41 (48%) | 0.8 |
| Glucosamine/chondroitin | 16 (11%) | 12 (14%) | 0.7 |
| Intraarticular injection | 18 (12%) | 20 (24%) | 0.1 |
| Less than three medications | 64 (45%) | 48 (56%) | 0.6 |
NSAIDs = nonsteroidal antiinflammatory drugs.
Of the 234 knees included in this study, at a mean followup of 11 years ± 4.7 years, 35 (15%) had radiographic progression of arthritis (Table 3) in the opposite compartment (five knees) or in the patellofemoral joint (27 knees). In the 35 knees with progressive disease, nine had primary chondrocalcinosis (nine of 85; 15%), eight had secondary chondrocalcinosis (eight of 63; 13%). At the last followup, 17 knees with chondrocalcinosis (17/148; 11%) had radiographic progression of arthritis in the opposite compartment and 15 had degenerative arthritis alone (15/86; 17%); radiographic progression was similar (p = 0.42) in knees without and with chondrocalcinosis. Three of the 35 knees with progression of arthritis needed revision to TKA. This degeneration in the opposite femorotibial compartment leading to revision occurred in three knees with medial UKA that had overcorrection of the deformity in valgus.
Table 3.
Evolution at the most recent followup
| Parameter | Osteoarthritis N = 86 |
Chondrocalcinosis N = 148 |
|
|---|---|---|---|
| Primary = 85 | Secondary = 63 | ||
| Overcorrection of the deformity | 4 | 2 | 2 |
| Radiographic progression in the opposite compartment | 3 | 1 | 1 |
| Revision for progression in the opposite compartment | 1 | 0 | 0 |
| Radiographic progression in the patellofemoral joint | 9 | 18 | 8 |
| Revision for progression in the patellofemoral joint | 1 | 1 | 0 |
| Revision for loosening | 7 | 8 | 6 |
With a similar posterior slope in the tibial plateau (3º versus 2º, respectively) for knees with a normal ACL and the 50 knees with extensive precipitate deposition of the CPPD in the synovial lining of the ACL at the time of surgery, there was no difference (p = 0.42) in tibial translation: 4.1 mm (3–6 mm) for the knees with chondrocalcinosis compared with 3.8 mm (2–6 mm) for knees without chondrocalcinosis. We observed no rupture of the ACL during evolution.
Of the 234 knees included in this study, at a mean followup of 11 years ± 4.7 years, at last assessment, 20 tibial components and one femoral component failed and had revision (Table 3); 33 others had nonprogressive radiolucent lines. The frequency of aseptic loosening was similar (p = 0.24) between the knees with (14/148; 8%) and without chondrocalcinosis (seven of 86; 8%).
The 15-year cumulative survival rates were 90% and 87% for knees without and with chondrocalcinosis, respectively, using the end point of revision to tricompartmental knee arthroplasty. The 95% CI for the two groups without and with chondrocalcinosis overlapped at 15 years (log-rank analysis p value = 0.64), indicating no difference in survivorship between groups. The timing of revision in both groups was similar (p = 0.12), although patients with chondrocalcinosis tended to have revision later than others.
Discussion
Deposition of CPPD crystals in fibrocartilage and hyaline cartilage is a common and predominantly age-related phenomenon [21, 31]. Several studies show more clinical and radiographic progression for CPPD than for OA [2, 25, 27] and some surgeons consider chondrocalcinosis a contraindication to UKA [5, 32]. Studies comparing knees with and without chondrocalcinosis are not consistent in showing complications during long-term followup. We therefore determined (1) the frequency of radiographic chondrocalcinosis during followup and whether medial or lateral fixed-bearing UKA in the presence or absence of chondrocalcinosis differed in (2) clinical outcome, (3) use of NSAIDs, (4) joint space narrowing in the opposite femorotibial compartment or the patellofemoral joint, (5) ACL rupture during followup, (6) loosening of components, and (7) long-term (10–15 years) survivorship.
