Abstract
Background
Pseudotumors are sterile inflammatory lesions found in the soft tissues surrounding metal-on-metal (MOM) and metal-on-polyethylene hip arthroplasties. In patients with MOM hip arthroplasties, pseudotumors are thought to represent an adverse reaction to metal wear debris. However, the pathogenesis of these lesions remains unclear. Currently, there is inconsistent evidence regarding the influence of adverse cup position and increased wear in the formation of pseudotumors.
Questions/purposes
We therefore determined whether pseudotumor formation was associated with (1) adverse cup position, (2) raised metal ion levels, and (3) increased wear rates of the retrieved components.
Methods
We retrospectively reviewed all 352 patients for whom we had retrieved specimens from revisions of a current-generation MOM hip prosthesis between February 2008 and September 2010; of these, 105 met our inclusion criteria. We used multivariate logistic regression analysis to compare acetabular orientation, metal ion levels before revision, and component wear rates between patients with (n = 72) and without (n = 33) pseudotumors, according to findings on metal artifact reduction sequence MRI.
Results
The proportion of patients demonstrating evidence of a pseudotumor in well-positioned hips was similar to those with adverse cup positions (67% and 66%, respectively). Patients revised with pseudotumors had similar whole-blood metal ion levels and component wear rates to those who were not revised.
Conclusions
Pseudotumors were not associated with increased wear or metal ion levels, suggesting patient susceptibility is likely to be more important.
Introduction
Data from the seventh annual report of the National Joint Registry for England and Wales has shown high failure rates for current-generation metal-on-metal (MOM) hip prostheses [34], which has led to the market recall of a leading current-generation design, the DePuy ASR™ (both the Resurfacing and XL Systems) (DePuy International Ltd, Leeds, UK) [31].
The Medicines and Healthcare products Regulatory Agency has suggested follow up of patients with MOM hips, to include cross-sectional imaging (metal artifact reduction sequence [MARS]-MRI or ultrasound) for the detection of pseudotumors [5, 35, 37, 39]. Pseudotumors are sterile inflammatory masses in the soft tissues surrounding MOM [35, 37] and metal-on-polyethylene (MOP) [1, 19] hip prostheses. In patients with a MOM hip prosthesis, these are thought to be the result of an adverse response to metal wear debris and elevated chromium and cobalt levels [15, 35]. Previous studies had only described these masses in symptomatic patients [15, 25, 26], but a recent study showed during routine followup they were observed in 6.5% of patients with asymptomatic, well-functioning, well-positioned prostheses [23].
Retrieval studies of current-generation MOM hips have suggested edge loading, resulting from adverse cup orientation, leads to a higher wear of the components and raised blood metal ion levels [7, 27, 30, 32]. In vivo studies have suggested disparities in certain design features, such as the clearance [41] and arc of cover [27], are important factors affecting component wear rates and the risk of edge loading. Several studies reported an association between elevated blood metal ion levels and pseudotumors [15, 25, 35], but only two studies associated them directly with high component wear rates [22, 25]. The largest published studies of wear [22] and metal ion levels [26] in patients with adverse soft tissue reactions, or pseudotumors, concerned only 30 and 37 patients, respectively.
Given that the pathogenesis of pseudotumors remains unclear and that several recent studies have shown cases in well-functioning, well-positioned, and low-wearing prostheses [8, 23], it is important to confirm the roles of component position and wear in larger series of patients.
We therefore determined whether adverse cup position is associated with (1) increased incidence of pseudotumor, (2) raised blood metal ion levels, and (3) increased component wear rates.
Patients and Methods
We retrospectively reviewed all 352 patients who underwent revision for a failed MOM hip implant and from whom we collected explanted components between February 2008 and September 2010. The retrieved hips came from 93 surgeons in 56 hospitals throughout the United Kingdom and represented the market distribution of MOM hip implants in the United Kingdom [34]. To be included, we required that patients had full preoperative assessment, including MARS-MRI to visualize the periprosthetic soft tissues, CT scan to measure cup orientation, and dynamic reaction cell inductively coupled mass spectrometry (DRC-ICPMS) to measure whole-blood metal ion levels. We included hips of several current-generation designs, all with monoblock cups and a large internal diameter (> 38 mm) suitable for either resurfacing or large-diameter MOM THA. For the purposes of wear analysis, we required that all hips were implanted for a minimum of 12 months to minimize the confounding effect of the bedding-in process [18]. All patients revised with a diagnosis of infection were excluded to ensure all pseudotumors were sterile inflammatory masses. We excluded 247 revisions of MOM hips that did not meet these inclusion criteria, leaving 105 patients whose implants were sent to our retrieval laboratory. Of these patients, 29 were men and 76 were women, with a median age of 56 years (range, 22–83 years) at the time of primary surgery. The median time to revision was 39 months (range, 12–95 months). Of the 105 patients, 69 were hip resurfacings and 36 were large-diameter THA. The local ethical committee approved this study and all patients consented to their inclusion. The laboratory operated with approval of the Human Tissue Authority and did not have a contract with any implant manufacturer that prevented publication of any data.
There were six patients with Adept® implants (Finsbury Orthopaedics Ltd, Leatherhead, UK), 19 with ASR™ implants (DePuy, Leeds, UK), 51 with BHR™ implants (Smith &Nephew UK Ltd, Warwick, UK), 15 with Cormet™ (Corin Group PLC, Cirencester, UK), seven with Durom® implants (Zimmer GmbH, Winterthur, Switzerland), and seven with M2a-Magnum™ implants (Biomet UK Ltd, Bridgend, South Wales, UK). The reason for revision was determined according to the categories used by the National Joint Registry of England and Wales [34] (Table 1). The reasons for revision were component mismatch (n = 2), fracture (n = 3), aseptic acetabular loosening (n = 9), aseptic femoral loosening (n = 6), malalignment (n = 2), and unexplained pain (n = 76).
Table 1.
