Abstract
Background
The use of dual-mobility cups has increased because of a low rate of dislocations combined with a 96% 15-year survival rate. However, late cup migrations have been attributed to their fixation (tripod - exact fit with two pegs and one extraacetabular screw) and the absence of porous coating. In a second-generation device, the designs were modified to achieve press-fit fixation and a layer of titanium beads was sintered on stainless steel cups.
Questions/purposes
We therefore (1) determined the midterm survival of press-fit, grit-blasted, second-generation cups with or without additional screws, compared with original tripod and (2) compared survival of grit-blasted dual-mobility cups with bimetallic porous-coated cups.
Methods
From a multiinstitutional trial, we reviewed 2408 patients with osteoarthritis implanted with 2601 prostheses of seven designs of a second-generation dual-mobility cup. The criteria for failure were migration, widening radiolucencies in any zone of the interface, or revision for cup loosening. The minimum followup was 5 years (mean, 7.7 years; range, 5–11 years).
Results
The 8-year survival rate of press-fit, grit-blasted cups was lower than that for press-fit, grit-blasted cups fixed with screws (91% versus 100%) and for tripod fixation (98%). The 8-year survival rate of press-fit, grit-blasted cups was less than that for press-fit, porous-coated cups made of the same alloy (91% versus 95%).
Conclusions
The data suggested primary fixation of grit-blasted dual-mobility cups should be secured with screws. Porous coating sintered on the convex side improved midterm survivorship. No deleterious effect of metallosis resulted from sintered titanium beads on stainless steel. Long-term followup is required to confirm these findings.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Farizon et al. [12] introduced the concept of mobile bearings for THA in the late 1970s to reduce the risk of dislocation. The concept was reported by others [14, 17, 26]. Contrary to conventional fixed-bearing cups, the mobile polyethylene insert, designed to be head retaining, is left free to rotate in its metallic shell. In extreme movements provoking femoral neck impingement, inserts behave like a large-diameter head and are more difficult to dislocate than a conventional femoral head.
With revision for aseptic loosening or dislocation as an end point, the 10-year survivorship of first-generation dual-mobility cups was reportedly 94%, with a 15-year survival of 90% [27]. The devices were not associated with substantial wear [27], particularly on the convex side of the mobile insert [1]. Although primary fixation was secured by a tripod system (two pegs at the bottom, pubis, and ischium, with one screw at the top of the cup) (Fig.1A), these first-generation dual-mobility cups were associated with a 4% rate of late cup loosening occurring from the ninth to the fifteenth postoperative years that particularly affected patients younger than 65 years [27]. These failures were attributable to delamination of their alumina coating sintered on a nonporous surface, resulting in third-body wear.
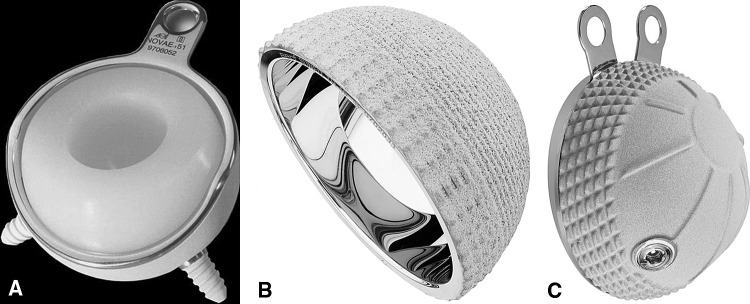
Fig. 1A–C.
(A) A tripod grit-blasted cup designed according to the original Bousquet model (Novae® cup, photograph published with permission from Serf dedienne santé, Décines, France), (B) a press-fit cup designed for equatorial press fit (Sunfit®, photograph published with permission from Serf dedienne santé, Décines, France), and (C) a grit-blasted cup with flanges allowing extraacetabular screw fixation (POLARCUP®, photograph published with permission from Smith & Nephew Orthopaedic AG, Aarau, Switzerland) are shown.
Second-generation cups became available in the late 1990s and were designed to correct the concerns of first-generation shells by replacing their alumina coating with hydroxyapatite [18, 30]. The design and outer surfaces were modified to achieve press-fit fixation followed by bone ingrowth; the inner surface had to permit low friction with the convex side of the mobile polyethylene insert, which required proper surface finish and tribologic properties. Some shells were designed for impaction alone (Fig. 1), whereas others incorporated extra fixation devices, such as flanges (Fig. 1C), allowing insertion of additional extraarticular screws to secure fixation if needed. From a metallurgic point of view, titanium was not recommended for femoral heads because of its poor tribologic properties affecting polyethylene bearings [6]. Therefore, cobalt-chromium and stainless steel were judged to be more appropriate for mobile-bearing shells, although the manufacturing techniques available in France did not allow sintering their surfaces with beads made of the same alloy. Therefore, although most shells had grit-blasted surfaces, manufacturers coated some stainless steel cups with titanium beads. These variations allowed seven types of shells: (1) press-fit grit-blasted stainless steel, (2) press-fit grit-blasted cobalt-chromium, (3) press-fit porous-coated stainless steel, (4) press-fit grit-blasted stainless steel with screws, (5) press-fit porous-coated stainless steel with screws, (6) tripod grit-blasted, and (7) tripod porous-coated.
