Abstract
Ability to form new associations between unrelated items is particularly sensitive to aging, but the reasons for such differential vulnerability are unclear. In this study, we examined the role of objective and subjective factors (working memory and beliefs about memory strategies) on differential relations of age with recognition of items and associations. Healthy adults (N = 100, age 21 to 79) studied word pairs, completed item and association recognition tests, and rated the effectiveness of shallow (e.g., repetition) and deep (e.g., imagery or sentence generation) encoding strategies. Advanced age was associated with reduced working memory (WM) capacity and poorer associative recognition. In addition, reduced WM capacity, beliefs in the utility of ineffective encoding strategies, and lack of endorsement of effective ones were independently associated with impaired associative memory. Thus, maladaptive beliefs about memory in conjunction with reduced cognitive resources account in part for differences in associative memory commonly attributed to aging.
Keywords: aging, memory, self-efficacy, strategy, working memory
Declines in episodic memory are associated with advanced age (Verhaeghen, Marcoen, & Goossens, 1993), but the magnitude of this association depends on the nature of test materials used to investigate memory. For example, age differences in recognition of associations between stimuli are greater than those in memory for single items (Old & Naveh-Benjamin, 2008). According to the associate deficit hypothesis (ADH, Naveh-Benjamin, 2000), the phenomenon reflects reduced ability for binding separate elements at encoding (Chalfonte & Johnson, 1996) and retrieval of bound representations. Such associative deficits are more strongly related to age-related difficulties in rejection of associative foils, particularly those consisting of recombined associations, than impairments in correct associative recognition (Bender, Naveh-Benjamin, & Raz, 2010; Shing, Werkle-Bergner, Li, & Lindenberger, 2008, 2009).
The mechanisms of ADH are unclear and several cognitive, metacognitive, and health-related factors have been proposed to account for them. Among the candidates are reduced cognitive resources such as speed of processing (Salthouse, 1996), impaired attentional control (Craik & Byrd, 1982; Kim & Giovanello, 2011), weakening of executive control (Moscovitch & Winocur, 1992; West, 1996), and reduction in attentional resources (Anderson, Craik, & Naveh-Benjamin, 1998; Craik, 1983; Craik & McDowd, 1987; Rabinowitz, Craik, & Ackerman, 1982). Age-related changes in metamemory (Connor, Dunlosky, & Hertzog, 1997; McDonald-Miszczak, Hertzog, & Hultsch, 1995; see Hertzog & Dunlosky, 2004 for a review) can also contribute to poor mnemonic performance. Relevant aspects of metamemory include memory self-efficacy (Bandura, 1997; Rebok & Balcerak, 1989), goal setting and goal-directed beliefs (West & Yassuda, 2004), metacognitive monitoring (Dunlosky & Connor, 1997; Hertzog, Kidder, Powell-Moman, & Dunlosky, 2002), metacognitive control processes (Berry, West, & Dennehey, 1989; Dunlosky & Connor, 1997), control beliefs (Lachman & Andreoletti, 2006), and strategy use (Kausler 1994; see Light, 1991; Hertzog & Dunlosky, 2004 for reviews).
Reductions in memory self-efficacy (Bandura, 1997; Rebok & Balcerak, 1989), and implicit beliefs in age-related declines of memory (Lineweaver & Hertzog, 1998; Miller & Lachman, 1999; Ryan, 1992; Ryan & Kwong-See, 1993) among older adults may negatively bias performance on memory tests (Hess, Auman, Colcombe, & Rahhal, 2003) by interfering with strategy selection (Hertzog, McGuire, & Lineweaver, 1998) or through stereotype threat (Rahhal, Hasher, Colcombe, 2001). Alternately, advanced age may be accompanied by deficits in deployment of effective strategic mediators (Kausler, 1994). However, such metacognitive processes do not account for the bulk of age-related variance in memory performance (Light, 1996; Salthouse, 1991). Indeed, attentional and inhibitory processes also must be brought to bear in a goal-directed manner in order to encode information from working memory (Baddeley & Hitch, 1974) into long-term stores (see Kane & Engle, 2002; West, 1996 for reviews). Thus, both associative and strategic processes appear necessary for successful associative memory performance (Shing et al., 2008).
In recognition memory tasks, older adults may improve to the level of their young counterparts if they deploy effective strategies at encoding and retrieval (Naveh-Benjamin, Brav, & Levy, 2007). Unfortunately, older adults do not consistently use such beneficial strategies even when instructed by experimenters (Hertzog & Dunlosky, 2004; Taconnat, Raz, Toczé, et al., 2009). It is unclear what prevents older adults from using effective strategies, but it is possible that the problem lies in high processing overhead that may be associated with utilizing effective mnemonic strategies during encoding (Souchay, Isingrini, & Espagnet, 2000; Souchay & Isingrini, 2004; Verhaeghen & Marcoen, 1994). Moreover, multiple modifiers of memory in older adults (e.g., poor strategy use and poor cognitive resources) may act in synergy and reinforce each other’s impact (Shing et al., 2008, 2009; Souchay & Isingrini, 2004).
Although all above-mentioned modifiers of mnemonic performance have been studied in the past, they are rarely, if at all, examined together. Thus, the main objective of this study was to gauge relative contributions and mutual influences of age, cognitive resources, and metamemory on recognition, while identifying differences in factors contributing to recognition of items and associations. With that goal in mind, we administered tests of recognition memory for items and associations and measures of working memory to healthy participants across the adult life span. Prior to testing, the participants received no information about strategy, but after the experiment they were presented with a list of possible strategies and questioned about their beliefs regarding the effectiveness of each strategy on that list for learning word pairs. We hypothesized that advanced age, low working memory capacity, and erroneous beliefs about strategy efficacy would be associated with poor memory performance. Moreover, in accord with the ADH, we expected that those factors would show a stronger link to recognition of associations compared to recognition of items.
Methods
Participants
Participants came from a large metropolitan area in the United States, and were part of an ongoing longitudinal study of aging, brain, and cognition. All participants signed an informed consent form approved by the University Human Investigations Committee and completed a self-report questionnaire to screen for history of health problems. Exclusion criteria were neurological or psychiatric disorders, cardiovascular diseases other than medically controlled hypertension, cancers other than basal cell carcinoma, head trauma with loss of consciousness for more than five minutes, thyroid disorder, diabetes, drug and alcohol abuse, or taking more than three alcoholic drinks per day. Persons not meeting those eligibility criteria or reporting current use of anticonvulsive, anxiolytic, antipsychotic, antihyperglycemic, or antidepressant medications were not included in the study.