Criteria used for diagnosing the reason for revision.
| Diagnosis | Diagnostic criteria |
|---|---|
| Unexplained (+/− pain) | Absence of: |
| • Intraoperative loosening of components | |
| • Infection (see below) | |
| • Gross malalignment (see below) | |
| • Component size mismatch | |
| • Fracture (on imaging/seen intraoperatively) | |
| Acetabular loosening | Intraoperative diagnosis (preoperative imaging has a high false negative rate) |
| Femoral loosening | Intraoperative diagnosis (preoperative imaging has a high false negative rate) |
| Infection | Positive if: |
| • Postoperative cultures positive for infection | |
| Negative if: | |
| • Preoperative CRP less than 10 mg/L or | |
| • Preoperative CRP greater than 10 mg/L, but postoperative culture was negative for infection | |
| Dislocation | Patient reported (+/- radiographic evidence) |
| Periprosthetic fracture | Radiographic evidence |
| Malalignment | Imaging (CT or x-ray) shows: |
| • Cup inclination greater than 70° | |
| • Cup version associated with impingement | |
| Component mismatch | Postoperative assessment of components |
We assigned patients to one of two groups according to findings on MARS-MRI (Table 2). The first group of patients consisted of those who had no apparent soft tissue abnormalities, and the second group consisted of patients with a pseudotumor. We compared clinical and component variables between the two groups. This included cup orientation, metal ion levels, and component wear rates.
Table 2.
Patient demographics and relevant clinical data*
| Demographics and clinical data | No pseudotumor | Pseudotumor |
|---|---|---|
| Number of patients | 33 | 72 |
| Number of components | 66 | 144 |
| Age (range) (years) | 51 (22–68) | 58 (33–83) |
| Gender (men/women) | 8/25 | 21/51 |
| Time to revision (range) (months) | 37 (12–69) | 40 (13–95) |
| Femoral diameter (range) (mm) | 46 (40–54) | 46 (38–58) |
| Prosthesis type (resurfacing/THA) | 21/12 | 48/24 |
| Implant type | ||
| Adept (Finsbury Orthopaedics) | 1 | 5 |
| ASR (DePuy) | 7 | 12 |
| BHR (Smith & Nephew) | 13 | 38 |
| Cormet (Corin Group Plc.) | 5 | 10 |
| Durom (Zimmer) | 5 | 2 |
| M2a-Magnum (Biomet) | 2 | 5 |
| Acetabular inclination (range) (°) | 44° (27º–63º) | 50° (33°–78°) |
| Acetabular version (range) (°) | 17° (−34° to 42°) | 19° (−14° to 45°) |
| Reason for Revision§ | ||
| Component mismatch | 0 | 2 |
| Fracture | 2 | 1 |
| Infection | 0 | 0 |
| Loosening (acetabular) | 5 | 4 |
| Loosening (femoral) | 2 | 4 |
| Malalignment | 1 | 1 |
| Unexplained pain | 23 | 53 |
| Whole blood cobalt (range) (μg/L) | 2.9 (0.5–162.3) | 11.0 (1.0–386.5) |
| Whole blood chromium (range) (μg/L) | 3.2 (0.4–50.0) | 6.7 (0.8–179.0) |
| Cup wear rate (range) (μm/year) | 2.2 (0.0–64.3) | 6.8 (0.0–180.0) |
| Head wear rate (range) (μm/year) | 2.0 (0.0–62.1) | 5.3 (0.0–84.1) |
§Seven patients in the pseudotumor group had insufficient data to diagnose the reason for revision.
* The clinical cause of failure was diagnosed according to the UK National Joint Registry classification.
Before undertaking the study, we performed a power analysis to determine the sample size needed to detect a significant difference in component wear rates between the two groups. Due to differences in methods and reporting of wear rates in the literature, we based our calculation on our own previous experience of wear measurement [14, 30]. Using Altman’s nomogram, we determined the minimum number of components required in each group to show a 5-μm/year difference in wear rate. A difference of 5 μm was chosen as this represents a twofold increase in the predicted average steady-state wear rate of MOM hips [9]. The sample size required for a power of 90% and a statistical significance of 0.05 was 54 patients (27 patients in each group). We also performed a two-proportion power analysis to determine the sample size needed to determine a difference in the prevalence of pseudotumors in well- and poor-positioned hips. Significance was set at a p value of 0.05 and power at 90%. Based on previous literature [23, 37], we estimated the prevalence of pseudotumors to be 60% in failed, adversely positioned MOM hips and 4% in well-positioned MOM hips, similar to that in well-functioning patients [23]. This resulted in a minimum sample size of 26 patients (13 patients in each group).
All 105 patients underwent prospective MARS-MRI of the hip using a 1.5-T scanner (MAGNETOM 1.5T; Siemens Medical, Erlangen, Germany) according to a previously described protocol [15]. Two experienced musculoskeletal radiologists (AWM, KS), blinded to all other clinical data and wear analysis, interpreted the images by consensus agreement. They recorded the presence or absence of a pseudotumor and, if present, characterized the lesion (Table 3), noting the wall, shape, and contents. The wall was classified according to its thickness, with a cutoff of 2 mm. The lesion’s shape rather than its size was used because this overcame the difficulty of quantifying a complicated three-dimensional shape using two-dimensional, cross-sectional imaging. The contents of soft tissue lesions were characterized according to their signal intensity on T1- and T2-weighted images. We noticed three patterns, which were likely to represent fluid, proteinaceous, or solid contents. Solid masses were classified separately.
Table 3.
Method of classification of pseudotumours found on MARS-MRI
| Pseudotumour category | Wall | Contents | Shape |
|---|---|---|---|
| Class 1 | Thin walled | Fluid like: | Flat with walls mainly in apposition |
| T1 - hypointense | |||
| T2 - hyperintense | |||
| Class 2A | Thick walled or irregular | Fluid like: | Not flat and more than 50% of the walls are not in apposition |
| T1 - hypointense | |||
| T2 - hyperintense | |||
| Class 2B | Thick walled or irregular | Atypical fluid: | Any size |
| T1 - hyperintense | |||
| T2 - variable | |||
| Class 3 | Solid | Mixed signal | Any size |
MARS = metal artifact reduction sequence.
As a part of the clinical care, all patients underwent low-radiation dose CT scanning with 0.75-mm collimation and artifact minimization, both of which enabled observation of the detail required to separate the metallic cup face from the large-diameter metallic femoral head. We reconstructed DICOM images using a three-dimensional reconstruction software package (Robin’s 3D Image Rendering Software; Robin Richards, Medical Physics, UCL, London, UK). Two of us (JH, AH) aligned the pelvis with the anterior pelvic plane and measured the angles of acetabular inclination and version using a previously published method [14–16]. By aligning the pelvis with the anterior pelvic plane, we measured acetabular orientation against a standard frame of reference, and factors such as pelvic tilt, which affects the position of the pelvis, did not affect our measurements. Supine CT scans were taken; however, we accepted this method was limited in assessing functional cup position in the standing position. Cup angles are reported using the radiographic definition [33]. There was no standard definition of an acceptable cup position for MOM THA, so we used the definition described by Lewinnek et al. [28] but included the spread of our results so that the readers could interpret cup position using their own criteria.