Our purposes were to (1) determine the mid-term survival of press-fit, grit-blasted second-generation cups with and without extraarticular screws compared with original tripod, and (2) compare survival of second-generation dual-mobility cups in terms of fixation for grit-blasted and bimetallic porous-coated cups.
Patients and Methods
We retrospectively reviewed all 3210 patients who underwent 3474 THAs between 1998 and 2003 in 16 French centers. Of these, 2408 patients underwent 2601 THAs for primary osteoarthritis. To provide a more homogeneous group, we reviewed only patients with primary osteoarthritis. With that particular etiology, indications for THA using a dual-mobility cup were: (1) advanced osteoarthritis involving complete joint narrowing and disabling pain, but conservation of some walking abilities, and (2) patients older than 55 years. The contraindications were: (1) mild osteoarthritis with incomplete joint narrowing, (2) patients younger than 55 years, and (3) patients unable to walk. Mean age was 72 ± 9 years (range, 55–92 years). There were 1429 women and 979 men. Although we asked physicians to recall patients for this study, 650 patients (696 hips or 27%) had less than 5 years followup: 416 patients (17%) (436 hips) died and 234 patients (10%) (264 hips) were lost to followup or had incomplete data. This left 1758 patients (1905 hips) with a minimum followup of 5 years (mean, 7.7 years; range, 5–11 years).
Preoperatively, we classified the preoperative mobility status using the method of Charnley [5] and classified comorbidities using the classification of the American Society of Anesthesiologists [10]. From the records, we collected demographic variables and compared qualitative variables (gender, Charnley class) between the seven prosthesis groups using the chi-square test. We found differences between the seven groups regarding sex and Charnley class distribution (p < 0.001) (Table 1). The group of press-fit porous-coated cups included the highest proportion of men (52%), and the highest proportion of patients with Charnley Class C (30%).
Table 1.
Demographics of different groups according to cup type in patients with primary osteoarthritis
| Cup type | Number of hips | Mean age (years) (± SD) |
BMI (± SD) |
Sex ratio female/male (p < 0.001) |
Charnley Class A/B/C (p < 0.001) |
|---|---|---|---|---|---|
| Press-fit stainless steel | 402 | 72 ± 10 | 26 ± 5 | 1.40 | 261/110/31 |
| Press-fit cobalt-chromium | 191 | 75 ± 8 | 26 ± 4 | 1.85 | 142/30/19 |
| Press-fit porous-coated | 404 | 71 ± 7 | 27 ± 5 | 0.91 | 148/132/124 |
| Press-fit stainless steel with additional screw fixation | 590 | 72 ± 10 | 26 ± 4 | 1.68 | 370/142/78 |
| Press-fit porous-coated with additional screws | 136 | 74 ± 8 | 26 ± 5 | 2.07 | 60/61/15 |
| Tripod grit-blasted | 797 | 72 ± 8 | 26 ± 4 | 1.58 | 399/286/112 |
| Tripod porous- coated | 81 | 71 ± 10 | 26 ± 5 | 1.45 | 19/42/20 |
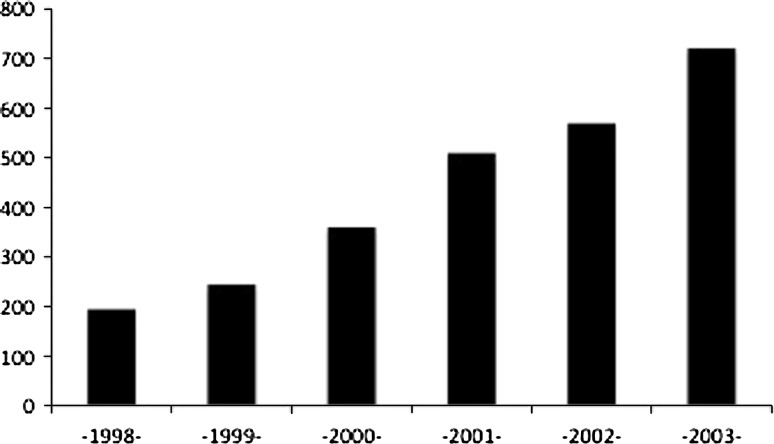
An increasing number of patients were operated on in the osteoarthritis group from 1998 to 2003 (Fig. 2). Design modifications preceded surface modifications, explaining that porous-coated cups had shorter followup than grit-blasted cups. We classified cups according to fixation type (press-fit, press-fit with additional extraarticular screws, tripod) and coating (grit-blasted, titanium porous-coating). All were covered with an osteoconductive layer (hydroxyapatite or double-layer hydroxyapatite-alumina) (Table 1). We used 22-mm diameter femoral heads in 1058 (41%) hips, and 28-mm diameter heads in 1543 (59%) hips. The bearings were made of metal-polyethylene in 1222 (47%) hips, alumina-polyethylene in 1170 (45%) hips, and zirconium-polyethylene in 209 (8%) hips.