The sample included 108 native English-speaking adults (21 to 79 years of age, 72 women) recruited through ads placed in local newspapers, flyers, and word of mouth. All participants had completed a prior wave in the study, approximately 24 months earlier. The sample’s distribution of age by decade was as follows: six persons 18–30 years (4 women), seven persons 31–40 years (5 women), 18 persons 41–50 years (15 women), 31 persons 51–60 (21 women), 20 persons 61–70 years (13 women), and 18 persons over 70 years (11 women). All participants were screened at baseline and follow-up for problems in near, far, and color vision (Optec 2000 Vision Tester, Stereo Optical Co., Inc., Chicago, IL), speech-range auditory deficits (MA27 Audiometer, Maico Diagnostics, Eden Prairie, MN), depressed mood (Center for Epidemiological Studies on Depression, CES-D, Radloff, 1977, cut-off score of 15), and cognitive impairment (Mini Mental State Examination, MMSE; Folstein, Folstein, & McHugh, 1975; cut-off score 26). Participants who did not meet baseline eligibility requirements, or who developed health problems in the follow-up period were not included in the present study. Out of 108 longitudinal study participants originally tested, eight were excluded from analyses due to changes in health status, including development of hypothyroidism (2), cancer (3), and transient ischemic attack (1), as well as prescription of anti-depressant (1) or antihyperglycemic medication (1). All participants reported education of high school diploma or equivalence, and mean education in the sample neared four years of college (15.7 ± 2.6 years). Male and female participants did not differ in mean age, years of education, prevalence of hypertension, or MMSE scores (see Table 1).
Table 1.
Demographic, Metacognitive, and Cognitive Measures by Sex
| Women | Men | |||
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | t or χ2 | p | |
| Age (years) | 55.0 ± 13.8 | 57.8 ± 14.1 | 0.91 | .360 |
| Years Education | 15.9 ± 2.3 | 15.5 ± 2.8 | 0.70 | .485 |
| MMSE Score | 29.0 ± 1.1 | 28.6 ± 0.9 | 1.66 | .102 |
| Hypertensive | 19 (28%) | 12 (39%) | 1.25 | .263 |
| Shallow Strategy | 4.3 ± 1.8 | 4.3 ± 1.8 | 0.03 | .971 |
| Deep Strategy | 8.0 ± 1.6 | 7.2 ± 1.9 | 2.00 | .056 |
| Size Judgment Span | 9.2 ± 2.0 | 9.1 ± 1.5 | 0.45 | .657 |
| Correct nonverbal 3-back | 11.2 ± 4.9 | 9.7 ± 3.8 | 1.62 | .142 |
| Correct verbal 3-back | 15.6. ± 3.2 | 13.7 ± 4.3 | 2.40 | .018 |
| WM Composite | 0.1 ± 0.8 | −0.2 ± 0.7 | 1.81 | .074 |
Note: Strategy = encoding strategy effectiveness rating
After providing informed consent and before the start of cognitive testing, an experimenter measured the participant’s blood pressure with an analog mercury sphygmomanometer (Model 12–525; Country Technology, Gays Mills, WI) using a left arm brachial cuff. The measurements were taken on three separate days with the participant comfortably seated in a quiet room; blood pressure was averaged across measurement occasions. Hypertension was operationally defined as either a diagnosis with prescription of anti-hypertensive medication, or measured blood pressure above threshold (systolic BP >140 mm Hg or diastolic BP > 90 mm Hg; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, 2004).
Design and Procedure
Recognition Tests
The tests of item and association recognition memory were based on Experiment 2 from Naveh-Benjamin’s (2000) study (see Bender et al., 2010 for details). Participants were individually tested on a recognition memory task that included presentation of two word lists (26 word pairs each), each followed by recognition tests for items and associations. All participants in the present study had previously completed the same task during baseline testing two years earlier. The two lists in the present study included one repeated list on which participants had been tested at baseline and one new list. Following administration of each list, the experimenter administered separate recognition memory tests for individual words (items) and pairs of words (associations). During study, word pairs were serially presented in a white, sans-serif font against a black background at the rate of 5500 ms per pair with a 200 ms inter-stimulus interval, on a 17-inch color CRT computer monitor (at resolution of 800 × 600). The two administered word pair lists were randomly drawn from a subset of six lists taken from a pool of 12 lists. Order of presentation, both of the two study lists and the item and association tests, was counterbalanced across participants. The relative position of the words in each pair (left or right) was randomized across participants. Participants responded by pressing one of two keys on a computer keyboard. A “YES” label was placed on the V key and a “NO” label on the N key.
There was no assigned strategy or orienting task. The participants were told they would see a series of 26 word pairs and that they should remember the individual words and the word pairs. Thus, study instructions were intentional, and participants were told they would be tested later on their memory for the words and pairings. To minimize rehearsal following the study phase, the testing program instructed participants to count backwards by threes from a randomly generated 900 number for 60 seconds. Following the distractor task, participants were administered separate recognition memory tests for items and paired associates. The tests were self-paced and participant response was required for the next trial to begin. Test items were visible on the screen for up to 3 s, unless a response was faster. A black screen was shown during the 200 ms inter-trial interval. Before testing, participants heard instructions for each test, studied a practice list of 6 word pairs, counted backwards, and completed four practice trials on each test. Before each test, the experimenter reminded participants which test was to be administered, and that half of the test items were previously studied and half were not. After study, distractor, and test phases, the procedure was repeated for the second word pair list.
Item recognition trials serially displayed 16 words (8 targets / 8 foils). The experimenter told participants to make a ‘YES’ response if the word was among those on the study lists, and to press ‘NO’ if the word was previously unstudied. The associative recognition test serially presented 16 word pairs (8 intact / 8 recombined). The experimenter instructed participants to respond ‘YES’ if the pair was intact, and to respond ‘NO’ if the pair was recombined and not as shown during the study phase. Words from study only appeared on one of the tests.
Memory Strategy Efficacy Beliefs: Personal Encoding Preferences Questionnaire
After completion of study and test phases for both lists, participants completed a modified version of the Personal Encoding Preferences (PEP) Questionnaire (Hertzog & Dunlosky, 2004). This modified PEP Questionnaire instructed participants to rate seven encoding strategies for learning word pairs, using a Likert-type scale ranging from 1 (least effective) to 10 (most effective). The PEP questionnaire covered seven separate strategies: repetition, saying the pair in one’s mind, focusing on the word until it is clearly visible in one’s mind, sentence generation, interactive visualization of the words, inferring personal relevance, and rhyming.
