Abstract
Background
Surface damage of the tibial polyethylene insert in TKA is thought to diminish with increasing conformity, based on computed lower contact stresses. Added constraint from higher conformity may, however, result in greater forces in vivo.
Questions/purposes
We therefore determined whether increased conformity was associated with increased surface pitting, delamination, creep, and polishing in a group of retrieved tibial inserts.
Methods
We compared 38 inserts with a dished articular surface (conforming group) with 31 inserts that were unconstrained and nonconforming in the sagittal plane (less conforming group). The two groups had identical polyethylene composition and processing history. The articulating surfaces were scored for pitting, delamination, deformation/creep, and polishing. Evidence of edge loading and the presence of embedded bone cement were also recorded.
Results
The conforming inserts were associated with higher delamination and pitting scores but lower polishing scores, even after adjusting for the effects of sex, age, insert thickness, and implantation duration. Long implantation duration and male sex were also associated with increased delamination, pitting, and polishing, whereas long shelf life was associated only with increased delamination. The conforming group also had approximately a fourfold greater prevalence of edge loading and approximately a threefold greater prevalence of embedded bone cement. The latter was associated with higher scores and proportions of delamination and pitting.
Conclusions
These findings suggest more conformity may increase surface fatigue damage in TKA. Higher constraint-induced stresses during secondary motions and more possibility for edge loading and bone cement capture on a dished surface may account for these results.
Clinical Relevance
The selection of materials with high fatigue resistance may be particularly important for high-conformity/constraint tibial inserts. In addition, awareness of the benefits and trade-offs with conformity may allow better matching of TKA design to patient.
Introduction
Clinical experience with TKAs shows generally good patient satisfaction [39], good performance in young patients [21], and high survivorship rates up to the second decade after implantation [21, 28]. Wear of the UHMWPE tibial insert and the resulting mechanical and biologic complications remain a problem [20], however, and constitute a recognized cause of failure [33, 40]. Many factors influence this wear [33]. Some are related to the material, such as type of resin, method of consolidation, manufacturing process, method of sterilization, shelf life, and level of oxidation [22]. Other factors include the patient (eg, activity, gait), surgical technique (eg, implant alignment, soft tissue balance), and prosthetic design (eg, liner thickness, articular surface geometry) [49].
The optimal degree of conformity between tibial plateau and femoral condyle is still a matter of debate [16, 18]. The wear of polyethylene (PE) in prosthetic components may be considered as one of the following three types: (1) microscopic or uniform wear by adhesion and abrasion, which is manifest by polishing of the surface; (2) abrasive wear due to third bodies, such as cement pieces (third-body wear); and (3) surface fatigue wear, the latter entailing pitting and delamination. Uniform wear is controlled by sliding distance, load, and shear direction changes [2, 9, 25, 43], whereas fatigue wear is commonly assumed to be controlled by the magnitude of the contact stresses [3, 4, 23, 31]. Consequently, it is perceived that less conforming geometries from flatter tibial surfaces result in increased tibiofemoral contact stresses and thus increased PE fatigue wear [3, 4, 23].
The mechanisms of wear in TKAs are complex, and in contrast to THAs, surface fatigue due to sliding and rolling kinematics [48] and imposed constraints hindering the natural envelope of motion play important roles [8]. In a constrained design, hindering motion may lead to zones of unusually high contact stress that are not taken into account when evaluating the design with idealized knee motions [27]. On the other hand, because the friction coefficient decreases with contact stress [18], mitigating the shear contact forces, the effect of higher but controlled contact stresses in a less conforming contact may be more than offset by avoiding edge loading-type situations. We thus hypothesized, given the same materials and manufacturing processes, a more conforming and thereby more constrained tibial articulating surface would exhibit more severe wear-related surface damage than one that is less conforming and less constrained.
We therefore answered the following research questions with regard to two groups of tibial inserts fabricated using the same combination of PE and manufacturing method and for which we examined three surface damage modes (delamination, pitting, deformation/creep) and polishing: (1) Did conformity have an effect on the prevalence and intensity of the damage modes? (2) Are tibial shelf time and implantation duration associated with increased surface damage and polishing? (3) Do other factors contribute to surface damage and polishing? (4) Does the prevalence of edge loading and embedded bone cement depend on conformity? And (5) are edge loading and embedded cement associated with surface damage?
Materials and Methods
We compared two groups of retrieved tibial inserts, one with a conforming articular geometry (38 inserts) and one with a less conforming articular geometry (31 inserts), for articular surface damage quantified with pitting, delamination, and deformation/creep scores. They were also compared for polishing scores, a measure of uniform wear coverage. All of the inserts in both groups were fabricated by one manufacturer (Zimmer, Inc, Warsaw, IN, USA) using GUR 4150 PE resin with 0.05% calcium stearate additive and sterilized by gamma radiation in air. The two groups were matched for patient age, sex, implantation time, component shelf time, and component material (Table 1). In addition to the pairwise univariate comparisons, the effect of implant conformity on surface damage was also examined with multivariate analysis to simultaneously take into account the effect of other variables. We also compared the two groups with respect to the prevalence of edge loading and embedded bone cement because we believed these two features could partly explain the genesis of the surface damage. The number of inserts used (38 conforming and 31 less conforming designs) was limited to those that were available at the commencement of the study. For a univariate comparison using a two-tailed t-test, these numbers of inserts in the two study groups permitted the detection of an effect size of approximately 0.72 with a power level of 0.8 at a confidence level of 95%, which was deemed adequate.
Table 1.
Demographic and implant data for the conforming and less conforming groups
| Group | TKA design | Number of retrievals | Patient age (years)* | Implantation duration (months)* | Patient sex (male/female) | Surgery side (left/right) | Primary/revision cases | Insert thickness (mm)*,† | Insert shelf time (months)*,‡ | Cemented femoral and/or tibial component | Material of femoral component§ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Conforming | CCK/PS Insall/Burstein® ∥ | 38 | 68.0 ± 12.6 (37–86) |
18.6 ± 16.1 (0.8–59.7) |
12/26 | 25/13 | 16/21 | 16.0 ± 5.5 (8.0–25.0) |
12.1 ± 10.6 (1.6–46.2) |
All yes | All Co-Cr-Mo |
| Less conforming | MG® ∥ | 31 | 62.8 ± 11.3 (39–81) |
19.9 ± 17.4 (0.4–64.3) |
13/18 | 16/15 | 28/3 | 12.7 ± 4.1 (9.0–23.0) |
10.1 ± 8.9 (0.9–36.1) |
24 yes 7 no | 20 Co-Cr-Mo, 11 Ti-6Al-4 V |
| p value∥ | 0.399 | 0.106 | 0.760 | 0.373 | 0.233 | < 0.001 | 0.006 | 0.497 | 0.002 | < 0.001 |
* Values are expressed as mean ± SD, with the range in parentheses; †minimum thickness; ‡known for 17 conforming and 25 less conforming inserts; §all the Co-Cr alloy and Ti alloy femoral components were cemented and uncemented, respectively; ∥comparing the combined conforming group (CCK + PS) with the less conforming group; the two-tailed t-test was used for the continuous variables and the chi-square test for the frequency values; PS = posterior-stabilized; CCK = constrained condylar knee.
