Abstract
Trop-2 is a transmembrane glycoprotein upregulated in several human carcinomas, including prostate cancer (PrCa). Trop-2 has been suggested to regulate cell-cell adhesion, given its high homology with the other member of the Trop family, Trop-1/EpCAM, and its ability to bind the tight junction proteins claudin-1 and claudin-7. However, a role for Trop-2 in cell adhesion to the extracellular matrix has never been postulated.
Here, we show for the first time that Trop-2's expression in PrCa cells correlates with their aggressiveness. Using either shRNA-mediated silencing of Trop-2 in cells that endogenously express it, or ectopic expression of Trop-2 in cells that do not express it, we show that Trop-2 inhibits PrCa cell adhesion to fibronectin (FN). In contrast, expression of another transmembrane receptor, αvβ5 integrin, does not affect cell adhesion to this ligand. We find that Trop-2 does not modulate either protein or activation levels of the prominent FN receptors, β1 integrins; however, it promotes β1 association with the adaptor molecule RACK1, and causes significant redistribution of RACK1 to the cell membrane. As a result of Trop-2 expression, we also observe activation of Src and FAK, known to occur upon β1-RACK1 interaction. These enhanced Src and FAK activities are not mediated by changes in either the activity of IGF-IR, which is known to bind RACK1, or IGF-IR's ability to associate with β1 integrins.
In summary, our data demonstrate that the transmembrane receptor Trop-2 is a regulator of PrCa cell adhesion to FN through the β1 integrin-RACK1-Src-FAK signaling axis.
Keywords: Src, Focal adhesion kinase, Insulin-like Growth Factor I Receptor
Introduction
Integrins are transmembrane receptors for extracellular matrix (ECM) ligands, and are critical regulators of cell adhesion to ECM (DeRoock et al., 2001; Hynes, 2002; Alam et al., 2007). Several signaling molecules, including focal adhesion kinase (FAK), Src, extracellular signal-regulated kinase (ERK) and myosin light-chain kinase (MLCK) participate in the regulation of dynamic assembly and disassembly of focal adhesions, macromolecular platforms that contribute to stabilize cell attachment to the substrate (Kanchanawong et al., 2010). Aberrant integrin signaling, coupled with intensive proteolytic activity to degrade the surrounding ECM, is frequently detected in several diseases, including cancer metastasis. Moreover, regulation of the physical link between integrins and the actin cytoskeleton is critical for the spatio-temporal control of cell detachment from the substrate, cell migration and metastasis.
Trop-1/EpCAM and Trop-2 are members of a transmembrane glycoprotein family, which is upregulated in several human carcinomas as compared with their normal counterparts (Ohmachi et al., 2006; Zanna et al., 2007; Fong et al., 2008; Guerra et al., 2008; Trerotola et al., 2010). Trop-1 regulates epithelial cell-cell adhesion through homotypic protein-protein interactions (Balzar et al., 2001; Trebak et al., 2001) and modulates the activity of cadherins (Litvinov et al., 1997) through PI3K (Winter et al., 2007). Trop-2 is a Ca2+ signal transducer (Ripani et al., 1998), and has been suggested to resemble Trop-1 in cell-cell adhesion, since it binds the tight junction proteins claudin-1 and claudin-7 (Nakatsukasa et al., 2010). The use of Trop-2 for identification and isolation of stem cells in normal and neoplastic prostate has been reported (Goldstein et al., 2008; Goldstein et al., 2010), suggesting a role for this molecule in cancer initiation and progression. This is in line with previous literature correlating Trop-2 expression with poor prognosis in several human carcinomas (Ohmachi et al., 2006; Fong et al., 2008), and also upregulation during prostate cancer (PrCa) progression (Calvo et al., 2002).
Signaling pathways previously shown to affect integrin-mediated cell adhesion and cytoskeletal rearrangements involve a cross-talk between FAK, Src and the receptor for activated C kinase 1 (RACK1). While FAK and Src have intrinsic kinase activities (Lu et al., 1997; Marx et al., 2001; Yeatman, 2004; Mitra et al., 2005), RACK1 is an adaptor molecule known to bind several key signaling proteins, such as activated PKCs (Ron et al., 1994), FAK (Kiely et al., 2009; Serrels et al., 2010) and Src (Chang et al., 2001). RACK1 activity as regulator of central, stable focal adhesions has been recently reported (Cox et al., 2003; Serrels et al., 2010), suggesting a critical function of this molecule in cell-ECM interaction. Focal adhesions bring together cytoskeletal and signalling proteins during the processes of cell attachment and spreading on ECM, and phosphorylation of FAK is a rapid event associated with the formation of these adhesive structures (Mitra and Schlaepfer, 2006). Tyr397 is a major autophosphorylation site in FAK, and phosphorylated FAK promotes binding and activation of Src (Mitra and Schlaepfer, 2006). However, in FAK-null cells reconstituted with Y397F–FAK or A430V-Src constitutively inactive mutants, adhesion disassembly is significantly slower than in control cells (Webb et al., 2004), indicating that an intact FAK-Src signaling axis is critical for inducing disassembly and turnover of integrin-based adhesion sites, an essential step in the metastatic cascade (Cox et al., 2006; Natarajan et al., 2006). A complex between RACK1, FAK and the cAMP-degrading phosphodiesterase PDE4D5 has been recently reported to localize selectively in nascent focal contacts, rather than in mature adhesions that are formed in cells stably attached to ECM, and to promote cell polarity (Serrels et al., 2010). It has been shown that the N-terminal region of RACK1 contains the FAK binding site (Kiely et al., 2009); RACK1 has been also demonstrated to associate with the cytoplasmic domain of several integrins, among them β1 (Liliental and Chang, 1998; Buensuceso et al., 2001; Besson et al., 2002). The integrin binding domain of RACK1 is localized in the C-terminal portion; in this region, the Tyr-246 residue has been identified as a Src binding site (Chang et al., 2001). Finally, RACK1 has been demonstrated to associate with the Insulin-like Growth Factor I (IGF-I) receptor (IGF-IR), suggesting a role in the IGF signaling network (Hermanto et al., 2002; Kiely et al., 2002). IGF-I and IGF-IR, known to play a positive role in PrCa (Wu et al., 2006; Zoubeidi et al., 2010), stimulate many signaling pathways upon binding to each other. Recent reports have shown that IGF-I binding to IGF-IR, which promotes the interaction between β1 integrins and RACK1 in adherent cells (Kiely et al., 2005), induces displacement of Src from RACK1 (Kiely et al., 2002); when Src is dissociated from RACK1, its kinase activity is increased (Cox et al., 2003).
Here, we describe for the first time that Trop-2 inhibits cell adhesion to fibronectin (FN) in PrCa cells, and we identify the β1 integrin-RACK1-FAK-Src as major signaling axis involved in this function. This study demonstrates that the transmembrane glycoprotein Trop-2 is a critical mediator of cell-ECM interaction. We propose that the mechanisms underlying these events may involve the control of cytoskeletal dynamics and the turnover of focal adhesions.
Materials and Methods
Reagents and Antibodies
FN – purified from whole blood as described in (Engvall and Ruoslahti, 1977) – and Poly-L-Lysine (PLL) (Sigma-Aldrich) were diluted in sterile phosphate buffer saline (PBS) for coating. IGF-I was purchased from R&D Systems. Antibodies (Abs) used for flow cytometry (FACS) analysis were as follows: mouse monoclonal Ab (mAb) 162.46.2 (ATCC number: HB-187) against human Trop-2; mouse mAbs TS2/16 (ATCC number: HB-243) and K20 (M0889, Dako Cytomation), and rat mAb 9EG7 (550531, BD Biosciences), all against β1 integrins. For immunofluorescence the mAb C20 (610177, BD Transduction Laboratories) against RACK1 was used. Abs used for immunoprecipitation (IP) were as follows: mAb against β1 (clone K20), and rabbit polyclonal Ab (pAb) against IGF-IRβ (C20; sc-713, Santa Cruz Biotechnology). Abs used for immunoblotting (IB) were as follows: mAbs against β1 integrins (clone C18; 610468, BD Transduction Laboratories), head domain of talin (talin-H) (clone TA205; MAB1676, Chemicon), RACK1 (C20), and total Src (clone L4A1, 2110, Cell Signaling); rabbit pAbs against Tyr397 phospho-FAK (pFAK) (44-624G, Invitrogen), FAK (C-20; sc-558, Santa Cruz Biotechnology), Tyr416 phospho-Src (pSrc) (2101, Cell Signaling), and pY20 (sc-508, Santa Cruz Biotechnology); a goat pAb against human Trop-2 (R&D Biosystems). Negative control Abs were: purified non-immune mouse IgG (Pierce/Thermo Fisher Scientific) and purified normal goat IgG (Santa Cruz Biotechnology).
