Abstract
Background
While modular femoral heads have been used in THA for decades, a recent innovation is a second neck-stem taper junction. Clinical advantages include intraoperative adjustment of leg length, femoral anteversion, and easier revision, all providing flexibility to the surgeon; however, there have been reports of catastrophic fracture, cold welding, and corrosion and fretting of the modular junction.
Questions/purposes
We asked whether (1) the neck-stem junction showed the same degradation mechanisms, if any, as the head-neck junction, (2) the junction contributed to THA revision, (3) the alloy affected the degree of degradation, and (4) the trunion machine finish affected the degradation mechanisms.
Methods
We compared 57 retrievals from seven total hip modular designs, three cobalt-chromium-molybdenum and four titanium based: Bionik® (four), GMRS® (four), Margron® (22), Apex® (five), M-series® (five), ZMR® (two), and S-ROM® (15). Macroscopic inspection, microscopy, and micro-CT were conducted to determine the effects of materials and design.
Results
The cobalt-chromium-molybdenum components showed crevice corrosion and fretting of the neck-stem taper, whereas the titanium components had less corrosion; however, there were several cases of cold welding where disassembly could not be achieved in theater.
Conclusions
Even with modern taper designs and corrosion-resistant materials, corrosion, fretting, and particulate debris were observed to a greater extent in the second neck-stem junction. Titanium-based modular arthroplasty may lessen the degree of degradation, but cold welding of the components may occur.
Clinical Relevance
Degradation of the second junction contributed to 8 cases of metallosis and two cases of aseptic lymphocyte-laminated vascular-associated lesions contributing to revision.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-011-2155-9) contains supplementary material, which is available to authorized users.
Introduction
Modular THAs incorporating a double-taper (neck-head and neck-stem) design are a recent innovation in hip arthroplasty and have distinct advantages compared to single neck-head taper or monolithic designs [7, 10]. The ability to select a femoral stem and independently attach a femoral neck allows the surgeon to accommodate clinical cases where standard hip design with a fixed neck geometry and head may not give optimum stability and soft tissue balance. Intraoperative flexibility is clearly increased by using a modular-neck device. Clinical advantages of modular necks include the adjustment of leg length and offset via the head-neck taper, femoral anteversion via the neck-stem taper, easier revision when there is no clinical need to revise a well-fixed stem, and optimal restoration of soft tissue tension and patient biomechanics [7]. As a consequence of introducing a second taper junction, it has been reported, in single-patient case studies, multiple modular taper junctions present an additional site for failure [1, 11, 23–26].
Numerous studies report failure mechanisms of modular hip designs with respect to the head-neck taper, the most common failure mechanisms being crevice corrosion and/or fretting corrosion, particularly in devices with a titanium alloy (Ti) stem coupled with a cobalt-chromium-molybdenum (Co-Cr-Mo) femoral head [5, 6, 12, 21, 24, 27]. The advantage of adding femoral head modularity, which facilitates revising an articulation while retaining a well-fixed stem, has outweighed the reported failures, albeit with some noted cases of femoral neck fracture [24].
The innovation of modular-neck THA has found a clinical niche, where there has been a philosophical shift from early modular prostheses of simply fitting and filling the proximal femur to promoting the idea of restoring hip biomechanics [7]. In doing so, the proposed advantages include reducing the dislocation rate, reducing impingement, and better restoration of leg length and prosthesis offset.
Several case reports have reported difficulties with second taper junctions [1, 11, 16]. Fracture of a modular neck was reported in a 30-year-old man who after uncomplicated surgery fell and fractured the neck [1]. In a comparison of modular-neck arthroplasty and resurfacing prostheses, the serum Co and Cr levels were 10 times and 2.6 times higher, respectively, in the modular-neck devices, likely due to pitting corrosion of the taper [11]. In addition, a previous study from our retrieval laboratory reported on 16 cases of corrosion in a double-tapered neck design [16]. There is also other evidence: eight fractured necks of a double-tapered design reported in the US Food and Drug Administration (FDA) adverse event database [24] and higher revision rates in the Australian Orthopaedic Association Hip Registry [2].
We therefore asked whether (1) the neck-stem junction in a retrieved modular THA device showed the same degradation mechanisms, if any, as the head-neck junction, (2) the junction contributed to THA revision, (3) the alloy affected the degree of degradation, and (4) the trunion machine finish affected the degradation mechanisms.
Materials and Methods
From our retrieval collection (n = 6700), we identified 57 retrievals of seven total hip modular designs (Bionik® [n = 4], GMRS® [n = 4], Margron® [n = 22], Apex® [n = 5], M-series® [n = 5], ZMR® [n = 2], S-ROM® [n = 15]) (Table 1). The patient demographics, time in situ, and clinical reasons for revision are summarized (Table 2). The devices were retrieved from 33 men and 23 women with an average implantation age of 23 months (range, 1–204 months) with all devices included in the study regardless of time in situ, so as not to arbitrarily introduce a cutoff time in situ and inadvertently remove devices from the study with substantial degradation mechanisms, similar to other studies [6, 8, 12, 16]. Mean time in situ was 19 months for the Co-Cr-Mo devices and 12 months for the Ti devices. Inclusion of the S-ROM® data resulted in an average time in situ of 25 months for the Ti devices. While the S-ROM® cannot strictly be considered a double-tapered neck design, it is multicomponent and has the longest clinical history of a modular device in our retrieval collection and thus was added to the study to elucidate the consequences of longer-term implantation. These devices are distinguished by their materials: Co-Cr-Mo (Fig. 1) and Ti alloy based (Fig. 2). The machine surface finishes of the stem trunions and head trunions were either ground or gramophone (Table 1).