Whole-blood samples were collected in trace-element blood tubes (K2EDTA; Becton, Dickinson and Co, Franklin Lakes, NJ, USA) using the Vacutainer® system (Becton, Dickinson and Co). These trace element tubes were certified low for metals and did not cause metal contamination. We measured cobalt and chromium ion levels according to a previously described protocol [15] using DCR-ICPMS (Elan® DRCII; PerkinElmer Inc, Waltham, MA, USA). We analyzed all samples before revision of the MOM hip implant. There was no standard definition of an acceptable metal ion level after MOM THA. We referred to the 7-μg/L definition given by the Medicines and Healthcare products Regulatory Agency but included the spread of our data so that the readers could interpret the metal ion levels using their own criteria.
We measured linear wear of all explanted acetabular and femoral components using a roundness-measuring machine (Talyrond 265; Taylor Hobson Ltd, Leicester, UK). We mounted and rotated the components on a spindle (spindle accuracy, ± 0.02 μm), while a stylus (2-mm-diameter ruby), in contact with the surface of the component, measured deviations from a perfect circle (resolution of stylus gauge, 10 nm). Using a previously described method [30], we took three sets of measurements for each hip. For the cup components, we measured the out-of-roundness along lines of latitude and longitude. We took latitudinal measurements at 1-mm increments, descending the cup from the rim, and took longitudinal measurements at 15° increments. For the head components, we measured out-of-roundness along the lines of longitude at increments of 15°. We analyzed raw data using the Ultra software package (Taylor Hobson Ltd). We calculated linear wear rates from the time to revision and defined edge wear as when the maximum wear depth occurred at the rim of the cup.
The recommended manufacturing tolerance for roundness of current-generation MOM hip components is only less than 10 μm. Therefore, it was essential that wear be distinguished from the manufactured shape or form. Form errors referred to a generalized uniform deviation from the nominal spherical shape of the component, whereas wear was the loss or transfer of material from the bearing surface manifesting as localized (nonuniform) deviation from round. By superimposing all of the measured roundness profiles for a given component, it was possible to estimate the unworn (manufactured) shape of the component and distinguish this from wear. We wrote a custom program in MATLAB® (MathWorks Inc, Natick, MA, USA) to superimpose all of the roundness measurements for a given component. From the superimposed profiles, it was possible to identify uniform deviation from round on all measurement profiles and estimate the unworn (as manufactured) shape of the component. Additionally, it was possible to identify out-of-roundness extending across only few measurement profiles, these representing localized areas of component wear. The linear wear depth was then measured by subtracting any measureable form error from those roundness deviations identified as wear. In this way, our method accounted for manufacturing tolerances.
We assessed the normality of component wear rates and whole-blood metal ion levels before revision using the Shapiro-Wilk test and found they were not normally distributed. A multivariate logistic regression analysis was used to test for group differences in metal ion levels, component wear rates, and cup position, adjusting the odds ratio for sex, type of prosthesis (resurfacing or THA), femoral diameter, time to revision, and manufacturer (variables associated with either the development of a pseudotumor [11] or to influence the rate of revision [34]). Additionally, we included cup position in our model when comparing the groups for metal ion levels and component wear rates. For all statistical analyses, tests were two-sided, and all analyses were carried out using the SAS® 9.1.3 software package (SAS® Institute Inc, Cary, NC, USA).
Results
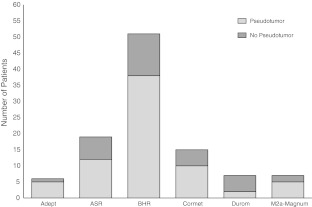
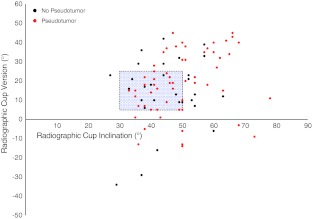
Seventy-two of 105 (69%) patients had evidence of a pseudotumor on MARS-MRI before revision. According to our MARS-MRI classification of pseudotumors, 13 patients were classified as Type 1 (Fig. 1), 42 patients as Type 2A (Fig. 2), 15 patients as Type 2B (Fig. 3), and 2 patients as Type 3 (Fig. 4). Patients with all brands of MOM hip prostheses demonstrated evidence of a pseudotumor on MARS-MRI (Fig. 5). Pseudotumors were not associated with adverse cup position. A similar proportion of patients with and without pseudotumors had acceptable cup position, with an adjusted odds ratio of 0.81 (95% CI, 0.3–2.6; p = 0.73). The proportion of patients with and without pseudotumors with acceptable cup inclination and version angles was similar, with odds ratios of 0.72 (95% CI, 0.2–2.7; p = 0.40) and 0.90 (95% CI, 0.3–2.4; p = 0.75), respectively. Overall, thirty-seven percent of patients had an acceptable cup position. Of these, 67% had a pseudotumor. In the remaining patients with an adverse cup position, 66% had a pseudotumor. There was a wide range of cup positions (Fig. 6).
Fig. 1.
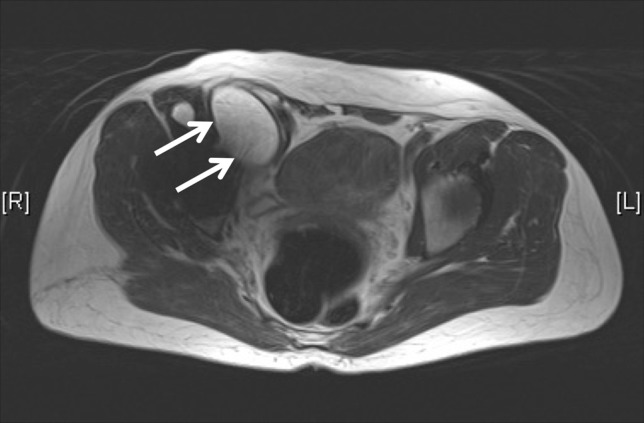
An axial MARS MR image shows a Type 1 pseudotumor (arrows).
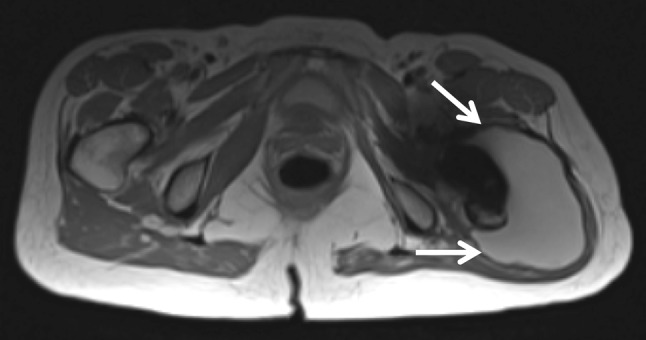
Fig. 2.