The conforming group consisted of 38 tibial inserts from posterior cruciate-substituting TKAs (Insall/Burstein® II; Zimmer, Inc). This group entailed two subgroups, namely 15 posterior-stabilized (PS) components and 23 constrained condylar knee (CCK) components that had identically dished surfaces; they were conforming in the coronal plane and partially conforming in the sagittal plane, with constraint determined both by the shape of the articulating surfaces and the presence of the post-cam mechanism. The less conforming group comprised 31 inserts from one posterior cruciate-retaining (CR) TKA design (MG II®; Zimmer, Inc) that were unconstrained and nonconforming in the sagittal plane but flat and fully conforming against a flat femoral surface in the coronal plane. Defining conformity as the ratio of the femoral to tibial contact radii [34], the average conformity at 0° of flexion was 0.50 for the CR inserts versus 0.92 for the PS and CCK inserts (Table 2). Another, perhaps more rigorous comparison of conformity is the ratio of the CR to PS/CCK peak Hertzian stresses, which was 2.3 at 300 N and 3.4 at 3000 N, computed [36] using a modulus of elasticity of 1.02 MPa for the PE [4]. The reasons for revision are given (Table 3).
Table 2.
Nominal conformity for the two design groups
| Aspect | Conforming design | Less conforming design | ||||
|---|---|---|---|---|---|---|
| Femoral component radius (mm) | Tibial insert radius (mm) | Conformity index | Femoral component radius (mm) | Tibial insert radius | Conformity index | |
| Frontal | 15 | 16 | 0.94 | Plane | Plane | 1 |
| Sagittal | 44 | 49 | 0.90 | 39 | Plane | 0 |
| Average | 0.92 | 0.50 | ||||
Table 3.
Reason for revision
| Group | Implant design | Reason for revision (number of implants)* | ||||||
|---|---|---|---|---|---|---|---|---|
| Infection | Loosening | Instability | Fracture | Patellar problems | Other | Unknown | ||
| Conforming | CCK Insall/Burstein® II | 11 (48%) | 1 (4%) | 1 (4%) | 1 (4%) | 1 (4%) | 5 (22%) | 3 (13%) |
| PS Insall/Burstein® II | 6 (40%) | 1 (7%) | 1 (7%) | 0 (0%) | 0 (0%) | 3 (20%) | 4 (27%) | |
| Less conforming | MG® II | 8 (26%) | 2 (6%) | 4 (13%) | 3 (10%) | 7 (23%) | 3 (10%) | 4 (13%) |
* Excluding patellar problems, a known issue with the MG II® system, the components in the conforming and less conforming groups were removed for similar (p = 0.25) reasons; PS = posterior-stabilized; CCK = constrained condylar knee.
The PS and CCK inserts were combined in one group because of their identical articular surface geometry and the lack of differences between them with respect to each of the variables considered, except for ratio of primary to revision cases (Table 4). The two insert groups were not different with respect to the damage-related outcome variables described below (delamination, pitting, deformation/creep, p ≥ 0.173) and the prevalence of embedded bone cement (p = 0.740) and edge loading (p = 0.192).
Table 4.
Demographic and implant data for the CCK and PS tibial inserts
| Implant design | Number of explants | Patient age at surgery (years)* | Implantation time (months)* | Sex (male/female) | Side (left/right) | Primary/revision cases | Component thickness (mm)* | Shelf time before implantation (months)*,† | Cemented femoral and/or tibial component | Material of femoral component |
|---|---|---|---|---|---|---|---|---|---|---|
| CCK Insall/Burstein® II | 23 | 68.6 ± 13.8 (37–86) |
19.7 ± 17.9 (0.8–59.7) |
8/15 | 14/9 | 3/19 | 16.8 ± 4.8 (10–25) |
15.1 ± 10.8 (6.2–46.2) |
All yes | All Co-Cr-Mo |
| PS Insall/Burstein® II | 15 | 66.9 ± 10.1 (45–76) |
16.1 ± 12.3 (3.4–39.8) |
4/11 | 11/4 | 13/2 | 14.8 ± 6.3 (8–25) |
5.1 ± 5.9 (1.6–15.4) |
All yes | All Co-Cr-Mo |
| p value‡ | 0.194 | 0.729 | 0.581 | 0.599 | 0.429 | < 0.001 | 0.728 | 0.074 | 1 | 1 |
* Values are expressed as mean ± SD, with the range in parentheses; †known for 12 CCK and five PS components; ‡comparing the CCK and PS groups; the two-tailed t-test was used for the continuous variables and the chi-square test for the frequency values; CCK = constrained condylar knee; PS = posterior-stabilized.
Wear damage to the medial and lateral articulating surfaces of the inserts was inspected visually and, where necessary, at ×5 to ×7 magnification. We adopted an earlier established system of damage identification for this study and examined the following wear and damage modes: polishing, pitting, delamination, and deformation/creep [6, 19, 45]. Wear maps on previously prepared design-based templates were drawn to score the surfaces consistently. Three observers, including one of the authors (JDH) and two others (TS, VS), independently performed the visual examination and scoring of the tibial articular components. We found an average correlation coefficient, r, of 0.707 (p < 0.0001) and 0.823 (p < 0.0001), respectively. The span of the deformation/creep score values, 0 to 2 with an average of 0.21, was too small to obtain a good correlation among the three observers (r = −0.073, p = 0.53), but a paired sign test of the scores revealed no difference among the observers (p = 0.428–0.734). The correlation among observers for the polishing score ranks was r = 0.256 (p = 0.040). Scores were based on the product of extent and severity of the damage. Extent took the size of a specific damage feature into account and was rated in 25 percentiles on a scale of 0 to 4 dependent on plateau coverage (Table 5). Severity was rated from 0 to 3 (Table 5). In addition, edge loading and the presence of embedded bone cement were noted by visual inspection. Edge loading was defined as plastic deformation/creep along the circumference of the implant (Fig. 1). The visual scores obtained by the three observers were averaged to produce the final score.