Limitations of this study include the following. First, we had nonrandomized groups with incomplete matching, resulting in different group sizes and numbers of patients lost to followup or deaths attributable to the older age of the patients. However 112 patients with more than 10 years followup (mean, 15 years; range, 10–20 years) and the same arthroplasty could be assessed in this study. Second, the size of the group without chondrocalcinosis decreased during followup. Since the goal of our study was to observe the influence of chondrocalcinosis on the survivorship of UKA, we did not consider the decreasing size a concern. Third, we did not blindly interpret the radiographs with multiple observers. Given the interpretations of some data (eg, presence of chondrocalcinosis on radiographs, narrowing of joint space in the opposite compartment) are subject to interobserver and intraobserver error, there could be some error in the groups. However, as patients had radiographs that were evaluated each year (by differing individuals), we believe the risk of error was limited. Finally, we had no joint aspiration or histology at the most recent followup to confirm secondary chondrocalcinosis but rather relied on radiographs. Therefore, we might have underestimated the total number of knees with chondrocalcinosis.
Our observations confirm that CPPD disease is frequent in knees with severe degenerative arthritis. Other studies show an incidence of 27% to 50% in patients with OA depending on the method of crystal detection [8–14] and age of the patient. The incidence in our patients is similar to that reported by Felson et al. [12], who reported the prevalence of radiographic knee chondrocalcinosis in a population aged 63 to 93 years to be 33%, in a population younger than 70 years to be 27%, and in population older than 80 years to be 44%. Although several reports analyze long-term results of UKA, they do not usually report chondrocalcinosis frequency [4–8]. However, another study of patients undergoing knee replacement surgery, representing the same population as ours, also showed an incidence of CPPD as much as 50% [34]. In our patients, secondary chondrocalcinosis was present in both knees on radiographs and was directly related to the age of the patient.
We found no difference in the ability of patients with or without chondrocalcinosis in performing activities of daily living, including cooking, standing from a sitting position, using restrooms, going upstairs, and going shopping. We believe that in patients with degenerative arthritis, the presence of CPPD crystals did not worsen their functional ability.
Treatment received by these two groups of patients was similar regarding number and types of medications and rehabilitative regimens. As previous studies show more disability and severity with CPPD disease as compared with OA [28–30], we studied if patients with CPPD disease use more medications than patients with OA. We found similar treatment regimens were used.
Despite chondrocalcinosis observed at the most recent followup in 148 knees, only 17 (11%) showed progression of the disease into the other compartments. Although patellofemoral arthrosis did progress, progressive joint space loss of the contralateral tibiofemoral compartment occurred in only four knees in this series. Degeneration in the opposite compartment and in the patellofemoral joint [15] is a common cause of UKA failure and occurs in 15% to 40% of patients when there is overcorrection of the deformity [4, 5, 7]. The alignment objective of UKA is slight undercorrection of normal leg alignment [4, 5, 16], defined as a straight line running from the center of the femoral head to the center of the ankle, falling just medial to, as opposed to through, the center point of the tibia at the knee. Chondrocalcinosis was not reported [4, 5, 16] as a cause of degeneration in the opposite compartment; however, Hernigou and Deschamps [16] concluded overcorrection of the joint alignment, which transfers increased forces to the uninvolved compartment, would accelerate degeneration. We suspect the overall undercorrection in our series may be responsible for the low rate of degeneration in the contralateral compartment. Other studies show more radiographic progression with CPPD than for OA [18–20]. In contrast, patients with CPPD who underwent arthroplasty in our study had no more degeneration in the other compartments than patients without CPPD. Our observations suggest chondrocalcinosis does not predispose to degenerative arthritis in the opposite compartment of knees that have undergone UKA without overcorrection, and contradict the theory that chondrocalcinosis predisposes to progression of arthritis in the knee. Therefore, we conclude, like Woods et al. [34], that chondrocalcinosis is not a contraindication to UKA. Our study is also consistent with a longitudinal MRI study that showed no association between the presence of chondrocalcinosis in knees and cartilage loss [26].
Reasons for failure leading to revision in our patients are similar to those reported in other studies [4, 5, 7, 8], with progression of arthritis and aseptic loosening being responsible in the majority of our cases. In our study, the predominant indication in knees with chondrocalcinosis is aseptic loosening, occurring at an average of 2 years later than knees without chondrocalcinosis. This may be a bias related to the older age of patients with chondrocalcinosis.
The 15-year cumulative survival rates are 90% and 87% for the knees without and with chondrocalcinosis, respectively, using the end point of revision to tricompartmental knee arthroplasty. These survivorship figures are similar to those reported for other fixed-bearing devices [4, 5, 8, 32].