Cells and Culture Conditions
LNCaP (ATCC) and C4-2B (UroCor, Inc.) cells were cultured in RPMI 1640 medium supplemented with 5% fetal bovine serum (FBS) (Gemini Bioproducts), 1% non-essential aminoacids, 1 mM sodium pyruvate, 10 mM Hepes pH 7.3. PC3-1 and PC3-2 are sublines of the original PC3 line from ATCC, and have been described in (Akech et al., 2010); these cells were cultured in RPMI 1640 supplemented with 10% FBS. DU145 (ATCC) cells were cultured in DMEM medium supplemented with 10% FBS.
Constructs and Cell Transfection
The vector for expression of Trop-2 in mammalian cells was pEGFP-N1 from Clontech (Palo Alto, CA), devoid of the coding sequence of EGFP (pΔEGFP). The human TROP2 cDNA (Fornaro et al., 1995) was amplified by PCR with primers FT2FP (gcgattctcgagtccggtccgcgttcc – XhoI) and RT2FP (gcgccggtaccaagctcggttcctttc – KpnI) and subcloned in the pΔEGFP vector. Transfection of PC3-2 and LNCaP cells was performed with Lipofectamine 2000 reagent (Invitrogen) following manufacturer instructions. Stable transfectants were selected and maintained in complete RPMI supplemented with G418 (100 μg/ml). Enrichment for stable transfectants was performed by flow cytometry, using a fluorescence activated cell sorter (FACSVantage, Becton Dickinson). PC3-2/β5 cells were generated by transfection of the human β5 integrin cDNA subcloned in the pBABE retroviral vector, followed by selection with puromycin (0.5 μg/ml). To generate DU145 cells with stable knockdown of endogenous Trop-2, two human Trop-2-directed pLKO.1 lentiviral small hairpin RNA (shRNA) plasmids were obtained from OpenBiosystems (Cat. n. RHS3979-9623602 and RHS3979-9623605). To generate lentiviral particles, HEK293FT cells in 6-well plates were co-transfected with 1.85 μg of pLKO.1-Trop-2-shRNA vector, 1.85 μg of pLP1 (gag/pol fusion protein), 0.75 μg of pLP2 (Rev protein) and 0.4 μg of pLP/VSVG (VSV-G envelope glycoprotein) plasmids using Lipofectamine 2000. Lentivirus-containing supernatants were harvested 48 h after transfection, followed by filtration through a 0.45 μm pore diameter filter, and used to infect cultures of DU145 cells. Stably infected cells were cultured in the presence of 1 μg/ml puromycin for at least one week, and expression of Trop-2 was analyzed by IB to confirm downregulation. One of the two Trop-2 shRNA plasmids (Open Biosystems Cat. n. RHS3979-9623602) was used as control shRNA for further experiments since it did not downregulate Trop-2 expression.
Flow Cytometry
Human cell lines and transfectants were subjected to single staining with primary Abs, followed by fluorescein isothiocyanate (FITC)-conjugated goat anti mouse (GAM) secondary Abs. Fluorescence intensity was determined by FACS analysis on a FACSCalibur or a FACS LSRII instrument interfaced with CellQuest Pro software (BD Biosciences).
Activation of β1 integrins in PC3-2 cell transfectants
The β1 integrin activation assay was performed as described (Green et al., 2009). Briefly, PC3-2/Trop-2, PC3-2/Mock and PC3-2/β5 cell transfectants cultured in complete medium were washed once with PBS without Ca2+/Mg2+, and cultured for 5 h in serum-free medium. Cells were then harvested using 1 mM EDTA in PBS without Ca2+/Mg2+. After extensive washes with PBS without Ca2+/Mg2+, cells were incubated for 30 min in Hepes buffer [20 mM Hepes pH 7.4, 150 mM NaCl, 1% bovine serum albumin, (BSA)]. Hepes buffer devoid of divalent cations was compared with buffer supplemented with 1 mM CaCl2 / 1mM MgCl2, or supplemented with 0.2mM MnCl2. Then, cells were incubated for 30 min on ice in the same buffers described above, in the presence of the Ab K20 to quantify total β1 integrin expression levels, or the Ab 9EG7 to quantify the levels of active β1. A mouse IgG was used as a negative control Ab.
Generation of Protein Lysates and IB
Protein lysates were prepared by scraping cells in 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1mM CaCl2, 1mM MgCl2, 1% NP-40, 1 mM benzamidine, 10 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride, 1 μg/ml pepstatin A, 1 μM calpain inhibitor, 1 mM Na3VO4, 1 mM Na4O7P2. After 15 min incubation on ice, lysates were centrifuged at 12,000 g for 10 min. Supernatants were collected and protein content was determined using the DC Protein Assay Kit (Bio-Rad). Samples were subjected to 10% SDS-PAGE under reducing conditions and transferred onto polyvinylidene difluoride membranes for IB.
Immunoprecipitation
IP experiments were performed as follows: cells were lysed in the lysis buffer described above and pre-clearing was performed by two consecutive incubations with protein G-Sepharose at 4°C for 45 min. Binding to the specific Abs (see above) was performed by incubation at 4°C for 3 h, followed by incubation with protein G-Sepharose for 1 h at 4°C. After six washes with lysis buffer, immunocomplexes were separated by reducing SDS-PAGE.
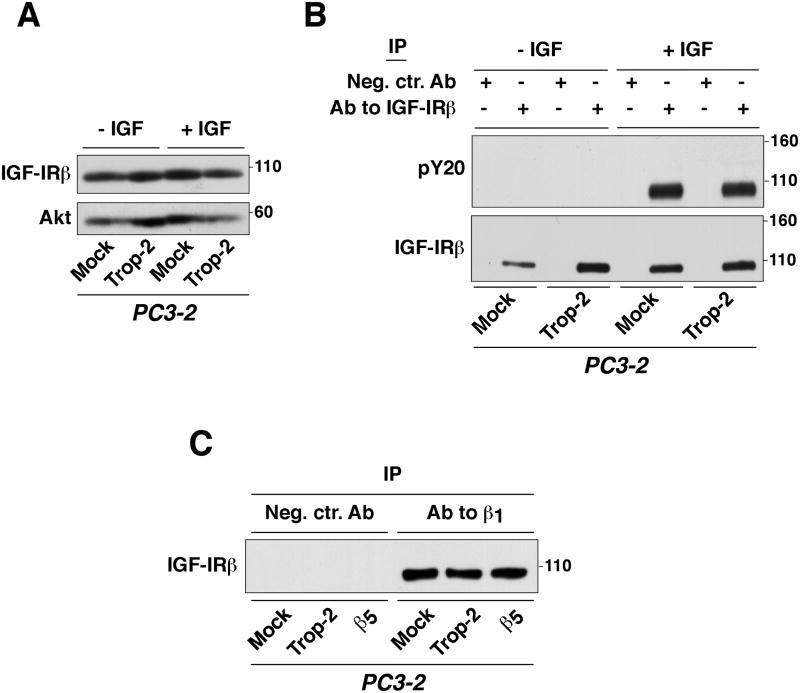
Activation of IGF-IR in PC3-2 cell transfectants
PC3-2/Trop-2, PC3-2/Mock and PC3-2/β5 cell transfectants were subjected to stimulation with IGF-I as described (Goel et al., 2004). Briefly, cells were starved in serum-free RPMI for 24h, then IGF-I was added at 100 ng/ml for 3 min. Cells were lysed as described above, and IP was performed using an Ab against IGF-IRβ. The immunocomplexes were then separated by SDS-PAGE and analyzed by IB, using an Ab against phosphorylated tyrosine residues (pY20) and against IGF-IRβ.