Table 5.
Scoring system for extent and severity
| Score | Extent | Severity | |||
|---|---|---|---|---|---|
| Pitting* | Delamination | Creep/Deformation | Polishing | ||
| 0 | No damage | No damage | No damage | No damage | No damage |
| 1 | 1%–25% of the surface damaged | Rare, small pits | Color change, no surface involvement | Minor visible creep | Machine marks still visible |
| 2 | 26%–50% of the surface damaged | Abundant small to medium pits | Surface involvement, no material loss | Palpable surface/edge shape change | No machine marks, reflection of lighting source visible |
| 3 | 51%–75% of the surface damaged | Abundant medium to large pits | Material loss | Gross surface shape change | Detailed reflection of lighting source |
| 4 | 76%–100% of the surface damaged | NA | NA | NA | NA |
* Embedded cement was not counted as pitting; NA = not applicable.
Fig. 1.
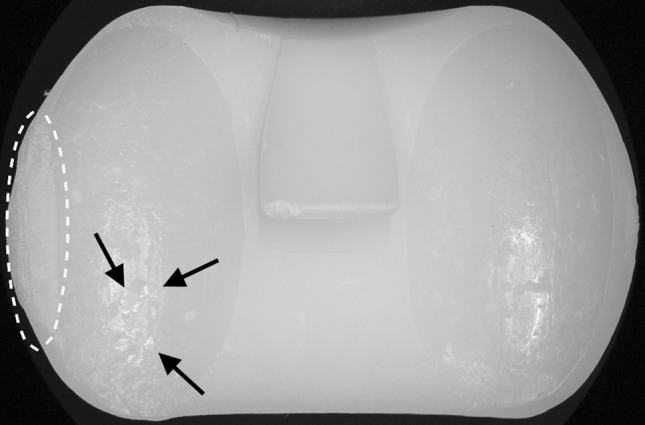
A photograph shows an example of edge loading, here shown on the lateral aspect of an Insall/Burstein® II PS tibial insert, and highlighted with an oval. The arrows point to embedded bone cement.
We determined differences in the prevalence of delamination, pitting, deformation/creep, and polishing between the two insert groups using the chi-square test or, if the expected frequency values were less than five, the Fisher exact test. Differences in the intensity of the damage modes between the two insert groups were determined using the two-sample t-test, except for the deformation/creep scores, which were analyzed with the Mann-Whitney test because they showed departure from normality. In addition to these univariate comparisons, the effect of implant conformity on surface damage was examined using multivariate analyses, namely, analysis of covariance (delamination, pitting, polishing) or logistic regression (deformation/creep) to simultaneously take into account the effect of other variables, such as sex, patient age, and insert thickness. The effects of insert shelf time and implantation duration on delamination on surface damage and polishing were examined on a subset of 40 components for which both the shelf time and implantation duration were known, using multivariate analyses that paralleled those for the larger group. The effect of other factors, namely, sex, patient age, and insert thickness, were assessed from the multivariate analyses for the larger group. The dependence of prevalence of embedded bone cement and edge loading on conformity was analyzed using the chi-square/Fisher exact test. We evaluated differences in surface damage scores between inserts with embedded cement and those without and between inserts with edge loading and those without using two-sample t-tests. For the covariance analyses, the total scores (medial + lateral) were used, variance equality was checked with Levene’s test, and normality was assessed with Q-Q plots of the studentized residuals. Statistical analyses were performed in Excel® (Microsoft Corp, Redmond, CA, USA) and SPSS® (SPSS Inc, Chicago, IL, USA). All reported p values are two-sided. Reported data are displayed as mean ± SD unless noted otherwise.
Results
The pitting and delamination scores and the delamination prevalence were higher for the conforming group, the polishing scores were greater for the less conforming group, and the deformation/creep scores and prevalence were not different for the two groups. The prevalence of the damage modes and the associated visual scores for the two groups are given in Table 6 (pitting and delamination) and in Table 7 (deformation/creep and polishing). Pitting was the most common mode of surface damage, affecting 84% of all the components, followed by delamination (54%) and deformation/creep (22%). Pitting also occurred on inserts associated with noncemented knees, although at a lower prevalence than on their cemented counterparts (27% versus 90%). Nevertheless, the pitting scores remained higher (p = 0.007) for the conforming group compared with the less conforming group even if the noncemented knees were excluded. All of the components exhibited polishing on the articular surfaces. The medial and lateral aspect scores were not different, except for delamination in the conforming group, for which the score was higher medially than laterally (Table 6). After adjusting for the effects of sex, age, insert thickness, and implantation duration, the conforming inserts were still associated with higher delamination scores (p = 0.025) and higher pitting scores (p < 0.001) but lower polishing scores (p = 0.022). Conformity, on the other hand, was not associated with deformation/creep (p = 0.891). These results are similar to those for the univariate comparisons (Tables 6, 7, bottom row).
Table 6.
Prevalence of and scores for pitting and delamination
| Parameter | Pitting | Delamination | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medial | Lateral | Both sides | Medial | Lateral | Both sides | |||||||
| Conforming | Less conforming | Conforming | Less conforming | Conforming | Less conforming | Conforming | Less conforming | Conforming | Less conforming | Conforming | Less conforming | |
| Prevalence* | 84% | 74% | 82% | 71% | 87% | 81% | 63% | 29% | 55% | 19% | 71% | 32% |
| p value† | 0.303 | 0.299 | 0.484 | 0.005 | 0.002 | 0.001 | ||||||
| Mean score | 2.22 | 1.09 | 2.18 | 1.06 | 4.39 | 2.15 | 1.38 | 0.57 | 0.96 | 0.57 | 2.33 | 1.14 |
| SD | 1.54 | 1.11 | 1.58 | 1.09 | 3.00 | 2.00 | 1.48 | 1.12 | 1.10 | 1.34 | 2.45 | 2.36 |
| p value† | 0.001 | 0.001 | < 0.001 | 0.015 | 0.194 | 0.045 | ||||||
* Percentage of components with a score > 0; †comparing the conforming and less conforming designs, obtained with a chi-square test for prevalence values and a two-tailed t-test for the mean scores.
Table 7.
