Abstract
Introduction
Patients with aggressive lower extremity musculoskeletal tumors may be candidates for either above-knee amputation or limb-salvage surgery. However, the subjective and objective benefits of limb-salvage surgery compared with amputation are not fully clear.
Questions/Purposes
We therefore compared functional status and quality of life for patients treated with above-knee amputation versus limb-salvage surgery.
Methods
We reviewed 20 of 51 patients aged 15 years and older treated with above-knee amputation or limb-salvage surgery for aggressive musculoskeletal tumors around the knee between 1994 and 2004 as a retrospective cohort study. At last followup we obtained the Physiological Cost Index, the Reintegration to Normal Living Index, SF-36, and the Toronto Extremity Salvage Score questionnaires. The minimum followup was 12 months (median, 56 months; range, 12–108 months).
Results
Compared with patients having above-knee amputation, patients undergoing limb-salvage surgery had superior Physiological Cost Index scores and Reintegration to Normal Living Index. The Toronto Extremity Salvage scores and SF-36 scores were similar in the two groups.
Conclusion
These data suggest that limb-salvage surgery offers better gait efficiency and return to normal living compared with above-knee amputation, but does not improve the patient’s perception of quality of life.
Level of Evidence
Level III, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Limb-salvage surgery (LSS) is considered the cornerstone of treatment for musculoskeletal sarcoma of the extremities if a functional limb can be attained and no oncologic contraindications are present [5]. However, the specific advantages of LSS over amputation for tumors around the knee remain unclear. Several studies [12, 28, 29] suggest no major disadvantage in terms of overall survival when comparing LSS with primary amputation of osteosarcoma, although local recurrence is consistently more frequent with LSS. Despite limited data for comparison, increased use of LSS for osteosarcoma does not appear to be associated with a decline in survival [3]. The effects of surgical treatment of sarcomas on quality of life, however, remain controversial [22]. Studies attempting to compare overall quality of life between the two treatment modalities have tended to show no differences [2, 23, 25, 30, 34]. Psychological acceptance of LSS and amputation is reportedly similar [21, 27, 28, 32]. Several studies, however, provide evidence for superior function in patients who have undergone LSS; this includes subjective questionnaire data as assessed with the Musculoskeletal Tumor Society (MSTS) score [2, 15, 16, 26, 28, 34] and objective measurements of functional capacity [4, 10]. Given the limited information, particularly regarding objective functional measurements, we thought it important to confirm these observations.
We therefore asked: (1) Does modern LSS for sarcoma about the knee provide patients with quality-of-life benefits compared with above-knee amputation (AKA)? (2) Do patients self-report superior physical functionality in either of the groups? (3) Does either treatment group provide patients with improved physical gait efficiency as measured by the Physiological Cost Index (PCI)?
Patients and Methods
We retrospectively identified 68 patients with sarcomas or aggressive tumors around the knee treated with AKA (n = 24) or LSS (n = 44) between March 1994 and July 2004. We excluded 48 of the 68 patients: 17 patients owing to age younger than 15 years at the time of the latest followup; two patients had not yet reached the 12-month minimum followup at the time the study was conducted; 19 patients were no longer alive at the time of followup; and 10 patients were lost to followup with last visits between 6 and 62 months postoperatively (two with AKA, eight with LSS). These exclusions left 20 of the 51 eligible patients older than 15 years (39%) with sarcomas or aggressive tumors around the knee who underwent AKA (n = 6) or LSS (n = 14) (Table 1). There were eight females and 12 males ranging in age from 15 to 76 years (mean, 34 years). Parental consent for participation was obtained from all participants younger than 18 years. Osteosarcoma was the most frequent diagnosis (nine patients), followed by Ewing’s sarcoma (four patients), giant cell tumor (three patients), pigmented villonodular synovitis (two patients), and chondrosarcoma (two patients). The minimum followup was 12 months (median, 56 months; range, 12–108 months). No patients were recalled specifically for this study; all data were obtained from medical records.
Table 1.