Working Memory
N-Back tests
Participants completed two separate computerized n-back tests to gauge working memory storage and maintenance (Hultsch, Hertzog, & Dixon, 1990; modeled after Dobbs & Rule, 1989). In the verbal n-back test, the participants viewed single-digit numbers, and in the nonverbal n-back test they viewed abstract shapes on a computer screen. The relative position of the target item in the list was held constant in separate presentations for 1-, 2-, and 3-back tests. Following presentation of items, the participants were asked to indicate which item had been presented in the specified serial position. No specific instructions regarding speed or accuracy were given. For the verbal n-back, participants pressed the key on the computer keyboard number pad that corresponded to the digit presented in the indicated position. Following presentation of the list of items in each trial on the nonverbal test, participants were presented with a screen that included all items with a digit under each item; participants pressed the key on the number pad that corresponded to the item presented in the indicated serial position. Presentation order of the three subtasks was counterbalanced across participants in a Latin square design. Performance indices included response time and number of correct responses. The tasks’ estimated reliability coefficients are .91 for the verbal and .88 for the nonverbal tests (Salthouse, Hancock, Meinz, & Hambrick, 1996). As the participants have been administered that test at the baseline of the longitudinal study, we used only the repeated administration scores concurrent with the association memory and strategy questionnaire. Test-retest reliability observed in our longitudinal study was r = .58 for the verbal and r = .73 for the nonverbal test after correction for attenuation (Raz, unpublished data).
Size Judgment Span
In this working memory task designed by Cherry & Park (1993), an experimenter reads a list of items for participants to hold in primary memory, perform mental comparisons, and recall the items in order of ascending physical size. Upon successful completion of each three-trial set, the number of items increases by one. The task’s estimated reliability coefficient is .79 (Cherry & Park, 1993), which corresponds to the disattenuated test-retest correlation observed in our longitudinal study (Raz, unpublished data).
Data Conditioning
We excluded recognition trials from analyses if response times were shorter than 200 ms or longer than 10 s, as those responses may be erroneous or reflect memory processes other than recognition. Hit rate (HR) and false alarm rate (FAR) were calculated as the proportion of correct target and incorrect lure responses, respectively. Both HR and FAR were arcsine-transformed to correct for significant skewness in their respective distributions.
The seven items from the PEP were submitted to an exploratory factor analysis (EFA) using Mplus 6.0 (Muthén & Muthén, 2010). The EFA iteratively fit solutions from one to four factors using CF-varimax rotation. Only the two-factor model showed acceptable fit: χ2(8) = 13.4, p = .1; RMSEA = .082, SRMR = .038. Rotated factor loadings were consistent with the notion of deep or effective vs. shallow or ineffective associative encoding strategies (Table 2). Scores were averaged across each of the two factors to yield mean deep and shallow strategy ratings. A quadratic function (x2 + 10) transformation addressed significant skew in the distribution of mean deep strategy ratings. The transformed strategy values were then centered and standardized to z-scores. The two strategy rating indices were not correlated (r =−.05, p > .6), and therefore we performed separate item analyses on the PEP for shallow and deep strategy items. Cronbach’s α was .63 for shallow items and .58 for deep strategy items.
Table 2.
Factor Loadings for Seven PEP Items
| Factor Loadings | |||
|---|---|---|---|
| Factor 1 | Factor 2 | ||
| Item | Strategy | "Deep" | "Shallow" |
| 1 | Repetition (shallow) | −0.176 | 0.513 |
| 2 | Read or say pair in mind (shallow) | 0.109 | 0.514 |
| 3 | Personal meaning (deep) | 0.691 | 0.031 |
| 4 | Stare and see in your minds eye (shallow) | −0.043 | 0.657 |
| 5 | Visual imagery / scene construction (deep) | 0.581 | −0.129 |
| 6 | Sentence construction (deep) | 0.457 | 0.128 |
| 7 | Rhyming Pair (shallow) | 0.079 | 0548 |
Working Memory Composite
We hypothesized that individual differences contributing to decrements in working memory may also contribute to differences in long-term memory performance and beliefs about strategy effectiveness. Results of an unrotated principal components analysis demonstrated that number correct from the 3-back condition of the verbal and nonverbal n-back tests and total number correct on the Size Judgment Span test loaded onto a single common component (all component loadings > .74). We therefore formed a working memory (WM) composite composed of the averaged, standardized scores from the three tests.
Statistical Analyses
We used a structural equation modeling (SEM) approach to partition the age-related variance by way of shared associations among individual differences in multiple continuous variables. To test hypotheses regarding partitioning of the age-related variance in memory performance between WM and strategic beliefs, we conducted a path analysis (Mplus 6.0, Muthén & Muthén, 2010) using maximum likelihood (ML) estimation. The path analysis variables included the four arcsine-transformed HR and FAR scores (item, association), mean-centered age, WM composite scores, and mean ratings of shallow and deep encoding strategies. Indirect effects were specified to test hierarchical partitioning of age-ascribed variance in associative recognition (HR and FAR for associations) between WM and strategy beliefs. In this hierarchy constructed on the bases of extant theories of cognitive aging reviewed above, we postulated that strategic beliefs explained variance in recognition memory and WM explained variance in strategic beliefs, thus partitioning the variance in memory initially attributed to age alone. We used bias-corrected bootstrap re-sampling with 1000 draws to generate estimates and 95% confidence intervals (CIs) of indirect effects (Cheung & Lau, 2008; Shrout & Bolger, 2002; Table 5), as such an approach is considered the most reliable method for estimation of indirect effects in SEM (MacKinnon, Lockwood, Hoffman, West, & Sheets, 2002; MacKinnon, Lockwood, & Williams, 2004).
Table 5.
Indirect Effects from the Final Path Model
| Indirect Effect | Std. Estimate |
Est. / S.E. |
p | 95% CI |
|---|---|---|---|---|
| Age → WM → Shallow Strategy → Association HR | −.034 | −2.00 | .043 | −.067 to −.001 |
| WM→ Shallow Strategy → Association HR | .070 | 1.94 | .050 | .000 to .129 |
| Age → WM → Deep Strategy → Association HR | −.034 | −2.13 | .032 | −.065 to −.003 |
| WM → Deep Strategy → Association HR | .070 | 2.33 | .020 | .011 to .129 |
| Age → WM → Association FAR | .135 | 2.81 | .005 | .041 to .229 |
| Age → WM → Shallow Strategy | .141 | 3.20 | .001 | .055 to .227 |
| Age → WM → Deep Strategy | −.115 | −2.45 | .014 | −.207 to −.023 |
S.E. = Standard error, WM – working memory, HR – hit rate, FAR – false alarm rate, CI – confidence interval.