An axial MARS MR image shows a Type 2A pseudotumor (arrows).
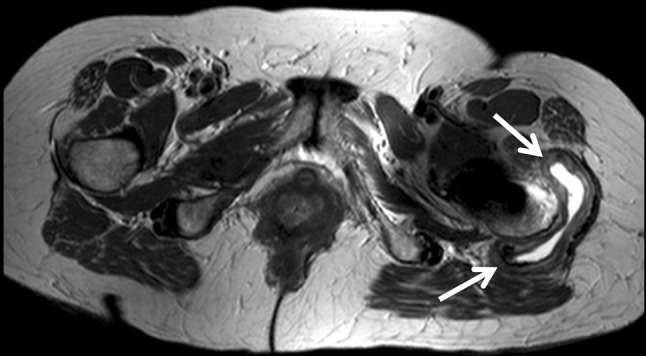
Fig. 3.
An axial MARS MR image shows a Type 2B pseudotumor (arrows).
Fig. 4.
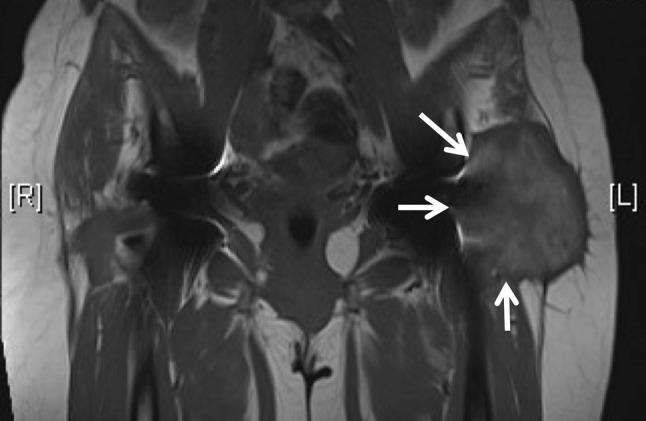
An coronal MARS MR image shows a Type 3 pseudotumor (arrows).
Fig. 5.
This graph shows the distribution of pseudotumor and nonpseudotumor patients according to the prosthesis type (manufacturer).
Fig. 6.
This graph shows the acetabular orientation of each hip, as measured from CT scan. Hips in red indicate those associated with a pseudotumor. The blue box demonstrates the “safe zone” for cup position as described by Lewinnek et al. [28].
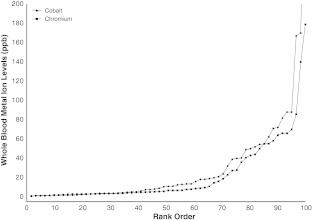
When compared to patients without pseudotumors, patients revised with pseudotumors had similar cobalt and chromium levels before revision, with adjusted odds ratios of 0.96 (95% CI, 0.9–1.0; p = 0.13) and 1.04 (95% CI, 0.9–1.1; p = 0.40), respectively. More than 40% of patients had both cobalt and chromium levels of less than 7 μg/L (Fig. 7).
Fig. 7.
This graph shows the distribution of patients (rank order, %) revised with pseudotumors according to blood cobalt and chromium levels.
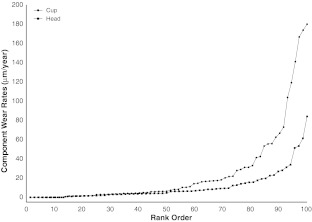
When compared to the retrieved components of patients without pseudotumors, those with pseudotumors had similar acetabular cup and femoral head wear rates, with adjusted odds ratios of 1.1 (95% CI, 0.9–1.2; p = 0.08) and 1.0 (95% CI, 0.9–1.1; p = 0.41), respectively. We recorded the spread of component wear rates from patients revised with pseudotumors (Fig. 8). More than 40% of retrieved components were wearing less than 5 μm/year.
Fig. 8.
This graph shows the distribution of patients (rank order, %) revised with pseudotumors according to component wear rates.
For all other variables included in the multivariate logistic regression, the only group difference was time to revision. When compared to patients without pseudotumors, those with pseudotumors underwent revision after a longer interval (p = 0.02) from primary arthroplasty, with an adjusted odds ratio of 1.0 (95% CI, 0.9–1.3). The groups had similar sex, prosthesis type (resurfacing/THA), manufacturer ratios, and femoral diameter.
Discussion
Numerous have reported pseudotumors in patients with current-generation MOM hip implants [11, 12, 15, 17, 22, 23, 35–37]. Pseudotumors have been found in patients with hip resurfacing and large-diameter THAs [37] and were associated with a poor outcome after revision surgery [12]. Some authors suggested that pseudotumors occurred as a result of an adverse reaction to metal wear debris and raised metal ion levels [15, 17, 35, 36], with several small studies finding a direct association with increased component wear rates [22, 25] and raised levels of chromium and cobalt ions [15, 35]. However, recent evidence suggests pseudotumors are common in patients with asymptomatic MOM THA [23] and in patients with low metal ion levels and a well-positioned cup [8]. The roles of excess wear and patient-specific sensitivity to metal wear debris in the pathogenesis of pseudotumors remains unclear. Therefore, we determined whether pseudotumors are associated with (1) adverse cup position, (2) raised metal ion levels, and (3) increased component wear rates.
There were several limitations to our study. First was the relatively low number of patients without pseudotumors (n = 33); however, this was sufficient to provide suitable power based on our sample size calculation. The trend towards raised metal ion levels and increased component wear being associated with pseudotumor formation should encourage further work on a larger number of patients. Thus, our findings should be interpreted with caution. Second, we used a radiographic definition for pseudotumor that included both fluid-filled (cystic) and solid lesions [11]. We did not investigate the immunologic or histologic changes in the tissues of our patients. Studies have used several radiographic, operative, and histopathologic terms to describe the sterile inflammatory reactions reported in patients with MOM and MOP hip implants. The radiographic terms include pseudotumor [35], fluid collection [38], inflammatory bursa [35], inflammatory lesion [15, 37], and effusion [2]. Studies described operative appearances as metallosis [6], fluid collection [38], and solid mass [35]. Histopathologic terms include aseptic lymphocytic vasculitis-associated lesion [3, 42], lymphocyte-dominant immune reaction [42], and adverse reaction to metal wear debris [25]. The variation in terminology made it difficult to correlate our findings, particularly with other studies that used histologic, immunologic, or operative definitions for soft tissue reactions. Future work should focus on a consensus definition of soft tissue reactions to MOM hips. Third, although the distribution of resurfacings and THAs was similar between the study groups, we did not investigate the influence of wear and corrosion occurring at the modular junctions of MOM THA. This likely had some effect on the accumulation of metal wear debris, although we have shown, in a previous study, metal ion levels were similar before revision of these two types of hip prostheses [30]. Finally, patients in this retrieval study may not have represented the general population of patients with MOM hip prostheses. Therefore, we compared our patient population with the largest published single-center series of patients with current-generation MOM hip prostheses [4, 20, 29, 40, 43] (Table 4). We also compared the outcome variables of this study with all of the published retrieval studies of current-generation MOM hip implants [10, 14, 22, 25, 30, 32, 41] (Table 5).