Activation of FAK in PC3-2 cell transfectants
For FAK activation assay, PC3-2 cell transfectants were trypsinized, and trypsin was inactivated by soybean trypsin inhibitor. After three washes with serum-free RPMI/1% BSA, cells were maintained in suspension for 30 min at 37°C. Then, cells were seeded on plastic dishes coated with FN (10 μg/ml) or PLL (1 mg/ml), and incubated for 15 min at 37°C. After two washes with PBS, cells were lysed and separated by reducing SDS-PAGE; then, IB analysis was performed using an Ab against pFAK (Tyr397) and total FAK.
Cell Adhesion Assay
Coating of plastic surfaces was performed using FN (10 μg/ml) or PLL (1 mg/ml) overnight at 4°C. Cell adhesion assays were performed as described (Zheng et al., 1999). Briefly, cells were trypsinized, and trypsin was inactivated by soybean trypsin inhibitor (Roche). After three washes with serum-free RPMI/1% BSA, cells were counted and seeded on 96-well plates for 1 h at 37°C. Cells were later fixed with 3.7% paraformaldehyde (PFA) and stained with 0.1% crystal violet. Cell adhesion was quantified by absorbance at 562 nm using a microtiter plate reader (ICN Titertek Multiskan Bichromatic). Fisher's exact test was used for statistical analysis. Each experiment was performed in triplicate. The assays were repeated at least three times, and similar results were observed.
Immunofluorescence and Confocal Microscopy
Cells were seeded on FN-coated glass coverslips for 1 h at 37°C. Then, fixation with 3.7% PFA was performed for 15 min at room temperature, followed by quencing with 50 mM NH4Cl. Cells were permeabilized by incubation with PBS / 0.2% Triton X-100 for 5 min, and then incubated for 30 min at RT with the blocking solution (PBS / 5% BSA). Staining was performed incubating samples with the primary mAb to RACK1 for 20 min at room temperature, followed by incubation with a secondary Ab (Alexa Fluor 488-Rabbit-anti-mouse) for 20 min at room temperature. After three washes, coverslips were mounted on glass slides using Pro-Long anti-fade reagent (Invitrogen), and slides were analyzed on an inverted confocal microscope (LSM510, Carl Zeiss) using Plan-Apochromat 63× (1.4 NA) or Plan-Neofluar 100× (1.3 NA) lenses.
Results
Trop-2 inhibits cell adhesion to fibronectin
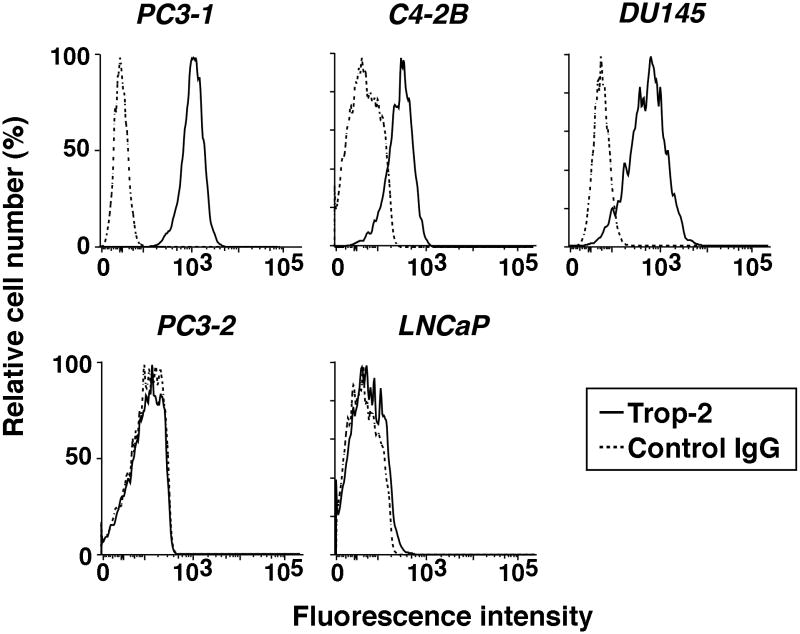
We have previously shown that Trop-2, whose gene is designated TACSTD2 (Fornaro et al., 1995), is upregulated in human PrCa (Trerotola et al., 2010; Trerotola et al., 2012); this upregulation is consistent with earlier reports demonstrating increased expression of Trop-2 in a murine model of PrCa progression (Calvo et al., 2002). As depicted in Figure 1, we extensively analyzed the expression levels of Trop-2 in five human PrCa cell lines. Three aggressive cell lines: PC3-1 [also designated PC3-H in (Akech et al., 2010)], C4-2B and DU145; two less aggressive cell lines: PC3-2 [also designated PC3-L in (Akech et al., 2010)] and LNCaP. Trop-2 expression is found to be high in aggressive DU145 and PC3-1 cells, intermediate in C4-2B, and undetectable in PC3-2 and LNCaP. Thus, Trop-2's expression levels may reflect the aggressive phenotype of PrCa cells.
Fig. 1.
Trop-2 expression in PrCa cell lines. Surface expression profiles of Trop-2 were obtained by FACS analysis in five human PrCa cell lines: PC3-1, C4-2B, DU145, PC3-2 and LNCaP. Fluorescence intensity average values up to 103 and over 103 were chosen as thresholds to designate “intermediate” and “high” expression levels, respectively; “undetectable” expression was designated for profiles overlapping the ones obtained by staining with a negative control Ab. Profiles obtained by staining with a mAb to Trop-2, continuous lines. Profiles obtained by staining with a mouse IgG (negative control Ab), dotted lines.
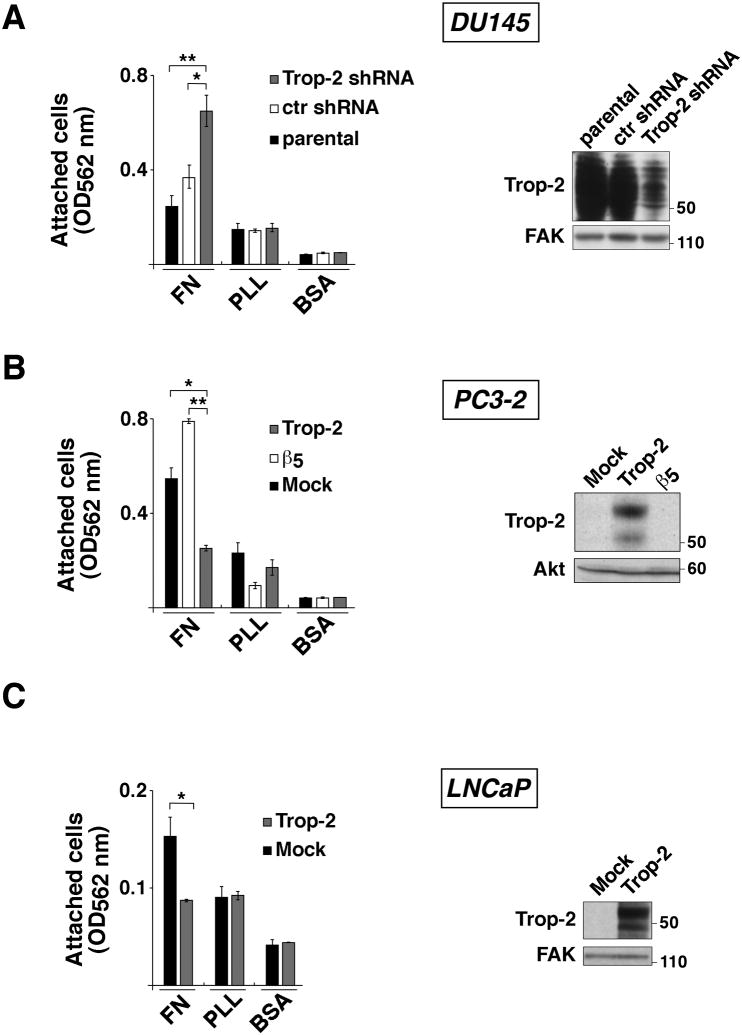
We silenced Trop-2 in DU145 cells using shRNA expressing lentiviruses; in parallel, we ectopically expressed Trop-2 in PC3-2 and LNCaP cells, as shown by IB (Fig. 2, right panels). Then, we seeded these cells on FN – a major component of the ECM – in order to evaluate Trop-2's effect on cell adhesion to extracellular substrates. As shown in Figure 2, we observe that shRNA-mediated silencing of Trop-2 enhances adhesion of DU145 cells to FN as compared with parental (157.3 ± 38.6%) and control shRNA cells (77.4 ± 19.3%) (Fig. 2A, left). Consistently, we observe that ectopic expression of Trop-2 significantly inhibits adhesion of PC3-2 cells to FN as compared with Mock (55.8±4.8%) or β5 (68.0±4.0%) transfectants (Fig. 2B, left). Since αvβ5 integrin is able to bind FN (Pasqualini et al., 1993), PC3-2/β5 transfectants were used as a control group in addition to PC3-2/Mock cells. We also observe that expression of Trop-2 in LNCaP cells inhibits adhesion to FN by 46.6±5.2% as compared with Mock transfectants (Fig. 2C, left). In all cases, BSA was used as negative control and PLL as loading control.
