Abstract
We evaluated the effect of acute and chronic graft-versus-host disease (GVHD) on relapse and survival after allogeneic haematopoietic stem cell transplantation (HSCT) for multiple myeloma (MM) using non-myeloablative conditioning (NMA) and reduced-intensity conditioning (RIC). The outcomes of 177 HLA-identical sibling HSCT recipients between 1997 and 2005 following NMA (n=98) or RIC (n=79) were analyzed. In 105 patients, autografting was followed by planned NMA/RIC allogeneic transplantation. The impact of GVHD was assessed as a time-dependent covariate using Cox models. The incidence of acute GVHD (grades I–IV) was 42% (95% confidence interval (CI) 35 – 49%) and of chronic GVHD at five years was 59% (95% CI 49 – 69%), with 70% developing extensive chronic GVHD. In multivariate analysis, acute GVHD (≥ grade I) was associated with an increased risk of TRM (relative risk (RR)=2.42; p=0.016), whereas limited chronic GVHD significantly decreased the risk of myeloma relapse (RR=0.35, p=0.035) and was associated with superior event-free survival (RR=0.40, p=0.027). Acute GVHD had a detrimental effect on survival, especially in those receiving autologous followed by allogeneic HSCT (RR=3.52; p=0.001). The reduction in relapse risk associated with chronic GVHD is consistent with a beneficial graft-versus-myeloma effect, but this did not translate into a survival advantage.
Keywords: Graft-versus-host disease, reduced intensity, allogeneic, myeloma
INTRODUCTION
Some studies suggest a graft-versus-myeloma effect after allogeneic haematopoietic stem cell transplantation (HSCT) for multiple myeloma (MM)(1–4). For example, donor lymphocyte infusions (DLI) have induced remission in patients with recurrent MM after HSCT. In recipients of allogeneic HSCT after traditional myeloablative conditioning, the graft-versus-myeloma effect is suggested by the fact that chronic GVHD correlates with complete remission(5). However, other studies report no correlation (6). Despite the beneficial graft-versus-myeloma effect, the high treatment-related mortality (TRM), mainly related to graft-versus-host disease (GVHD), has made myeloablative HSCT unattractive compared with autologous transplants or new drugs (7–9).
Recently, allogeneic transplantations have been used earlier in the course of MM and with reduced conditioning intensity, in an attempt to reduce TRM after HSCT(10). A promising approach is the combination of high-dose chemotherapy and autologous transplant, followed by reduced-intensity HSCT(11). This approach relies on a maximal disease control strategy with autologous transplantation followed by lower-intensity conditioning allogeneic HSCT to achieve an immune-mediated graft-versus-myeloma effect(6, 11–14).
Two randomised studies in high risk MM patients indicated that autologous followed by allogeneic HSCT had similar outcomes compared with tandem autologous transplantation(13, 14). Studies not limited to high risk MM patients with autologous followed by allogeneic approach, compared with tandem autologous transplantation, have shown discordant results with an earlier Italian study showing a survival advantage, whereas the recently reported Bone Marrow Transplant Clinical Trials Network 0102 study showed no benefit to allogeneic transplantation(12, 15).
With reduction in conditioning intensity, any beneficial effect of allogeneic HSCT is likely to be derived from an immune-mediated graft-versus-MM effect, but the relative impact of this effect has been difficult to characterize. A retrospective study by Crawley et al. showed that chronic GVHD was associated with superior survival in patients treated with reduced-intensity allogeneic transplantation (16). Another prospective study suggested no correlation between chronic GVHD and response in patients undergoing autologous followed by allogeneic HSCT for MM(6). Interestingly, the study by Crawley et al. did not specifically address the upfront planned autologous followed by allogeneic HSCT approach(16).
We analyzed the impact of acute and chronic GVHD on outcomes in myeloma patients undergoing allogeneic HSCT following reduced-intensity conditioning, both in the planned autologous followed by allogeneic (auto-allo) and the single upfront allogeneic HSCT (not preceded by autotransplant) settings.
PATIENTS AND METHODS
Patient Selection
Recipients of HLA-identical sibling bone marrow and/or peripheral blood stem cell allogeneic transplants for MM within 18 months of diagnosis, between 1997 and 2005 and reported to the CIBMTR (Center for International Blood and Marrow Transplant Research) were identified. Reduced-intensity regimens were defined and classified as non-myeloablative conditioning (NMA) or reduced-intensity conditioning (RIC) based on standard definitions(17). The patients were grouped into those receiving a single allogeneic HSCT (allo only) and those receiving a planned autologous followed by allogeneic HSCT (auto-allo). Patients who received an autologous HSCT followed by an unplanned allogeneic HSCT at progression (n=16) were excluded from the study.
Data Source
The CIBMTR is a research organisation of more than 450 transplant centers worldwide, that contribute detailed data on consecutive transplants. Patients are followed longitudinally, with yearly follow-up. Computerised checks for errors, physician reviews of submitted data and on-site audits of participating centers ensure data quality.