Fig. 2.
A histogram shows the number of dual mobility cups implanted per year.
Nine hundred ninety-seven cups (929 patients) designed for press-fit fixation alone had a peripheral rim for equatorial press fit. An 80-μm hydroxyapatite layer (Collegia™, Wright Medical EMEA, Amstelveen, the Netherlands) covered 402 press-fit, grit-blasted stainless steel cups (n = 91) or a bilayer of hydroxyapatite and alumina (Novae Sunfit™, Serf, Decines, France) (n = 311). Four hundred four stainless steel cups were porous coated with titanium beads, giving 150 to 400 μm diameter pores (Polarcup™, Smith & Nephew Orthopaedics AG, Rotkreuz, Switzerland [n = 301]; Saturne™, Amplitude, Valence, France [n = 103]). One hundred ninety-one cups were made of cobalt-chromium and covered with a 100-μm hydroxyapatite layer (Evora™, Science et Médecine, Montrouge, France). They were grit-blasted and supplied with rotational stabilizers (fins or small spikes).
A second group of 726 stainless steel cups (680 patients) was supplied with flanges, allowing the insertion of additional extraarticular screws. Five hundred ninety cups were grit blasted (Gyros, DePuy, Warsaw, IN, USA), while 136 were porous coated, with a plasma-sprayed layer of titanium beads (Fig. 1C [Polarcup™, Smith & Nephew Orthopaedics AG, Memphis, TN, USA]).
Finally, a third group of 878 cups (799 patients) were designed for tripod fixation according to the original model of Bousquet, as described by Farizon et al. [12], in which the majority had a grit-blasted surface covered with an hydroxyapatite-alumina bilayer (797 cups [Novae, Serf, Decines, France]) (Fig. 1A), and the rest were covered with a porous surface (81 cups [EOL™, Ceramconcept, Paris, France]).
Prostheses were implanted through a posterolateral approach. The acetabulum was prepared using reamers of growing size and press fit was achieved by implanting the cup with a 2-mm greater diameter than that of the last reamer. At the end of the operation, repair of the external rotator muscles was performed in 10% of the cases. Additional screw fixation then was performed in implants with flanges.
Patients were allowed immediate weightbearing as tolerated using crutches. No other physiotherapy other than walking assistance was provided during the hospital stay. We typically discharged patients between the sixth and eighth postoperative days; patients either were sent home or to a convalescent home until they recovered independence. They were encouraged to avoid high flexion combined with adduction of the involved hip for 6 weeks postoperatively, after which we recommended they stop using their crutches and resume their usual activities.
Patients were clinically examined for walking distance, pain, and motilities 6 weeks, 6 months, and yearly after surgery. Early and late complications were recorded and classified according to Dindo et al. [9]. At each visit an AP radiograph of the pelvis was taken with the lower limbs internally rotated and including the entire implant. Magnification was calculated by the ratio between the measured and the known cup diameter. We compared these radiographs with the immediate postoperative radiographs taken using the same technique.
There were missing data for the 650 patients whose followup was less than 5 years. However, we included these patients in the first intervals of the survivor analysis (0 to 5 years) because four of these patients had early cup mobilization, which was included in the radiographic failure end point.
Six of us (VO, RP, and four observers who also were the treating physicians) independently evaluated all radiographs for cup migration and widening radiolucencies in any zone of the fixation interface. We measured cup migration on comparable AP pelvic views according to criteria established by Massin et al. [23], based on a minimum 5-mm variation in perpendicular vertical distance between the obturator line and the teardrop line for 10% magnification. Compared with the gold standard, radiostereometry, such manual measurements on selected radiographs provided an accuracy of 4.4 mm, with an interobserver error of 3.2 mm [19]. We evaluated radiolucencies in the three zones of DeLee and Charnley [8] with the following criteria for lucencies: (1) radiolucent lines greater than 2 mm in width, (2) developing at least in acetabular Zone 3 of DeLee and Charnley, and (3) they were not present on immediate postoperative radiographs. We defined mechanical radiographic failure as cup center migration in either direction greater than 3 mm and/or variations of cup inclination greater than 10°, and/or by widening and extensive radiolucencies after the first postoperative year. We examined AP and lateral hip radiographs for osteolysis.
We performed Kaplan-Meier survivorship analysis [2] based on successive 1-year intervals (life table), until the latest interval including a minimum of 10 patients at risk, and using radiographic failure as a primary end point. Secondary end points were revision for any cause and revision for failure of fixation. We calculated the 95% CI according to Peto et al. [25]. We compared 8-year survival of grit-blasted cups with different modes of primary fixation, but the same outer surface: tripod, press-fit alone, and press-fit with additional extraarticular screws. We compared survival of cups with the same primary fixation type, but different surfaces (grit-blasted versus porous-coating) to address whether outer cup surface influenced biologic fixation. Thus, we compared survival of press-fit, grit-blasted stainless steel cups with press-fit, porous-coated cups made of the same alloy, and of tripod grit-blasted cups with tripod porous-coated cups. We compared survivor curves using the log-rank test [22]. We used version 12 of the SPSS® software (SPSS, IBM® Inc, Chicago, IL, USA) for statistical analysis.