Table 1.
Devices included in the retrieval analysis
| Style | Manufacturer | Ti or Co-Cr-Mo | Standard | Head trunion finish | Stem trunion finish |
|---|---|---|---|---|---|
| Apex® | Global Orthopaedics, Portsmouth, UK, USA | Ti | ISO 5832-3 | Gramophone | Ground |
| Bionik® | Eska Orthodynamics GmbH, Lübeck, Germany | Co-Cr-Mo | ISO 5832-4 | Gramophone | Gramophone |
| GMRS® | Stryker Corp, Mahwah, NJ, USA | Co-Cr-Mo | ISO 5832-4 | Ground | Ground |
| M-series® | Exactech, Inc, Gainesville, FL, USA | Ti | Forged Ti | Ground (2/5) Gramophone (3/5) | Ground |
| Margron® | Portland Orthopaedics, Atlanta, GA, USA | Co-Cr-Mo | ISO 5832-4 | Ground | Ground |
| S-ROM® | DePuy Orthopaedics, Warsaw, IN, USA | Ti | ISO 5832-3 | Ground | Ground |
| ZMR® | Zimmer, Inc, Warsaw, IN, USA | Ti | ISO 5832-3 | Gramophone | Ground |
Table 2.
Demographic data of retrievals and reasons for revision
| Style | Number | Average age (years) | Male | Female | Time in situ (months) | Pain/loosening | Infection | Periprosthetic | Metallosis | Dislocation |
|---|---|---|---|---|---|---|---|---|---|---|
| Bionik® | 4 | 61 | 0 | 4 | 20 | 3 | 0 | 1 | 2‡ | |
| GMRS® | 4 | 57 | 3 | 1 | 15 | 2 | 2 | 0 | 1 | |
| Margron® | 22 | 60 | 13 | 9 | 31 | 6† | 6 | 2 | 3 | 7 |
| Apex® | 5 | 63 | 4 | 1 | 8 | 3 | 0 | 1 | 1 | |
| M-series® | 5 | 69 | 4 | 1 | 14 | 2 | 1 | 1 | 0 | 2 |
| S-ROM® | 15 | 65 | 9 | 6 | 61 | 7 | 6 | 0 | 1 | 3 |
| ZMR® | 2 | 65 | 1 | 1 | 17 | 1 | 1 | 1 | 0 | |
| Total | 57 | 63* | 34 | 23 | 22.5* | 24 | 16 | 6 | 8 | 12 |
* Average of all retrievals; †one case of neck retroversion; ‡two cases of aseptic lymphocyte-laminated vascular-associated lesions.
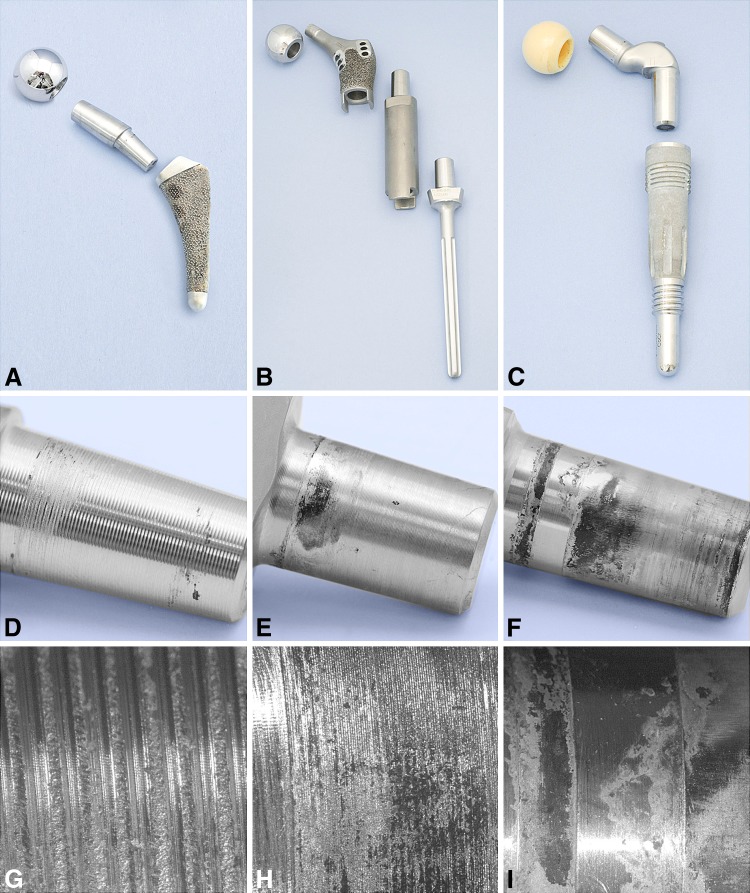
Fig. 1A–I.