Outcomes
Overall survival was defined as the time from date of transplant to date of death, with survivors censored at the time of last contact. Transplant-related mortality (TRM) was defined as death occurring in the absence of relapse/progressive disease and summarized by the cumulative incidence estimate with relapse as the competing risk.
Relapse/progression was defined as the time to first evidence of laboratory recurrence or progression of myeloma according to the standard EBMT/IBMTR criteria(18) and summarized by the cumulative incidence estimate with TRM as the competing risk. Event-free survival (EFS) was defined as survival without progressive disease or relapse from complete remission (CR). Progressive disease, relapse from CR and death in remission were considered events. Probabilities of survival and EFS were calculated using the Kaplan–Meier estimator and compared using the log-rank test. The incidence and stage of acute GVHD (aGVHD) were measured by standard criteria(19). The incidence of chronic GVHD (cGVHD) was measured according to the standard criteria(20).
Statistical Analysis
Multivariate analyses were performed using Cox proportional hazards models. A stepwise model-building approach was used to identify the significant risk factors associated with outcomes of TRM, relapse, EFS and overall survival. The variables considered in the model-building procedures were as follows: age at transplant (<50 years vs. ≥50 years), gender (male vs. female), Karnofsky performance score (<90 vs. ≥90 vs. unknown), Durie-Salmon stage at diagnosis (I/II vs. III), disease status and sensitivity of MM to chemotherapy prior to transplant (sensitive vs. not sensitive vs. others), prior lines of chemotherapy (≤1 line vs. 2 lines vs. >2 lines), type of transplant (allo only vs. auto+allo), donor-recipient sex match (male-to-male vs. male-to-female vs. female-to-male, vs. female-to-female), conditioning (NMA vs. RIC), year of transplant (≤ 2001 vs. >2001), acute and chronic GVHD. At the time of transplantation, it is unknown who will and who will not develop GVHD. Therefore GVHD was treated as a time-dependent covariate. Since acute and chronic GVHD effects are the main interests of this study, they were included in each step of model building. Factors that were significant at a 5% level were kept in the final model. The potential interactions between main effects and all significant risk factors were tested. The relative risks of significant covariates based on final models were reported. In addition to considering GVHD as a time-dependent covariate, we used a landmark analysis method to compute outcomes stratified by patients who developed acute GVHD within 100 days. Patients surviving longer than 100 days were included in acute GVHD landmark analysis. A similar landmark study for those who developed chronic GVHD within 1 year of transplant was also performed. Landmark analyses results are presented in figures.
RESULTS
Pre-transplant Characteristics
Table 1 summarizes patient, disease and transplant-related variables of interest. Fifty-five percent of the patients had IgG MM and 63% had Salmon-Durie stage III. Seventy-two percent of the patients were in complete or partial remission at the time of transplantation. Fifty-six percent of the patients received NMA. The most common immunosuppressive protocols were cyclosporine combined with mycophenolate mofetil, or cyclosporine combined with methotrexate.
Table 1.
Characteristics of patients
| Variables | N (%) |
|---|---|
| Patient-related | |
| Number of patients | 177 |
| Number of centers | 65 |
| Age, median (range), years | 51 (24 – 69) |
| Male sex | 102 (58) |
| Karnofsky score at transplant ≥ 90% | 121 (68) |
| Disease-related | |
| Immunochemical subtype of MM | |
| IgG | 97 (55) |
| IgA | 23 (13) |
| IgD | 4 (2) |
| Light chain | 39 (22) |
| Non secretory/Other | 14 (8) |
| Salmon-Durie stage at diagnosis | |
| I | 8 (4) |
| II | 39 (22) |
| III | 111 (63) |
| Missing | 19 (11) |
| Albumin at diagnosis <3.5 g/dL | 47 (26) |
| Prior lines of chemotherapy | |
| 1 | 79 (45) |
| 2 | 43 (24) |
| ≥3 | 25 (14) |
| Missing/Unknown | 30 (17) |
| Disease status prior to transplant | |
| Complete remission/Partial remission | 127 (72) |
| Minimal response | 10 (6) |
| No response/Stable disease | 16 (9) |
| Progression | 2 (1) |
| Missing | 22 (12) |
| Bortezomib pre transplant | 8 (5) |
| Thalidomide pre transplant | 44 (25) |
| Transplant-related | |
| Conditioning regimen | |
| Reduced-intensity conditioning | |
| TBI based | 2 (1) |
| Melphalan≤150 mg/m^2 | 37 (21) |
| Busulfan≤9 mg/kg | 13 (7) |
| Cyclophosphamide | 27 (15) |
| Non-myeloablative conditioning | |
| TBI=200cGY | 54 (31) |
| Fludarabine+TBI=200cGY | 26 (15) |
| Other | 18 (10) |
| Donor age, median (range), years | 46 (16 – 73) |
| Female donor/male recipient | 52 (29) |
| Donor-recipient CMV serostatus, −/− | 43 (24) |
| Peripheral blood stem cells | 173 (98) |
| GVHD prophylaxis | |
| CSA based ± MTX | 52 (29) |
| FK506 based ± MTX | 23 (13) |
| CSA + MMF | 86 (49) |
| FK506 + MMF | 2 (1) |
| Campath ± other | 2 (1) |
| Other/Unknown | 12 (7) |
| Year of transplant | |
| 1997–1999 | 6 (4) |
| 2000 | 17 (9) |
| 2001 | 28 (16) |
| 2002 | 24 (14) |
| 2003 | 18 (10) |
| 2004 | 44 (25) |
| 2005 | 40 (22) |
| Median (range) follow-up of survivors, months | 29 (3 – 98) |
Abbreviations: TBI = total body irradiation; CsA = cyclosporine; MTX = methotrexate; FK506 = tacrolimus; MMF = mycophenolate.