Three alternate models were tested. The first, referred to here as “age-partialed” model specified direct paths from age to all variables. The second, a “reversed-path” model was predicated on the assumption that variance in WM performance might be explained by strategic beliefs or the unknown factors from which such beliefs act as proxies. In the reversed-path model, the directional relations between WM and strategic beliefs were reversed, i.e. beliefs acting as a source, and WM as a downstream variable. The third alternative was the “correlational” model in which we tested the possibility that the relationships among the variables without specific directionality may fit the data as well. All alternative models were specified based on the final path model with no re-specification except for adding direct age paths in the age-partial model, reversing the paths between cognitive and metacognitive variables in the reversed-path model and substitution of correlations for direct paths in the correlational model. It must be noted that in these analyses, we did not seek to test any causal links or hypotheses about change as correlational methods such as SEM are unsuitable for those objectives with cross-sectional data (Lindenberger & Pötter, 1998; Hofer, Flaherty, & Hoffman, 2006; Maxwell & Cole, 2007; Lindenberger, von Oertzen, Ghisletta, & Hertzog, 2011; Raz & Lindenberger, 2011).
Because the PEP was administered only at follow-up, we limited our analyses of cognitive measures to the scores obtained at follow-up as well. For the same reason, the present analyses of data from the recognition task were limited to the novel word pair list not previously presented at baseline.
Based on the hypotheses, the initial model specified paths from age to WM, deep and shallow strategic beliefs, and the four memory indices; direct paths were also specified from WM to deep and shallow strategy ratings, from WM to HR and FAR for both item and associations, and from both strategy composites to the two HR and two FAR indices. Although neither correlational relationships nor constraints were initially specified, the model was subsequently reduced to remove unreliable paths (p > .15). In addition, a single constraint was imposed on the correlation between associative FAR and item HR (r = .004, p = .96).
Results
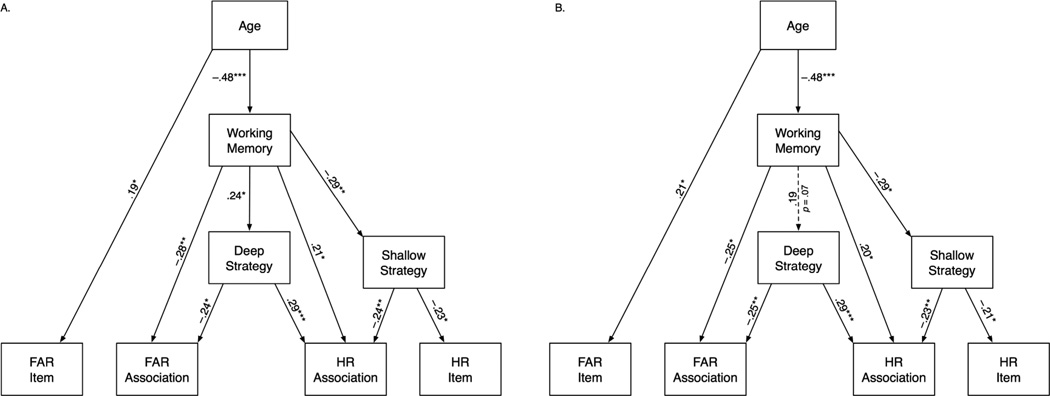
Zero-order correlations (Table 3) showed moderate age differences in hit rate for associations, but not for individual items, and somewhat weaker but significant age differences in the false alarm rates for both. Both the final (reduced) path model (Figure 1a) and the age-partialed model (Figure 1b) had excellent fit according to all indices (Table 4). Although the reversed-path model demonstrated a marginally acceptable fit (Table 4), it was inferior to that of the final model (Figure 1) on all indices. The correlation-only model was over-identified (df = 15) and had worse fit than the final and the reversed-paths models. Thus, the reduced model (Figure 1a) and age-partialed model (Figure 1b) provided the best fit to the data.
Table 3.
Zero-order and Partial Correlations among the Age, Working Memory, Strategy Beliefs and Memory
| Age | WM | Shallow | Deep | Item HR | Association HR | Item FAR | Association FAR | |
|---|---|---|---|---|---|---|---|---|
| Age | — | |||||||
| WM | −.48*** | — | .26* | .17 | .17 | .33*** | −.03 | −.31** |
| Shallow Strategy | .15 | −.29* | — | −.02 | −.22* | −.31** | .05 | .24* |
| Deep Strategy | −.19 | .24 | −.05 | — | .02 | .35*** | −.09 | −.31** |
| Item HR | −.16 | .23 | −.24 | −.05 | — | .27** | 0.13 | −.06 |
| Assoc. HR | −.36*** | .44 | −.33** | .30* | .30** | — | −.15 | −.35*** |
| Item FAR | .21* | −.13 | .08 | −.16 | −.16 | −.21* | — | .20 |
| Association FAR | .26** | −.39*** | .26** | −.34* | −.09 | −.40*** | .24* | — |
Zero-order correlations are below the main diagonal; partial correlations (controlling for age) are below the main diagonal. WM – working memory; HR – hit rate; FAR – false alarm rate.
p < .001,
p < .01,
p < .05
Figure 1.
Only significant paths (***p < .001, **p < .01, *p < .05) are depicted. FAR is false alarm rate and HR is hit rate. A. Reduced path model depicting relations among age, working memory composite scores, mean ratings of shallow and deep encoding strategies, and proportions of hits and false alarms for items and associations. B. Path model maintaining specified direct paths from age to all other modeled variables.
Table 4.
Goodness-of-Fit Indices for Path Models
| Model | χ2 | df | p | χ2/df | CFI | TLI | AIC | Adjusted BIC |
RMSEA | SRMR |
|---|---|---|---|---|---|---|---|---|---|---|
| Final | 5.9 | 11 | .880 | 0.54 | 1.00 | 1.12 | 1554.5 | 1537.3 | .000 | .045 |
| Age-partialed | 2.8 | 7 | .900 | 0.40 | 1.00 | 1.15 | 1559.4 | 1540.5 | .000 | .025 |
| Normotensives only | 5.6 | 11 | .899 | 0.51 | 1.00 | 1.17 | 1018.2 | 989.8 | .000 | .042 |
| Alternate (reversed) | 19.4 | 14 | .151 | 1.38 | 0.94 | 0.89 | 1565.9 | 1549.4 | .062 | .093 |
| Correlational | 25.4 | 15 | .018 | 0.04 | 0.91 | 0.83 | 1570.0 | 1553.9 | .083 | .112 |
In both models, significant direct effects indicated the link between accurate recognition of associations (high HR, low FAR) and higher WM scores and, independently, between memory for associations and a correct belief in effectiveness of deep encoding strategies. Moreover, incorrect belief in effectiveness of shallow strategies was related to low hit rate for associations and items alike. Notably, endorsement of deep strategies was associated with higher WM scores in the reduced model (Figure 1a), but became independent of age and WM (a nonsignificant path with p = .074), once age-related variance is removed (Figure 1b).