Prevalence of and scores for deformation/creep and polishing
| Parameter | Deformation/Creep | Polishing | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medial | Lateral | Both sides | Medial | Lateral | Both sides | |||||||
| Conforming | Less conforming | Conforming | Less conforming | Conforming | Less conforming | Conforming | Less conforming | Conforming | Less conforming | Conforming | Less conforming | |
| Prevalence* | 11% | 13% | 16% | 6% | 24% | 19% | 100% | 100% | 100% | 100% | 100% | 100% |
| p value† | 0.759 | 0.273 | 0.665 | 1 | 1 | 1 | ||||||
| Mean score | 0.09 | 0.10 | 0.12 | 0.05 | 0.21 | 0.15 | 2.91 | 3.12 | 2.67 | 3.33 | 5.58 | 6.45 |
| SD | 0.31 | 0.26 | 0.30 | 0.21 | 0.47 | 0.32 | 1.04 | 0.99 | 0.87 | 1.19 | 1.67 | 2.06 |
| p value† | 0.745 | 0.237 | 0.669 | 0.406 | 0.009 | 0.056 | ||||||
* Percentage of components with a score > 0; †comparing the conforming and less conforming designs, obtained with a chi-square test for prevalence values, a Kruskal-Wallis test for the deformation/creep scores, and a two-tailed t-test for the polishing scores.
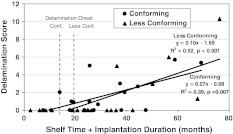
Shelf time increased delamination (p = 0.030) but not pitting (p = 0.545), deformation/creep (p = 0.366), and polishing (p = 0.928), whereas implantation duration had an effect on increasing delamination (p = 0.003), pitting (p = 0.020), and polishing (p = 0.009) but not on deformation/creep (p = 0.447). The onset of visible delamination occurred 5.5 months sooner for the conforming group based on shelf life plus implantation duration (Fig. 2). Excluding the inserts for noncemented knees did not alter these onset times.
Fig. 2.
Delamination scores versus shelf time plus implantation duration are shown in this graph. Also shown are the results for onset of delamination for conforming (Conf.) and less conforming (Less Conf.) inserts, defined as the time between insert manufacture and discovery of delamination.
Sex influenced delamination (p = 0.032), polishing (p = 0.041), and deformation/creep (p = 0.032), all three surface features having higher scores with males, but insert thickness, patient age, and side of implantation (left, right) had no effect on any of the damage modes or polishing. There were no differences (p = 0.141) between revision and primary TKA components on the damage scores.
The prevalence of edge loading and embedded bone cement was higher for the conforming group (Table 8). The medial and lateral aspects were comparable except that edge loading was more prevalent medially than laterally for the conforming group (Table 8), consistent with the corresponding medial-lateral difference in the delamination scores noted above.
Table 8.
Prevalence of edge loading and embedded bone cement
| Parameter | Edge loading | Embedded bone cement* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conforming | Less conforming | Conforming | Less conforming | |||||||||
| Medial | Lateral | Both | Medial | Lateral | Both | Medial | Lateral | Both | Medial | Lateral | Both | |
| Prevalence† | 34% | 13% | 37% | 10% | 10% | 10% | 45% | 39% | 50% | 17% | 13% | 17% |
| p value‡ | 0.002 | 1 | 0.642 | 0.705 | ||||||||
| p value§ | 0.009 | 0.008 | ||||||||||
* Only components with a bone-cement interface were included; †percentage of components with a score > 0; ‡chi-square test p values comparing the medial and lateral aspects within a design group; §chi-square test p values comparing the conforming and less conforming designs using the prevalence for the whole insert (“both”).
Edge loading was associated with a higher prevalence of delamination and higher pitting and delamination scores (Table 9), whereas embedded bone cement was associated with both a higher prevalence and higher scores for pitting and delamination (Table 10). Embedded bone cement was also associated with lower polishing scores.
Table 9.
Prevalence of and scores for tibial inserts (conforming and less conforming) with and without edge loading
| Parameter | Pitting | Delamination | Creep | Polishing | ||||
|---|---|---|---|---|---|---|---|---|
| No edge loading | Edge loading | No edge loading | Edge loading | No edge loading | Edge loading | No edge loading | Edge loading | |
| Prevalence* | 81% | 94% | 42% | 88% | 19% | 29% | 100% | 100% |
| p value† | 0.270 | 0.001 | 0.499 | 1.000 | ||||
| Mean score | 2.90 | 4.88 | 1.15 | 3.78 | 0.17 | 0.24 | 6.02 | 5.82 |
| SD | 2.65 | 2.83 | 1.85 | 3.07 | 0.41 | 0.40 | 2.04 | 1.38 |
| p value‡ | 0.010 | 0.003 | 0.553 | 0.714 | ||||
* Percentage of components with a score > 0; †obtained with the chi-square or Fisher exact test; ‡obtained with the two-tailed t-test.
Table 10.
Prevalence of and scores for tibial inserts (conforming and less conforming) with and without embedded bone cement
| Parameter | Pitting | Delamination | Deformation/Creep | Polishing | ||||
|---|---|---|---|---|---|---|---|---|
| No embedded bone cement | Embedded bone cement | No embedded bone cement | Embedded bone cement | No embedded bone cement | Embedded bone cement | No embedded bone cement | Embedded bone cement | |
| Prevalence* | 76% | 100% | 30% | 100% | 22% | 22% | 100% | 100% |
| p value† | 0.007 | < 0.001 | 1 | 1 | ||||
| Mean score | 1.99 | 6.17 | 0.46 | 4.46 | 0.17 | 0.20 | 6.36 | 5.19 |
| SD | 1.88 | 2.24 | 0.97 | 2.39 | 0.42 | 0.40 | 2.01 | 1.36 |
| p values‡ | < 0.001 | < 0.001 | 0.784 | 0.006 | ||||
* Percentage of components with a score > 0; †obtained with the chi-square or Fisher exact test; ‡obtained with the two-tailed t-test.
Discussion
Surface damage of the tibial PE insert in TKA is thought to diminish with increasing conformity [3, 4, 15, 23]. Nevertheless, because high constraint can lead to high contact stresses, we hypothesized, given the same materials and manufacturing processes, a more conforming and thereby more constrained tibial articulating surface could exhibit more severe wear-related surface damage than one that is less conforming and less constrained.