Outcomes
Table 2 summarizes the univariate outcomes after allogeneic HSCT. Table 3 summarizes the results of the multivariate analysis.
Table 2.
Univariate outcomes of GVHD, TRM, EFS and OS after Allogeneic HSCT
| Outcomes | N Eval | Probability (95% CI) |
|---|---|---|
| Maximum overall acute GVHD grade, N (%) | 177 | |
| 0 | 92 (52) | |
| I | 32 (18) | |
| II | 27 (15) | |
| III | 23 (13) | |
| IV | 3 (2) | |
| Acute GVHD (grade I – IV) @ 100 days | 177 | 42 (35 – 49) |
| Chronic GVHD | 176 | |
| @ 1 yr | 45 (37 – 52) | |
| @ 5 yrs | 59 (49 – 69) | |
| Extensive cGVHD | 59 (70%) | |
| Any GVHD @ 5 yrs | 177 | 72 (65 – 79) |
| 100-day mortality | 177 | 8 (5 – 13) |
| Treatment-related mortality | 177 | |
| @ 1 yr | 15 (10 – 20) | |
| @ 5 yrs | 25 (17 – 34) | |
| Relapse/progression | 177 | |
| @ 1 yr | 22 (16 – 28) | |
| @ 5 yrs | 52 (41 – 63) | |
| Event-free survival | 177 | |
| @ 1 yr | 64 (57 – 71) | |
| @ 5 yrs | 22 (13 – 34) | |
| Overall survival | 177 | |
| @ 1 yr | 75 (69 – 82) | |
| @ 5 yrs | 38 (26 – 50) |
Table 3.
Multivariate analysis
| Outcome | Relative Risk (95% CI) | P-value |
|---|---|---|
| TRMb | ||
| Acute GVHD | ||
| No | 1.00a | |
| Yes | 2.42 (1.18 – 4.96) | 0.016 |
| Relapsec | ||
| Type of transplant | ||
| First allogeneic | 1.00a | |
| Planned autologous+allogeneic | 0.59 (0.36 – 0.98) | 0.043 |
| Chronic GVHD | ||
| No | 1.00a | |
| Limited | 0.35 (0.13 – 0.93) | 0.035 |
| Extensive | 0.58 (0.29 – 1.19) | 0.14 |
| Event-free survival (RR of relapse/death)d | ||
| Type of transplant | ||
| First allogeneic | 1.00a | |
| Planned autologous+allogeneic | 0.57 (0.38 – 0.86) | 0.008 |
| Chronic GVHD | ||
| No | 1.00a | |
| Limited | 0.40 (0.19 – 0.90) | 0.027 |
| Extensive | 0.81 (0.47 – 1.41) | 0.56 |
| Overall survival (RR of death)e | ||
| Acute GVHD | ||
| First allogeneic | ||
| No | 1.00a | |
| Yes | 0.90 (0.48 – 1.70) | 0.75 |
| Planned autologous+allogeneic | ||
| No | 1.00a | |
| Yes | 3.52 (1.67 – 7.45) | 0.001 |
Reference group
GVHD impact (yes vs. no) on outcomes where not significant summarized below:
| bLimited chronic GVHD | RR=0.65 (0.17–2.47) | p=0.53 |
| bExtensive chronic GVHD | RR=1.50 (0.61–3.70) | p=0.37 |
| cAcute GVHD | RR=0.79 (0.47–1.36) | p=0.40 |
| dAcute GVHD | RR=1.10 (0.72–1.68) | p=0.66 |
| eLimited cGVHD | RR=0.45 (0.18–1.13) | p=0.09 |
| eExtensive cGVHD | RR=1.18 (0.66–2.10) | p=0.59 |
| eTest interaction: p(first allogeneic=planned autologous+allogeneic) = 0.005 | ||
Graft-versus-host disease
The cumulative incidence of acute GVHD grades I–IV at 100 days was 42% (95% CI, 35–49%). Overall acute GVHD grades II–IV was observed in 53 patients (30%) (Table 2). Chronic GVHD at one year was 45% (95% CI, 37–52%). At five years, it was 59% (95% CI, 49–69%) with 70% of cGVHD was extensive.