The analysis of indirect effects (Table 5) suggested that different variables explained the variance in correct and false recognition of intact and recombined word pairs. Indirect effects are multiplicative and therefore are inherently smaller than the direct effects they comprise. Regardless, the 95% confidence intervals (Table 5) of all significant indirect effects did not overlap with zero. In the final reduced model (Figure 1a), endorsement of ineffective, shallow strategies and lack of belief in the effective, deep strategies, were independently linked to low hit rate for associations. However, both strategic beliefs were significantly associated with WM: negatively (shallow) and positively (deep). High WM scores and belief in effectiveness of deep strategy were independently linked to lower FAR in recognition of associations. In addition, an indirect path from age to WM to associative FAR was the only significant indirect effect involving false recognition.
In the age-partialed model (Figure 1b), age-related differences in WM accounted for a significant share of variance in recognition of associations and endorsement of shallow strategies. In both cases, age-related reduction in WM was linked to increased likelihood of false recognition and false beliefs in effectiveness of ineffective strategies. Partialling out age from all variables resulted in minor reduction of correlations between two indices of memory for associations and endorsement of deep and shallow encoding strategies (see Table 3 for partial correlations). Somewhat greater reduction was observed in associations between WM and memory variables, although in that case, the correlations remain significant. Thus, even with age variance removed, WM and beliefs about strategy effectiveness were independently associated with recognition memory for word associations.
Selection of the best model at this stage of analyses is based on theoretical considerations. As the first model does not remove age-related variance from all variables, it is essentially a mediation model of age effects, with all the problems outlined above (e.g., Lindenberger & Pötter, 1998; Hofer et al., 2006; Raz & Lindenberger, 2011). The second, age-partialed, model presents a complete control for age-related variance and is more suitable for testing individual differences hypotheses.
To test whether inclusion of participants with hypertension unduly affected the relationships among variables observed in the final model, hypertensive persons (n = 31) were excluded and the final model was re-evaluated. Correlations between item and association indices were no longer significant for HR or FAR, likely due to removal of 31% of the sample. As shown in Table 4, the fit did not change from the final path model that included hypertensive participants, and there were no changes in direct effects.
Discussion
The results of this study show that age-related differences in recognition memory for associations can be explained by objective and subjective factors: reduced processing resource (working memory) and deficient metamemory (belief in ineffective encoding strategies). Stronger endorsement of effective, deep encoding strategies was linked to more correct and fewer false recognitions of associations. Belief in inefficient shallow strategies was associated with poor hit rate for items and associations alike. Notably, working memory, an age-sensitive cognitive resource, is related to metamemory: reduced accuracy of performance on WM tasks is linked to increased likelihood of belief in inefficient encoding strategies, and probably to reduced belief in effective deep strategies. Age-related deficits in memory for associations have been widely replicated (see Old & Naveh-Benjamin, 2008 for a review), and impaired cognitive resources and poor metacognitive functioning have been suggested as possible explanations (Naveh-Benjamin, Craik, Guez, & Dori, 1998; Naveh-Benjamin, Craik, Guez, & Kreuger, 2005; Naveh-Benjamin et al., 2007). Our results support that view, although it remains unclear which additional cognitive and metacognitive factors can explain age-related variance.
To interpret our results in the context of extant literature, it is important to draw a distinction between belief in strategy effectiveness as measured here and strategic behavior. It is plausible that the latter indeed follows from the former, as strategic metamemory functioning is associated with belief in the utility of effort or skill in successful memory performance (Riggs, Lachman, & Wingfield, 1997). However, although the link between negative personal control beliefs about strategies and their reduced use has been shown in some studies (Lachman, Weaver, Bandura, & Elliott, 1995), it is not universally upheld (Hertzog, McGuire, Horhota, & Jopp, 2010; see Hertzog & Dunlosky, 2004 for a review). Moreover, according to Hertzog and Dunlosky (2004), strategy knowledge (i.e., semantic understanding regarding practical aspects of strategy implementation) and strategy beliefs (i.e., memory self-efficacy) are proximal but separate influences on strategic behavior in associative recall. Although the present study does shed light on the association between memory performance and self-efficacy beliefs, it does not directly inform about actual strategic behavior or knowledge. Nevertheless, because strategic behavior is the most plausible link between strategy beliefs and memory performance, it is important to consider certain task characteristics that may modify such behavior.
In contrast to recall measures, the lack of feedback in the recognition task may limit performance monitoring important for updating beliefs and knowledge about strategies (Hertzog & Dunlosky, 2004). It is also possible that specific aspects of the encoding task we used may have increased the difficulty of engaging strategic behaviors. The encoding task’s presentation rate of five seconds per pair may be too fast a pace for older adults or those with limited WM resources to spontaneously engage effective strategies such as sentence generation (Hertzog & Dunlosky 2004). In addition, several studied words represented intangible items (e.g., “belief,” “purpose,” or “effect”). Such nouns, unlike names of concrete objects, could discourage strategic production of mental imagery for recognition of associations, and thus might have further hindered generation of effective mediators for associative encoding. Therefore, even greater effort may have been required to use effective encoding strategies under the aforementioned constraints.
An important distinction between strategic behaviors and beliefs is that deployment of effective strategies may require additional effort, whereas no such cost is associated with merely holding a belief. However, in the context of a recognition paradigm, with participants uniformed about and therefore unbiased to strategy use, the degree to which post-test measured beliefs reflect strategy knowledge and selection remains unclear. These findings also highlight a distinction between beliefs in effective and ineffective strategies: the two were not correlated, and thus neither mutually dependent, nor exclusive. The participants who rated both types of strategies as effective could potentially be disabused of their inefficacious beliefs and benefit from training or instruction to reinforce positive metacognitive processes. Furthermore, the models in Figure 1 show that wrongful belief in ineffective strategies was only linked to deficient recognition of intact word pairs, but not false alarms to recombined pairs. High and low beliefs in the utility of effective strategies, however, were associated with greater recognition of intact and increased false alarms to recombined word pairs, respectively. Thus, whereas shallow encoding strategies do not involve mediator production and do little to enhance distinctiveness of studied word pairs, effective strategic beliefs appear to positively modulate the distinctiveness of bound representations and thereby reduce false recognition of recombined word pairs.
Shing and colleagues (2008) proposed that older adults’ elevated false alarms in recognition of recombined word pairs are due to impaired strategic and associative processes. The model presented in Figure 1 supports such a dissociation, in which cognitive resources explain some but not all variance in strategy beliefs and both are independently linked to associative memory. The extant literature indicates that cognitive resources, of which WM is a fundamental component (Knudsen, 2007), are reliably associated with memory performance (Butler, McDaniel, Dornburg, Price, & Roediger, 2004; Troyer, Graves, & Cullum, 1994; see Fletcher & Henson, 2001 for a review). Moreover, cognitive resources play a significant role in operations involved in associative binding (Castel & Craik, 2003; Craik & Byrd, 1982); our finding of the relationship between WM and associative recognition is in accord with those reports. Furthermore, age was associated with false recognition of individual foils, independent of WM and metacognitive functioning, One possibility is age-related reductions in representational distinctiveness of items (Li, Naveh-Benjamin, & Lindenberger, 2005) may be independent of WM and strategy processes that are critical for inter-item binding. Similarly, failure to reject items as novel may be more linked to age-impaired familiarity processes, whereas associative memory essential for recognizing intact and recombined pairs may inherently rely on recollection (Yonelinas, 2002).