Co-Cr-Mo modular devices are shown: (A) Bionik®, (B) GMRS®, and (C) Margron®. Macroscopic images are shown of (D) the Bionik® neck-stem trunion, (E) the GMRS® proximal neck-stem and distal trunion, and (F) the Margron® neck-stem trunion. Microscopic images (original magnification, ×10) are shown of (G) the Bionik® neck-stem trunion, (H) the GMRS® neck-stem trunion, and (I) the Margron® neck-stem trunion.
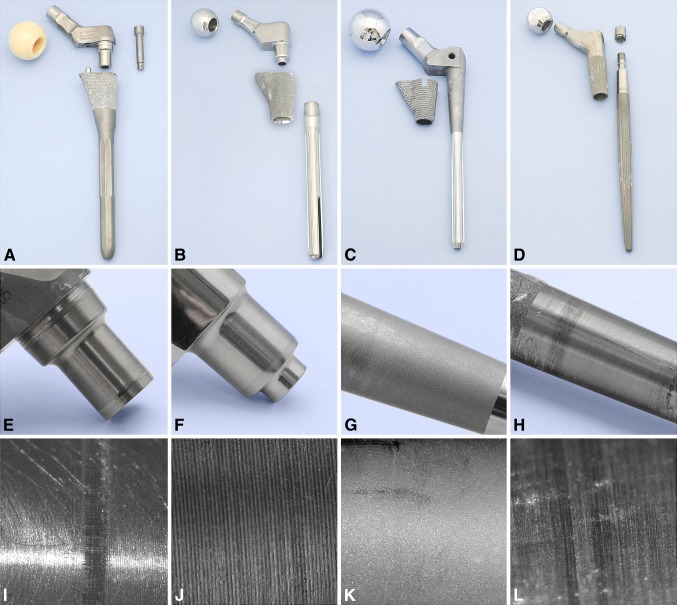
Fig. 2A–L.
Ti modular devices are shown: (A) Apex®, (B) M-series®, (C) S-ROM®, and (D) ZMR®. Macroscopic images are shown of (E) the Apex® neck-stem trunion, (F) the M-series® neck-stem trunion, (G) the S-ROM® collar-stem taper, and (H) the ZMR® neck-stem trunion. Microscopic images (original magnification, ×15) are shown of (I) the Apex® neck-stem trunion, (J) the M-series® neck-stem trunion, (K) the S-ROM® stem, and (L) the ZMR® neck-stem trunion.
Evaluation of the devices incorporated the following: (1) qualitative macroanalysis of the retrieved components in terms of degradation mechanisms [1, 6, 8, 12, 16, 21]; (2) optical microscopy [16]; (3) micro-CT scanning of tapers to determine taper “fit”; and (4) microstructural assessment [16].
We performed macroanalysis using a stereomicroscope (Leitz MZ10; Leitz, Wetzlar, Germany) at maximum ×40 magnification. Two of the authors (AMK, ES) made qualitative observations, including assessing the corrosion, determining whether fretting was present, and correlating the clinically noted cold welding with surface-surface seizure. The trunions were assessed according to a modification of a previously described four-level scoring system [12]. That scoring system was modified to include an additional level between no corrosion and mild corrosion; thus, 0 = no corrosion; 1 = corrosion present but minimal; 2 = mild corrosion; 3 = moderate corrosion; and 4 = extensive corrosion of mating surfaces. Fretting was assessed as present (yes) or no evidence of fretting (no). In addition, an observational comment of mechanical movement was added where it was clear the damage to the taper resulted from the initial application of seating load to the taper or during removal, especially in the case of the Ti devices, which have bolts or nuts to secure the components. This damage was observed as long scratches measuring millimeters in length. None of the Co-Cr-Mo devices had secondary locking mechanisms.
Further optical microscopy was possible on trunions using reflected light microscopy (Leitz Orthoplan) as the machined surfaces, even in the corroded state, were of sufficiently high reflectance to render them suitable for viewing. This was particularly important when assessing the role of fretting in the trunions with a gramophone finish. Magnification up to x400 was used to view the trunions.
We performed micro-CT using a Vtomex 240D CT scanner (General Electric Phoenix X-ray; General Electric Co, Fairfield, CT) with a 240-kV direct tube. Samples were sectioned through the neck trunion and reassembled before scanning in fast scan mode with the parameters being optimized according to density, thickness, and size. Imaging was performed using Mimics® (Materialise NV, Leuven, Belgium). We observed both transverse, coronal and sagittal images for possible discrepancies in the two mating surfaces, primarily observing whether there were gaps. Alignment of the tapers was assessed by comparing the gap, if any, at a point of the mating surface and then 180° around (transverse view) or directly opposite (coronal view) of the CT images. At the time of the micro-CT, no Bionik® stems had been sent for retrieval analysis and the S-ROM® stems were not available; thus, we had no micro-CT data on these stems.
Devices with substantial corrosion were sectioned, polished, and etched for reflected light microstructural assessment (Appendix 1; supplemental materials are available with the online version of CORR).