Transplant-related mortality
At one year, TRM was 15% (95% CI, 10–20%), and at five years it was 25% (95% CI, 17–34%). In multivariate analysis, acute GVHD was associated with an increased risk of TRM (Table 3, RR 2.42, p=0.016). Chronic GVHD, whether limited or extensive, had no significant impact on TRM. Figures 1a and 1b represent the landmark analyses for TRM in those developing aGVHD by day 100 vs. those who did not, and those developing cGVHD within 1 year vs. those who did not.
Figure 1.
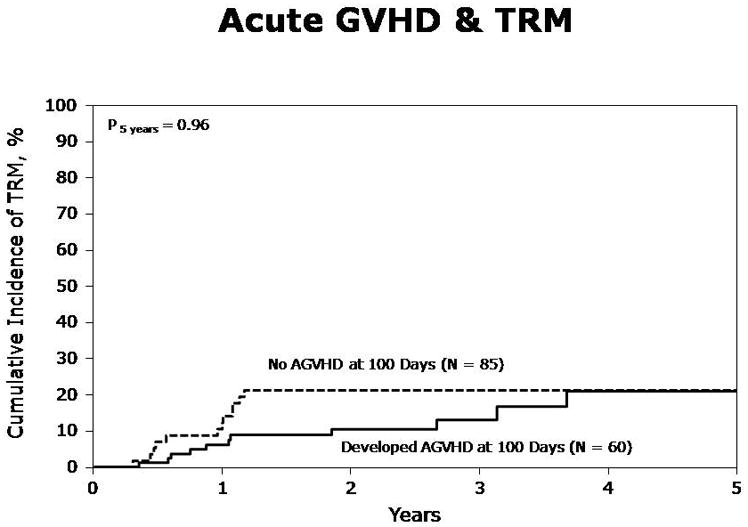
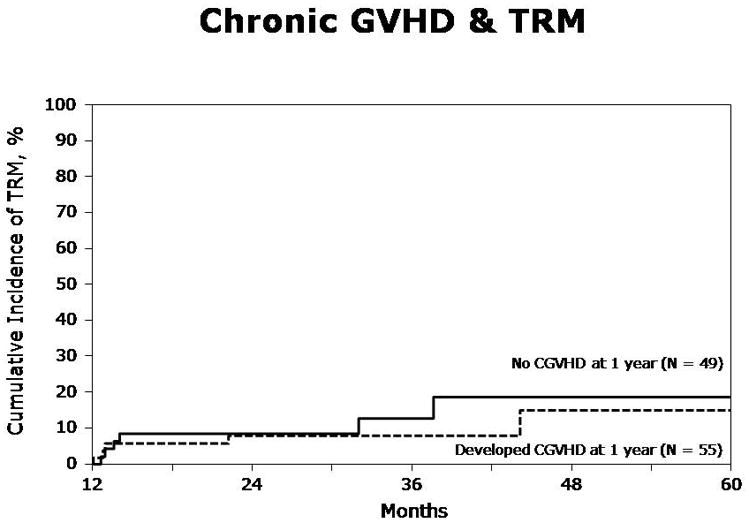
Figure. 1a. Acute GVHD and transplant-related mortality (TRM) in patients with (grades I–IV) and without any acute GVHD by day 100 (Landmark analysis)
Figure. 1b. Chronic GVHD and transplant-related mortality (TRM) in patients with and without chronic GVHD within one year (Landmark analysis)
Relapse
Cumulative incidence of relapse at one year was 22% (95%CI, 16–28%). At five years, the incidence of relapse was 52% (95%CI, 41–63%). Acute GVHD had no statistically significant effect on the risk of relapse. Chronic GVHD overall was associated with a reduced risk of relapse in the multivariate analysis, but the beneficial effect was confined to those with limited cGVHD (RR=0.35, p=0.035) but was not statistically significant in those with extensive chronic GVHD (RR=0.58, p=0.14) (Table 3). Figure 2 represents the additional landmark analysis for relapse in those who developed any cGVHD within 1 year of HSCT vs. those who did not.
Figure 2.