Table 4.
Comparison of retrieval studies concerning current-generation large-diameter metal-on-metal hip arthroplasties
| Study | Number of patients in study | Number of retrieved components | Implant type | Retrievals with pseudotumor (number) | Cup inclination (°) | Cup version (°) | Cobalt (μg/L) | Chromium (μg/L) | Head wear rate (μm/year) | Cup wear rate (μm/year) |
|---|---|---|---|---|---|---|---|---|---|---|
| Ebramzadeh et al. [10] | 433 (wear measured in 306) | ASR™ | 55 | Pseudotumor:52 | Pseudotumor:23.8 | NA | NA | Pseudotumor:8.00 | Pseudotumor:10.02 | |
| BHR™ | ||||||||||
| Conserve® Plus | No Pseudotumor:44.1 | No Pseudotumor:18.9 | No pseudotumor:5.25 | No pseudotumor:4.15 | ||||||
| Cormet™ 2000 | ||||||||||
| Durom® | ||||||||||
| McMinn | ||||||||||
| Metasul® | ||||||||||
| ReCap® | ||||||||||
| Wagner | ||||||||||
| Wright Medical | ||||||||||
| Hart et al. [14] | 45 | 90 | ASR™ | NA | 50.1 (43.3–58.6) | 24.5 (10–36) | NA | NA | 5.6 (3.2–27.2) | 2.7 (0.0–8.4) |
| BHR™ | ||||||||||
| Cormet™ 2000 | ||||||||||
| Durom® | ||||||||||
| M2a-Magnum™ | ||||||||||
| Kwon et al. [22] | 30 | 30 | BHR™ | 9 | NA | NA | NA | NA | Pseudotumor:8.1 (2.8–25.4) | Pseudotumor:7.4 (1.6–24.9) |
| Conserve® Plus | ||||||||||
| Cormet™ 2000 | ||||||||||
| Control:1.8 (0.8–4.2) | Control:1.3 (0.2–3.3) | |||||||||
| Langton et al. [25] | 660 | 15 | ASR™ | 8 (with diagnosis of ARMD) | ASR™:48.5 (31–70) | ASR™:20.4 (3–39) | ASR™:2.7 (0.4–271.0) | ASR™:4.2 (1.5–69.8) | 11.8 (1.4–137.7) | 12.8 (5.9–96.0) |
| BHR™ | ||||||||||
| BHR™:48.3 (32–70) | BHR™:19.9 (−5–39) | BHR™:1.8 (0.6–147.0) | BHR™:4.2 (2.4–40.0) | |||||||
| Matthies et al. [30] | 120 | 240 | Adept® | NA | MOM THA:50 (24–65) Resurfacing:49 (30–70) |
MOM THA:12º (−29–37.5) Resurfacing:22 (−47–48) |
MOM THA:9.6 (0.5–91.0) Resurfacing:11.1 (0.9–167.0) |
MOM THA:4.2 (0.1–58.2) Resurfacing:6.5 (0.4–183.0) |
MOM THA:2.7 (0.0–51.3) Resurfacing:3.5 (0.0–84.7) |
MOM THA:3.9 (0.0–119.2) Resurfacing:4.7 (0.0–173.8) |
| ASR™ | ||||||||||
| BHR™ | ||||||||||
| Cormet™ | ||||||||||
| Durom® | ||||||||||
| M2a-Magnum™ | ||||||||||
| Morlock et al. [32] | 32 | 58 | Unknown(All MOM hip resurfacings) | NA | Nonedge worn:51 (42–62) | NA | NA | NA | Combined volumetric wear rate reported (mm3/day): | |
| Edge worn:58 (45–75) | Nonedge worn: 0.001 (0.00–0.01) | |||||||||
| Edge worn: 0.08 (0.01–0.19) | ||||||||||
| Underwood et al. [41] | 130 | 260 | ASR™ | NA | ASR™:51 (15–82) | ASR™:15 (−8–48) | ASR™:13.5 (0.5–167.0) | ASR™:9.8 (0.2–119.0) | ASR™:6.0 (0.0–84.7) | ASR™:9.2 (0.0–245.6) |
| BHR™ | ||||||||||
| BHR™:51 (24–73) | BHR™:23 (−47–50) | BHR™:10.2 (0.0–67.0) | BHR™:4.8 (0.4–183.0) | BHR™:3.5 (0.7–52.4) | BHR™:4.2 (0.0–153.8) | |||||
| Witzleb et al. [43] | 8 | 10 | BHR™ | NA | 49 (44º–70º) | NA | NA | NA | 7.3 (1.9–22.4) | 20.3 (9.2–31.5) |
| Current study | 105 | 210 | Adept® | 72 | Pseudotumor:50 (33–78) | Pseudotumor:19 (−14–45) | Pseudotumor:11.0 (1.0–386.5) | Pseudotumor:6.7 (0.8–179.0) | Pseudotumor:5.3 (0.0–84.1) | Pseudotumor:6.8 (0.0–180.0) |
| ASR™ | ||||||||||
| BHR™ | ||||||||||
| Cormet™ 2000 | Control:44 (27–63) | Control:17 (−34–42) | Control:2.9 (0.5–162.3) | Control:3.2 (0.4–50.0) | Control:2.0 (0.0–62.1) | Control:2.2 (0.0–64.3) | ||||
| Durom® | ||||||||||
| M2a-Magnum™ | ||||||||||
Values are expressed as median, with range in parentheses; ARMD = adverse reaction to metal wear debris; NA = not available.
Table 5.