Results
Sixty-two percent of Co-Cr-Mo components exhibited corrosion of the trunion, with 90% showing fretting (Table 3). In contrast, 30% of Ti-based components showed corrosion, with 50% showing fretting (Table 3). Two devices had minimal corrosion (Level 1), being in situ for 1 month. There were six cases of cold welding, all Ti based, where it was difficult to disengage the tapers. Seventy percent of the Co-Cr-Mo head trunions showed some sign of corrosion, with 66% of cases having a severity of 1 and 34% a severity of 2. Similarly, but to a lesser degree, only 44% of the Ti head trunions showed corrosion. Representative photographs of retrieved stems, necks, and femoral heads are shown (Co-Cr-Mo: Fig. 1; Ti based: Fig. 2).
Table 3.
Observational results of the modular components
| Style | Number | Average time in situ (months) | Junction | Head type | Corrosion severity (number) | Fretting % | Cold welding (number) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | Yes | No | ||||||
| Bionik® | 4 | 20 | Neck-head | Co-Cr-Mo | 2 | 2 | 67 | 33 | ||||
| Neck-stem | 4 | 100 | 0 | |||||||||
| GMRS®* | 4 | 15 | Neck-head | Co-Cr-Mo | 3 | 1 | 0 | 100 | ||||
| Stem proximal | 1 | 2 | 1 | 50 | 50 | |||||||
| Stem distal | 1 | 1 | 1 | 67 | 33 | |||||||
| Margron® | 22 | 31 | Neck-head | 21 Al2O3 1 Co-Cr-Mo |
13 | 7 | 1 | 1 | 36 | 64 | ||
| Neck-stem | 10 | 4 | 2 | 2 | 4 | 55 | 45 | |||||
| Apex® | 5 | 8 | Neck-head | 5 Al2O3 | 5 | 0 | 100 | |||||
| Neck-stem | 3 | 2 | 40 | 60 | 3 | |||||||
| M-series® | 5 | 14 | Neck-head | 2 Al2O3 3 Co-Cr-Mo |
5 | 0 | 100 | |||||
| Neck-stem | 2 | 3 | 0 | 60 | 40 | 1 | ||||||
| S-ROM® | 15 | 61 | Neck-head | 2 Al2O3 11 Co-Cr-Mo 2 ZTA |
13 | 2 | 62 | 48 | ||||
| Stem/sleeve | 12 | 3 | 67 | 33 | 2 | |||||||
| ZMR® | 2 | 17 | Neck-head | 2 Co-Cr-Mo | 2 | 0 | 100 | |||||
| Neck-stem | 1 | 1 | 0 | 100 | ||||||||
* GMRS® has a head, proximal, and distal taper (Fig. 1); one GMRS® was revised without removing the distal trunion; ZTA = zirconia-toughened alumina.
The reasons for revision in order of importance were pain associated with loosening, infections, dislocations, and metallosis (Table 2). Periprosthetic fracture was also recorded as contributing to revision in one case. Pain and loosening are common reasons for removal in our retrieval collection (n = 6800), yet the number of cases of metallosis in this group is noteworthy. There were 8 cases of metallosis, two confirmed also to have aseptic lymphocyte-laminated vascular-associated lesions (ALVALs) for the Co-Cr-Mo devices compared to a single case of metallosis for the Ti-based components. Metallosis in these cases is noteworthy as none had a metal-on-metal articulation.
We observed a greater degree of degradation of the Co-Cr-Mo devices compared to the Ti-based devices (Fig. 3). The mean time in situ for the Co-Cr-Mo devices was 23 months, compared to 39 months for the Ti-based devices; thus, comparatively there was greater degradation (Table 4). For the Co-Cr-Mo components, there were substantial areas of surface irregularity with associated black debris, pits, and etch marks associated with crevice corrosion, and most showed fretting, indicative of movement at the taper junction. The striped nature of the corrosion on the Margron® trunions is due to there being a relief step in the stem below the proximal contact point (Fig. 4). For the Bionik® trunions, corrosion was preferentially observed at the base of each machining groove (Fig. 1D, 1G). The GMRS® components exhibited preferential corrosion at the top of the mating trunion surfaces. In comparison, the Ti-based components did not show extensive corrosion along the neck mating surfaces and was only obvious when contact with the stem occurred (Fig. 2E–H). The average time of the S-ROM® stems in vivo was 5 years (Table 3). Sixty percent of these stems showed corrosion, with one stem at a severity of 4 and the remainder at a severity of 1. All microstructures complied with ASTM and ISO standards and were not implicated in degradation.
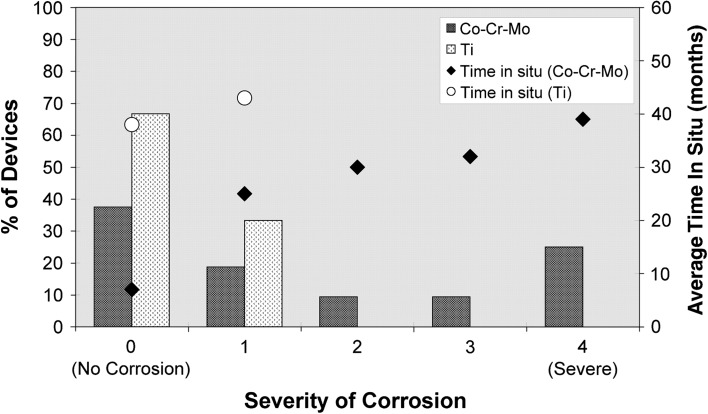
Fig. 3.