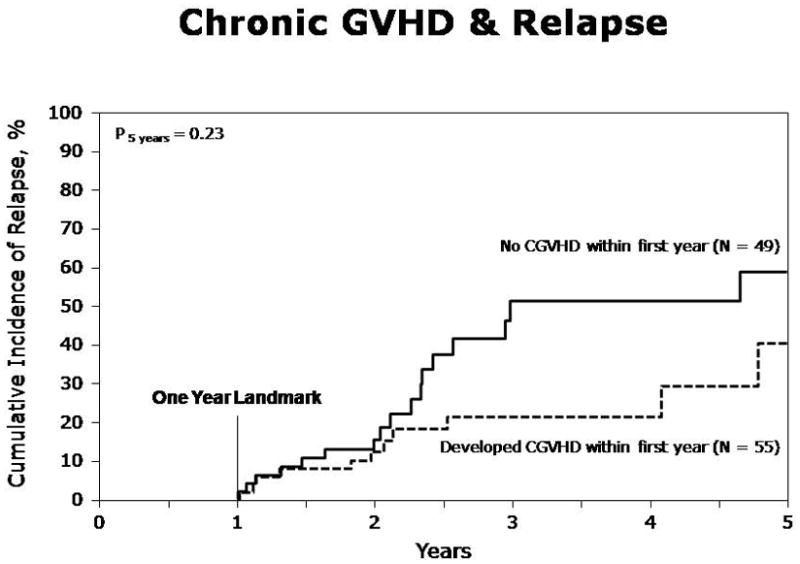
Relapse in patients with and without chronic GVHD within one year (Landmark analysis)
The cumulative incidence of relapse at 1 year was 32% (95% CI 21–43%) in the allo only group vs.15% (95% CI 8–22%) in the auto-allo group. The auto-allo group had a significantly lower risk of relapse in multivariate analysis, compared to the allo-only group (Table 3, RR 0.59; p=0.043).
Event-free survival
At one year, EFS was 64% (95% CI, 57–71%), and at five years, it was 22% (95% CI, 13–34%). In the multivariate analysis, aGVHD and cGVHD overall did not impact EFS (Table 3). However, limited cGVHD was associated with superior EFS (RR for relapse/death =0.40, p=0.027), while extensive cGVHD had no statistically significant impact on EFS. Figure 3 depicts a landmark analysis of EFS in those developing any cGVHD within one year of HSCT vs. those who did not. At one year, EFS was 48% (95% CI 36–60%) in the allo only group, compared to 74% (95% CI 66–83%) in the auto-allo group. At five years, EFS was 17% (95% CI 7–29%) and 24% (95% CI 7–48%) in the two groups, respectively. In the multivariate analysis, the auto-allo group had superior EFS (Table 3, RR=0.57, p=0.008).
Figure 3.
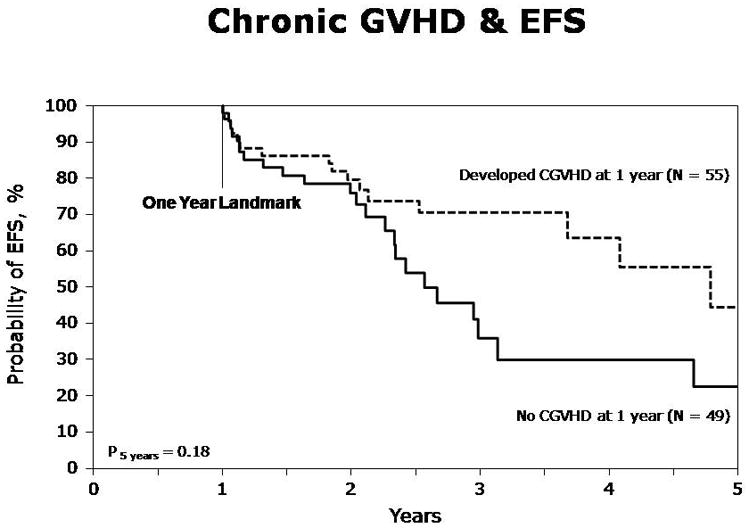
Event-free survival (EFS) in patients with and without chronic GVHD within one year (Landmark analysis)
Overall survival
At one year, survival was 75% (95%CI, 69–82%), and at five years it was 38% (95%CI, 26–50%). Acute GVHD was not associated with survival in the allo-only cohort (Table 3, RR of death =0·90, p=0·75). In the Auto-allo cohort, acute GVHD was associated with a higher risk of death (RR=3·52, p=0·001). Chronic GVHD on the other hand, had no significant impact on survival.
Causes of Death
The most common cause of death was relapsed or progressive MM in 33% patients, followed by infections and organ failure.
DISCUSSION
This aim of this analysis was to define the impact of GVHD on outcomes after allogeneic HSCT for MM. Acute GVHD is the major underlying cause of morbidity and TRM following allogeneic HSCT in patients with MM(6). High TRM, mainly related to GVHD, made myeloablative HSCT unacceptable for most patients with MM(8, 21). In addition, only a limited number of myeloma patients are candidates for myeloablative allogeneic HSCT, because of age, non-availability of HLA-matched donors and pre-transplant co-morbidities. The advent of reduced-intensity conditioning has led to increased numbers of patients becoming eligible for HSCT as well as hope of reduced risk of TRM. However, the success of this modality is dependent on immune mediated graft-versus-myeloma effect since anti-neoplastic effect derived from the conditioning regimen is modest. We attempted to evaluate the relative impact of aGVHD and cGVHD on TRM, relapse and survival endpoints.