Clinical demographics
| Type of treatment | Age of patients (years) | Gender | Diagnosis | Followup (months) |
|---|---|---|---|---|
| Limb-salvage surgery | 22 | M | Osteosarcoma | 32 |
| 47 | M | Chondrosarcoma | 35 | |
| 20 | F | Ewing’s sarcoma | 12 | |
| 19 | F | Osteosarcoma | 16 | |
| 60 | M | Giant cell tumor | 83 | |
| 18 | F | Ewing’s sarcoma | 54 | |
| 37 | F | Giant cell tumor | 44 | |
| 76 | F | Pigmented villonodular synovitis | 84 | |
| 47 | M | Giant cell tumor | 106 | |
| 30 | M | Osteosarcoma | 108 | |
| 21 | M | Osteosarcoma | 105 | |
| 17 | F | Osteosarcoma | 57 | |
| 15 | M | Osteosarcoma | 37 | |
| 16 | M | Osteosarcoma | 105 | |
| Above-knee amputation | 16 | M | Ewing’s sarcoma | 17 |
| 51 | F | Chondrosarcoma | 12 | |
| 39 | F | Osteosarcoma | 21 | |
| 20 | M | Ewing’s sarcoma | 72 | |
| 34 | M | Osteosarcoma | 96 | |
| 72 | M | Pigmented villonodular synovitis | 75 |
Four patients, all of whom were treated with LSS, were younger than 18 years at the time of the surgery. There were 15 patients with malignant tumors and five with nonmalignant but aggressive tumors (Table 1). Distribution of age was similar between the two groups (LSS mean, 32 years, CI, 27–37 years; AKA mean, 39 years, CI, 30–47 years) On average, time to followup was longer in the LSS group (LSS mean, 63 months, CI, 53–58 months; AKA mean, 49 months, CI, 34–64 months). The AKA group had a greater percentage of female patients (six of 14 patients in the LSS group; two of six patients in the AKA group).
Surgical procedures were performed according to the size and location of the lesions. Decisions to primarily amputate were based on the inability to attain a functional limb after adequate resection. Encasement of neurovascular structures also was considered an indication for primary amputation. AKA was performed using a standard technique as previously described [11]. For LSS, distal femur or proximal tibial replacement prostheses were used (Stryker, Kalamazoo, MI, USA). Additional soft tissue procedures were performed when resection did not allow for adequate coverage.
All patients underwent physical therapy and occupational therapy, while hospitalized, for daily 30-minute observed sessions of moderate intensity. Weightbearing status varied depending on the surgical procedure, need for flap coverage, and the amount of resected bone. Patients who had amputations were not permitted to bear weight until the surgical wound was well healed and nontender. Patients who had limb salvage with cemented implants were given a weightbearing status based on the amount of resected bone that ranged from immediate full weightbearing to nonweightbearing for 6 weeks. Patients requiring musculocutaneous flap coverage were allowed limited weightbearing based on the need to protect the flap for as much as 6 weeks. After the 2-week followup, patients began an outpatient physical therapy program of three weekly visits for 4–6 weeks.
For patients who had amputations, consultation with a prosthetist was initiated early during the initial hospital stay. Prosthetic fitting was performed after the 6-week clinical followup if the wound was well healed and nontender. Physical therapy was continued for prosthetic training with three weekly visits for an additional 4 weeks.
Patients returned for clinical followup monthly for the first year after surgery, with a physical examination and chest radiographs performed at every visit. Surveillance CT scans were performed quarterly. Patients returned every 3 months for Years 2 through 5, then on a yearly basis. Endpoint measures included the SF-36 quality-of-life measure [31], Reintegration to Normal Living Index (RNL) [33], were the Toronto Extremity Salvage Score (TESS) [7], and PCI [20]. These measures assessed at an appointment for regular postoperative followup at a minimum of 12 months after surgery (median, 56 months; range, 12–108 months). Assessment of the PCI score was performed using a simple walking test. Use of assistive walking devices was permitted. Before walking, the patient was allowed to rest for 5 minutes and an average resting heart rate was calculated during the subsequent 2 minutes. The patient then was instructed to walk at a comfortable walking speed for 5 minutes. A pulse monitor was used to record the walking heart rate during the final 2 minutes of this period. The PCI was calculated as the difference between walking and resting heart rate divided by the distance (meters) walked. This score has high test-retest reproducibility among individuals with lower-limb amputations and healthy adults, and acceptable intraclass correlation [13]. In a study by Fredrickson et al. [9], comparing healthy adults and stroke victims, the PCI was validated as an appropriate proxy for the oxygen cost of walking with a correlation coefficient to oxygen consumption of r = 0.83; the correlation coefficient of the PCI to walking speed was reported as r = −0.461.