A graph shows severity of corrosion of Co-Cr-Mo and Ti devices as a function of percentage of devices showing no corrosion (severity of 0) through to severe corrosion (severity of 4). Time in situ for the Co-Cr-Mo and Ti devices is depicted on the second y axis. Despite a shorter implantation time, a greater degree of degradation is seen in the Co-Cr-Mo devices than in the Ti-based devices.
Table 4.
Severity of corrosion and fretting as a function of alloy type
| Alloy type | Severity of corrosion | Fretting | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | Yes | No | |
| Co-Cr-Mo | 12 | 6 | 3 | 3 | 9 | 19 | 13 |
| Average time in situ (months) | 7 | 25 | 30 | 32 | 39 | ||
| Ti | 18 | 9 | 0 | 0 | 0 | 13 | 14 |
| Average time in situ (months) | 38 | 43 | 0 | 0 | 0 | ||
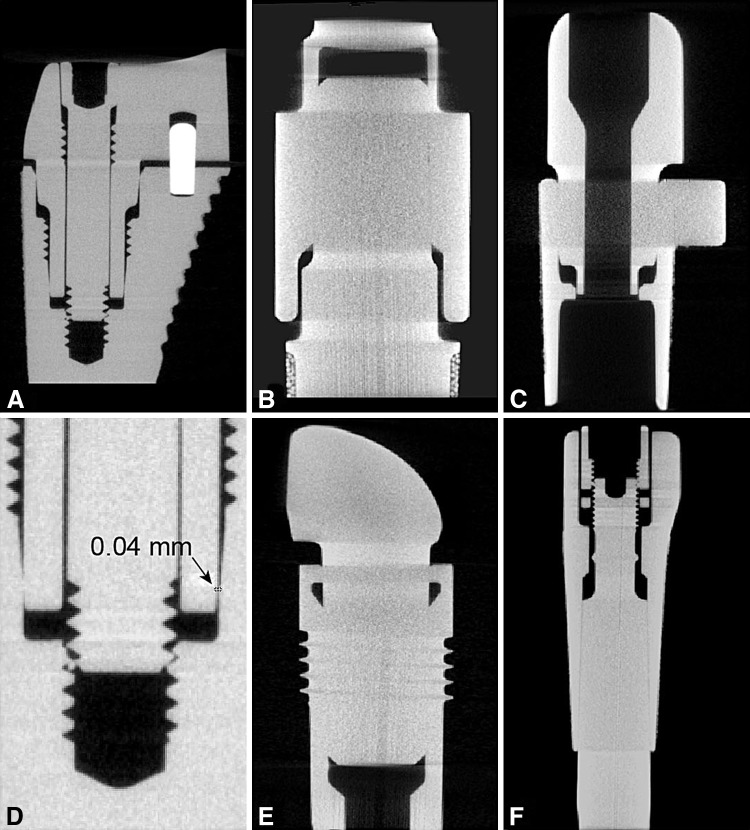
Fig. 4A–F.
Sagittal micro-CT images (original magnification, ×1.4) of the modular devices show proximal sections through the neck-stem junction: (A) Apex®, (B) GMRS®, (C) M-series®, (D) Apex® (higher magnification showing gap of 0.04 mm; original magnification, ×30), (E) Margron®, and (F) ZMR®.
Trunions for the Co-Cr-Mo devices were more severely corroded with a gramophone finish (100% Level 4) compared to the ground devices (26% Level 4) (Table 3). In contrast, the Ti-based devices showed a greater propensity for corrosion with the ground finish (50% Level 1) compared to the gramophone finish (6% Level 1). All gaps between mating surfaces of all trunions measured less than 0.05 mm (Fig. 4A–E). We observed different taper designs with contact over the entire mating surfaces for Co-Cr-Mo devices, while the Ti differed by THA style, with the Apex® and M-series® having contact only proximally and distally and the ZMR® having the largest contact area (Fig. 4F).
Discussion
Modular femoral heads have been in common use for decades, with the head taper junction being widely accepted as a standard feature of THAs. More recently, prostheses with a double-taper modular neck have allowed operative flexibility via on-table adjustment of anteversion and leg length [1, 11, 18]. Modularity may also reduce the likelihood of postoperative dislocation by reducing neck/cup impingement, which remains a serious clinical problem in arthroplasty surgery [3, 5, 23]. Even so, we asked whether (1) the degradation mechanisms of the neck-stem junction were similar to the head-neck junction, (2) the second junction contributed to revision, (3) the alloy type affected the degree of degradation, and (4) the machined finish of the junction affected the degradation mechanisms.
There are several limitations to our study. First, all retrieval studies are retrospective and data may come from a small number of devices from one manufacturer; thus, results may be biased or of limited application. In the present study, however, seven modular styles from seven manufacturers, in all 57 devices, were analyzed to compare and contrast failure mechanisms. Second, corrosion testing of samples taken from the retrieved devices was not conducted to ascertain the propensity for surface corrosion. Third, we did not perform in vitro testing to elucidate the mechanism of fretting, micromotion, or corrosion peculiar to each of the modular designs. This type of testing is technically challenging, however, and commonly such in vitro studies have concluded modular-junction degradation is of little clinical relevance [17, 18, 22, 25], which contrasts to the findings of this retrieval study. Finally, we could not retrospectively collate metal ion levels, which may indicate the degree of systemic metal exposure. In the present case, we relied on surgeon observations at the time of revision and in some cases tissue pathology to identify metallosis or ALVALs. In any case, metal ion levels in fluids (serum, blood, urine) just before revision surgery may be of limited value if baseline measures before primary implantation or subsequently in the followup period of a well-functioning device were not taken.