Several studies reported links between age-related impairment in executive functions and weakened metacognitive control (Souchay et al., 2000; Souchay & Isingrini, 2004) and between age-associated cognitive decrements in impaired strategy production (Verhaeghen & Marcoen, 1994). Comparison of the reduced path analysis model with age-partialed model and partial correlation analysis reveals that contributions of the cognitive resource (WM) to memory and metamemory depend on age differences in WM scores. In contrast, the share of metamemory in memory variance appears to do little with age differences in either. Experimental studies show that negative beliefs about effectiveness may bias older adults’ choice of strategy (Hertzog et al., 1998) or induce stereotype threat (Rahhal et al., 2001), leading to further memory impairments. Thus, it is plausible that declines in metamemory drive reduction in objective WM measures. Our analyses argue against that explanation, as the models postulating reversed or correlative links between beliefs and cognitive resources did not reflect the data as well as those that presumed the flow of variance from WM to metamemory variables. However, when the effects of age are taken into account (Figure 1b), only the linkage from WM to shallow strategy ratings remains significant, further supporting the proposition that reduced WM capacity is one factor that may lead individuals to believe in the efficacy of ineffective strategies.
The mechanisms by which age-related reduction in cognitive resources affects metacognition are also unclear. In one view, memory self-efficacy beliefs stem from experience in cognitive monitoring (see Hertzog & Hultsch, 2000 for a review; Jopp & Hertzog, 2007), and may be rooted in awareness of one’s own cognitive limitations (Fernandez-Duque, Baird, & Posner, 2000). As a recent meta-analysis demonstrated, memory self-efficacy may be especially important for memory tasks requiring significant allocation of cognitive resources (Beaudoin & Desrichard, 2011). This is supported by prior evidence showing a link between high cognitive abilities, strategic behavior, and associative memory (Kyllonen & Christal, 1990; Kyllonen, Tirre, & Christal, 1991). If participants in the present study felt they were unable to successfully invoke an effective encoding strategy, they might have simply resorted to a less effective, but available shallow one.
This study has several limitations. Although it is a part of longitudinal project, we have only one set of belief measures, administered at follow-up. Statistical methods based on variance partitioning (e.g., path analysis) are appropriate only for evaluation of individual differences; they do not provide valid estimates of change (Lindenberger et al., 2011). Thus, whereas the findings reported here highlight the roles of certain variables in age-related differences in successful and false recognition, they do not inform about the process through which they emerged. Another important limitation is that we examined only the role of strategic beliefs in learning unrelated word pairs, and not the effect of actual strategy use. Because the participants have performed the same memory test before, familiarity with the task could have minimized age-related differences (Ferrer, Salthouse, Stewart, & Schwartz, 2004), although the interval length may mitigate such benefit (Salthouse, 2009). In addition, although the sample size was adequate for testing the hypotheses of cognitive and metacognitive influences on associative recognition, it was nevertheless too small for examining the modification of age differences by vascular risk or sex.
In conclusion, we observed that cross-sectional age differences in associative memory covary with both objective and subjective factors. Establishing whether the observed differences reflect true change and whether reduction in resources precedes strategic decline is an important goal for a longitudinal study. Future efforts to remediate age-related memory deficits may need to pay special attention to designing interventions that alleviate maladaptive metamemory beliefs within the constraints of reduced cognitive resources.
Acknowledgments
This study was supported in part by a grant from the National Institutes of Health (R37-AG-011230). We thank an anonymous reviewer for pointing out the importance of partial correlation analysis.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/XXX
Contributor Information
Andrew R. Bender, Department of Psychology & Institute of Gerontology, Wayne State University
Naftali Raz, Department of Psychology & Institute of Gerontology, Wayne State University.
References
- Anderson ND, Craik FI, Naveh-Benjamin M. The attentional demands of encoding and retrieval in younger and older adults: Evidence from divided attention costs. Psychology and Aging. 1998;13(3):405–423. doi: 10.1037//0882-7974.13.3.405. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch GJ. Working memory. In: Bower GH, editor. The psychology of learning and motivation. Vol. 8. New York: Academic Press; 1974. pp. 47–89. [Google Scholar]
- Bandura A. Self-efficacy: The exercise of control. New York: Freeman; 1997. [Google Scholar]
- Beaudoin M, Desrichard O. Are memory self-efficacy and memory performance related? A meta-analysis. Psychological Bulletin. 2011;137(2):211–241. doi: 10.1037/a0022106. [DOI] [PubMed] [Google Scholar]
- Bender AR, Naveh-Benjamin M, Raz N. Associative deficit in recognition memory in a lifespan sample of healthy adults. Psychology and Aging. 2010;25(4):940–948. doi: 10.1037/a0020595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry JM, West RL, Dennehy DM. Reliability and validity of the memory self-efficacy questionnaire. Developmental Psychology. 1989;25:701–713. [Google Scholar]
- Butler KM, McDaniel MA, Dornburg CC, Price AL, Roediger HL., III Age differences in veridical and false recall are not inevitable: The role of frontal lobe function. Psychonomic Bulletin & Review. 2004;11(5):921–925. doi: 10.3758/bf03196722. [DOI] [PubMed] [Google Scholar]
- Castel AD, Craik FIM. The effects of aging and divided attention on memory for item and associative information. Psychology and Aging. 2003;18(4):873–885. doi: 10.1037/0882-7974.18.4.873. [DOI] [PubMed] [Google Scholar]
- Chalfonte BL, Johnson MK. Feature memory and binding in young and older adults. Memory and Cognition. 1996;24(4):403–416. doi: 10.3758/bf03200930. [DOI] [PubMed] [Google Scholar]
- Cherry KE, Park DC. Individual difference and contextual variables influence spatial memory in younger and older adults. Psychology and Aging. 1993;8(4):517–526. doi: 10.1037//0882-7974.8.4.517. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8292280. [DOI] [PubMed] [Google Scholar]
- Cheung GW, Lau RS. Testing mediation and suppression effects of latent variables: Bootstrapping with structural equation models. Organizational Research Methods. 2008;11(2):296–325. [Google Scholar]