Data for the three primary measures were evaluated using descriptive statistics to assess data distribution. We determined differences in SF-36, RNL, TESS, and PCI scores between patients with LSS and AKA using nonparametric Mann-Whitney U tests. Statistical analysis was performed using SPSS Statistics 17.0 (IBM; Armonk, NY, USA).
Results
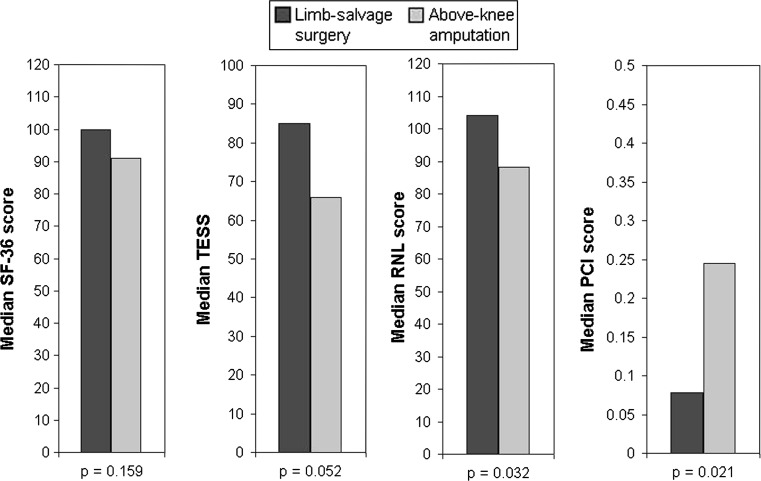
We observed no difference (p = 0.176) in the SF-36 scores between the LSS group (median, 100) and the AKA group (median, 91). RNL scores were higher (p = 0.032) in the LSS group (median, 104) than in the AKA group (median, 88).
TESS scores were similar (p = 0.051) between the LSS and AKA groups (LSS = 85; AKA = 66).
The PCI scores showed more efficient (p = 0.021) gait in the LSS group: the median PCI was 0.078 beats m−1 in the LSS group and 0.245 beats m−1 in the AKA group (Fig. 1). The distance walked also was greater in the LSS group (Table 2).
Fig. 1.
The median scores in both treatment groups are shown graphically.
Table 2.
Physiological Cost Index (PCI)
| Measurement | LSS | AKA | p value |
|---|---|---|---|
| Median distance walked (m) | 321 | 107 | 0.011 |
| Median increase in heart rate (beats per minute) | 27 | 31 | 0.741 |
| Median PCI (beats per minute/m) | 0.078 | 0.245 | 0.021 |
LSS = limb-salvage surgery; AKA = above-knee amputation.
Discussion
Despite a large body of literature evaluating the relationship of LSS to patients’ quality of life, the specific benefits to patients remain unclear. Our study, in addition to reexploring general quality-of-life measures, was intended to quantify the previously described positive effects of LSS on physical function. Subjective measures of quality of life, return to normal living, and musculoskeletal functionality were obtained in addition to an objective measure of functional gait efficiency. This information adds to the pool of clinical cases by using established subjective scoring systems. Although not previously cited in the limb-salvage literature, the PCI is a useful low-cost instrument that provides practicing clinician-scientists with a simple method to quantify the energy expenditure associated with mobility.
As a retrospective study, our study has certain limitations common to all retrospective designs. First, the high mortality associated with musculoskeletal tumors and wide geographic catchment area that orthopaedic oncologists serve inherently limits the numbers of patients available for medium- and long-term followup. Among our original cohort only 39% of the potentially eligible patients 15 years or older were available for 1-year followup. Although this represents a limitation with a high risk of selection bias, it is generally similar to that of related retrospective series [15, 28]. Second, to allow patients to reach a functional plateau, a minimum followup of 1 year was set. This might have resulted in a skewed patient collective, as patients with poorer functional status are more likely to be lost to followup owing to decreased survivorship or reduced mobility. The 10 patients lost to followup were distributed between groups in a manner generally proportionate to the group sizes (two in the AKA group; eight in the LSS group).