Results
Overall, the 10-year survival rate for all revisions was 95% (95% CI, 91%–99%). When the end point was limited to revisions for failure of fixation only, the cumulative survival rate was 95% (95% CI, 91%–99%). There were several complications (Table 2). In the 23 patients who had revision surgery for cup loosening, only one had revision of acetabular and femoral components. Six other patients had the components revised for recurrent dislocation (one patient), groin pain attributed to psoas impingement (one patient), deep infection (one patient), and intraprosthetic dislocations (three patients respectively occurring at 32, 63, and 85 months postoperatively).
Table 2.
Classification of complications according to Dindo et al. [9] (of a total of 2601 hips)
| Grade | Complications | Number of hips |
Percentage of hips |
|---|---|---|---|
| Grade I | Regressive sciatic palsies | 5 | 0.2% |
| Psoas impingement | 75 | 3% | |
| Leg length discrepancy 1 cm or greater | 279 | 11% | |
| Grade II (nonrevised) | Symptomatic deep venous thrombosis | 21 | 0.8% |
| Cup migration | 10 | 0.4% | |
| Grade III (revised) | Early dislocations (reduction under GA) | 10 | 0.4% |
| Recurrent dislocation | 1 | 0.04% | |
| Deep infection | 24 | 0.9% | |
| Hematomas | 53 | 2% | |
| Psoas impingement | 1 | 0.04% | |
| Intraprosthetic dislocations | 3 | 0.1% | |
| Periprosthetic fractures | 31 | 1.2% | |
| Cup loosening | 23 | 0.9% | |
| Grade IV (life threatening) | Symptomatic pulmonary embolism | 13 | 0.5% |
| Ischemic stroke/brain hemorrhage | 3 | 0.1% | |
| Grade V (death) | Mesenteric ischemia | 1 | 0.04% |
| Heart failure | 1 | 0.04% |
GA = general anesthesia.
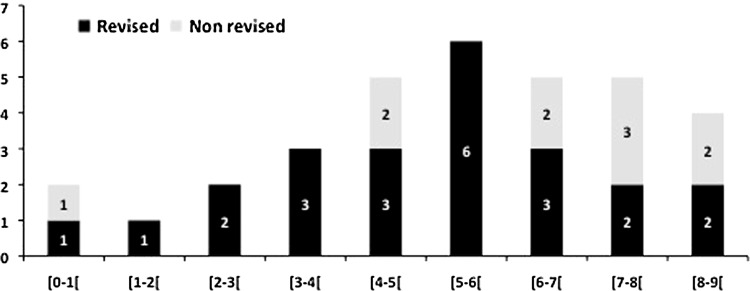
The 8-year survival of press-fit, grit-blasted stainless steel cups was lower (p = 0.05) than that of tripod grit-blasted cups made of the same alloy: 91% versus 98%, respectively (Table 3). Eight-year survival of the latter was, in turn, lower (p = 0.03) than that of grit-blasted cups with flanges and secured with additional screws: 98% versus 100%. Finally, among press-fit cups, 8-year survival of cobalt-chromium cups was greater (p = 0.03) than that of stainless steel cups with no screw fixation: 100% versus 91%. The failure rate was high in the group of press-fit grit-blasted cups with no additional screw fixation (15 failures, 3.7%) (Fig. 3). After a symptom-free initial period (1–9 years), the cups tilted acutely, although no radiographic signs of osteolysis or radiolucency were apparent in all but three cases. We easily removed these 15 press-fit, grit-blasted cups and noted no membrane or bone defect except in three cases with osteolysis. At revision, the hydroxyapatite layer was completely resorbed, exposing the noncoated convex side of the metallic shell. We performed revision without reconstruction in these cases, using primary cups of a slightly larger diameter. Eleven failures (1.3%) occurred in the group of tripod grit-blasted cups, with all but one occurring after 5 years postoperatively. In contrast to press-fit, grit-blasted cups, we observed widening radiolucencies around the pegs or at the cup dome in eight of these 11 cases (Fig. 4), indicating osteolysis.
Table 3.
Cumulative survival rates of different dual-mobility cup groups (%)
| Followup (years) | Press-fit, grit-blasted cobalt-chromium (n = 191) | Press-fit, grit-blasted stainless steel (n = 402) (± 95% CI) | Press-fit grit-blasted stainless steel with screws (n = 590) | Press-fit porous-coated stainless steel (n = 404) (± 95% CI) | Press-fit porous-coated stainless steel with screws (n = 136) (± 95% CI) | Tripod grit-blasted (n = 797) (± 95% CI) | Tripod porous-coated (n = 81) (± 95% CI) |
|---|---|---|---|---|---|---|---|
| 6 | 100% | 97% ± 3% | 100% | 99% ± 2% | 100% | 99% ± 1% | 100% |
| 7 | 100% | 96% ± 4% | 100% | 98% ± 3% | 100% | 99% ± 1% | 98% ± 4% |
| 8 | 100% | 91% ± 6% | 100% | 98% ± 3% | 91% ± 16% | 98% ± 2% | 90% ± 14% |
| 9 | 84% ± 13% | 98% ± 2% | |||||
| 10 | 98% ± 5% | ||||||
| p value (comparison grit-blasted/tripod) | 0.05 | 0.03 | * | ||||
| p value (comparison grit-blasted/porous-coated | 0.002 | * | * | 0.04 | * |
* Reference for 8-year survival comparison.