In the present study, patients receiving allogeneic HSCT for MM had a significant late risk of relapse (52% at 5 years). A striking finding is the high number of late relapses, especially among the patients who did not develop chronic GVHD (Fig. 2). This is especially striking when we do a landmark analysis, because relapses occurring during the first year are not included in the figure. The continuous increase in relapses is not specific for this study, but is often seen in patients undergoing HSCT for myeloma (6, 8, 10, 22).
There were significant risks of acute and chronic GVHD consistent with previous observations(22). The probability of grade III–IV acute GVHD after RIC/NMA was 15%. In this group, mortality from GVHD is typically high(23). Similar to previous studies in leukemia patients, acute GVHD was associated with a significant increase in risk of TRM (Table 3) whereas chronic GVHD overall was not associated with increased TRM (Table 3, Fig. 1b). The negative impact of aGVHD on survival was marked in the planned Auto-allo cohort.
Several small studies have suggested a graft-versus-MM effect in patients receiving allogeneic HSCT after myeloablative conditioning (1–5, 16). Our study demonstrates that in the setting of RIC or NMA, chronic GVHD especially limited cGVHD is associated with beneficial impact with a decreased risk of myeloma relapse and superior EFS (Table 3). Acute GVHD on the other hand had no impact on relapse. This is in keeping with most studies of the graft-versus-leukemia effect, showing that chronic GVHD has the strongest association with decreased relapse, whereas the effect of acute GVHD on relapse was manifest in some, but not all studies (24–27).
A reduced relapse risk was significantly associated with limited but not extensive chronic GVHD. This is in contrast to a study in patients with acute leukemia, showing that there was no difference in relapse in patients with limited or extensive chronic GVHD (25). There may be several reasons why we didn’t find a reduced relapse risk in patients with extensive chronic GVHD. First, this is a multicenter study and there may be difficulties associated with the distinction between limited and extensive disease. Furthermore, there is a limited number of patients included and there may not have been sufficient patient numbers to find a significant effect in patients with extensive chronic GVHD. We may also speculate that patients with extensive chronic GVHD are treated with more heavy immunosuppressive therapy that may abrogate the graft-versus-myeloma effect to a larger extent than the milder immunosuppression used in patients with limited disease.
In the comparison between allo-only and auto-allo cohorts, there were significantly lower early relapses and superior EFS in the auto-allo group, compared with the allo-only group (Table 3). The reason for the reduction in early relapse and improved EFS in the auto-allo group may be due to a selection bias favoring more high risk patients proceeding to an initial allogeneic transplant without a preceding autograft (supplemental data not shown). The allo-only group had markers of worse prognosis at baseline, including a higher proportion of patients with light chain and non-secretory disease (p<0.001), those with three or more lines of pretransplant chemotherapy (p=0.01), and fewer patients with chemotherapy sensitive disease compared with the auto-allo group. The allo-only group also received RIC including Melphalan (p<0.001) based conditioning more often, suggesting a higher intensity of conditioning within the reduced-intensity category. This is also consistent with the notion that some of these patients were selected since they had more advanced disease and were considered for more “intensive conditioning” within the RIC spectrum.
There was no increase in TRM associated with chronic GVHD (Table 3). This suggests that any graft-versus-MM effect induced by chronic GVHD not only decreased the probability of relapse, but had no adverse effect on survival. A study by Crawley et al showed that chronic GVHD was associated with improved EFS(16). EFS was also not significantly impacted by acute GVHD despite its association with higher risk of TRM. The reason for this mitigating effect may be an association between acute and chronic GVHD (p-value = 0.03). The increased mortality risk associated with aGVHD was statistically significant in the Auto-allo group but not in the allo-only group. The reason for this may also be due to the selection of higher risk patients in the allo-only group.
Chronic GVHD had no impact on overall survival despite lower relapse and unchanged TRM. Also the impact of chronic GVHD on quality of life and co-morbidities cannot be measured in this analysis. This also suggests that currently the role of allotransplantation in MM remains limited by lack of adequate long term disease control, a persistent risk of relapse and death from recurrent myeloma. These findings are consistent with emerging data from randomized studies such as the BMTCTN 0102 study(15).
In conclusion, our analysis demonstrates a beneficial effect on relapse risk reduction associated with limited chronic GVHD without an increased risk of TRM. These findings have implications for clinical practice and future trials in allogeneic HSCT for MM. In this study, 59% of the patients with acute GVHD developed chronic GVHD and 30% of them had limited chronic GVHD. In clinical practice, this figure may be increased by early discontinuation of immunosuppression in the absence of GVHD(28). However, early immunosuppression should be the best available to prevent acute GVHD, since it was associated with an increased risk of TRM and decreased survival. These findings could also prompt wider use of donor lymphocyte infusions to induce graft-versus-myeloma effect in selected settings. Despite the promise of a graft-versus-myeloma effect, the major current shortcoming of allogeneic transplantation in MM is the ongoing risk of relapse. These are best addressed in prospective trials incorporating more novel conditioning and maintenance strategies.