Fig. 2.
Trop-2 inhibits cell adhesion to FN. (A) Adhesion assays were performed using DU145/Trop-2 shRNA cells seeded on FN, PLL and BSA, as described in Materials and Methods. Parental DU145 cells or DU145/ctr shRNA (infected with a non-silencing shRNA) were used as negative controls. Error bars, SEM. *, P=0.021. **, P=0.004. Silencing of Trop-2 was evaluated by IB (right panel). FAK, control of protein loading. (B) Adhesion assays were performed using PC3-2/Trop-2 cell transfectants seeded on FN, PLL and BSA. PC3-2/Mock and PC3-2/β5 transfectants were used as negative control groups. Error bars, SEM. *, P=0.0095; **, P=0.0011. Ectopic expression of Trop-2 obtained by transfection of PC3-2 cells was evaluated by IB (right panel). Akt, control of protein loading. (C) Adhesion assays were performed using LNCaP/Trop-2 versus LNCaP/Mock transfectants. Error bars, SEM. *, P=0.030. Ectopic expression of Trop-2 obtained by transfection of LNCaP cells was evaluated by IB (right panel). FAK, control of protein loading.
This experimental evidence indicates that Trop-2 regulates PrCa cell adhesion to FN, suggesting that its activity may impinge on signaling networks regulated by FN receptors, namely β1 integrins (Pytela et al., 1985).
Trop-2 does not affect β1 integrin activation
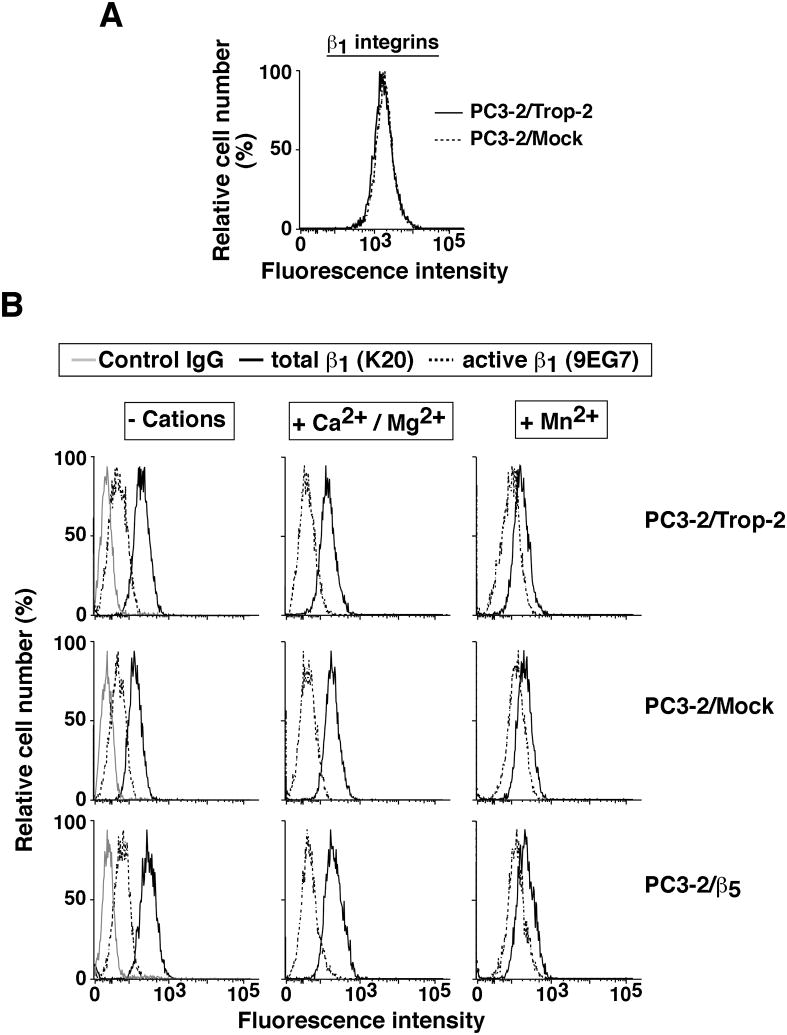
We ruled out the possibility that β1 integrin levels would be altered upon Trop-2 expression in PC3-2 transfectants. As shown in Figure 3A, we do not detect significant changes in β1 protein levels on the cell surface of PC3-2/Trop-2 transfectants as compared with control cells. Therefore, we hypothesized that the effect of Trop-2 on cell adhesion to FN might be a consequence of Trop-2-induced modulation of β1 integrin affinity for FN. A conformational switch from low-affinity or inactive form to high-affinity or active form occurs upon integrin binding to their ligands, but can alternatively be induced with activation stimuli such as Mn2+ ions (Hynes, 2002). This conformational switch exposes CLIBS (cation-and-ligand-influenced binding site) epitopes that can be recognized by specific antibodies, such as 9EG7 (Bazzoni et al., 1995). We performed a cation-mediated β1 activation assay by flow cytometry, using PC3-2 transfectants incubated in the presence of either Ca2+/Mg2+ ions (that do not activate β1) or Mn2+ ions (that do activate β1) (Bazzoni et al., 1995). As depicted in Figure 3B, we do not observe significant changes of the 9EG7 epitope's levels in Trop-2 transfectants versus negative controls, indicating that ion-mediated activation of β1 is not affected by Trop-2. Thus, other mechanisms occur to induce the Trop-2-dependent anti-adhesive phenotype of PrCa cells.
Fig. 3.
Ectopic expression of Trop-2 does not affect either β1 integrin levels or activation. (A) β1 integrin surface levels were analyzed by FACS. Continuous and dotted lines represent β1 integrin surface expression profiles in PC3-2/Trop-2 and PC3-2/Mock cells, respectively. (B) Activation of β1 integrins was evaluated by FACS analysis on PC3-2/Trop-2 (top panels) versus PC3-2/Mock (middle panels) and PC3-2/β5 (bottom panels) cells. Continuous black lines, total β1 integrin expression profiles as measured by staining with K20 Ab. Dotted black lines, active β1 integrin levels as measured by staining with 9EG7 Ab. Continuous gray lines, profiles obtained by staining with a negative control Ab. Left column, β1 integrin expression profiles obtained by incubating cells in a buffer devoid of divalent cations. Middle column, β1 integrin expression profiles obtained by incubating cells in a buffer supplemented with CaCl2 and MgCl2. Right column, β1 integrin expression profiles obtained by incubating cells in a buffer supplemented with MnCl2.