Our findings of the neck-stem taper support those of other studies of the head-neck taper junction (Table 5: comparison to Goldberg et al. [12]; Table 6); furthermore, longer implantation time has resulted in a greater degradation [12, 21]. We found the severity was greater at the neck-stem junction than at the head-neck junction. This is not unexpected as the forces at the head-neck junction are transferred through a spherical bearing resulting in relatively low contact stresses, whereas there is eccentric loading at the neck-stem junction, and depending on the offset and length of the neck, these stresses can be high [7, 17, 22] (Table 6). In this regard, one case study of 5000 Ti modular-neck prostheses reported an implant neck fracture rate of 1.4% [7]. A further study by Atwood et al. [1] stated there had been eight reported cases of modular-neck fracture in the FDA adverse event report database since 2006. As such, it is not unexpected to observe fretting at the neck-stem trunion in the majority of retrieved cases (Table 6). It is known the assembly method of modular components can affect the resultant disassembly force; however, loads imposed by the patient are equivalent to hammer blows to fix the neck components [19]. It is thus unlikely the fretting could have resulted from inadequate location of the neck into the stem component.
Table 5.
Comparison of head/stem corrosion in our study with that in Goldberg et al. [12]
| Study | Stem material | Head material | Number of heads | Average time in situ (months) | % corrosion severity | |||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||
| Goldberg et al. [12] | Co-Cr-Mo | Co-Cr-Mo | 132 | 44 (1–156) | 38 | 34 | 20 | 8 |
| Current study | Co-Cr-Mo | Co-Cr-Mo | 7 | 26 (4–103) | 100 | |||
| Goldberg et al. [12] | Ti | Co-Cr-Mo | 89 | 44 (1–156) | 18 | 4 | 30 | 12 |
| Current study | Ti | Co-Cr-Mo | 15 | 21 (3–204) | 87 | 13 | ||
Our data were redistributed for comparison with Goldberg et al. [12]; thus, severity scores of 0 and 1 were combined to be equivalent to a Goldberg score of 1.
Table 6.
Comparison of our study and other studies in the literature evaluating modular devices
| Study | Study results | Our results |
|---|---|---|
| Goldberg et al. [12] | Modular head-neck taper Corrosion on taper n = 231 Corrosion increases with implantation time |
Figure 3 depicts implantation time with severity of corrosion |
| Salvati et al. [21] | n = 48 Corrosion correlates with implantation time |
As above |
| Dunbar [7] | Neck stress dependent on length and offset and relates to propensity of fretting and corrosion | Determination of fretting at the junction in response to higher applied stresses |
| Kretzer et al. [17] | Simulation study (n = 5) Mechanical loading of neck-stem junction leads to higher stresses |
As above and when compared to the lack of fretting at the head-taper junction |
| Schramm et al. [22] | Simulation study of three devices Observations of fretting and corrosion | As above |
| Dunbar [7] | Neck fracture 1.4% (n = 5000) | As above, supporting our observations of the propensity for fretting in the joints |
| Atwood et al. [1] | n = 1 Fracture of a modular hip prosthesis Also cites FDA database from 2006 |
Again supports fretting at the junction, which also can lead to corrosion |
| Pallini et al. [19] | Load imposed by patient are equivalent to hammer blows | Premise that fretting is unlikely to occur as a function of inadequate location of the neck in theater |
| Jacobs et al. [13] | n = 20 Increased concentrations of circulating metal degradation products derived from orthopaedic implants may have deleterious biologic effects over the long term that warrant investigation |
Linking the observed corrosion, fretting, and particulates in our study to a clinical consequence |
| Jacobs et al. [14] | n = 10 Debris that has a deleterious biologic effect comes from modular junctions |
8 of 57 had metallosis including 2 ALVALs |
| Patntirapong et al. [20] | Laboratory study Effect of soluble Co and Cr ions on osteoclast differentiation and activation |
Relates to our study with respect to the significance of the corrosion findings |
| Cameron [4] | Cites use of alternative methods to lock the taper | Even with secondary locking mechanisms (n = 27), fretting was observed (n = 15) |
| Fraitzl et al. [8] | S-ROM® devices (n = 22): cold welding 27% (n = 5) | Ti devices (n = 27): cold welding 22% (n = 4) S-ROM® devices (n = 15): cold welding 13% (n = 2) |
FDA = US Food and Drug Administration; ALVAL = aseptic lymphocyte-laminated vascular-associated lesion.
In our study, three of 57 devices (5%) were primarily revised due to the second junction, two as a consequence of ALVALs and one due to a loose/retroverted neck in a Margron® prosthesis removed at 4 months. In addition, there were four cases (7%) of metallosis directly associated with degradation of the second junction that contributed to revision of the device. Several studies show degradation products from metallic devices may have deleterious local and systemic biologic and clinical effects over the short and long term [13, 14, 20] (Table 6). Metal release likely emanates from modular head-neck junctions, rather than passive surface dissolution [14]. The additional taper junction, subject to high stress levels, may therefore increase metal ion and debris concentration locally and systemically and contribute to device revision.