Our study suffered from several limitations. The first is a series of limitations stemming from the retrospective nature of this study. The implantation time was relatively brief, with a maximum of 5.4 years (Table 1). The inserts were manufactured from ram-extruded GUR 4150 PE and gamma-sterilized in air, an obsolete sterilization technique that makes the material prone to oxidation. PE oxidation has been associated with delamination and fatigue fracture both in vivo [5, 12, 13] and in vitro [35, 42]. The results may nevertheless be relevant for PE irradiated in an inert atmosphere because in vivo oxidation is still possible [14]. Thus, these components may be viewed as providing an accelerated path for detecting any effects of conformity and constraint associated with PE oxidation. The influence of insert shelf time could only be analyzed on a subset of 40 components for which shelf time and implantation duration were both known. However, shelf time was similar for the two groups (Table 1), so we do not expect this factor to bias the results. The conforming group had a greater proportion of revision components (Table 1), but whether the component was from a primary or revision TKA had no effect on the average damage and polishing scores or on the prevalence of edge loading and embedded bone cement. The influence of other relevant factors, such as patient activity [26] and patient weight [29], could not be assessed due to insufficient data. There was also insufficient radiographic information to provide meaningful correlations between the quality of the cemented interface and the damage on the articulating surfaces. The second limitation was that we did not determine volumetric wear, a key factor in osteolysis, along with particle size [31, 38]. The third limitation was that the study was confined to the analyses of the articulating surfaces, ignoring backside wear damage and, for the conforming group, post wear, both of which can be substantial [17, 37, 41, 44, 46].
The association of higher pitting score and delamination score with higher degree of conformity is particularly noteworthy because it challenges the prevalent wisdom that the lower contact stress from a more congruent contact will mitigate fatigue-related failure [4, 15]. Our findings are in agreement with those of Blunn et al. [8] but not with those of Collier et al. [11] and Willie et al. [47] (Table 11). Both studies did not control for material, which might be the source of the discrepancy because the susceptibility of PE to fatigue-related damage is influenced by consolidation quality [24, 50] and resistance to oxidative degradation [5, 14, 51]. As expected, polishing, as a normal wear feature, occurred on all the articular surfaces of the retrieved tibial PE inserts.
Table 11.
Previous retrieval studies evaluating the influence of conformity on tibial polyethylene surface damage
| Study | Designs* | Number | Implantation duration (years) | UHMWPE type† | Damage scores‡,§ | Comments/Conclusions | ||
|---|---|---|---|---|---|---|---|---|
| Delamination | Pitting | Deformation | ||||||
| Collier et al. [11] (1991) | Fully conforming: LCS® rp (DP), LCS® mb (DP), Insall-Burstein® II (Z1) | 38 | Not specified | 0.4 | 1.6 | 1.1 | There was a positive correlation between the intensity of wear and the level of contact stress Noncongruent designs had greater wear than fully congruent ones Greater wear in the thinner inserts was observed for the noncongruent design |
|
| Moderately conforming curved-on-curved: Synatomic® (DP), Kinemax® (H), PCA® II (H) | 42 | Not specified | 1.0 | 1.8 | 1.3 | |||
| Moderately conforming flat-on-flat: MG® (Z1), MG® II (Z1), PCA® I, Ortholoc® III (DC), Natural Knee® (I), Kinematic® (H) | 42 | Not specified | 0.9 | 1.5 | 1.1 | |||
| Blunn et al. [8] (1997) | Minns Meniscal® TKA (Z2) | 30 | 4.4 | Not specified | 5.6 | 3.0 | 3.5 | Delamination was the principal wear type In medium- and long-term retrieved specimens of the designs with moderately high conformity, delamination wear was associated with restriction of rotational movement of the femoral component or with abrupt changes in the radius of the tibial component Wear attributed to cement abrasion or entrapment occurred on the more conforming designs |
| Kinematic® TKA (H) | 60 | 5.1 | Not specified | 7.0 | 4.7 | 1.7 | ||
| PCA® TKA (H) | 17 | 5.1 | Not specified | 9.0 | 3.4 | < 1.0 | ||
| Total Condylar® TKA (H) | 22 | 3.8 | Not specified | 3.3 | 3.6 | < 1.0 | ||
| Attenborough® TKA (Z2) | 15 | 8.4 | Not specified | 11 | < 1.0 | < 1.0 | ||
| Willie et al. [47] (2008) | 10 congruent 8 ultracongruent (knee system was not specified) |
18 | GUR 1020, slab consolidation molded, gamma-in-air | 5 (11) E 3 (6) O |
0 (0) E 0 (0) O |
The focus was on comparing the highly crosslinked polyethylene to the conventional polyethylenes | ||
| 3 congruent 7 flat (knee system was not specified) |
10 | GUR 4150, ram extruded, gamma-in-air | 32 (28) E 35 (25) O |
2 (2) E 1 (2) O |
Used melt technique to distinguish between plastic deformation and actual wear damage Premelt: conformity did not have an effect on surface damage for both polyethylenes Postmelt: the flat geometry was associated with higher damage for conventional polyethylene |
|||
| 8 ultracongruent 5 congruent (knee system was not specified) |
13 | Durasul® | 0 (0) E 0 (0) O |
0 (0) E 0 (0) O |
||||
| Current study | Conforming: CCK/PS Insall-Burstein® II (Z1) | 38 | GUR 4150, ram extruded, gamma-in-air | 2.3 (2.4) | 4.4 (3) | 0.21 (0.47) | Conforming design was associated with more delamination, pitting, edge loading, and embedded bone cement | |
| Less conforming: MG® II (Z1) | 31 | Same | 1.1(2.4) | 2.2 (2) | 0.15 (0.32) | |||
* Manufacturer code in parentheses: DP = DePuy (Warsaw, IN, USA); H = Howmedica (Rutherford, NJ, USA); R = Richards (Memphis, TN, USA); W = Waldemar Link (Hamburg, Germany); Z1 = Zimmer, Inc (Warsaw, IN, USA); Z2 = Zimmer UK (Swindon, UK); †Durasul®: GUR 1050, slab compression molded, ~95-kGy electron beam, melted, ethylene oxide); ‡values are expressed as mean, with SD in parentheses; §E = premelt surface damage area (%) and O = postmelt surface damage area (%) for Willie at al. [47]; PS = posterior-stabilized; CCK = constrained condylar knee.