Trop-2 stimulates the association of β1 integrins with RACK1, and the activity of Src
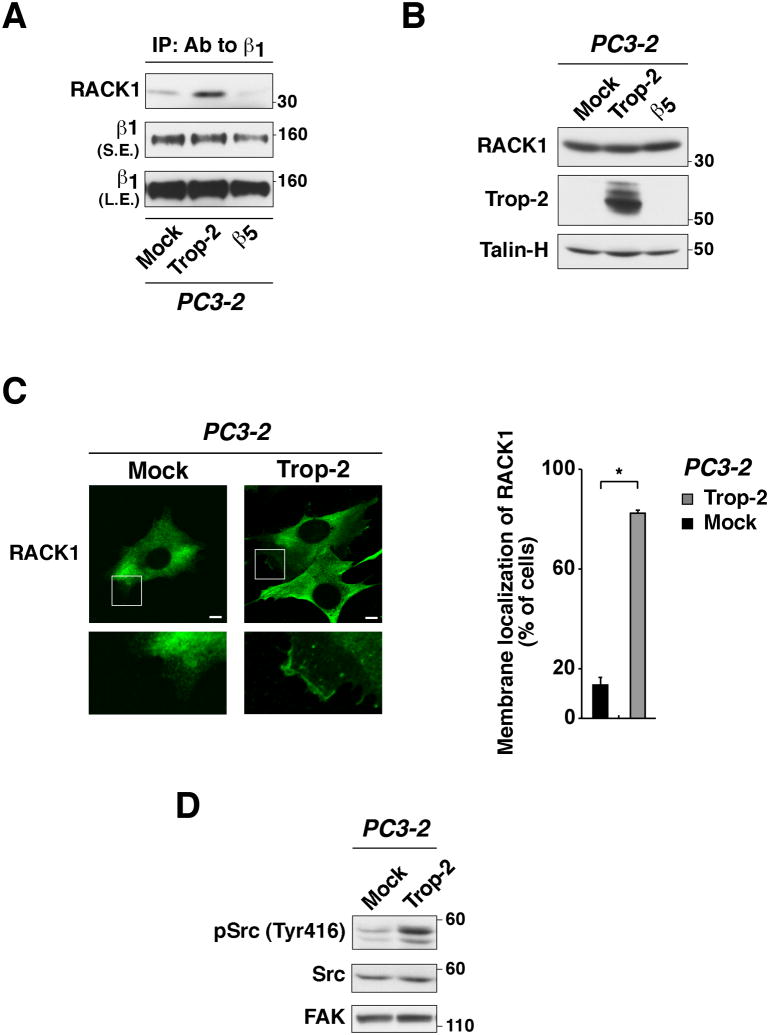
RACK1 is an adaptor molecule that has been previously shown to regulate integrin-dependent cell adhesion to ECM ligands (Serrels et al., 2010), and to associate with β1 integrins (Liliental and Chang, 1998; Besson et al., 2002). We show that upon ectopic expression of Trop-2 in PC3-2 cells, the association of RACK1 to β1 integrins is strikingly enhanced (Fig. 4A), although RACK1's expression levels remain constant (Fig. 4B). We also performed IP of Trop-2, but we did not detect interaction with RACK1 (data not shown). We then analyzed the localization of RACK1 in PC3-2 transfectants by immunofluorescence (Fig. 4C). Our data show that RACK1 accumulates in membrane compartments in PC3-2/Trop-2 cells, whereas it appears to be diffuse in the cytoplasm of PC3-2/Mock transfectants (Fig. 4C, left). The percentage of cells in which RACK1 is localized in membrane regions was as follows: 82.5±0.9 per cell in PC3-2/Trop-2 (n=140 cells) as compared with 13.1±2.3 per cell in PC3-2/Mock (n=195 cells) transfectants (Figure 4C, right). Thus, these data suggest that Trop-2 induces accumulation of RACK1 in membrane regions, where this adaptor molecule may form a tight complex with β1 integrins. Recent reports have shown that IGF-I, required for RACK1 to bind β1 integrins (Kiely et al., 2005), induces displacement of Src from RACK1 (Kiely et al., 2002) with increase in its kinase activity (Cox et al., 2003). Therefore, we tested Src activity by immunoblotting using an Ab specifically targeting the active form of this kinase, pSrc (Tyr416); as depicted in Figure 4D, we find that the levels of pSrc (Tyr416) are enhanced in PC3-2/Trop-2 transfectants as compared with PC3-2/Mock cells. This appears to be consistent with a role of Trop-2 as a promoter of β1-RACK1 interaction.
Fig. 4.
Trop-2 stimulates the association of β1 integrins with RACK1, and activates Src. (A) β1 integrins were immunoprecipitated using PC3-2 transfectants, and IB was performed on the immunoprecipitates using an Ab against RACK1. PC3-2/Trop-2 cells were compared with Mock and β5 integrin transfectants in order to ensure specificity. IB using a mAb against β1 was performed on the same immunoprecipitates as control of loading. (B) Expression levels of RACK1 were evaluated by IB in PC3-2/Trop-2 versus PC3-2/Mock and PC3-2/β5 transfectants. Talin-H, control of protein loading. (C, left) PC3-2/Mock and PC3-2/Trop-2 transfectants were seeded on FN and analyzed by IF staining with a mAb against RACK1. High magnification boxed areas (bottom) show membrane regions with specific localization of RACK1. Bars, 10 μm. (C, right) The presence of RACK1 in membrane rims was evaluated. The bar graph indicates the percent fraction of cells showing membrane staining of RACK1 in PC3-2/Mock versus PC3-2/Trop-2 transfectants. Error bars, SEM. *, P=0.0092. (D) The activation of Src was analyzed by IB using an Ab against pSrc (Tyr416). PC3-2/Trop-2 transfectants were compared with PC3-2/Mock. Total Src and FAK, controls of protein loading.
Trop-2 stimulates the phosphorylation of FAK
Adhesion signals and IGF-I promote association between IGF-IR, RACK1 and β1 integrins (Kiely et al., 2005). Since IGF-IR plays a critical role in the regulation of RACK1's binding to several partners, we hypothesized that Trop-2 might regulate the activity of IGF-IR, or alternatively its interaction with β1 integrins (Goel et al., 2004). As shown in Figure 5A, we observe that IGF-IRβ expression levels are not affected by Trop-2. In addition, we find that Trop-2 does not alter the activity of IGF-IR. Indeed, when we incubate PC3-2 transfectants with IGF-I, rapid phosphorylation of IGF-IRβ is detected, independently on Trop-2's expression (Fig. 5B). Consistently, the association between β1 and IGF-IR was again unaffected when comparing PC3-2/Trop-2 with PC3-2/Mock and PC3-2/β5 cells (Fig. 5C).
Fig. 5.
Trop-2 does not affect either the activity of IGF-IR or its association with β1 integrins. (A) Expression levels of IGF-IRβ were assessed by IB using PC3-2 transfectants stimulated with IGF-I for 3 min. (B) IGF-IRβ was immunoprecipitated, and its phosphorylation levels were assessed by IB using a pY20 Ab. IB using an Ab against IGF-IRβ was performed to confirm that the receptor was pulled-down from both non-stimulated and IGF-I-stimulated PC3-2 transfectants. (C) The presence of the β1 integrin/IGF-IR complex was investigated in PC3-2/Trop-2 cells compared with negative control groups by IP of β1 and analysis of IGF-IRβ by IB.
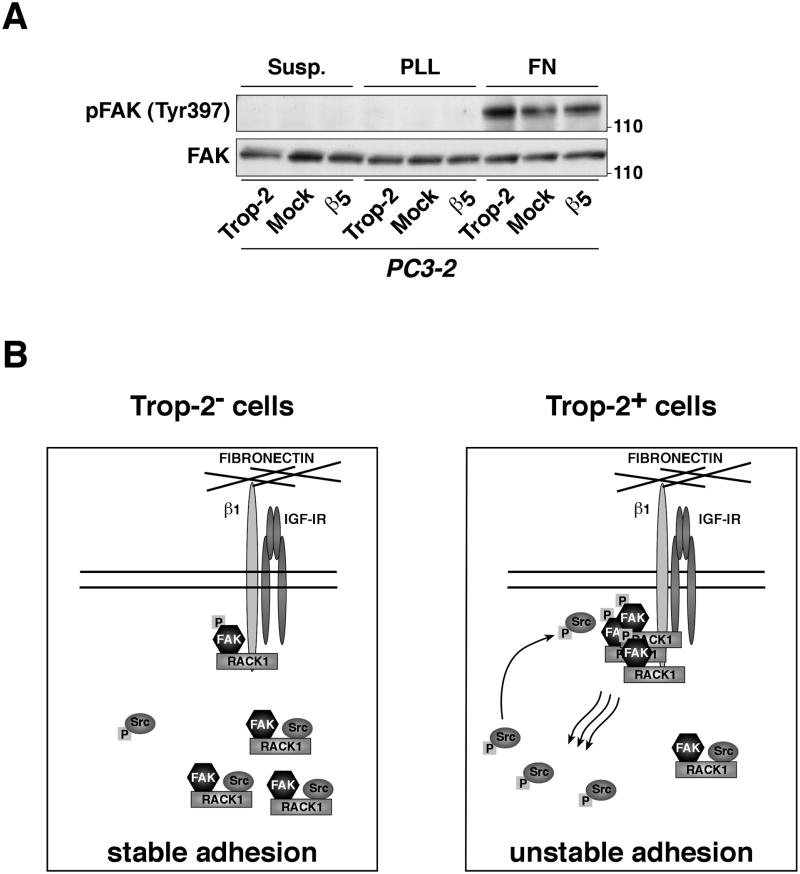
Reduced cell adhesion to ECM substrates occurs through modulation of focal adhesion turnover, an event affected by FAK, a kinase known to associate with RACK1. Therefore, we investigated whether Trop-2 affects the activation of FAK in PC3-2 transfectants upon adhesion to FN. We observe that ectopic expression of Trop-2 induces phosphorylation of FAK (Tyr397) on FN, whereas reduced levels of phosphorylated FAK (Tyr397) are observed in PC3-2/Mock and PC3-2/β5 transfectants (Fig. 6A). Thus, Trop-2 promotes an anti-adhesive phenotype of PrCa cells modulating the β1 integrin-RACK1-FAK-Src signaling axis.