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Aetna; American Society for Blood and Marrow Transplantation; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US, Inc.; Baxter International, Inc.; Bayer HealthCare Pharmaceuticals; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical, Inc.; Biovitrum AB; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Buchanan Family Foundation; Canadian Blood and Marrow Transplant Group; Caridian BCT; Celgene Corporation; CellGenix, GmbH; Centers for Disease Control and Prevention; Children’s Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Cubist Pharmaceuticals; Cylex Inc.; CytoTherm; DOR BioPharma, Inc.; Dynal Biotech, an Invitrogen Company; Eisai, Inc.; Enzon Pharmaceuticals, Inc.; European Group for Blood and Marrow Transplantation; Gamida Cell, Ltd.; GE Healthcare; Genentech, Inc.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery Co., Ltd.; The Leukemia & Lymphoma Society; Merck & Company; The Medical College of Wisconsin; MGI Pharma, Inc.; Michigan Community Blood Centers; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; New York Blood Center; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Pall Life Sciences; Pfizer Inc; Saladax Biomedical, Inc.; Schering Corporation; Society for Healthcare Epidemiology of America; Soligenix, Inc.; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex America, Inc.; THERAKOS, Inc.; Thermogenesis Corporation; Vidacare Corporation; Vion Pharmaceuticals, Inc.; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Olle Ringdén is supported by grants from the Swedish Cancer Society, the Children’s Cancer Foundation, the Swedish Research Council, the Cancer Society in Stockholm, and Karolinska Institutet.
References
- 1.Aschan J, Lonnqvist B, Ringden O, Kumlien G, Gahrton G. Graft-versus-myeloma effect. Lancet. 1996;348(9023):346. doi: 10.1016/s0140-6736(05)64525-4. [DOI] [PubMed] [Google Scholar]
- 2.Lokhorst HM, Schattenberg A, Cornelissen JJ, van Oers MH, Fibbe W, Russell I, et al. Donor lymphocyte infusions for relapsed multiple myeloma after allogeneic stem-cell transplantation: predictive factors for response and long-term outcome. J Clin Oncol. 2000;18(16):3031–7. doi: 10.1200/JCO.2000.18.16.3031. [DOI] [PubMed] [Google Scholar]
- 3.Tricot G, Vesole DH, Jagannath S, Hilton J, Munshi N, Barlogie B. Graft-versus-myeloma effect: proof of principle. Blood. 1996;87(3):1196–8. [PubMed] [Google Scholar]
- 4.Verdonck LF, Lokhorst HM, Dekker AW, Nieuwenhuis HK, Petersen EJ. Graft-versus-myeloma effect in two cases. Lancet. 1996;347(9004):800–1. doi: 10.1016/s0140-6736(96)90871-5. [DOI] [PubMed] [Google Scholar]
- 5.Le Blanc R, Montminy-Metivier S, Belanger R, Busque L, Fish D, Roy DC, et al. Allogeneic transplantation for multiple myeloma: further evidence for a GVHD-associated graft-versus-myeloma effect. Bone Marrow Transplant. 2001;28(9):841–8. doi: 10.1038/sj.bmt.1703253. [DOI] [PubMed] [Google Scholar]
- 6.Bruno B, Rotta M, Patriarca F, Mattei D, Allione B, Carnevale-Schianca F, et al. Nonmyeloablative allografting for newly diagnosed multiple myeloma: the experience of the Gruppo Italiano Trapianti di Midollo. Blood. 2009;113(14):3375–82. doi: 10.1182/blood-2008-07-167379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barlogie B, Kyle RA, Anderson KC, Greipp PR, Lazarus HM, Hurd DD, et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321. J Clin Oncol. 2006;24 (6):929–36. doi: 10.1200/JCO.2005.04.5807. [DOI] [PubMed] [Google Scholar]
- 8.Bjorkstrand BB, Ljungman P, Svensson H, Hermans J, Alegre A, Apperley J, et al. Allogeneic bone marrow transplantation versus autologous stem cell transplantation in multiple myeloma: a retrospective case-matched study from the European Group for Blood and Marrow Transplantation. Blood. 1996;88(12):4711–8. [PubMed] [Google Scholar]
- 9.Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370(9594):1209–18. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 10.Gahrton G, Svensson H, Cavo M, Apperly J, Bacigalupo A, Bjorkstrand B, et al. Progress in allogenic bone marrow and peripheral blood stem cell transplantation for multiple myeloma: a comparison between transplants performed 1983–93 and 1994–8 at European Group for Blood and Marrow Transplantation centres. Br J Haematol. 2001;113(1):209–16. doi: 10.1046/j.1365-2141.2001.02726.x. [DOI] [PubMed] [Google Scholar]
- 11.Maloney DG, Molina AJ, Sahebi F, Stockerl-Goldstein KE, Sandmaier BM, Bensinger W, et al. Allografting with nonmyeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood. 2003;102(9):3447–54. doi: 10.1182/blood-2002-09-2955. [DOI] [PubMed] [Google Scholar]