We found a high incidence of CPPD crystals in patients undergoing TKA. We compared UKA with and without chondrocalcinosis with reference to the clinical outcomes, progression of disease in the opposite compartment, and survivorship. Patients with these crystals experienced similar difficulties in performing daily activities after knee arthroplasty and received similar treatment to patients with severe OA without CPPD crystals. Both groups had pain relief and restoration of function with durable implant survival. The reasons for revision were similar for both groups with aseptic loosening being predominant. Our observations suggest chondrocalcinosis is not a contraindication to UKA.
Footnotes
Each author certifies that he has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Hospital Henri Mondor, Creteil, France.
References
- 1.Ahlback S. Osteoarthritis of the knee: a radiological investigation. Acta Radiol. 1968;39(suppl 277):7–22. [PubMed] [Google Scholar]
- 2.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 3.Altman RD, Fries JF, Bloch DA, Carstens J, Cooke TD, Genant H, Gofton P, Groth H, McShane DJ, Murphy WA, et al. Radiographic assessment of progression in osteoarthritis. Arthritis Rheum. 1987;30:1214–1225. doi: 10.1002/art.1780301103. [DOI] [PubMed] [Google Scholar]
- 4.Argenson JN, Chevrol-Benkeddache Y, Aubaniac JM. Modern unicompartmental knee arthroplasty with cement: a three to ten-year follow-up study. J Bone Joint Surg Am. 2002;84:2235–2239. [PubMed] [Google Scholar]
- 5.Barrett WP, Scott RD. Revision of failed unicondylar unicompartmental knee arthroplasty. J Bone Joint Surg Am. 1987;69:1328–1335. [PubMed] [Google Scholar]
- 6.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 7.Carr A, Keyes G, Miller R, O’Connor J, Goodfellow J. Medial unicompartmental arthroplasty: a survival study of the Oxford meniscal knee. Clin Orthop Relat Res. 1993;295:205–213. [PubMed] [Google Scholar]
- 8.Chesnut WJ. Preoperative diagnostic protocol to predict candidates for unicompartmental arthroplasty. Clin Orthop Relat Res. 1991;273:146–150. [PubMed] [Google Scholar]
- 9.Cheung HS, Halverson PB, McCarty DJ. Release of collagenase, neutral protease, and prostaglandins from cultured mammalian synovial cells by hydroxyapatite and calcium phosphate dihydrate crystals. Arthritis Rheum. 1981;24:1338–1344. doi: 10.1002/art.1780241102. [DOI] [PubMed] [Google Scholar]
- 10.Dieppe PA, Alexander GJ, Jones HE, Doherty M, Scott DG, Manhire A, Watt I. Pyrophosphate arthropathy: a clinical and radiological study of 105 cases. Ann Rheum Dis. 1982;41:371–376. doi: 10.1136/ard.41.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derfus BA, Kurian JB, Butler JJ, Draft LJ, Carrera GF, Ryan LM, Rosenthal AK. The high prevalence of pathologic calcium crystals in pre-operative knees. J Rheumatol. 2002;29:570–574. [PubMed] [Google Scholar]
- 12.Felson DT, Anderson JJ, Naimark A, Kannel W, Meenan RF. The prevalence of chondrocalcinosis in the elderly and its association with knee osteoarthritis: the Framingham Study. J Rheumatol. 1989;16:1241–1245. [PubMed] [Google Scholar]
- 13.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly: the Framingham Osteoarthritis Study. Arthritis Rheum. 1987;30:914–918. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 14.Gibilisco PA, Schumacher HR, Jr, Hollander JL, Soper KA. Synovial fluid crystals in osteoarthritis. Arthritis Rheum. 1985;28:511–515. doi: 10.1002/art.1780280507. [DOI] [PubMed] [Google Scholar]
- 15.Hernigou P, Deschamps G. Patellar impingement following unicompartmental arthroplasty. J Bone Joint Surg Am. 2002;84:1132–1137. doi: 10.2106/00004623-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Hernigou P, Deschamps G. Alignment influences wear in the knee after medial unicompartmental arthroplasty. Clin Orthop Relat Res. 2004;423:161–165. doi: 10.1097/01.blo.0000128285.90459.12. [DOI] [PubMed] [Google Scholar]