Fig. 6.
Trop-2 activates FAK. (A) The activation of FAK was investigated using PC3-2/Trop-2 versus PC3-2/Mock and PC3-2/β5 cells seeded on FN for 10 min. Cells seeded on FN- or PLL-coated plastic dishes were washed with PBS, lysed and subjected to analysis by IB using an Ab against pFAK (Tyr397). Total FAK, control of protein loading. (B) Schematic drawing of Trop-2-mediated inhibition of PrCa cell adhesion to FN through the β1 integrin-RACK1 signaling axis. (B, left) In cells that do not express Trop-2, RACK1 is mostly localized in the cytoplasm, where it binds FAK and Src, and prevents Src to be activated; the functional consequence is a decreased focal adhesion turnover, with stabilized cell adhesion to FN. (B, right) In Trop-2-expressing cells, increased binding of RACK1 to β1 brings FAK in close proximity to β1, and this results in activation of FAK by autophosphorylation of its Tyr397 residue, release of Src from the complex, and Src activation by phosphorylation of the Tyr416 residue. Active Src and FAK interact with each other and stimulate downstream events that finally determine faster turnover of adhesive structures and destabilized cell attachment to FN.
Discussion
In this study, we demonstrate that Trop-2, a transmembrane protein that is frequently upregulated in human carcinomas, inhibits PrCa cell adhesion to FN. The mechanism underlying Trop-2's function in cell adhesion involves modulation of the β1 integrin-RACK1-FAK-Src signaling axis.
This study describes a function for Trop-2 that has never been demonstrated before, in inhibiting cell adhesion to FN. This observation is relevant to the understanding of PrCa mechanisms since Trop-2 expression correlates with, and appears to promote, an aggressive phenotype of PrCa cells. Since it is conceivable that more aggressive cells need to be poorly adhesive and more motile than less aggressive cells, we conclude that Trop-2 expression is a requirement for cancer cells which need to detach from the ECM. Finally, this finding is also relevant to PrCa since β1 integrins, receptors for FN, are known to be upregulated in this disease (Goel et al., 2008); thus, we speculate that Trop-2 expression is also required in distant sites, where FN is upregulated (Ruoslahti, 1999).
The signaling pathway, described here, by which Trop-2 affects PrCa cell adhesion to FN is also novel for the following reasons. First, it is independent of changes occurring in β1 affinity, as shown by analysis of β1 integrins from a “closed” conformation that does not allow binding to their ligands to an “open” conformation, resulting in increased affinity for the ligands (Hynes, 2002). Second, we demonstrate that Trop-2's effects on PrCa cell adhesion to FN comprise relocalization of the adaptor molecule RACK1 from cytoplasm to the cell membrane, and, more importantly, enhanced association between β1 integrins and RACK1. RACK1 has been reported to bind other integrin β subunits, including β5 (Liliental and Chang, 1998; Buensuceso et al., 2001); however, ectopic expression of β5 integrin in PC3-2 cells did not result in significant effects either on cell adhesion to FN or on the β1-RACK1 binding. This evidence indicates that Trop-2's effect is specific and cannot be obtained by expression of another transmembrane receptor. Third, Trop-2 does not alter the association between β1 and IGF-IR, known to occur in PrCa cells (Goel et al., 2004), but its effect on β1-RACK1 appears to resemble either non-adhesive phenotypes (Kiely et al., 2005), or the ectopic expression of a defective Y1250/1251F IGF-IR mutant that does not bind RACK1, which make the β1-RACK1 association constitutive and insensitive to IGF-I stimulation (Kiely et al., 2005). We thus propose that cells may use Trop-2 to increase the association between β1 integrins and RACK1 when they need to detach and invade the surrounding ECM. Finally, the novelty of our observation is that Trop-2 activates a RACK1-mediated signal from β1 integrin-dependent adhesion to ECM ligands, which converges in the activation of the motility kinases FAK and Src (Chang et al., 1998; Mitra et al., 2005; Kiely et al., 2009; Serrels et al., 2010). This is consistent with a previous report showing that enhanced β1-RACK1 association contributes to activate FAK and Src (Kiely et al., 2005). Furthermore, additional studies have shown that the N-terminal region of RACK1 contains the FAK binding site (Kiely et al., 2009), whereas the C-terminal portion of RACK1 mediates interactions with β1 integrins (Liliental and Chang, 1998; Buensuceso et al., 2001; Besson et al., 2002) as well as Src (Chang et al., 2001). Thus, while FAK is constitutively associated with RACK1 (Kiely et al., 2009), β1 integrins and Src compete with each other for binding to the same region. As seen in the schematic model in Figure 6B, we propose that, as a consequence of Trop-2-mediated increase of the association between RACK1 and β1, FAK is translocated in close proximity of the β1 cytoplasmic tail. Then, FAK is autophosphorylated in response to β1 signaling (Mitra and Schlaepfer, 2006), and Src is released in the cytoplasm and its activity is induced. Increased levels of pFAK (Tyr397) stimulate binding to active Src, that can further phosphorylate FAK in additional sites, such as Tyr925, to induce full activation and focal adhesion turnover. The less adhesive phenotype of PrCa cells observed upon expression of Trop-2 is consistent with earlier studies showing that a fully-functional FAK-Src pathway is necessary for disassembly and turnover of focal adhesions, and for a dynamic regulation of cell-ECM interaction (Webb et al., 2004), one of the earliest steps in the metastatic cascade. FAK has been described to control microtubule polarization within cells (Palazzo et al., 2004), and this phenotype has been linked to the cross-talk between FAK and Rho GTPases (Ren et al., 2000). Rac/Rho/Cdc42 GTPases control formation and disassembly of actin cytoskeletal structures (such as stress fibers, lamellipodia and filopodia) (Clark et al., 1998; Kuhn et al., 1998; Price et al., 1998; Hirsch et al., 2002), and support cell motility (Symons and Settleman, 2000). Rho-family GTPases are also known to promote cell migration (Wells et al., 2010) by activating p21-activated Ser/Thr kinases (PAK)s (Kumar et al., 2006; Acconcia et al., 2007), which cross-talk with Src (Siu et al., 2010). Given our evidence that Trop-2 impinges on networks regulated by FAK/Src, we envision a role for Trop-2 in PrCa cell migration through cross-talk with these major signaling hubs. Although the pathway described above appears to be predominant, other important signaling molecules like Pten, Akt (Goswami et al., 2005) and AR (Lamoureux et al., 2011) are critical for development and progression of PrCa. These molecules are therefore promising candidates for future investigations of additional molecular mechanisms underlying Trop-2-mediated events in advanced PrCa.
In summary, we describe a novel molecular mechanism of regulation of PrCa cell adhesion to FN, whereby the transmembrane glycoprotein Trop-2, previously suggested to have a role in cell-cell adhesion, modulates cell-ECM attachment by controlling the β1 integrin-RACK1 complex and consequently FAK and Src. Further investigations are needed to delineate additional roles for Trop-2 in integrin-related functions, such as migration and invasion, and will allow to develop potential innovative drugs for target therapy of advanced PrCa.
Acknowledgments
We thank Dr. A. Dutta, Dr. T.J. Fitzgerald, Dr. H.L. Goel, Dr. E. Guerra, Dr. K.M. Havas, Dr. A. Sayeed, Dr. T. Wang, for constructive discussion; Dr. L.W. Chung for providing PC3-1 cells; and L. Tang and L. Griffith for helping with the preparation of the manuscript.