Fig. 3.
A histogram shows the number of mechanical fixation failures (revised or not) in relation to postoperative followup.
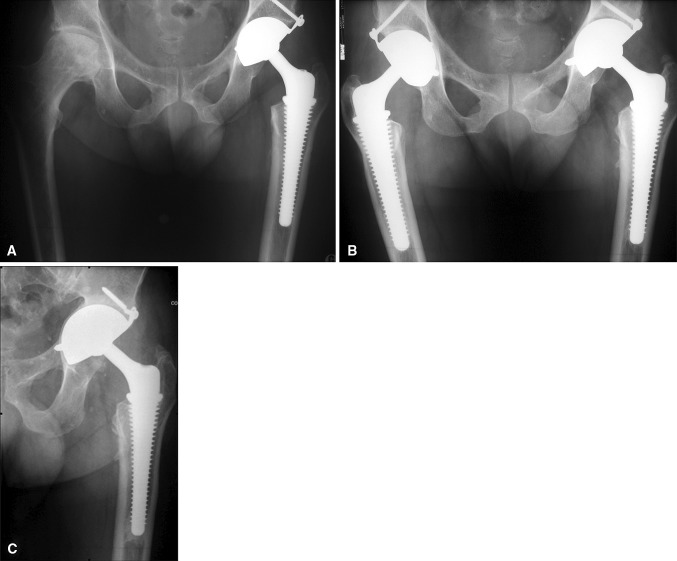
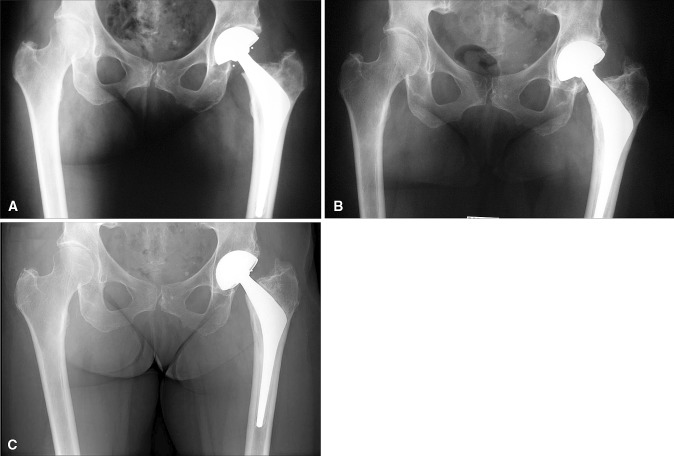
Fig. 4A–C.
(A) A postoperative radiograph shows a press-fit, grit-blasted tripod dual-mobility cup implanted in a 65-year-old active woman during THA for advanced primary osteoarthritis. (B) Seven years later, the patient reported excellent hip function and radiographic control looked optimal. (C) In the eighth postoperative year, hip function deteriorated slightly, and the cup migrated medially. The upper fixation screw broke, while bone density at the acetabular roof increased. The patient has declined reoperation.
The outer surface effect (grit-blasted versus porous-coated) affected the midterm fixation of press-fit dual-mobility cups. The 8-year cumulative survival of press-fit, grit-blasted cups (with or without screws) was lower (p = 0.002) than that of porous-coated cups (with or without screws): 91% versus 95%, respectively. In contrast, tripod grit-blasted cups were fixed more durably (p = 0.04) than porous-coated cups: 98% versus 90% 8-year survival, respectively.
Discussion
Second-generation dual-mobility cups were expected to correct the defaults of the first-generation tripod cups described by Farizon et al. [12]. The delamination of the alumina layer coated on a grit-blasted surface jeopardized the fixation of the initial design. The first modification consisted of substitution of the alumina with hydroxyapatite that initially was coated on grit-blasted surfaces. At the same time, tripod fixation was converted to a press-fit fixation and eventually secured with additional extraacetabular screws. Later, titanium plasma-sprayed beads were sintered on the outer surface of stainless steel cups. To assess the modified devices we (1) determined the mid-term survival of press-fit, grit-blasted second-generation cups with or without additional screws compared with original tripod cups, and (2) compared survival of grit-blasted dual-mobility cups with bimetallic porous-coated cups.