Third, the minimum followup of 12 months represents a shorter followup than for other studies evaluating functionality and quality of life [2, 23]. Although this limits the long-term conclusions that can be drawn from this study, it provides additional insight into the midterm postoperative period after initial rehabilitation while possibly including a wider range of patient functionality. Fourth, patients who underwent AKAs are likely to have had more advanced and/or widespread disease, potentially reducing their physical abilities and quality of life. However, the lack of observed differences on overall quality of life between the two groups makes this potential source of bias less likely. Fifth, the sample size of this study is relatively small and the risk of Type II error must be discussed. It is possible that differences were present between the two groups in terms of quality of life (SF-36) and TESS, but that the study was not adequately powered to detect these differences. Despite this, there is broad agreement among our data and those of other studies showing no major differences in terms of overall quality of life and TESS scores between the LSS and AKA groups [2, 8, 14, 22, 23, 34]. Sixth, the ability to compare functional ability and quality of life in different age categories is unclear. Some experts believe pediatric patient populations adapt to amputation better and will have different functionality and psychological response [24]; however, we did not have enough pediatric patients to make such a comparison. A minimum age of 15 years was set to allow for inclusion of more patients in their second decade of life—a frequently affected age group—while removing prepubescent and skeletally immature patients from analysis owing to the emotional and physical differences in these patients. Seventh, none of our patients who had AKA used any of the new advanced prosthetic legs such as the C-leg (Otto Bock; Duderstadt, Germany). Kahle et al. [17] reported that use of the C-leg improves performance and quality of life and can increase community ambulation level. This will add to the argument of defining the line between amputation and limb salvage. Eighth, the objective PCI measurement has not been validated specifically in a cohort with soft tissue sarcoma, for whom chemotherapy toxicity seriously affects cardiovascular health [19]. Finally, the heterogeneity of diagnoses among the patients introduces some amount of uncertainty into the conclusions drawn from this work. Followup was limited to a minimum of 1 year to reduce the influence of preoperative chemotherapy or other disease-specific factors that could affect functionality and quality of life.
A literature review was performed to compare our data with data from prior studies. The National Library of Medicine’s MEDLINE database was searched using a combination of the subject heading “sarcoma/surgery” with the keywords “limb salvage” and “lower extremity” with multiple synonyms and alternate terms. This resulted in 527 English-language articles published from 1990 to present, from which 11 articles were identified that compared functional status of limb salvage with amputation (Table 3). Only one of 11 publications observed a difference in quality-of-life scores between the two groups [15]. Three publications included an objective, quantitative functional measure [4, 10, 15]. The one publication that compared AKA only and LSS using an objective measure identified superior physical function in the LSS group [10]. Our study provides additional data to support the lack of quality-of-life difference between these treatment groups. In addition, a validated, objective measurement shows functional quality-of-life improvements in patients who underwent LSS.
Table 3.
Literature comparing functional and quality of life outcomes between lower extremity amputation and limb salvage surgery
| Study | Number | End points | Comparison | Minimum followup (months) |
Conclusions | Limitations | |
|---|---|---|---|---|---|---|---|
| Objective | Subjective | ||||||
| Rougraff et al. [28] | 78 | None | MSTS/KSS | AKA versus limb salvage | 24 | Better function with LSS; no difference in quality of life | No objective endpoints |
| Davis et al. [6] | 36 | None | TESS/SF-36/RNL | All LE amputations versus limb salvage | 12 | Better function with LSS | Combined all LE amputations |
| Renard et al. [26] | 77 | None | MSTS | All LE amputations versus limb salvage | 28 | Better function with LSS | Only included young patients |
| Zahlten-Hingurange et al. [34] | 124 | None | MSTS/QLQ-C30/FLZ | All LE amputations versus limb salvage | 12 | No difference in quality of life | Combined all LE amputations |
| Nagarajan et al. [23] | 528 | None | TESS/QOL-CS | All LE amputations versus limb salvage | 60 | No difference in function or quality of life | Combined all LE amputations |
| Hopyan et al. [15] | 54 | Uptime* | MSTS/SF-36/TESS | All LE amputations versus limb salvage | 60 | Limb salvage showed better quality of life | Only included young patients |
| Ginsberg et al. [10] | 91 | FMA | MSTS/SF-36/TESS | AKA versus limb salvage | 12 | Better function with LSS | Only included young patients |
| Akahane et al. [1] | 22 | None | MSTS/SF-36 | AKA/BKA versus reconstruction versus rotationplasty | 12 | Better function with rotationplasty | Combined AKA/BKA |
| Aksnes et al. [2] | 118 | None | MSTS/SF-36/TESS | AKA/BKA versus limb salvage | 60 | No difference in quality of life; better MSTS scores with LSS | No objective endpoints |
| Robert et al. [27] | 57 | None | TESS/QOL-CS/ABIS | All LE amputations versus limb salvage | 24 | No difference in quality of life, body image, or function | Combined all LE amputations |
| Bekkering et al. [4] | 82 | Multiple† | TESS/Baecke | AKA/BKA/rotationplasty versus limb salvage | 12 | Better function with LSS | Combined AKA/BKA/rotationplasty |
| Current study | 20 | PCI | TESS/SF-36/RNL | AKA versus limb salvage | 12 | Better function with LSS; no difference in quality of life | Small sample size |
LE = lower extremity; LSS = limb salvage surgery; ABIS = Amputee Body Image Score; FLZ = Fragebogen zur Lebenszufriedenheit [Questionnaire for Life Satisfaction]; FMA = Functional Mobility Assessment; KSS = Knee Society score; MSTS = Musculoskeletal Tumor Society Rating Scale; QLQ-C30 = European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; PCI = Physiological Cost Index; QOL-CS = Quality of Life for Cancer Survivors; RNL = Return to Normal Living Index; TESS = Toronto Extremity Salvage Score; AKA = above-knee amputation; BKA = below-knee amputation; * measurement of upright time using a remote activity sensor; †validated measures included timed up and down stairs test, timed up and go test, 6-minute walking test, and remote activity monitoring.