Comparison of reported variables in single-center studies
| Study | Number of patients | Implant type | Patient age at primary surgery (years)* | Sex (male/female) | Femoral diameter (mm)* | Cup inclination (°)* | Cup version (°)* | Number of revisions (%) | Followup (years)* | Reasons for revision (% of total revisions) |
|---|---|---|---|---|---|---|---|---|---|---|
| Carrothers et al. [4] | 5000 | BHR™ | 52.5 (13–87) | 3346/1654 | NA | NA | NA | 182 (3.6%) | NA | Fracture: 29.6% |
| Cup loosening: 17.6% | ||||||||||
| AVN: 16.5% | ||||||||||
| Femoral loosening: 10.4% | ||||||||||
| Infection: 9.3% | ||||||||||
| Unexplained pain: 8.2% | ||||||||||
| Combined loosening: 2.7% | ||||||||||
| Dislocation: 2.7% | ||||||||||
| Malalignment: 1.6% | ||||||||||
| Unknown: 1.4% | ||||||||||
| Jameson et al. [20] | 192 | ASR™ | 56 (28–74) | 107/85 | 49 (41–59) | 49 (31–67) | 20 (3–29) | 12 (5.4%) | 3.6 (2.5–4.8) | Unexplained pain: 42% |
| Fracture: 42% | ||||||||||
| AVN: 16% | ||||||||||
| Long et al. [29] | 180 | Durom® | Male patients: 55.8 | 61/119 | (44–50) | 41.3 | 20.2 | 30 (16%) | 1.6 (1.0–2.0) | Loosening: 72% |
| Female patients: 63.4 | Unreported: 28% | |||||||||
| Treacy et al. [40] | 144 | BHR™ | 52 (17–76) | 107/37 | 50 (42–58) | 49 | NA | 10 (6.9%) | 10.8 (10.2–12.2) | Infection: 30% |
| AVN: 30% | ||||||||||
| Fracture: 20% | ||||||||||
| Dislocation: 10% | ||||||||||
| Other: 10% | ||||||||||
| Current study | 105 | Adept® | 56 (22–83) | 29/76 | 46 (38–58) | 48 (2778) | 18 (−34–45) | NA | NA | Unexplained pain: 72% |
| ASR™ | Cup loosening: 8.5% | |||||||||
| BHR™ | Unknown: 6.6% | |||||||||
| Cormet™ | Femoral loosening: 5.7% Fracture: 2.8% | |||||||||
| Durom® | Component mismatch: 1.9% | |||||||||
| M2a-Magnum™ | Malalignment: 1.9% |
* Values are expressed as mean, with range in parentheses, except for the current study, in which the values were expressed as median, with range in parentheses; AVN = avascular necrosis; NA = not available.
We found 72 of 105 patients (69%) had evidence of a pseudotumor before revision of their hip arthroplasty for any cause. The majority of these reactions were cystic with only 2 patients demonstrating evidence of a solid mass. We observed pseudotumors in patients with hip resurfacing and large-diameter THAs and in patients with each of the six current-generation designs in this study. There are currently no other studies regarding the incidence of pseudotumors in patients with MOM hip prostheses revised for any reason. However, our results were in keeping with previous work concerning patients with symptomatic MOM hip implants, in which the authors estimate the incidence at almost 65%, with cystic lesions most commonly found [37].
Our study of retrieved MOM THA implants revealed pseudotumors occurred as frequently in patients with well-positioned cups as in those with a cup position outside of the safe zone described by Lewinnek et al. [28]. This was unexpected given that the literature suggests that steep cup inclination is associated with increased release of metal wear debris [7, 27, 30, 31] and increased wear is associated with pseudotumor formation [15, 22]. However, there is some recent evidence of high rates of devastating soft tissue reactions in patients with well-positioned MOM hip prostheses [8], which supports our findings. Importantly, our results were unlikely due to the low validity of radiographic measurement of cup position [13, 21] because we used three-dimensional CT to measure cup position in all cases. Future work may focus on other surgical positioning factors, such as horizontal femoral offset, femoral version, and leg length discrepancy.
We saw no significant association between the presence of a pseudotumor at revision and either increased metal ion levels or component wear rates. Furthermore, almost ½ of the pseudotumor cases demonstrated acceptable metal ion levels (< 7 μg/L) and component wear rates (< 5 μm/year). Again, this was an unexpected finding and contradicted the currently accepted theory proposed by Kwon et al. [23] and Langton et al. [25, 26]: pseudotumors occur as a result of a reaction to increased metal wear debris. These studies were of a maximum of 37 patients with soft tissue reactions and were likely underpowered. The study by Kwon et al. [23] contained no data concerning acetabular orientation, and this may have been a confounder for higher wear rates in the eight pseudotumor patients. The studies by Langton et al. [25, 26] used an umbrella term, adverse reaction to metal wear debris, to describe a wide range of complications and not just sterile inflammatory masses. This may have also contributed to variations in the findings.
Pseudotumors were common in patients with well-positioned prostheses and were not necessarily associated with high wear or raised metal ion levels. The trend toward increased material loss in patients with pseudotumors suggests, in some cases, there may have been a dose-response inflammatory reaction, but in almost ½ of patients revised with a pseudotumor, low material loss suggests there may have been an underlying patient susceptibility to metal wear debris. Our observations suggest pseudotumors may occur in patients with any hip design, and although some designs, such as the ASR™, were more susceptible to high wear [40] and a dose-response reaction, susceptible patients may suffer a reaction irrespective of the type or design of prosthesis used. It is nonetheless important to emphasize patients with high-wearing hip designs, such as the ASR™ [25, 40], are likely to be at an increased risk of developing a soft tissue reaction. Although some studies have suggested metal hypersensitivity (Type 4) may be an important factor [3, 36, 42], there is contradictory evidence surrounding this hypothesis [24], and further characterization of this group of patients is needed to understand the pathogenesis of pseudotumors.
Acknowledgments
The authors thank Dr Adam W. Mitchell and Dr Keshthra Satchithananda for interpreting MR images, Mr Peter Wilkinson for his assistance with data analysis, and Gwynneth Lloyd for her coordination of the retrieval center.
Footnotes
One or more of the authors (AH, JS) received funding from the British Orthopaedic Association through an industry consortium of nine manufacturers: DePuy International Ltd (Leeds, UK), Zimmer GmbH (Winterthur, Switzerland), Smith & Nephew UK Ltd (Warwick, UK), Biomet UK Ltd (Bridgend, South Wales, UK), JRI Ltd (London, UK), Finsbury Orthopaedics Ltd (Leatherhead, UK), Corin Group PLC (Cirencester, UK), Mathys Orthopaedics Ltd (Alton, UK), and Stryker UK Ltd (Newbury, UK). No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Imperial College London, UK.