Our study has some limitations. First, because it was a large multicenter study, it involved numerous surgeons using different techniques. Although this introduced heterogeneity, it likely enhanced the generalizability of the data. Second, we lost a substantial number of patients to followup who were included in the early intervals of the survival analysis, but which decreased the number of patients at risk after 5 years postoperatively. Nonetheless, owing to the large number of cases, we observed differences between the implant types, some of them related to the type of primary fixation, others to the type of outer surface; however, we cannot say whether the differences we observed would apply to the entire group. Third, four late failures impacted the long-term survival of tripod grit-blasted cups, which we did not consider in the survival comparisons that stopped before 9 years because the number of patients at risk in the other groups of implants was too low. Such late failures indicated either suboptimal fibrous biologic fixation, late osteolysis, or both.
Survival was worst among grit-blasted, press-fit stainless steel cups in which the primary fixation was not fixed with additional extraarticular screws. Survivorship was lower among these cups, which we initially stabilized by press-fit impaction alone. This probably was related to the absence of bone ongrowth, explaining some late acute migration or tilting that occurred in patients who previously reported optimal function for years (Fig. 5). Stainless-steel cups that were fixed using additional screws were associated with higher survival and were not subject to late tilting.
Fig. 5A–C.
(A) A postoperative radiograph shows a press-fit, grit-blasted, dual-mobility cup implanted in a 72-year-old active woman for advanced primary osteoarthritis. The patient, who initially was pain free, reported acute groin pain during the third postoperative year. (B) The radiograph shows radiolucency at the bottom of the cup and bone condensation at its top, although there is no measurable migration. (C) She received a larger press-fit, porous-coated dual-mobility cup seen in this radiograph obtained 3 years after revision surgery. She acknowledged immediate and complete pain relief.
Thus, we believe additional extraarticular screws are required to secure the fixation of grit-blasted stainless steel cups to improve their mid-term survival. This kind of late fixation failure is not specific to mobile-bearing cups, but was reported with fixed-bearing cementless cups without porous coating [15, 16, 24, 28]. It is explained as mechanical failure of the fixation interface independent of particle-induced bone resorption and may be attributed to the lack of microinterlocking between bone and metal in the absence of porous coating. Dual-mobility cobalt-chromium cups had the best survival rate among press-fit cups. They had reliefs or macrostructures, such as grooves, pegs, or wings implanted on their dome, whereas the stainless steel cups had only some rough texture located at their periphery [18]. Thus, it appears that better survival with cobalt-chromium cups was attributable to their design rather than their metallurgy.
Porous coating appeared to be beneficial in press-fit, stainless steel cup fixation. In the literature, mechanical fixation failure rates of press-fit, porous-coated cups with fixed bearings were variable, ranging from 0% [3, 11, 13], 6% [7] to 17% [4], or 18% [20] (Table 4). It is difficult to distinguish between particle-induced osteolysis-related loosening and mechanical loosening resulting from suboptimal fibrous biologic fixation. With a followup similar to that in the current study, Manley et al. [21] compared cups of different outer surfaces (grit-blasted plasma-sprayed versus porous-coated) and reached similar conclusions. They found that 11% of the hydroxyapatite-coated, grit-blasted cups but only 2% of the porous-coated cups were revised because of aseptic loosening. More recently, Vicente et al. [29] compared long-term results of the two types of cups. Although they followed their patients longer, they found no difference. Particularly, they reported an 11.4% mechanical failure rate for the PCA cups (Howmedica, Rutherford, NJ, USA), which corresponded to the results of Bojescul et al. [4] and Malchau et al. [20] using the same implant. Thus, this failure rate is likely to be attributable to this particular cup design. In our study, the loosening rate of press-fit, porous-coated dual-mobility cups was in the lower range of those reported for fixed-bearing cups. In particular, we observed no deleterious effect of bimetallism (titanium beads coated on a stainless steel shell). In contrast, porous coating did not improve tripod-cup fixation. It is possible that incomplete penetration of the pegs prevented complete host bone/implant contact. We hypothesized that this may have increased the rate of fibrous encapsulation and subsequent particle migration and osteolysis at the fixation interface of tripod cups.
Table 4.
Comparisons of our mechanical fixation failure rates with those of fixed-bearing cementless cups
| Study | Grist-blasted cups % of mechanical fixation failures | Porous-coated cups % of mechanical fixation failures | Mean followup (years) or intervals of followup |
|---|---|---|---|
| Reikeras and Gunderson [28] | 25% | 7–10 | |
| Lai et al. [16] | 38% | 10 | |
| Miyakawa et al. [24] | 31% | 11–14 | |
| Kim et al. [15] | 14% | 7 | |
| Malchau et al. [20] | 18% | 6 | |
| Bohm and Bosche [3] | 0% | 11 | |
| Manley et al. [21] | 11% | 2% | 7.9 |
| D’Lima et al. [7] | 6% | 6 | |
| Bojescul et al. [4] | 17% | 15 | |
| Engh et al. [11] | 0,4% | 7–15 | |
| Garavaglia et al. [13] | 0% | 10 | |
| Vicente et al. [29] | 12.3% | 11.4% | 10–12 |
| Current study | 3.7% | 1% | 7.7 |
Fixation of second-generation dual-mobility cups still needs improvement. Press-fit, grit-blasted cups without additional screw fixation did not provide satisfactory midterm stability and should not be used. Macrostructures fixed on their dome may be a solution to optimize rotational stability. Titanium porous coating applied on the surface of press-fit cups appeared to be promising, but did not improve the fixation of tripod cups. Thus, it seems that the best design for receiving the porous layer would be press-fit hemispherical cups. Followup beyond 10 years is required before confirming the tolerance of bialloy titanium, stainless steel cups.