- Connor LT, Dunlosky J, Hertzog C. Age-related differences in absolute but not relative metamemory accuracy. Psychology and Aging. 1997;12(1):50–71. doi: 10.1037//0882-7974.12.1.50. [DOI] [PubMed] [Google Scholar]
- Craik FIM. On the transfer of information from temporary to permanent memory. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 1983;302(1110):341–359. [Google Scholar]
- Craik FIM, Byrd M. Aging and cognitive deficits: The role of attentional resources. Aging and Cognitive Processes. 1982;8:191–211. [Google Scholar]
- Craik FI, McDowd JM. Age differences in recall and recognition. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1987;13(3):474–479. [Google Scholar]
- Dobbs AR, Rule BG. Adult age differences in working memory. Psychology and Aging. 1989;4:500–503. doi: 10.1037//0882-7974.4.4.500. [DOI] [PubMed] [Google Scholar]
- Dunlosky J, Connor LT. Age differences in the allocation of study time account for age differences in memory performance. Memory & Cognition. 1997;25(5):691–700. doi: 10.3758/bf03211311. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque D, Baird J, Posner M. Executive attention and metacognitive regulation. Consciousness and Cognition. 2000;9:288–307. doi: 10.1006/ccog.2000.0447. [DOI] [PubMed] [Google Scholar]
- Ferrer E, Salthouse TA, Stewart WF, Schwartz BS. Modeling age and retest processes in longitudinal studies of cognitive abilities. Psychology and Aging. 2004;19(2):243–259. doi: 10.1037/0882-7974.19.2.243. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN. Frontal lobes and human memory: Insights from functional neuroimaging. Brain. 2001;124(Pt. 5):849–881. doi: 10.1093/brain/124.5.849. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11335690. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Dunlosky J. Aging, metacognition, and cognitive control. In: Ross BH, editor. Psychology of Learning and Motivation. San Diego: CA: Academic Press; 2004. pp. 215–251. [Google Scholar]
- Hertzog C, McGuire CL, Horhota M, Jopp D. Does believing in "use it or lose it" relate to self-rated memory control, strategy use, recall? The International Journal of Aging and Human Development. 2010;70(1):61–87. doi: 10.2190/AG.70.1.c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog C, Hultsch DF. Metacognition in adulthood and aging. In: Craik FIM, Salthouse TA, editors. Handbook of aging and cognition. 2nd ed. Mahwah, NJ: Lawrence Erlbaum; 2000. pp. 417–466. [Google Scholar]
- Hertzog C, Kidder DP, Powell-Moman A, Dunlosky J. Aging and monitoring associative learning: Is monitoring accuracy spared or impaired? Psychology and Aging. 2002;17(2):209–225. [PubMed] [Google Scholar]
- Hertzog C, McGuire CL, Lineweaver TT. Aging, attributions, perceived control, and strategy use in a free recall task. Aging, Neuropsychology, and Cognition. 1998;5(2):85–106. [Google Scholar]
- Hess TM, Auman C, Colcombe SJ, Rahhal TA. The impact of stereotype threat on age differences in memory performance. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2003;58(1):P3–P11. doi: 10.1093/geronb/58.1.p3. [DOI] [PubMed] [Google Scholar]
- Hofer SM, Flaherty BP, Hoffman L. Cross-sectional analysis of time-dependent data: Mean-induced association in age-heterogeneous samples and an alternative method based on sequential narrow age-cohort samples. Multivariate Behavioral Resarch. 2006;41(2):165–187. doi: 10.1207/s15327906mbr4102_4. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Dixon RA. Ability correlates of memory performance in adulthood and aging. Psychology and aging. 1990;5(3):356–368. doi: 10.1037//0882-7974.5.3.356. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2242240. [DOI] [PubMed] [Google Scholar]
- Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. (NIH Publication No. 04-5230) 2004 Retrieved from http://www.nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf.
- Jopp D, Hertzog C. Activities, self-referent memory beliefs, and cognitive performance: Evidence for direct and mediated relations. Psychology and Aging. 2007;22(4):811–825. doi: 10.1037/0882-7974.22.4.811. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychonomic Bulletin & Review. 2002;9(4):637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Kausler DH. Learning and memory in normal aging. New York: Academic Press; 1994. [Google Scholar]
- Kim SY, Giovanello KS. The effects of attention on age-related relational memory deficits: Evidence from a novel attentional manipulation. Psychology and Aging. 2011;26(3):678–688. doi: 10.1037/a0022326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI. Fundamental components of attention. Annual Review of Neuroscience. 2007;30(1):57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- Kyllonen PC, Christal RE. Reasoning ability is (little more than) working-memory capacity?! Intelligence. 1990;14(4):389–433. [Google Scholar]
- Kyllonen PC, Tirre WC, Christal RE. Knowledge and processing speed as determinants of associative learning. Journal of Experimental Psychology: General. 1991;120(1):57. [Google Scholar]
- Lachman ME, Andreoletti C. Strategy use mediates the relationship between control beliefs and memory performance for middle-aged and older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2006;61(2):P88–P94. doi: 10.1093/geronb/61.2.p88. Retrieved from http://psychsocgerontology.oxfordjournals.org/content/61/2/P88.full. [DOI] [PubMed] [Google Scholar]
- Lachman ME, Weaver SL, Bandura M, Elliott E. Assessing memory control beliefs: The memory controllability inventory. Aging and Cognition. 1995;2:67–68. [Google Scholar]
- Li S-C, Naveh-Benjamin M, Lindenberger U. Aging neuromodulation impairs associative binding: A neurocomputational account. Psychological Science. 2005;16(6):445–450. doi: 10.1111/j.0956-7976.2005.01555.x. [DOI] [PubMed] [Google Scholar]
- Light LL. Memory and aging: Four hypotheses in search of data. Annual Review of Psychology. 1991;42(1):333–376. doi: 10.1146/annurev.ps.42.020191.002001. [DOI] [PubMed] [Google Scholar]
- Light LL. Memory and aging. In: Bjork EL, Bjork RA, editors. Memory, Handbook of perception and cognition. 2nd ed. San Diego, CA: Academic Press; 1996. pp. 443–490. [Google Scholar]
- Lindenberger U, Pötter U. The complex nature of unique and shared effects in hierarchical linear regression: Consequences for cross-sectional developmental research. Psychological Methods. 1998;3:218–230. [Google Scholar]
- Lindenberger U, von Oertzen T, Ghisletta P, Hertzog C. Cross-sectional age variance extraction: What's change got to do with it? Psychology and Aging. 2011;26(1):34–47. doi: 10.1037/a0020525. [DOI] [PubMed] [Google Scholar]