We observed that most patients in both groups presented with high quality-of-life measures. This reflects the good quality of life commonly reflected in the literature, with one large series reporting mental dimensions of SF-36 and levels of physical activity to be comparable to those for the general population [2]. We found no difference of overall quality of life between the AKA and LSS groups as measured by the SF-36, which is in agreement with multiple prior studies (Table 3). However, we did find a difference in the RNL index in favor of the LSS group, suggesting superior return to normal living.
The higher gait efficiency reflected in higher PCI scores for patients treated with LSS suggests a superior level of physical function in these patients. Prior studies using objective measurements comparing patients who have had amputation and those who have had LSS have reported mixed data, with no differences in the amount of time spent upright, but superior scores in functional testing [4, 10, 15]. Our data add to the literature showing superior physical functionality with limb salvage treatment. The PCI may be a useful objective clinical tool for further investigations of LSS. It is simpler to measure than previously described methods of energy cost calculation [18] and requires no special equipment beyond a watch.
The matter of limb salvage versus amputation is a highly personal decision made in the context of the best available evidence. “How will amputating my leg affect my life?” remains a difficult question, particularly given the relative dearth of literature supporting improved quality of life in patients who have had LSS. Data supporting objective differences in physical functionality can provide the surgeon with more answers in an otherwise emotionally charged situation.
Footnotes
Each author certifies that he or she, or a member of their immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Akahane T, Shimizu T, Isobe K, Yoshimura Y, Fujioka F, Kato H. Evaluation of postoperative general quality of life for patients with osteosarcoma around the knee joint. J Pediatr Orthop B. 2007;16:269–272. doi: 10.1097/BPB.0b013e3280925670. [DOI] [PubMed] [Google Scholar]
- 2.Aksnes LH, Bauer HC, Jebsen NL, Folleras G, Allert C, Haugen GS, Hall KS. Limb-sparing surgery preserves more function than amputation: a Scandinavian sarcoma group study of 118 patients. J Bone Joint Surg Br. 2008;90:786–794. doi: 10.1302/0301-620X.90B6.19805. [DOI] [PubMed] [Google Scholar]
- 3.Ayerza MA, Farfalli GL, Aponte-Tinao L, Muscolo DL. Does increased rate of limb-sparing surgery affect survival in osteosarcoma? Clin Orthop Relat Res. 2010;468:2854–2859. doi: 10.1007/s11999-010-1423-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bekkering WP, Vliet Vlieland TP, Koopman HM, Schaap GR, Bart Schreuder HW, Beishuizen A, Jutte PC, Hoogerbrugge PM, Anninga JK, Nelissen RG, Taminiau AH. Functional ability and physical activity in children and young adults after limb-salvage or ablative surgery for lower extremity bone tumors. J Surg Oncol. 2011;103:276–282. doi: 10.1002/jso.21828. [DOI] [PubMed] [Google Scholar]
- 5.Borden EC, Baker LH, Bell RS, Bramwell V, Demetri GD, Eisenberg BL, Fletcher CD, Fletcher JA, Ladanyi M, Meltzer P, O’Sullivan B, Parkinson DR, Pisters PW, Saxman S, Singer S, Sundaram M, Oosterom AT, Verweij J, Waalen J, Weiss SW, Brennan MF. Soft tissue sarcomas of adults: state of the translational science. Clin Cancer Res. 2003;9:1941–1956. [PubMed] [Google Scholar]