The increase of delamination scores with tibial insert shelf time is in keeping with the well-established increase in oxidation with shelf time [14], which in turn increases the polymer’s susceptibility to delamination [5, 12, 14]. The absence of an effect of shelf time on pitting score is consistent with the previously reported finding that in vivo oxidation contributes to delamination but not pitting [32]. The prevalence of pitting and delamination increased with implantation duration, suggesting both wear types are connected with cumulative cyclic loading and/or time. The faster onset of delamination in the conforming group suggests effectively greater contact forces in this group, perhaps arising from the higher constraint imposed by the dished surface. Deformation/creep most likely reflected bedding-in rather than an ongoing damage process, consistent with the negligible dependence of deformation/creep on implant duration.
The association of both delamination score and deformation/creep score with being a male patient is not unexpected given that males are heavier than females [30]. Unfortunately, we lacked sufficient information to assess the effect of patient body weight directly. Insert thickness did not have an effect on surface damage, suggesting the minimum thickness of the inserts here (8 mm, Table 1) was sufficient to mitigate the stress-rising effect of the underlying metal tray [3].
The almost fourfold greater prevalence of edge loading in the conforming versus the less conforming inserts may be partly related to the retained PCL, which acts as a secondary varus-valgus constraint [10]. Perhaps even more important, the flat-on-flat design of the less constrained prosthesis provided a larger lever arm to resist any occurring varus torques during activities of daily living, which made lateral joint opening and insert edge loading less likely [1]. Edge loading was linked to higher delamination scores, suggesting unstable joint motions contribute to high stresses that produce severe PE wear.
The almost threefold greater prevalence of embedded bone cement in the conforming inserts may be because a dished design is more likely to trap particles than a flat design, as noted in previous studies [8, 24]. Embedded bone cement is undesirable, being associated here with approximately triple the prevalence of delamination and triple the pitting scores. The positive association of pitting scores with the amount of embedded bone cement implies third-body initiated damage, which would have taken place mostly early in the implantation period if it were purely based on indentation. However, the positive correlation of pitting with implantation duration suggests also cumulative cyclic fatigue, which can be explained by rolling bone cement particles acting as local stress raisers and causing ejection of PE debris [7] or facilitating the crack initiation process. Our results suggest, during surgery, great care should be taken to remove all cement extruding from the cement-bone interface and/or cement-implant interface.
The present observations of more pitting and delamination in the conforming group indicate optimizing surface geometry to reduce surface damage is not just a matter of minimizing the idealized contact stresses. In this study, edge loading and third-body ingress were associated with increasing surface damage. Also mitigating the effect of nonconformity is that the maximum value of the von Mises stress, which is related to fatigue failure, is limited by the nonlinear mechanical behavior of PE [4]. In addition, recent studies suggest increased conformity has limited or negative influence on uniform wear. Using a computational model, Fregly et al. [16] found conformity beyond approximately 0.4 had little effect on further decreasing wear, and sagittal but not frontal conformity had an effect. Galvin et al. [18] found, in a simulator study in a low-conforming, high-contact stress TKA design having a flat tibial insert, the wear was three times lower than was the case for a low-stress standard design having a curved insert, a result attributed to the decrease of the PE wear factor with increase in contact stress.
In conclusion, we found the prevalence and intensity of pitting and delamination were associated with tibial surface conformity. Further, surface damage increased with increasing shelf time and implantation duration for these gamma-in-air-sterilized components. The prevalence of edge loading and entrapped bone cement was higher in the more conforming inserts and contributed to fatigue damage. Polishing scores, on the other hand, were higher for the less conforming inserts. Our observations and those of other studies (Table 11) indicate the problem of wear of the tibial PE insert is complex and cannot be explained by a single variable. These findings challenge the common wisdom that the magnitude of the idealized contact stress is the most important determinant of fatigue-related wear in TKA. Overall, the study suggests the selection of PE materials with high fatigue resistance may be particularly important for inserts with more conformity and/or constraint.
Acknowledgments
The authors thank Thorsten Schwenke and Vivek Shekhawat who as two of the three independent observers performed the visual examination and scoring of the tibial articular components. We also thank Dr Sanjib Basu, Division of Statistics, Northern Illinois University, DeKalb, IL, USA, for providing statistical consultation and Dr Anne Mündermann for helpful editorial input. This manuscript is dedicated to Dr. Aivars Berzins, our friend and colleague who initiated this study but, sadly, was unable to complete it before his sudden death.
Footnotes
One or more of the authors (JJJ) have received funding from the NIH (NIH Grant AR39310) and the Crown Family Chair. One or more of the authors has or may receive payments or benefits, in any 1 year, an amount in excess of $10,000 (JJJ) or $1,000,0000 (JOG) from a commercial entity related to this work (Zimmer, Inc, Warsaw, IN, USA). The institution of the authors has received, in any 1 year, funding from Zimmer, Inc.