Ti-based devices have higher corrosion breakdown potentials than Co-Cr-Mo devices [15], ensuring the constructs are more corrosion resistant, and this was reflected in the retrieval data with the Co-Cr-Mo devices having a higher degree of degradation than the Ti-based devices. With respect to degradation and junction stability, a common feature of the retrieved Ti-based modular-neck THAs was an additional locking mechanism for the neck-stem junction in terms of a bolt or integrated thread and nut. In contrast, none of the Co-Cr-Mo THA devices had such a mechanism and relied on mechanical stability through the design of the trunion. Such additional locking mechanisms are not practicable for Co-Cr-Mo devices due to the inherent limitations of the alloy. Some Co-Cr-Mo designs incorporate an oblong taper to prevent rotating of the neck or use cogs for additional rotational resistance [4]. Whether such secondary locking mechanisms aid in reducing micromotion is unknown; however, our study suggests, despite these mechanisms, fretting rates were similar when comparing the Ti and Co-Cr-Mo THAs (Table 6). In contrast, the creation of a cold weld between the modular components may be beneficial in light of the reduced fretting and corrosion observed for Ti trunions; however, this presents problems when revising such a device. A recent study of S-ROM® THAs revealed 27% of retrieved devices could not be disassociated in theater, and while this may prevent the production of metallic debris, a key design feature of these devices is partial replacement in revision surgery [8]. We also found cold welding a problem, since we could not disassemble 22% of Ti devices without gripping them in a bench vice and hammering them repeatedly (Table 6). Consideration must be given in revision surgery of Ti modular-neck devices to the possibility that cold welding may be present and a well-fixed stem may need to be revised.
We found corrosion and fretting were not confined to either the ground finish or gramophone finish trunions. The only implant with a turned neck finish, the Bionik®, showed substantial corrosion in all four cases, which is notable due to the relatively short implantation time. We suspect the turned finish may exacerbate corrosion by creating ready-made crevices. The corrosion of the Bionik® trunions was preferentially observed at the root of the machined grooves, the peaks showing fretting via wear scars running perpendicular to the turned surface. Even so, extensive corrosion was also observed with the other Co-Cr-Mo devices that had a ground finish. Even though the analysis of micro-CT data of representative samples showed good congruency, crevices remain that may be subject to crevice corrosion or mechanically assisted crevice corrosion in the presence of fretting. In vitro studies described an insignificant amount of corrosion and fretting at the modular head-stem junction [9, 22] while a similar in vitro study of the effect of machine surface finish on fretting and corrosion showed a negative correlation with surface roughness but also concluded the degree of degradation is “within a clinically non-critical range” [17]. These studies are in direct contrast to our study where revision of some devices can be attributed to degradation at the neck-stem taper junction.
Modularity may come at a cost, with the neck-stem junction showing greater fretting and crevice corrosion compared to the head-neck junction and as a consequence increased metallic debris and soluble metallic ions, both locally and systemically [13, 27]. Clinically, eight of the retrieved devices had metallosis with two cases of ALVALs and one case of neck loosening/retroversion. The Co-Cr-Mo components had a higher degree of degradation compared to the Ti components and thus may be compromised in modular-neck designs. Micro-CT demonstrated congruency of trunion surfaces, although machine finish may have an effect on corrosion susceptibility and requires further investigation. An important question remains as to whether further optimization of the junction can take full advantage of modularity without sacrificing implant performance and long-term biocompatibility.
Electronic supplementary material
Acknowledgments
The authors thank all orthopaedic surgeons in Western Australia who continue to support the Royal Perth Hospital Retrieval Service and consistently send retrievals for analysis. We also acknowledge assistance from Dr David Morrison and Dr Andrew Campbell with regard to the micro-CT image manipulation.
Footnotes
Each author certifies that he or she, or a member of their immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Atwood SA, Patten EW, Bozic KJ, Pruitt LA, Ries MD. Corrosion-induced fracture of a double-modular hip prosthesis: a case report. J Bone Joint Surg Am. 2010;92:1522–1525. doi: 10.2106/JBJS.I.00980. [DOI] [PubMed] [Google Scholar]
- 2.Australian Orthopaedic Association. AOA National Joint Replacement Registry. Annual Report 2010. Available at: http://www.dmac.adelaide.edu.au/aoanjrr/documents/aoanjrrreport_2010.pdf. Accessed May 10, 2011.