- 17.Hernigou P, Deschamps G. Posterior slope of the tibial implant and the outcome of unicompartmental arthroplasty. J Bone Joint Surg Am. 2004;86:506–511. doi: 10.2106/00004623-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Jaovisidha K, Rosenthal AK. Calcium crystals in osteoarthritis. Curr Opin Rheumatol. 2002;14:298–302. doi: 10.1097/00002281-200205000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;453:457–481. [Google Scholar]
- 20.Ledingham J, Regan M, Jones A, Doherty M. Factors affecting radiographic progression of knee osteoarthritis. Ann Rheum Dis. 1995;54:53–58. doi: 10.1136/ard.54.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malaviya AN, Al-Shari IM, Al-Shayeb AR, Shehab D, Hussain MA, Al-Mutairy M, Roberts OM, Al-Ghuriear S. Calcium pyrophosphatase dihydrate (CPPD) crystal deposition disease in a teaching hospital in Kuwait. Ann Rheum Dis. 2001;60:416–419. doi: 10.1136/ard.60.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarty DJ, Hogan JM, Gatter RA, Grossman M. Studies on pathological calcifications in human cartilage: I. prevalence and types of crystal deposits in the menisci of two hundred fifteen cadavera. J Bone Joint Surg Am. 1966;48:309–325. [PubMed] [Google Scholar]
- 23.Menkes CJ, Simon F, Delrieu F, Forest M, Delbarre F. Destructive arthropathy in chrondrocalcinosis articularis. Arthritis Rheum. 1976;19(suppl 3):329–348. doi: 10.1002/1529-0131(197605/06)19:3+<329::AID-ART1780190706>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 24.Molloy ES, McCarthy GM. Basic calcium phosphate crystals: pathways to joint degeneration. Curr Opin Rheumatol. 2006;18:187–192. doi: 10.1097/01.bor.0000209433.43978.a8. [DOI] [PubMed] [Google Scholar]
- 25.Nalbant S, Martinez JA, Kitumnuaypong T, Clayburne G, Sieck M, Schumacher HR., Jr Synovial fluid features and their relations to osteoarthritis severity: new findings from sequential studies. Osteoarthritis Cartilage. 2003;11:50–54. doi: 10.1053/joca.2002.0861. [DOI] [PubMed] [Google Scholar]
- 26.Neogi T, Nevitt M, Niu J, LaValley MP, Hunter DJ, Terkeltaub R, Carbone L, Chen H, Harris T, Kwoh K, Guermazi A, Felson DT. Lack of association between chondrocalcinosis and increased risk of cartilage loss in knees with osteoarthritis: results of two prospective longitudinal magnetic resonance imaging studies. Arthritis Rheum. 2006;54:1822–1828. doi: 10.1002/art.21903. [DOI] [PubMed] [Google Scholar]
- 27.Pattrick M, Hamilton E, Wilson R, Austin S, Doherty M. Association of radiographic changes of osteoarthritis, symptoms, and synovial fluid particles in 300 knees. Ann Rheum Dis. 1993;52:97–103. doi: 10.1136/ard.52.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenthal AK. Calcium crystal deposition and osteoarthritis. Rheum Dis Clin North Am. 2006;32:401–412, vii. [DOI] [PubMed]
- 29.Rosenthal AK, Ryan LM. Pathogenesis of osteoarthritis: crystals and osteoarthritis. In: Brandt KD, Doherty M, Lohmander LS, editors. Osteoarthritis. New York, NY: Oxford University; 2003. pp. 120–124. [Google Scholar]
- 30.Rosenthal AK, Ryan LM. Calcium pyrophosphate crystal deposition disease, pseudogout and articular chondrocalcinosis. In: Koopman WJ, Moreland LW, editors. Arthritis and Allied Conditions. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. pp. 2373–2396. [Google Scholar]
- 31.Sokoloff L, Varma AA. Chondrocalcinosis in surgically resected joints. Arthritis Rheum. 1988;31:750–756. doi: 10.1002/art.1780310608. [DOI] [PubMed] [Google Scholar]
- 32.Stern SH, Becker MW, Insall JN. Unicondylar knee arthroplasty: an evaluation of selection criteria. Clin Orthop Relat Res. 1993;286:143–148. [PubMed] [Google Scholar]
- 33.Wilkins E, Dieppe P, Maddison P, Evison G. Osteoarthritis and articular chondrocalcinosis in the elderly. Ann Rheum Dis. 1983;42:280–284. doi: 10.1136/ard.42.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woods DA, Wallace DA, Woods CG, McLardy-Smith P, Carr AJ, Murray DW, Martin J, Gunther T. Chondrocalcinosis and medial unicompartmental knee arthroplasty. Knee. 1995;2:117–119. doi: 10.1016/0968-0160(95)00013-F. [DOI] [Google Scholar]