Acknowledgments
This work is a multicenter retrospective study conducted in 16 French centers: Bichat Claude Bernard Hospital, Paris Diderot University, France (V. Orain, P. Massin), North Hospital, Saint Etienne University, France (R. Philippot, F. Farizon), Lyon Sud Hospital, Lyon University, France (M. Fessy), Edouard Herriot Hospital, Lyon University, France (O. Guyen), Hautepierre Hospital, Strasbourg University, France (P. Adams, P. Corcos, M. Ehlinger), North Hospital, Amiens University, France (P. Mertl, F. Leiber), Rangueil Hospital, Toulouse University, France, Orthopaedic Department (J.M. Laffosse, J. Puget), Nancy University, France (O. Roche), Saint Roch Hospital, Cavaillon, France (P. Tracol), La Meynard Hospital, Fort de France University, France (J.L. Rouvillain), Côte de Nacre Hospital, Caen University, France (G. Burdin), Saint Martin Hospital, Caen (S. Leclercq), Salengro Hospital, Lille University, France (A. Combes, J. Girard, H. Migaud), Saint Georges Hospital, Nice, France (L. Descamps, G. Dehri), South Hospital, Rennes University, France (N. Barba, D. Huten), Saint Vincent de Paul Hospital, Bourgoin Jallieu, France (D. Noyer). We thank Drs P. Adams, N. Barba, G. Burdin, A. Combes, P. Corcos, L. Descamps, G. Dehri, M. Ehlinger, J. Girard, O. Guyen, S. Leclercq, D. Noyer, O. Roche, and P. Tracol for reviewing the patients and reading the radiographs. We also thank Ovid Da Silva for editing this manuscript, and the department of biostatistics (Lille University, Lille, France, Pr Duhamel) for the statistical analysis.
Footnotes
The authors (PM, RP, FF, MF) certify that they have received payments of royalties and benefits, an amount in excess of $10,000 from Serf, Decines, France (RP, FF, MF), Ceramconcept, Paris, France (PM, FF), and Wright Medical EMEA, Amstelveen, the Netherlands (PM), all companies related to this work.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Adam P, Farizon F, Fessy MH. Dual articulation retentive acetabular liners and wear: surface analysis of 40 retrieved polyethylene implants][in French. Rev Chir Orthop Reparatrice Appar Mot. 2005;91:627–636. doi: 10.1016/S0035-1040(05)84466-6. [DOI] [PubMed] [Google Scholar]
- 2.Bland JM, Altman DG. Survival probabilities (the Kaplan-Meier method) BMJ. 1998;317:1572. doi: 10.1136/bmj.317.7172.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohm P, Bosche R. Survival analysis of the Harris-Galante I acetabular cup. J Bone Joint Surg Br. 1998;80:396–403. doi: 10.1302/0301-620X.80B3.8349. [DOI] [PubMed] [Google Scholar]
- 4.Bojescul JA, Xenos JS, Callaghan JJ, Savory CG. Results of porous-coated anatomic total hip arthroplasty without cement at fifteen years: a concise follow-up of a previous report. J Bone Joint Surg Am. 2003;85:1079–1083. doi: 10.2106/00004623-200306000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Charnley J. The long-term results of low-friction arthroplasty of the hip performed as a primary intervention. J Bone Joint Surg Br. 1972;54:61–76. [PubMed] [Google Scholar]
- 6.Cotogno G, Holzwarth U, Franchi M, Rivetti S, Chiesa R. Tribological characterization of surface-treated commercially pure titanium for femoral heads in total hip replacement: a feasibility study. Int J Artif Organs. 2006;29:1174–1184. doi: 10.1177/039139880602901211. [DOI] [PubMed] [Google Scholar]
- 7.D’Lima DD, Yashar AA, Venn-Watson EJ, Colwell CW, Jr, Walker RH. The Harris-Galante Porous acetabular component at intermediate follow-up. Orthopedics. 2001;24:747–751. doi: 10.3928/0147-7447-20010801-16. [DOI] [PubMed] [Google Scholar]
- 8.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed] [Google Scholar]
- 9.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dripps RD, Lamont A, Eckenhoff JE. The role of anesthesia in surgical mortality. JAMA. 1961;178:261–266. doi: 10.1001/jama.1961.03040420001001. [DOI] [PubMed] [Google Scholar]