- 3.Berry DJ, Knoch M, Schleck CD, Harmsen WS. The cumulative long-term risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2004;86:9–14. doi: 10.2106/00004623-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Cameron HU. The proximal modular neck in THA: a bridge too far: opposes. Orthopedics. 2010;33:641. doi: 10.3928/01477447-20100722-27. [DOI] [PubMed] [Google Scholar]
- 5.Collier JP, Mayor MB, Williams IR, Surprenant VA, Surprenant HP, Currier BH. The tradeoffs associated with modular hip prostheses. Clin Orthop Relat Res. 1995;311:91–101. [PubMed] [Google Scholar]
- 6.Cook SD, Barrack R, Clemow AJ. Corrosion and wear at the modular interface of uncemented femoral stems. J Bone Joint Surg Br. 1994;76:68–72. [PubMed] [Google Scholar]
- 7.Dunbar MJ. The proximal modular neck in THA: a bridge too far: affirms. Orthopedics. 2010;33:640. doi: 10.3928/01477447-20100722-30. [DOI] [PubMed] [Google Scholar]
- 8.Fraitzl C, Buly R, Castellani L, Moya L. Corrosion at the sleeve-stem interface of a modular titanium alloy femoral component. J Bone Joint Surg Br. 2010;92(Suppl IV):514–515. doi: 10.1016/j.arth.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Fricker DC, Shivanath R. Fretting corrosion studies of universal femoral head prostheses and cone taper spigots. Biomaterials. 1990;11:495–500. doi: 10.1016/0142-9612(90)90064-W. [DOI] [PubMed] [Google Scholar]
- 10.Frost RB, Sekel R, Kandel L. The engineering design of the MARGRON® femoral hip prosthesis system. J Eng Design. 2003;14:115–127. doi: 10.1080/0954482031000091473. [DOI] [Google Scholar]
- 11.Garbuz DS, Tanzer M, Greidanus NV, Masri BA, Duncan CO. The John Charnley Award. Metal-on-metal resurfacing versus large diameter head metal-on-metal total hip arthroplasty: a randomized clinical trial. Clin Orthop Relat Res. 2010;468:318–325. doi: 10.1007/s11999-009-1029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg JR, Gilbert JL, Jacobs JJ, Bauer TW, Paprosky W, Leurgans SA. Multicenter retrieval study of the taper interfaces of modular hip prosthesis. Clin Orthop Relat Res. 2002;401:149–161. doi: 10.1097/00003086-200208000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs JJ, Skipor AK, Patterson LM, Hallab NH, Paprosky WG, Black J, Galante JO. Metal release in patients who have had a primary total hip arthroplasty: a prospective, controlled, longitudinal study. J Bone Joint Surg Am. 1998;80:1447–1458. doi: 10.2106/00004623-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs JJ, Urban RM, Gilbert JL, Skipor AK, Black J, Jasty M, Galante JO. Local and distant products from modularity. Clin Orthop Relat Res. 1995;319:94–105. [PubMed] [Google Scholar]
- 15.Khan MA, Williams RL, Williams DF. In-vitro corrosion and wear of titanium alloys in the biological environment. Biomaterials. 1996;17:2117–2126. doi: 10.1016/0142-9612(96)00029-4. [DOI] [PubMed] [Google Scholar]
- 16.Kop AM, Swarts E. Corrosion of a hip stem with a modular neck taper junction: a retrieval study of 16 cases. J Arthroplasty. 2009;24:1019–1023. doi: 10.1016/j.arth.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Kretzer JP, Jakubowitz E, Krachler M, Thomsen M, Heisel C. Metal release and corrosion effects of modular neck total hip arthroplasty. Int Orthop. 2009;33:1531–1536. doi: 10.1007/s00264-009-0729-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McTighe T. Cementless modular stems. 2002. Joint Implant Surgery and Research Foundation. Available at: http://www.jisrf.org/pdf files/Updated0402.pdf. Accessed May 10, 2011.
- 19.Pallini F, Cristofolini L, Traina F, Toni A. Modular hip stems: determination of disassembly force of a neck-stern coupling. Artif Organs. 2007;31:166–170. doi: 10.1111/j.1525-1594.2007.00359.x. [DOI] [PubMed] [Google Scholar]
- 20.Patntirapong S, Habibovic P, Hauschka PV. Effects of soluble cobalt and cobalt incorporated into calcium phosphate layers on osteoclast differentiation and activation. Biomaterials. 2009;30:548–555. doi: 10.1016/j.biomaterials.2008.09.062. [DOI] [PubMed] [Google Scholar]
- 21.Salvati EA, Lieberman JR, Huk OL, Evans BG. Complications of femoral and acetabular modularity. Clin Orthop Relat Res. 1995;319:85–93. [PubMed] [Google Scholar]
- 22.Schramm M, Wirtz DC, Holzwarth U, Pitto RP. The Morse taper junction in modular revision hip replacement—a biomechanical and retrieval analysis. Biomed Tech (Berl) 2000;45:105–109. doi: 10.1515/bmte.2000.45.4.105. [DOI] [PubMed] [Google Scholar]
- 23.Sporer SM, Valle C, Jacobs J, Wimmer M. A case of disassociation of a modular femoral neck trunion in total hip arthroplasty. J Arthroplasty. 2006;21:918–921. doi: 10.1016/j.arth.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 24.US Food and Drug Administration. MAUDE database. 2009. Available at: www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/search.cfm. Accessed May 10, 2011.
- 25.Viceconti M, Baleani M, Squarzoni S, Toni A. Fretting wear in a modular neck hip prosthesis. J Biomed Mater Res. 1997;35:207–216. doi: 10.1002/(SICI)1097-4636(199705)35:2<207::AID-JBM9>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 26.Viceconti M, Ruggeri O, Toni A, Giunti A. Design-related fretting in modular neck hip prosthesis. J Biomed Mater Res. 1996;30:181–186. doi: 10.1002/(SICI)1097-4636(199602)30:2<181::AID-JBM7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 27.Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Köster G, Lohmann CH. Metal-on-metal hypersensitivity in patients with artificial hip joints: a clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.