- Lineweaver TT, Hertzog C. Adults' efficacy and control beliefs regarding memory and aging: Separating general from personal beliefs. Aging, Neuropsychology, and Cognition. 1998;5(4):264–296. [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychological Methods. 2002;7(1):83. doi: 10.1037/1082-989x.7.1.83. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2819363/pdf/nihms-173350.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behavioral Research. 2004;39(1):99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell SE, Cole DA. Bias in cross-sectional analyses of longitudinal mediation. Psychological Methods. 2007;12(1):23–44. doi: 10.1037/1082-989X.12.1.23. [DOI] [PubMed] [Google Scholar]
- McDonald-Miszczak L, Hertzog C, Hultsch DF. Stability and accuracy of metamemory in adulthood and aging: A longitudinal analysis. Psychology and Aging. 1995;10(4):553–564. doi: 10.1037//0882-7974.10.4.553. [DOI] [PubMed] [Google Scholar]
- Miller LMS, Lachman ME. The sense of control and cognitive aging: Toward a model of mediational processes. In: Hess TM, Blanchard-Fields F, editors. Social cognition and aging. San Diego, CA: Academic Press; 1999. pp. 17–42. [Google Scholar]
- Moscovitch M, Winocur G. The neuropsychology of memory and aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Hillsdale: Lawrence Erlbaum Associates; 1992. pp. 315–372. [Google Scholar]
- Muthén L, Muthén B. Mplus User’s Guide. Sixth Edition. Los Angeles: Muthén & Muthén; 2010. [Google Scholar]
- Naveh-Benjamin M. Adult age differences in memory performance: Tests of an associative deficit hypothesis. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2000;26:1170–1188. doi: 10.1037//0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Brav TK, Levy O. The associative memory deficit of older adults: The role of strategy utilization. Psychology and Aging. 2007;22(1):202–208. doi: 10.1037/0882-7974.22.1.202. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Craik FI, Guez J, Dori H. Effects of divided attention on encoding and retrieval processes in human memory: Further support for an asymmetry. Journal of Experimental Psychology. Learning, Memory, and Cognition. 1998;24(5):1091–1104. doi: 10.1037//0278-7393.24.5.1091. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Craik FIM, Guez J, Kreuger S. Divided attention in younger and older adults: Effects of strategy and relatedness on memory performance and secondary task costs. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31(3):52–537. doi: 10.1037/0278-7393.31.3.520. [DOI] [PubMed] [Google Scholar]
- Old S, Naveh-Benjamin M. Differential effects of age on item and associative measures of memory: A meta-analysis. Psychology and Aging. 2008;23(1):104–118. doi: 10.1037/0882-7974.23.1.104. [DOI] [PubMed] [Google Scholar]
- Rabinowitz JC, Craik FIM, Ackerman BP. A processing resource account of age-differences in recall. Canadian Journal of Psychology. 1982;36(2):325–344. [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rahhal T, Hasher L, Colcombe S American Psychological Association. Instructional manipulations and age differences in memory: Now you see them, now you don't. Psychology and Aging. 2001;16(4):697–706. doi: 10.1037//0882-7974.16.4.697. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U. Only time will tell: Cross-sectional studies offer no solution to the Age-Brain-Cognition triangle—Comment on Salthouse (2011) Psychological Bulletin. 2011;137:790–795. doi: 10.1037/a0024503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebok GW, Balcerak LJ. Memory self-efficacy and performance differences in young and old adults: The effect of mnemonic training. Developmental Psychology. 1989;25(5):714. [Google Scholar]
- Riggs KM, Lachman ME, Wingfield A. Taking charge of remembering: Locus of control and older adults' memory for speech. Experimental Aging Research. 1997;23(3):237–256. doi: 10.1080/03610739708254282. [DOI] [PubMed] [Google Scholar]
- Ryan EB. Beliefs about memory changes across the adult lifespan. Journal of Gerontology: Psychological Sciences. 1992;47:P41–P46. doi: 10.1093/geronj/47.1.p41. [DOI] [PubMed] [Google Scholar]
- Ryan EB, Kwong-See SK. Age-based beliefs about memory changes for self and others across adulthood. Journal of Gerontology: Psychological Sciences. 1993;48:P199–P201. doi: 10.1093/geronj/48.4.p199. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Theoretical perspectives on cognitive aging. Mahwah, NJ: Erlbaum; 1991. [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. When does age-related cognitive decline begin? Neurobiology of Aging. 2009;30(4):507–514. doi: 10.1016/j.neurobiolaging.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Hancock HE, Meinz EJ, Hambrick DZ. Interrelations of age, visual acuity, and cognitive functioning. Journals of Gerontology Series B: Psychological Sciences. 1996;51B:P317–P330. doi: 10.1093/geronb/51b.6.p317. [DOI] [PubMed] [Google Scholar]
- Shing YL, Werkle-Bergner M, Li SC, Lindenberger U. Associative and strategic components of episodic memory: A life-span dissociation. Journal of Experimental Psychology: General. 2008;137(3):495–513. doi: 10.1037/0096-3445.137.3.495. [DOI] [PubMed] [Google Scholar]
- Shing YL, Werkle-Bergner M, Li SC, Lindenberger U. Committing memory errors with high confidence: Older adults do but children don't. Memory. 2009;17(2):169–179. doi: 10.1080/09658210802190596. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychological Methods. 2002;7(4):422–445. [PubMed] [Google Scholar]
- Souchay C, Isingrini M. Age related differences in metacognitive control: Role of executive functioning. Brain and cognition. 2004;56(1):89–99. doi: 10.1016/j.bandc.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Souchay C, Isingrini M, Espagnet L. Aging, episodic memory, feeling-of-knowing, and frontal functioning. Neuropsychology. 2000;14(2):299–309. doi: 10.1037//0894-4105.14.2.299. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10791869. [DOI] [PubMed] [Google Scholar]
- Taconnat L, Raz N, Toczé C, Bouazzaoui B, Sauzéon H, Fay S, Isingrini M. Aging and organisation strategies in free recall: The role of cognitive flexibility. European Journal of Cognitive Psychology, Special Issue: Aging, Neuropsychology and Cognition. 2009:347–365. [Google Scholar]
- Troyer AK, Graves RE, Cullum CM. Executive functioning as a mediator of the relationship between age and episodic memory in healthy aging. Aging, Neuropsychology, and Cognition. 1994;1(1):45–53. [Google Scholar]
- Verhaeghen P, Marcoen A. Production deficiency hypothesis revisited: Adult age differences in strategy use as a function of processing resources. Aging and Cognition. 1994;1:323–338. [Google Scholar]
- Verhaeghen P, Marcoen A, Goossens L. Facts and fiction about memory aging: A quantitative integration of research findings. Journal of Gerontology. 1993;48(4):P157–P171. doi: 10.1093/geronj/48.4.p157. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- West RL, Yassuda MS. Aging and memory control beliefs: Performance in relation to goal setting and memory self-evaluation. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2004;59(2):P56–P65. doi: 10.1093/geronb/59.2.p56. [DOI] [PubMed] [Google Scholar]
- Yonelinas A. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46(3):441–517. [Google Scholar]