Each author certifies that his or her institution has approved the human protocol for this investigation that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
References
- 1.Andriacchi TP. Dynamics of knee malalignment. Orthop Clin North Am. 1994;25:395–398. [PubMed] [Google Scholar]
- 2.Archard JF. Contact and rubbing of flat surface. J Appl Phys. 1953;24:981–988. doi: 10.1063/1.1721448. [DOI] [Google Scholar]
- 3.Bartel DL, Bicknell VL, Wright TM. The effect of conformity, thickness, and material on stresses in ultra-high molecular weight components for total joint replacement. J Bone Joint Surg Am. 1986;68:1041–1051. [PubMed] [Google Scholar]
- 4.Bartel DL, Rawlinson JJ, Burstein AH, Ranawat CS, Flynn WF., Jr Stresses in polyethylene components of contemporary total knee replacements. Clin Orthop Relat Res. 1995;317:76–82. [PubMed] [Google Scholar]
- 5.Bell CJ, Walker PS, Abeysundera MR, Simmons JM, King PM, Blunn GW. Effect of oxidation on delamination of ultrahigh-molecular-weight polyethylene tibial components. J Arthroplasty. 1998;13:280–290. doi: 10.1016/S0883-5403(98)90173-5. [DOI] [PubMed] [Google Scholar]
- 6.Berzins A, Jacobs JJ, Berger R, Ed C, Natarajan R, Andriacchi T, Galante JO. Surface damage in machined ram-extruded and net-shape molded retrieved polyethylene tibial inserts of total knee replacements. J Bone Joint Surg Am. 2002;84:1534–1540. doi: 10.2106/00004623-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Blunn GW, Joshi AB, Lilley PA, Engelbrecht E, Ryd L, Lidgren L, Hardinge K, Nieder E, Walker PS. Polyethylene wear in unicondylar knee prostheses: 106 retrieved Marmor, PCA, and St Georg tibial components compared. Acta Orthop Scand. 1992;63:247–255. doi: 10.3109/17453679209154776. [DOI] [PubMed] [Google Scholar]
- 8.Blunn GW, Joshi AB, Minns RJ, Lidgren L, Lilley P, Ryd L, Engelbrecht E, Walker PS. Wear in retrieved condylar knee arthroplasties: a comparison of wear in different designs of 280 retrieved condylar knee prostheses. J Arthroplasty. 1997;12:281–290. doi: 10.1016/S0883-5403(97)90024-3. [DOI] [PubMed] [Google Scholar]
- 9.Bragdon CR, O’Connor DO, Lowenstein JD, Jasty M, Syniuta WD. The importance of multidirectional motion on the wear of polyethylene. Proc Inst Mech Eng H. 1996;210:157–165. doi: 10.1243/PIME_PROC_1996_210_408_02. [DOI] [PubMed] [Google Scholar]
- 10.Burstein AH, Wright TM. Biomechanics. In: Insall JN, Windsor RE, Scott WN, Kelly MA, Aglietti P, editors. Surgery of the Knee. 2. New York, NY: Churchill Livingstone; 1993. pp. 43–62. [Google Scholar]
- 11.Collier JP, Mayor MB, McNamara JL, Surprenant VA, Jensen RE. Analysis of the failure of 122 polyethylene inserts from uncemented tibial knee components. Clin Orthop Relat Res. 1991;273:232–242. [PubMed] [Google Scholar]
- 12.Collier JP, Sperling DK, Currier JH, Sutula LC, Saum KA, Mayor MB. Impact of gamma sterilization on clinical performance of polyethylene in the knee. J Arthroplasty. 1996;11:377–389. doi: 10.1016/S0883-5403(96)80026-X. [DOI] [PubMed] [Google Scholar]
- 13.Costa L, Luda MP, Trossarelli L, Brach del Prever EM, Crova M, Gallinaro P. In vivo UHMWPE biodegradation of retrieved prosthesis. Biomaterials. 1998;19:1371–1385. doi: 10.1016/S0142-9612(98)00013-1. [DOI] [PubMed] [Google Scholar]
- 14.Currier BH, Currier JH, Mayor MB, Lyford KA, Citters DW, Collier JP. In vivo oxidation of gamma-barrier-sterilized ultra-high-molecular-weight polyethylene bearings. J Arthroplasty. 2007;22:721–731. doi: 10.1016/j.arth.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 15.D’Lima DD, Chen PC, Colwell CW., Jr Polyethylene contact stresses, articular congruity, and knee alignment. Clin Orthop Relat Res. 2001;392:232–238. doi: 10.1097/00003086-200111000-00029. [DOI] [PubMed] [Google Scholar]
- 16.Fregly BJ, Marquez-Barrientos C, Banks SA, DesJardins JD. Increased conformity offers diminishing returns for reducing total knee replacement wear. J Biomech Eng. 2010;132:021007. doi: 10.1115/1.4000868. [DOI] [PubMed] [Google Scholar]
- 17.Furman BD, Lipman J, Kligman M, Wright TM, Haas SB. Tibial post wear in posterior-stabilized knee replacements is design-dependent. Clin Orthop Relat Res. 2008;466:2650–2655. doi: 10.1007/s11999-008-0422-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galvin AL, Kang L, Udofia I, Jennings LM, McEwen HM, Jin Z, Fisher J. Effect of conformity and contact stress on wear in fixed-bearing total knee prostheses. J Biomech. 2009;42:1898–1902. doi: 10.1016/j.jbiomech.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Hood RW, Wright TM, Burstein AH. Retrieval analysis of total knee prostheses: a method and its application to 48 total condylar prostheses. J Biomed Mater Res. 1983;17:829–842. doi: 10.1002/jbm.820170510. [DOI] [PubMed] [Google Scholar]
- 20.Ingham E, Fisher J. Biological reactions to wear debris in total joint replacement. Proc Inst Mech Eng H. 2000;214:21–37. doi: 10.1243/0954411001535219. [DOI] [PubMed] [Google Scholar]
- 21.Keeney JA, Eunice S, Pashos G, Wright RW, Clohisy JC. What is the evidence for total knee arthroplasty in young patients? A systematic review of the literature. Clin Orthop Relat Res. 2011;469:574–583. doi: 10.1007/s11999-010-1536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtz SM. UHMWPE Biomaterials Handbook. 2. Boston, MA: Elsevier; 2009. [Google Scholar]
- 23.Kurtz SM, Bartel DL, Rimnac CM. Postirradiation aging affects stress and strain in polyethylene components. Clin Orthop Relat Res. 1998;350:209–220. doi: 10.1097/00003086-199805000-00029. [DOI] [PubMed] [Google Scholar]
- 24.Landy MM, Walker PS. Wear of ultra-high-molecular-weight polyethylene components of 90 retrieved knee prostheses. J Arthroplasty. 1988;3:S73–S85. doi: 10.1016/S0883-5403(88)80013-5. [DOI] [PubMed] [Google Scholar]
- 25.Laurent MP, Johnson TS, Yao JQ, Blanchard CR, Crowninshield RD. In vitro lateral versus medial wear of a knee prosthesis. Wear. 2003;255:1101–1106. doi: 10.1016/S0043-1648(03)00271-0. [DOI] [Google Scholar]
- 26.Lavernia CJ, Sierra RJ, Hungerford DS, Krackow K. Activity level and wear in total knee arthroplasty: a study of autopsy retrieved specimens. J Arthroplasty. 2001;16:446–453. doi: 10.1054/arth.2001.23509. [DOI] [PubMed] [Google Scholar]