- 11.Engh CA, Hopper RH, Jr, Engh CA., Jr Long-term porous-coated cup survivorship using spikes, screws, and press-fitting for initial fixation. J Arthroplasty. 2004;19(72):54–60. doi: 10.1016/j.arth.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Farizon F, Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility: a twelve-year follow-up study. Int Orthop. 1998;22:219–224. doi: 10.1007/s002640050246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garavaglia G, Lubbeke A, Barea C, Roussos C, Peter R, Hoffmeyer P. Ten-year results with the Morscher press-fit cup: an uncemented, non-modular, porous-coated cup inserted without screws. Int Orthop. 2011;35:957–963. doi: 10.1007/s00264-010-1059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyen O, Pibarot V, Vaz G, Chevillotte C, Bejui-Hugues J. Use of a dual mobility socket to manage total hip arthroplasty instability. Clin Orthop Relat Res. 2009;467:465–472. doi: 10.1007/s11999-008-0476-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SY, Kim DH, Kim YG, Oh CW, Ihn JC. Early failure of hemispheric hydroxyapatite-coated acetabular cups. Clin Orthop Relat Res. 2006;446:233–238. doi: 10.1097/01.blo.0000195923.28480.d4. [DOI] [PubMed] [Google Scholar]
- 16.Lai KA, Shen WJ, Chen CH, Yang CY, Hu WP, Chang GL. Failure of hydroxyapatite-coated acetabular cups: ten-year follow-up of 85 Landos Atoll arthroplasties. J Bone Joint Surg Br. 2002;84:641–646. doi: 10.1302/0301-620X.84B5.12384. [DOI] [PubMed] [Google Scholar]
- 17.Lautridou C, Lebel B, Burdin G, Vielpeau C. Survival of the cementless Bousquet dual mobility cup: minimum 15-year follow-up of 437 total hip arthroplasties][in French. Rev Chir Orthop Reparatrice Appar Mot. 2008;94:731–739. doi: 10.1016/j.rco.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Leclercq S, Benoit JY, Rosa JP, Euvrard P, Leteurtre C, Girardin P. Results of the Evora dual mobility socket: five years follow-up][in French. Rev Chir Orthop Reparatrice Appar Mot. 2008;94:37–42. doi: 10.1016/j.rco.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Malchau H, Karrholm J, Wang YX, Herberts P. Accuracy of migration analysis in hip arthroplasty: digitized and conventional radiography, compared to radiostereometry in 51 patients. Acta Orthop Scand. 1995;66:418–424. doi: 10.3109/17453679508995578. [DOI] [PubMed] [Google Scholar]
- 20.Malchau H, Wang YX, Karrholm J, Herberts P. Scandinavian multicenter porous coated anatomic total hip arthroplasty study: clinical and radiographic results with 7- to 10-year follow-up evaluation. J Arthroplasty. 1997;12:133–148. doi: 10.1016/S0883-5403(97)90059-0. [DOI] [PubMed] [Google Scholar]
- 21.Manley MT, Capello WN, D’Antonio JA, Edidin AA, Geesink RG. Fixation of acetabular cups without cement in total hip arthroplasty: a comparison of three different implant surfaces at a minimum duration of follow-up of five years. J Bone Joint Surg Am. 1998;80:1175–1185. doi: 10.2106/00004623-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 23.Massin P, Schmidt L, Engh CA. Evaluation of cementless acetabular component migration: an experimental study. J Arthroplasty. 1989;4:245–251. doi: 10.1016/S0883-5403(89)80020-8. [DOI] [PubMed] [Google Scholar]
- 24.Miyakawa S, Kawamura H, Mishima H, Yasumoto J. Grit-blasted and hydroxyapatite-coated total hip arthroplasty: an 11- to 14-year follow-up study. J Orthop Sci. 2004;9:462–467. doi: 10.1007/s00776-004-0806-3. [DOI] [PubMed] [Google Scholar]
- 25.Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG. Design and analysis of randomized clinical trials requiring prolonged observation of each patient: II. analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Philippot R, Camilleri JP, Boyer B, Adam P, Farizon F. The use of a dual-articulation acetabular cup system to prevent dislocation after primary total hip arthroplasty: analysis of 384 cases at a mean follow-up of 15 years. Int Orthop. 2009;33:927–932. doi: 10.1007/s00264-008-0589-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Philippot R, Farizon F, Camilleri JP, Boyer B, Derhi G, Bonnan J, Fessy MH, Lecuire F. Survival of dual mobility socket with a mean 17 years follow-up][in French. Rev Chir Orthop Reparatrice Appar Mot. 2008;94:43–48. doi: 10.1016/j.rco.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Reikeras O, Gunderson RB. Failure of HA coating on a gritblasted acetabular cup: 155 patients followed for 7–10 years. Acta Orthop Scand. 2002;73:104–108. doi: 10.1080/000164702317281503. [DOI] [PubMed] [Google Scholar]
- 29.Vicente JR, Ulhoa CA, Katz M, Addeo RD, Croci AT. A comparative study of “plasmacup” and “porous-coated” acetabular components: survival after 10 to 12 years of follow-up. Clinics (Sao Paulo) 2010;65:1111–1114. doi: 10.1590/S1807-59322010001100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vielpeau C, Lebel B, Ardouin L, Burdin G, Lautridou C. The dual mobility socket concept: experience with 668 cases. Int Orthop. 2011;35:225–230. doi: 10.1007/s00264-010-1156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]