Contributor Information
Ashley K. Matthies, Email: ashley.matthies09@imperial.ac.uk.
Alister J. Hart, Email: a.hart@imperial.ac.uk.
References
- 1.Berquist TH, Bender CE, Maus TP, Ward EM, Rand JA. Pseudobursae: a useful finding in patients with painful hip arthroplasty. AJR Am J Roentgenol. 1987;148:103–106. doi: 10.2214/ajr.148.1.103. [DOI] [PubMed] [Google Scholar]
- 2.Bin Nasser A, Beaule PE, O’Neill M, Kim PR, Fazekas A. Incidence of groin pain after metal-on-metal hip resurfacing. Clin Orthop Relat Res. 2010;468:392–399. doi: 10.1007/s11999-009-1133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell P, Ebramzadeh E, Nelson S, Takamura K, Smet K, Amstutz HC. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468:2321–2327. doi: 10.1007/s11999-010-1372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrothers AD, Gilbert RE, Jaiswal A, Richardson JB. Birmingham hip resurfacing: the prevalence of failure. J Bone Joint Surg Br. 2010;92:1344–1350. doi: 10.1302/0301-620X.92B10.23504. [DOI] [PubMed] [Google Scholar]
- 5.Cooper HJ, Ranawat AS, Potter HG, Foo LF, Jawetz ST, Ranawat CS. Magnetic resonance imaging in the diagnosis and management of hip pain after total hip arthroplasty. J Arthroplasty. 2009;24:661–667. doi: 10.1016/j.arth.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 6.Haan R, Campbell PA, Su EP, Smet KA. Revision of metal-on-metal resurfacing arthroplasty of the hip: the influence of malpositioning of the components. J Bone Joint Surg Br. 2008;90:1158–1163. doi: 10.1302/0301-620X.90B9.19891. [DOI] [PubMed] [Google Scholar]
- 7.Haan R, Pattyn C, Gill HS, Murray DW, Campbell PA, Smet K. Correlation between inclination of the acetabular component and metal ion levels in metal-on-metal hip resurfacing replacement. J Bone Joint Surg Br. 2008;90:1291–1297. doi: 10.1302/0301-620X.90B10.20533. [DOI] [PubMed] [Google Scholar]
- 8.Donell ST, Darrah C, Nolan JF, Wimhurst J, Toms A, Barker TH, Case CP, Tucker JK, Norwich Metal-on-Metal Study Group Early failure of the Ultima metal-on-metal total hip replacement in the presence of normal plain radiographs. J Bone Joint Surg Br. 2010;92:1501–1508. doi: 10.1302/0301-620X.92B11.24504. [DOI] [PubMed] [Google Scholar]
- 9.Dowson D, Hardaker C, Flett M, Isaac GH. A hip joint simulator study of the performance of metal-on-metal joints. Part II: design. J Arthroplasty. 2004;19(8 Suppl 3):124–130. doi: 10.1016/j.arth.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Ebramzadeh E, Campbell PA, Takamura KM, Lu Z, Sangiorgio SN, Kalma JL, Smet KA, Amstutz HC. Failure modes of 433 metal-on-metal hip implants: how, why, and wear. Orthop Clin North Am. 2011;42:241–250. doi: 10.1016/j.ocl.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Glyn-Jones S, Pandit H, Kwon YM, Doll H, Gill HS, Murray DW. Risk factors for inflammatory pseudotumor formation following hip resurfacing. J Bone Joint Surg Br. 2009;91:1174–1566. doi: 10.1302/0301-620X.91B12.22287. [DOI] [PubMed] [Google Scholar]
- 12.Grammatopolous G, Pandit H, Kwon YM, Gundle R, McLardy-Smith P, Beard DJ, Murray DW, Gill HS. Hip resurfacings revised for inflammatory pseudotumor have a poor outcome. J Bone Joint Surg Br. 2009;91:1019–1024. doi: 10.1302/0301-620X.91B8.22562. [DOI] [PubMed] [Google Scholar]
- 13.Hart AJ, Dandachli W, Schlueter-Brust K, Henckel J, Cobb J. Large ball metal on metal hips obscure cup angle measurement on plain radiographs. Hip Int. 2009;19:323–329. doi: 10.1177/112070000901900405. [DOI] [PubMed] [Google Scholar]
- 14.Hart AJ, Ilo K, Underwood R, Cann P, Henckel J, Lewis A, Cobb J, Skinner J. The relationship between the angle of version and rate of wear of retrieved metal-on-metal resurfacings. J Bone Joint Surg Br. 2011;93:315–320. doi: 10.2106/JBJS.K.00562. [DOI] [PubMed] [Google Scholar]
- 15.Hart AJ, Sabah S, Henckel J, Lewis A, Cobb J, Sampson B, Mitchell A, Skinner JA. The painful metal-on-metal hip resurfacing. J Bone Joint Surg Br. 2009;91:738–744. doi: 10.1302/0301-620X.91B6.21682. [DOI] [PubMed] [Google Scholar]
- 16.Hart AJ, Skinner J, Henckel J, Sampson B, Gordon F. Insufficient acetabular version increases blood metal ion levels after metal-on-metal hip resurfacing. Clin Orthop Relat Res. 2011;469:2590–2597. doi: 10.1007/s11999-011-1930-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harvie P, Giele H, Fang C, Ansorge O, Ostlere S, Gibbons M, Whitwell D. The treatment of femoral neuropathy due to pseudotumor caused by metal-on-metal resurfacing arthroplasty. Hip Int. 2008;18:313–320. doi: 10.1177/112070000801800408. [DOI] [PubMed] [Google Scholar]
- 18.Heisel C, Streich N, Krachler M, Jakubowitz E, Kretzer JP. Characterization of the running-in period in total hip resurfacing arthroplasty: an in vivo and in vitro metal ion analysis. J Bone Joint Surg Am. 2008;90(suppl 3):125–134. doi: 10.2106/JBJS.H.00437. [DOI] [PubMed] [Google Scholar]
- 19.Howie DW, Cain CM, Cornish BL. Pseudo-abscess of the psoas bursa in failed double-cup arthroplasty of the hip. J Bone Joint Surg Br. 1991;73:29–32. doi: 10.1302/0301-620X.73B1.1991769. [DOI] [PubMed] [Google Scholar]
- 20.Jameson SS, Langton DJ, Nargol AV. Articular surface replacement of the hip: a prospective single-surgeon series. J Bone Joint Surg Br. 2010;92:28–37. doi: 10.1302/0301-620X.92B1.22769. [DOI] [PubMed] [Google Scholar]