- 6.Davis AM, Devlin M, Griffin AM, Wunder JS, Bell RS. Functional outcome in amputation versus limb sparing of patients with lower extremity sarcoma: a matched case-control study. Arch Phys Med Rehabil. 1999;80:615–618. doi: 10.1016/S0003-9993(99)90161-2. [DOI] [PubMed] [Google Scholar]
- 7.Davis AM, Wright JG, Williams JI, Bombardier C, Griffin A, Bell RS. Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual Life Res. 1996;5:508–516. doi: 10.1007/BF00540024. [DOI] [PubMed] [Google Scholar]
- 8.Felder-Puig R, Formann AK, Mildner A, Bretschneider W, Bucher B, Windhager R, Zoubek A, Puig S, Topf R. Quality of life and psychosocial adjustment of young patients after treatment of bone cancer. Cancer. 1998;83:69–75. doi: 10.1002/(SICI)1097-0142(19980701)83:1<69::AID-CNCR10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 9.Fredrickson E, Ruff RL, Daly JJ. Physiological Cost Index as a proxy measure for the oxygen cost of gait in stroke patients. Neurorehabil Neural Repair. 2007;21:429–434. doi: 10.1177/1545968307300400. [DOI] [PubMed] [Google Scholar]
- 10.Ginsberg JP, Rai SN, Carlson CA, Meadows AT, Hinds PS, Spearing EM, Zhang L, Callaway L, Neel MD, Rao BN, Marchese VG. A comparative analysis of functional outcomes in adolescents and young adults with lower-extremity bone sarcoma. Pediatr Blood Cancer. 2007;49:964–969. doi: 10.1002/pbc.21018. [DOI] [PubMed] [Google Scholar]
- 11.Gottschalk F. Transfemoral amputation: biomechanics and surgery. Clin Orthop Relat Res. 1999;361:15–22. doi: 10.1097/00003086-199904000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Grimer RJ, Taminiau AM. Cannon SR; Surgical Subcomitte of the European Osteosarcoma Intergroup. Surgical outcomes in osteosarcoma. J Bone Joint Surg Br. 2002;84:395–400. doi: 10.1302/0301-620X.84B3.12019. [DOI] [PubMed] [Google Scholar]
- 13.Hagberg K, Tranberg R, Zügner R, Danielsson A. Reproducibility of the physiological cost index among individuals with a lower-limb amputation and healthy adults. Physiother Res Int. 2011;16:92–100. doi: 10.1002/pri.477. [DOI] [PubMed] [Google Scholar]
- 14.Hillmann A, Hoffmann C, Gosheger G, Krakau H, Winkelmann W. Malignant tumor of the distal part of the femur or the proximal part of the tibia: endoprosthetic replacement or rotationplasty. Functional outcome and quality-of-life measurements. J Bone Joint Surg Am. 1999;81:462–468. doi: 10.1302/0301-620X.81B3.9134. [DOI] [PubMed] [Google Scholar]
- 15.Hopyan S, Tan JW, Graham HK, Torode IF. Function and upright time following limb salvage, amputation, and rotationplasty for pediatric sarcoma of bone. J Pediatr Orthop. 2006;26:405–408. doi: 10.1097/01.bpo.0000203016.96647.43. [DOI] [PubMed] [Google Scholar]
- 16.Johansen R, Nielsen OS, Keller J. Functional outcome in sarcomas treated with limb-salvage surgery or amputation. Sarcoma. 1998;2:19–23. doi: 10.1080/13577149878118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahle JT, Highsmith MJ, Hubbard SL. Comparison of nonmicroprocessor knee mechanism versus C-Leg on Prosthesis Evaluation Questionnaire, stumbles, falls, walking tests, stair descent, and knee preference. J Rehabil Res Dev. 2008;45:1–14. doi: 10.1682/JRRD.2007.04.0054. [DOI] [PubMed] [Google Scholar]
- 18.Kawai A, Backus SI, Otis JC, Healey JH. Interrelationships of clinical outcome, length of resection, and energy cost of walking after prosthetic knee replacement following resection of a malignant tumor of the distal aspect of the femur. J Bone Joint Surg Am. 1998;80:822–831. doi: 10.2106/00004623-199806000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Lane JM, Kroll MA, Rossbach PG. New advances and concepts in amputee management after treatment for bone and soft-tissue sarcomas. Clin Orthop Relat Res. 1990;256:22–28. [PubMed] [Google Scholar]