Research in this publication includes work carried out using the Kimmel Cancer Center Bioimaging and Flow Cytometry Facilities, which are supported in part by NCI Cancer Center Support Grant P30 CA56036.
Contract grant sponsor: NIH
Contract grant number: NIH-R01CA109874 and NIH-P01CA140043-Project 2
Contract grant sponsor: Italian Association for Cancer Research (AIRC)
List of Abbreviations
- ECM
extracellular matrix
- FN
fibronectin
- IP
immunoprecipitation
- IB
immunoblotting
- IF
immunofluorescence
- FACS
flow cytometry
- FAK
focal adhesion kinase
- pFAK
phospho FAK
- Src
c-Src
- pSrc
phospho c-Src
- ERK
extracellular signal-regulated kinase
- IGF-I
Insulin-like Growth Factor I
- IGF-IR
IGF-I Receptor
- MLCK
myosin light-chain kinase
- RACK1
receptor for activated C kinase 1
- shRNA
small hairpin RNA
- siRNA
small interfering RNA
- PKC
protein kinase C
- PLL
poly-L-lysine
- PBS
phosphate buffer saline
- FITC
fluorescein isothiocyanate
- Ab
antibody
- mAb
monoclonal Ab
- pAb
polyclonal Ab
- FBS
fetal bovine serum
- GAM
goat anti mouse
- PFA
paraformaldehyde
- BSA
bovine serum albumin
- SEM
standard error of the mean
List of Abbreviations used in Figures and Legends
- Ab
Antibody
- BSA
Bovine Serum Albumin
- ctr shRNA
control shRNA
- FACS
flow cytometry
- FAK
focal adhesion kinase
- FN
fibronectin
- IB
immunoblotting
- IF
immunofluorescence
- IGF
insulin growth factor I
- IGF-IRβ
IGF-I Receptor β
- IgG
immunoglobulin G
- IP
immunoprecipitation
- mAb
monoclonal Ab
- Neg. ctr. Ab.
negative control Ab
- PBS
phosphate buffer saline
- pFAK
phospho-FAK
- PLL
Poly-L-Lysine
- PrCa
Prostate Cancer
- pSrc
phospho-Src
- SEM
standard error of the mean
- shRNA
small hairpin RNA
- Susp.
suspension
- Talin-H
talin-Head
Footnotes
The authors state no conflict of interest.
References
- Acconcia F, Barnes CJ, Singh RR, Talukder AH, Kumar R. Phosphorylation-dependent regulation of nuclear localization and functions of integrin-linked kinase. Proc Natl Acad Sci USA. 2007;104:6782–6787. doi: 10.1073/pnas.0701999104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akech J, Wixted JJ, Bedard K, van der Deen M, Hussain S, Guise TA, van Wijnen AJ, Stein JL, Languino LR, Altieri DC, Pratap J, Keller E, Stein GS, Lian JB. Runx2 association with progression of prostate cancer in patients: mechanisms mediating bone osteolysis and osteoblastic metastatic lesions. Oncogene. 2010;29:811–821. doi: 10.1038/onc.2009.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam N, Goel HL, Zarif MJ, Butterfield JE, Perkins HM, Sansoucy BG, Sawyer TK, Languino LR. The integrin-growth factor receptor duet. J Cell Physiol. 2007;213:649–653. doi: 10.1002/jcp.21278. [DOI] [PubMed] [Google Scholar]
- Balzar M, Briaire-de Bruijn IH, Rees-Bakker HA, Prins FA, Helfrich W, de Leij L, Riethmuller G, Alberti S, Warnaar SO, Fleuren GJ, Litvinov SV. Epidermal growth factor-like repeats mediate lateral and reciprocal interactions of Ep-CAM molecules in homophilic adhesions. Mol Cell Biol. 2001;21:2570–2580. doi: 10.1128/MCB.21.7.2570-2580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzoni G, Shih DT, Buck CA, Hemler ME. Monoclonal antibody 9EG7 defines a novel β1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J Biol Chem. 1995;270:25570–25577. doi: 10.1074/jbc.270.43.25570. [DOI] [PubMed] [Google Scholar]
- Besson A, Wilson TL, Yong VW. The anchoring protein RACK1 links protein kinase Cepsilon to integrin β chains. Requirements for adhesion and motility. J Biol Chem. 2002;277:22073–22084. doi: 10.1074/jbc.M111644200. [DOI] [PubMed] [Google Scholar]
- Buensuceso CS, Woodside D, Huff JL, Plopper GE, O'Toole TE. The WD protein Rack1 mediates protein kinase C and integrin-dependent cell migration. J Cell Sci. 2001;114:1691–1698. doi: 10.1242/jcs.114.9.1691. [DOI] [PubMed] [Google Scholar]
- Calvo A, Xiao N, Kang J, Best CJ, Leiva I, Emmert-Buck MR, Jorcyk C, Green JE. Alterations in gene expression profiles during prostate cancer progression: functional correlations to tumorigenicity and down-regulation of selenoprotein-P in mouse and human tumors. Cancer Res. 2002;62:5325–5335. [PubMed] [Google Scholar]
- Chang BY, Chiang M, Cartwright CA. The interaction of Src and RACK1 is enhanced by activation of protein kinase C and tyrosine phosphorylation of RACK1. J Biol Chem. 2001;276:20346–20356. doi: 10.1074/jbc.M101375200. [DOI] [PubMed] [Google Scholar]
- Chang BY, Conroy KB, Machleder EM, Cartwright CA. RACK1, a receptor for activated C kinase and a homolog of the β subunit of G proteins, inhibits activity of src tyrosine kinases and growth of NIH 3T3 cells. Mol Cell Biol. 1998;18:3245–3256. doi: 10.1128/mcb.18.6.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EA, King WG, Brugge JS, Symons M, Hynes RO. Integrin-mediated signals regulated by members of the rho family of GTPases. J Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BD, Natarajan M, Stettner MR, Gladson CL. New concepts regarding focal adhesion kinase promotion of cell migration and proliferation. J Cell Biochem. 2006;99:35–52. doi: 10.1002/jcb.20956. [DOI] [PubMed] [Google Scholar]
- Cox EA, Bennin D, Doan AT, O'Toole T, Huttenlocher A. RACK1 regulates integrin-mediated adhesion, protrusion, and chemotactic cell migration via its Src-binding site. Mol Biol Cell. 2003;14:658–669. doi: 10.1091/mbc.E02-03-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRoock IB, Pennington ME, Sroka TC, Lam KS, Bowden GT, Bair EL, Cress AE. Synthetic peptides inhibit adhesion of human tumor cells to extracellular matrix proteins. Cancer Res. 2001;61:3308–3313. [PubMed] [Google Scholar]
- Engvall E, Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977;20:1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Fong D, Moser P, Krammel C, Gostner JM, Margreiter R, Mitterer M, Gastl G, Spizzo G. High expression of TROP2 correlates with poor prognosis in pancreatic cancer. Br J Cancer. 2008;99:1290–1295. doi: 10.1038/sj.bjc.6604677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornaro M, Dell'Arciprete R, Stella M, Bucci C, Nutini M, Capri MG, Alberti S. Cloning of the gene encoding Trop-2, a cell-surface glycoprotein expressed by human carcinomas. Int J Cancer. 1995;62:610–618. doi: 10.1002/ijc.2910620520. [DOI] [PubMed] [Google Scholar]
- Goel HL, Fornaro M, Moro L, Teider N, Rhim JS, King M, Languino LR. Selective modulation of type 1 insulin-like growth factor receptor signaling and functions by β1 integrins. J Cell Biol. 2004;166:407–418. doi: 10.1083/jcb.200403003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel HL, Li J, Kogan S, Languino LR. Integrins in prostate cancer progression. Endocr Relat Cancer. 2008;15:657–664. doi: 10.1677/ERC-08-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. Identification of a cell of origin for human prostate cancer. Science. 2010;329:568–571. doi: 10.1126/science.1189992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AS, Lawson DA, Cheng D, Sun W, Garraway IP, Witte ON. Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. Proc Natl Acad Sci USA. 2008;105:20882–20887. doi: 10.1073/pnas.0811411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami A, Burikhanov R, de Thonel A, Fujita N, Goswami M, Zhao Y, Eriksson JE, Tsuruo T, Rangnekar VM. Binding and phosphorylation of par-4 by akt is essential for cancer cell survival. Mol Cell. 2005;20:33–44. doi: 10.1016/j.molcel.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Green JA, Berrier AL, Pankov R, Yamada KM. β1 integrin cytoplasmic domain residues selectively modulate fibronectin matrix assembly and cell spreading through talin and Akt-1. J Biol Chem. 2009;284:8148–8159. doi: 10.1074/jbc.M805934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra E, Trerotola M, Dell' Arciprete R, Bonasera V, Palombo B, El-Sewedy T, Ciccimarra T, Crescenzi C, Lorenzini F, Rossi C, Vacca G, Lattanzio R, Piantelli M, Alberti S. A bicistronic CYCLIN D1-TROP2 mRNA chimera demonstrates a novel oncogenic mechanism in human cancer. Cancer Res. 2008;68:8113–8121. doi: 10.1158/0008-5472.CAN-07-6135. [DOI] [PubMed] [Google Scholar]