- 21.Kalteis T, Handel M, Herold T, Perlick L, Paetzel C, Grifka J. Position of the acetabular cup—accuracy of radiographic calculation compared to CT-based measurement. Eur J Radiol. 2006;58:294–300. doi: 10.1016/j.ejrad.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Kwon YM, Glyn-Jones S, Simpson DJ, Kamali A, McLardy-Smith P, Gill HS, Murray DW. Analysis of wear of retrieved metal-on-metal hip resurfacing implants revised due to pseudotumors. J Bone Joint Surg Br. 2010;92:356–361. doi: 10.1302/0301-620X.92B3.23281. [DOI] [PubMed] [Google Scholar]
- 23.Kwon YM, Ostlere SJ, McLardy-Smith P, Athanasou N, Gil HS, Murray DW. “Asymptomatic” pseudotumors after metal-on-metal hip resurfacing arthroplasty: prevalence and metal ion study. J Arthroplasty. 2011;26:511–518. doi: 10.1016/j.arth.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 24.Kwon YM, Thomas P, Summer B, Pandit H, Taylor A, Beard D, Murray DW, Gill HS. Lymphocyte proliferation responses in patients with pseudotumors following metal-on-metal hip resurfacing arthroplasty. J Orthop Res. 2010;28:444–450. doi: 10.1002/jor.21015. [DOI] [PubMed] [Google Scholar]
- 25.Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg Br. 2010;92:38–46. doi: 10.1302/0301-620X.92B1.22770. [DOI] [PubMed] [Google Scholar]
- 26.Langton DJ, Joyce TJ, Jameson SS, Lord J, Orsouw M, Holland JP, Nargol AV, Smet KA. Adverse reaction to metal debris following hip resurfacing: the influence of component type, orientation and volumetric wear. J Bone Joint Surg Br. 2011;93:164–171. doi: 10.1302/0301-620X.93B2.25099. [DOI] [PubMed] [Google Scholar]
- 27.Langton DJ, Sprowson AP, Joyce TJ, Reed M, Carluke I, Partington P, Nargol AV. Blood metal ion concentrations after hip resurfacing arthroplasty: a comparative study articular surface replacement and Birmingham hip resurfacing arthroplasties. J Bone Joint Surg Br. 2009;91:1287–1295. doi: 10.1302/0301-620X.91B10.22308. [DOI] [PubMed] [Google Scholar]
- 28.Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60:217–220. [PubMed] [Google Scholar]
- 29.Long WT, Dastane M, Harris MJ, Wan Z, Dorr LD. Failure of the Durom Metasul acetabular component. Clin Orthop Relat Res. 2010;468:400–405. doi: 10.1007/s11999-009-1071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matthies A, Underwood R, Cann P, Ilo K, Nawaz Z, Skinner J, Hart AJ. Retrieval analysis of 240 metal-on-metal hip components, comparing modular total hip replacement with hip resurfacing. J Bone Joint Surg Br. 2011;93:307–314. doi: 10.1302/0301-620X.93B3.25551. [DOI] [PubMed] [Google Scholar]
- 31.Medicines and Healthcare products Regulatory Agency (MHRA). Medical device alert: ASR™ hip replacement implants manufactured by DePuy International Ltd (MDA/2010/069). Available at: http://www.mhra.gov.uk/. Accessed October 12, 2011.
- 32.Morlock MM, Bishop N, Zustin J, Hahn M, Ruther W, Amling M. Modes of implant failure after hip resurfacing: morphological and wear analysis of 267 retrieval specimens. J Bone Joint Surg Am. 2008;90(suppl 3):89–95. doi: 10.2106/JBJS.H.00621. [DOI] [PubMed] [Google Scholar]
- 33.Murray DW. The definition and measurement of acetabular orientation. J Bone Joint Surg Br. 1993;75:228–232. doi: 10.1302/0301-620X.75B2.8444942. [DOI] [PubMed] [Google Scholar]
- 34.National Joint Registry Centre. National Joint Registry UK 7th Annual Report 2010. Available at: http://www.njrcentre.org.uk/. Accessed October 12, 2011.
- 35.Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CL, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumors associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008;90:847–851. doi: 10.1302/0301-620X.90B7.20213. [DOI] [PubMed] [Google Scholar]
- 36.Pandit H, Vlychou M, Whitwell D, Crook D, Lugmani R, Ostlere S, Murray DW, Athanasou NA. Necrotic granulomatous pseudotumors in bilateral resurfacing arthroplasties: evidence for a type IV immune response. Virchows Arch. 2008;453:529–534. doi: 10.1007/s00428-008-0659-9. [DOI] [PubMed] [Google Scholar]
- 37.Sabah SA, Mitchell AW, Henckel J, Sandison A, Skinner JA, Hart AJ. Magnetic resonance imaging findings in painful metal-on-metal hips. J Arthroplasty. 2011;26:71–76. doi: 10.1016/j.arth.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Toms AP, Marshall TJ, Cahir J, Darrah C, Nolan J, Donell ST, Barker T, Tucker JK. MRI of early symptomatic metal-on-metal hip arthroplasty: a retrospective review of radiological findings in 20 hips. Clin Radiol. 2008;63:49–58. doi: 10.1016/j.crad.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Toms AP, Smith-Bateman C, Malcolm PN, Cahir J, Graves M. Optimization of metal artifact reduction (MAR) sequences for MRI of total hip prostheses. Clin Radiol. 2010;65:447–452. doi: 10.1016/j.crad.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 40.Treacy RB, McBryde CW, Shears E, Pynsent PB. Birmingham hip resurfacing: a minimum follow-up of ten years. J Bone Joint Surg Br. 2011;93:27–33. doi: 10.1302/0301-620X.93B1.24134. [DOI] [PubMed] [Google Scholar]
- 41.Underwood R, Matthies A, Cann P, Skinner JA, Hart AJ. A comparison of explanted articular surface replacement and Birmingham hip resurfacing components. J Bone Joint Surg Br. 2011;93:1169–1177. doi: 10.1302/0301-620X.93B9.26511. [DOI] [PubMed] [Google Scholar]
- 42.Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Koster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints: a clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 43.Witzleb W, Hanisch U, Ziegler J, Guenther K. In vivo wear rate of Birmingham Hip Resurfacing arthroplasty: a review of 10 retrieved components. J Arthroplasty. 2009;24:951–956. doi: 10.1016/j.arth.2008.06.022. [DOI] [PubMed] [Google Scholar]