- 20.MacGregor J. The objective measurement of physical performance with long-term ambulatory physiological surveillance equipment (LAPSE) In: Stott FD, Reftery EB, Goulding L, editors. ISAM 1979: Proceedings of the Third International Symposium on Ambulatory Monitoring. London, UK: Academic Press; 1979. pp. 29–39. [Google Scholar]
- 21.Meyers PA, Gorlick R. Osteosarcoma. Pediatr Clin North Am. 1997;44:973–989. doi: 10.1016/S0031-3955(05)70540-X. [DOI] [PubMed] [Google Scholar]
- 22.Nagarajan R. Quality of Life (QOL) in patients with osteosarcoma. Recent Results Cancer Res. 2009;179:339–344. doi: 10.1007/978-3-540-77960-5_21. [DOI] [PubMed] [Google Scholar]
- 23.Nagarajan R, Clohisy DR, Neglia JP, Yasui Y, Mitby PA, Sklar C, Finklestein JZ, Greenberg M, Reaman GH, Zeltzer L, Robison LL. Function and quality-of-life of survivors of pelvic and lower extremity osteosarcoma and Ewing’s sarcoma: the Childhood Cancer Survivor Study. Br J Cancer. 2004;91:1858–1865. doi: 10.1038/sj.bjc.6602220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagarajan R, Neglia JP, Clohisy DR, Robison LL. Limb salvage and amputation in survivors of pediatric lower-extremity bone tumors: what are the long-term implications? J Clin Oncol. 2002;20:4493–4501. doi: 10.1200/JCO.2002.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Ottaviani G, Robert RS, Huh WW, Jaffe N. Functional, psychosocial and professional outcomes in long-term survivors of lower-extremity osteosarcomas: amputation versus limb salvage. Cancer Treat Res. 2009;152:421–436. doi: 10.1007/978-1-4419-0284-9_23. [DOI] [PubMed] [Google Scholar]
- 26.Renard AJ, Veth RP, Schreuder HW, Loon CJ, Koops HS, Horn JR. Function and complications after ablative and limb-salvage therapy in lower extremity sarcoma of bone. J Surg Oncol. 2000;73:198–205. doi: 10.1002/(SICI)1096-9098(200004)73:4<198::AID-JSO3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 27.Robert RS, Ottaviani G, Huh WW, Palla S, Jaffe N. Psychosocial and functional outcomes in long-term survivors of osteosarcoma: a comparison of limb-salvage surgery and amputation. Pediatr Blood Cancer. 2010;54:990–999. doi: 10.1002/pbc.22419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rougraff B, Simon M, Kneisl J, Greenberg D, Mankin H. Limb salvage compared with amputation for osteosarcoma of the distal end of the femur: a long-term oncological, functional, and quality-of-life study. J Bone Joint Surg Am. 1994;76:649–656. doi: 10.2106/00004623-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 1986;68:1331–1337. [PubMed] [Google Scholar]
- 30.Sugarbaker PH, Barofsky I, Rosenberg SA, Gianola FJ. Quality of life assessment of patients in extremity sarcoma clinical trials. Surgery. 1982;91:17–23. [PubMed] [Google Scholar]
- 31.Ware JR, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Weddington WW, Jr, Segraves KB, Simon MA. Psychological outcome of extremity sarcoma survivors undergoing amputation or limb salvage. J Clin Oncol. 1985;3:1393–1399. doi: 10.1200/JCO.1985.3.10.1393. [DOI] [PubMed] [Google Scholar]
- 33.Wood-Dauphinee SL, Opzoomer MA, Williams JI, Marchand B, Spitzer WO. Assessment of global function: The Reintegration to Normal Living Index. Arch Phys Med Rehabil. 1988;69:583–590. [PubMed] [Google Scholar]
- 34.Zahlten-Hinguranage A, Bernd L, Ewerbeck V, Sabo D. Equal quality of life after limb-sparing or ablative surgery for lower extremity sarcomas. Br J Cancer. 2004;91:1012–1014. doi: 10.1038/sj.bjc.6602104. [DOI] [PMC free article] [PubMed] [Google Scholar]