- 12.Bruno B, Rotta M, Patriarca F, Mordini N, Allione B, Carnevale-Schianca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007;356(11):1110–20. doi: 10.1056/NEJMoa065464. [DOI] [PubMed] [Google Scholar]
- 13.Garban F, Attal M, Michallet M, Hulin C, Bourhis JH, Yakoub-Agha I, et al. Prospective comparison of autologous stem cell transplantation followed by dose-reduced allograft (IFM99-03 trial) with tandem autologous stem cell transplantation (IFM99-04 trial) in high-risk de novo multiple myeloma. Blood. 2006;107(9):3474–80. doi: 10.1182/blood-2005-09-3869. [DOI] [PubMed] [Google Scholar]
- 14.Rosinol L, Perez-Simon JA, Sureda A, de la Rubia J, de Arriba F, Lahuerta JJ, et al. A prospective PETHEMA study of tandem autologous transplantation versus autograft followed by reduced-intensity conditioning allogeneic transplantation in newly diagnosed multiple myeloma. Blood. 2008;112(9):3591–3. doi: 10.1182/blood-2008-02-141598. [DOI] [PubMed] [Google Scholar]
- 15.Krishnan A, Pasquini M, Ewell M, Stadtmauer EA, Edwin PAI, Antin JH, et al. Tandem Autologous Hematopoietic Stem Cell Transplants (AuHCT) with or without Maintenance Therapy (auto-auto) Versus Single AuHCT Followed by HLA Matched Sibling Non- Myeloablative Allogeneic HCT (auto-allo) for Patients with Standard Risk (SR) Multiple Myeloma (MM): Results From the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0102 Trial. ASH Annual Meeting Abstracts. 2010;116:41. [Google Scholar]
- 16.Crawley C, Lalancette M, Szydlo R, Gilleece M, Peggs K, Mackinnon S, et al. Outcomes for reduced-intensity allogeneic transplantation for multiple myeloma: an analysis of prognostic factors from the Chronic Leukaemia Working Party of the EBMT. Blood. 2005;105(11):4532–9. doi: 10.1182/blood-2004-06-2387. [DOI] [PubMed] [Google Scholar]
- 17.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–33. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102(5):1115–23. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 19.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–17. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 21.Crawley C, Iacobelli S, Bjorkstrand B, Apperley JF, Niederwieser D, Gahrton G. Reduced-intensity conditioning for myeloma: lower nonrelapse mortality but higher relapse rates compared with myeloablative conditioning. Blood. 2007;109(8):3588–94. doi: 10.1182/blood-2006-07-036848. [DOI] [PubMed] [Google Scholar]
- 22.Rotta M, Storer BE, Sahebi F, Shizuru JA, Bruno B, Lange T, et al. Long-term outcome of patients with multiple myeloma after autologous hematopoietic cell transplantation and nonmyeloablative allografting. Blood. 2009;113(14):3383–91. doi: 10.1182/blood-2008-07-170746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ringden O, Hermans J, Labopin M, Apperley J, Gorin NC, Gratwohl A. The highest leukaemia-free survival after allogeneic bone marrow transplantation is seen in patients with grade I acute graft-versus-host disease. Acute and Chronic Leukaemia Working Parties of the European Group for Blood and Marrow Transplantation (EBMT) Leuk Lymphoma. 1996;24(1–2):71–9. doi: 10.3109/10428199609045715. [DOI] [PubMed] [Google Scholar]
- 24.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555–62. [PubMed] [Google Scholar]
- 25.Ringden O, Labopin M, Gluckman E, Reiffers J, Vernant JP, Jouet JP, et al. Graft-versus-leukemia effect in allogeneic marrow transplant recipients with acute leukemia is maintained using cyclosporin A combined with methotrexate as prophylaxis. Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1996;18(5):921–9. [PubMed] [Google Scholar]
- 26.Sullivan KM, Weiden PL, Storb R, Witherspoon RP, Fefer A, Fisher L, et al. Influence of acute and chronic graft-versus-host disease on relapse and survival after bone marrow transplantation from HLA-identical siblings as treatment of acute and chronic leukemia. Blood. 1989;73(6):1720–8. [PubMed] [Google Scholar]
- 27.Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981;304(25):1529–33. doi: 10.1056/NEJM198106183042507. [DOI] [PubMed] [Google Scholar]
- 28.Carlens S, Aschan J, Remberger M, Dilber M, Ringden O. Low-dose cyclosporine of short duration increases the risk of mild and moderate GVHD and reduces the risk of relapse in HLA-identical sibling marrow transplant recipients with leukaemia. Bone Marrow Transplant. 1999;24(6):629–35. doi: 10.1038/sj.bmt.1701954. [DOI] [PubMed] [Google Scholar]