- 27.Liau JJ, Cheng CK, Huang CH, Lo WH. The effect of malalignment on stresses in polyethylene component of total knee prostheses—a finite element analysis. Clin Biomech (Bristol, Avon). 2002;17:140–146. doi: 10.1016/S0268-0033(01)00109-7. [DOI] [PubMed] [Google Scholar]
- 28.Lidgren L, Robertsson O, editors. The Swedish Arthroplasty Registry–Annual Report 2008. Lund, Sweden: Wallin & Dalholm AB; 2008. [Google Scholar]
- 29.McClung CD, Zahiri CA, Higa JK, Amstutz HC, Schmalzried TP. Relationship between body mass index and activity in hip or knee arthroplasty patients. J Orthop Res. 2000;18:35–39. doi: 10.1002/jor.1100180106. [DOI] [PubMed] [Google Scholar]
- 30.McDowell MA, Fryar CD, Ogden CL. Anthropometric reference data for children and adults: United States, 1988–1994. Vital Health Stat 11. 2009;249:1–68. [PubMed] [Google Scholar]
- 31.McGloughlin TM, Kavanagh AG. Wear of ultra-high molecular weight polyethylene (UHMWPE) in total knee prostheses: a review of key influences. Proc Inst Mech Eng H. 2000;214:349–359. doi: 10.1243/0954411001535390. [DOI] [PubMed] [Google Scholar]
- 32.Medel FJ, Kurtz SM, Parvizi J, Klein GR, Kraay MJ, Rimnac CM. In vivo oxidation contributes to delamination but not pitting in polyethylene components for total knee arthroplasty. J Arthroplasty. 2011;26:802–810. doi: 10.1016/j.arth.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naudie DD, Ammeen DJ, Engh GA, Rorabeck CH. Wear and osteolysis around total knee arthroplasty. J Am Acad Orthop Surg. 2007;15:53–64. doi: 10.5435/00124635-200701000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Pascau A, Guardia B, Puertolas JA, Gómez-Barrena E. Knee model of hydrodynamic lubrication during the gait cycle and the influence of prosthetic joint conformity. J Orthop Sci. 2009;14:68–75. doi: 10.1007/s00776-008-1287-6. [DOI] [PubMed] [Google Scholar]
- 35.Popoola OO, Yao JQ, Johnson TS, Blanchard CR. Wear, delamination, and fatigue resistance of melt-annealed highly crosslinked UHMWPE cruciate-retaining knee inserts under activities of daily living. J Orthop Res. 2010;28:1120–1126. doi: 10.1002/jor.21104. [DOI] [PubMed] [Google Scholar]
- 36.Popov VL. Contact Mechanics and Friction: Physical Principles and Applications. Berlin, Germany: Springer Verlag; 2010. [Google Scholar]
- 37.Puloski SK, McCalden RW, MacDonald SJ, Rorabeck CH, Bourne RB. Tibial post wear in posterior stabilized total knee arthroplasty—an unrecognized source of polyethylene debris. J Bone Joint Surg Am. 2001;83:390–397. doi: 10.2106/00004623-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Revell PA. The combined role of wear particles, macrophages and lymphocytes in the loosening of total joint prostheses. J R Soc Interface. 2008;5:1263–1278. doi: 10.1098/rsif.2008.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robertsson O, Dunbar M, Pehrsson T, Knutson K, Lidgren L. Patient satisfaction after knee arthroplasty: a report on 27,372 knees operated on between 1981 and 1995 in Sweden. Acta Orthop Scand. 2000;71:262–267. doi: 10.1080/000164700317411852. [DOI] [PubMed] [Google Scholar]
- 40.Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award Paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7–13. doi: 10.1097/00003086-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Surace MF, Berzins A, Urban RM, Jacobs JJ, Berger RA, Natarajan RN, Andriacchi TP, Galante JO. Coventry Award Paper. Backsurface wear and deformation in polyethylene tibial inserts retrieved postmortem. Clin Orthop Relat Res. 2002;404:14–23. doi: 10.1097/00003086-200211000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Toohey KS, Blanchet TA, Heckelman DD. Effect of accelerated aging conditions on resultant depth-dependent oxidation and wear resistance of UHMWPE joint replacement bearing materials. Wear. 2003;255:1076–1084. doi: 10.1016/S0043-1648(03)00270-9. [DOI] [Google Scholar]
- 43.Wang A, Stark C, Dumbleton JH. Mechanistic and morphological origins of ultra-high molecular weight polyethylene wear debris in total joint replacement prostheses. Proc Inst Mech Eng H. 1996;210:141–155. doi: 10.1243/PIME_PROC_1996_210_407_02. [DOI] [PubMed] [Google Scholar]
- 44.Wasielewski RC. The causes of insert backside wear in total knee arthroplasty. Clin Orthop Relat Res. 2002;404:232–246. doi: 10.1097/00003086-200211000-00037. [DOI] [PubMed] [Google Scholar]
- 45.Wasielewski RC, Galante JO, Leighty RM, Natarajan RN, Rosenberg AG. Wear patterns on retrieved polyethylene tibial inserts and their relationship to technical considerations during total knee arthroplasty. Clin Orthop Relat Res. 1994;299:31–43. [PubMed] [Google Scholar]
- 46.Wasielewski RC, Parks N, Williams I, Surprenant H, Collier JP, Engh G. Tibial insert undersurface as a contributing source of polyethylene wear debris. Clin Orthop Relat Res. 1997;345:53–59. doi: 10.1097/00003086-199712000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Willie BM, Foot LJ, Prall MW, Bloebaum RD. Surface damage analysis of retrieved highly crosslinked polyethylene tibial components after short-term implantation. J Biomed Mater Res B Appl Biomater. 2008;85:114–124. doi: 10.1002/jbm.b.30923. [DOI] [PubMed] [Google Scholar]
- 48.Wimmer MA, Andriacchi TP. Tractive forces during rolling motion of the knee: implications for wear in total knee replacement. J Biomech. 1997;30:131–137. doi: 10.1016/S0021-9290(96)00112-1. [DOI] [PubMed] [Google Scholar]
- 49.Wimmer MA, Andriacchi TP, Natarajan RN, Loos J, Karlhuber M, Petermann J, Schneider E, Rosenberg AG. A striated pattern of wear in ultrahigh-molecular-weight polyethylene components of Miller-Galante total knee arthroplasty. J Arthroplasty. 1998;13:8–16. doi: 10.1016/S0883-5403(98)90069-9. [DOI] [PubMed] [Google Scholar]
- 50.Wrona M, Mayor MB, Collier JP, Jensen RE. The correlation between fusion defects and damage in tibial polyethylene bearings. Clin Orthop Relat Res. 1994;299:92–103. [PubMed] [Google Scholar]
- 51.Yao JQ, Blanchard CR, Lu X, Laurent MP, Johnson TS, Gilbertson LN, Swarts DF, Crowninshield RD. Improved resistance to wear, delamination, and posterior loading fatigue damage of electron beam irradiated, melt-annealed, highly crosslinked UHMWPE knee inserts. In: Kurtz SM, Gsell RA, Martell J, editors. Standard Technical Publication 1445: Crosslinked and Thermally Treated Ultra-High Molecular Weight Polyethylene for Joint Replacements. West Conshohocken, PA: ASTM International; 2004. pp. 59–72. [Google Scholar]