- Hermanto U, Zong CS, Li W, Wang LH. RACK1, an insulin-like growth factor I (IGF-I) receptor-interacting protein, modulates IGF-I-dependent integrin signaling and promotes cell spreading and contact with extracellular matrix. Mol Cell Biol. 2002;22:2345–2365. doi: 10.1128/MCB.22.7.2345-2365.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch E, Barberis L, Brancaccio M, Azzolino O, Xu D, Kyriakis JM, Silengo L, Giancotti FG, Tarone G, Fassler R, Altruda F. Defective Rac-mediated proliferation and survival after targeted mutation of the β1 integrin cytodomain. J Cell Biol. 2002;157:481–492. doi: 10.1083/jcb.200111065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, Waterman CM. Nanoscale architecture of integrin-based cell adhesions. Nature. 2010;468:580–584. doi: 10.1038/nature09621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiely PA, Baillie GS, Barrett R, Buckley DA, Adams DR, Houslay MD, O'Connor R. Phosphorylation of RACK1 on tyrosine 52 by c-Abl is required for insulin-like growth factor I-mediated regulation of focal adhesion kinase. J Biol Chem. 2009;284:20263–20274. doi: 10.1074/jbc.M109.017640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiely PA, Leahy M, O'Gorman D, O'Connor R. RACK1-mediated integration of adhesion and insulin-like growth factor I (IGF-I) signaling and cell migration are defective in cells expressing an IGF-I receptor mutated at tyrosines 1250 and 1251. J Biol Chem. 2005;280:7624–7633. doi: 10.1074/jbc.M412889200. [DOI] [PubMed] [Google Scholar]
- Kiely PA, Sant A, O'Connor R. RACK1 is an insulin-like growth factor 1 (IGF-1) receptor-interacting protein that can regulate IGF-1-mediated Akt activation and protection from cell death. J Biol Chem. 2002;277:22581–22589. doi: 10.1074/jbc.M201758200. [DOI] [PubMed] [Google Scholar]
- Kuhn TB, Brown MD, Bamburg JR. Rac1-dependent actin filament organization in growth cones is necessary for β1-integrin-mediated advance but not for growth on poly-D-lysine. J Neurobiol. 1998;37:524–540. doi: 10.1002/(sici)1097-4695(199812)37:4<524::aid-neu3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer. 2006;6:459–471. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]
- Lamoureux F, Thomas C, Yin MJ, Kuruma H, Fazli L, Gleave ME, Zoubeidi A. A novel HSP90 inhibitor delays castrate-resistant prostate cancer without altering serum PSA levels and inhibits osteoclastogenesis. Clin Cancer Res. 2011;17:2301–2313. doi: 10.1158/1078-0432.CCR-10-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liliental J, Chang DD. Rack1, a receptor for activated protein kinase C, interacts with integrin β subunit. J Biol Chem. 1998;273:2379–2383. doi: 10.1074/jbc.273.4.2379. [DOI] [PubMed] [Google Scholar]
- Litvinov SV, Balzar M, Winter MJ, Bakker HA, Briaire-de Bruijn IH, Prins F, Fleuren GJ, Warnaar SO. Epithelial cell adhesion molecule (Ep-CAM) modulates cell-cell interactions mediated by classic cadherins. J Cell Biol. 1997;139:1337–1348. doi: 10.1083/jcb.139.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TT, Barreuther M, Davis S, Madri JA. Platelet endothelial cell adhesion molecule-1 is phosphorylatable by c-Src, binds Src-Src homology 2 domain, and exhibits immunoreceptor tyrosine-based activation motif-like properties. J Biol Chem. 1997;272:14442–14446. doi: 10.1074/jbc.272.22.14442. [DOI] [PubMed] [Google Scholar]
- Marx M, Warren SL, Madri JA. pp60(c-src) modulates microvascular endothelial phenotype and in vitro angiogenesis. Exp Mol Pathol. 2001;70:201–213. doi: 10.1006/exmp.2001.2358. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Nakatsukasa M, Kawasaki S, Yamasaki K, Fukuoka H, Matsuda A, Tsujikawa M, Tanioka H, Nagata-Takaoka M, Hamuro J, Kinoshita S. Tumor-associated calcium signal transducer 2 is required for the proper subcellular localization of claudin 1 and 7: implications in the pathogenesis of gelatinous drop-like corneal dystrophy. Am J Pathol. 2010;177:1344–1355. doi: 10.2353/ajpath.2010.100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan M, Stewart JE, Golemis EA, Pugacheva EN, Alexandropoulos K, Cox BD, Wang W, Grammer JR, Gladson CL. HEF1 is a necessary and specific downstream effector of FAK that promotes the migration of glioblastoma cells. Oncogene. 2006;25:1721–1732. doi: 10.1038/sj.onc.1209199. [DOI] [PubMed] [Google Scholar]
- Ohmachi T, Tanaka F, Mimori K, Inoue H, Yanaga K, Mori M. Clinical significance of TROP2 expression in colorectal cancer. Clin Cancer Res. 2006;12:3057–3063. doi: 10.1158/1078-0432.CCR-05-1961. [DOI] [PubMed] [Google Scholar]
- Palazzo AF, Eng CH, Schlaepfer DD, Marcantonio EE, Gundersen GG. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science. 2004;303:836–839. doi: 10.1126/science.1091325. [DOI] [PubMed] [Google Scholar]
- Pasqualini R, Bodorova J, Ye S, Hemler ME. A study of the structure, function and distribution of β5 integrins using novel anti-β5 monoclonal antibodies. J Cell Sci. 1993;105(Pt 1):101–111. doi: 10.1242/jcs.105.1.101. [DOI] [PubMed] [Google Scholar]
- Price LS, Leng J, Schwartz MA, Bokoch GM. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol Biol Cell. 1998;9:1863–1871. doi: 10.1091/mbc.9.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R, Pierschbacher MD, Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985;40:191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Ren XD, Kiosses WB, Sieg DJ, Otey CA, Schlaepfer DD, Schwartz MA. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J Cell Sci. 2000;113(Pt 20):3673–3678. doi: 10.1242/jcs.113.20.3673. [DOI] [PubMed] [Google Scholar]
- Ripani E, Sacchetti A, Corda D, Alberti S. Human Trop-2 is a tumor-associated calcium signal transducer. Int J Cancer. 1998;76:671–676. doi: 10.1002/(sici)1097-0215(19980529)76:5<671::aid-ijc10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Ron D, Chen CH, Caldwell J, Jamieson L, Orr E, Mochly-Rosen D. Cloning of an intracellular receptor for protein kinase C: a homolog of the β subunit of G proteins. Proc Natl Acad Sci USA. 1994;91:839–843. doi: 10.1073/pnas.91.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its integrin receptors in cancer. Adv Cancer Res. 1999;76:1–20. doi: 10.1016/s0065-230x(08)60772-1. [DOI] [PubMed] [Google Scholar]
- Serrels B, Sandilands E, Serrels A, Baillie G, Houslay MD, Brunton VG, Canel M, Machesky LM, Anderson KI, Frame MC. A complex between FAK, RACK1, and PDE4D5 controls spreading initiation and cancer cell polarity. Curr Biol. 2010;20:1086–1092. doi: 10.1016/j.cub.2010.04.042. [DOI] [PubMed] [Google Scholar]
- Siu MK, Chan HY, Kong DS, Wong ES, Wong OG, Ngan HY, Tam KF, Zhang H, Li Z, Chan QK, Tsao SW, Stromblad S, Cheung AN. p21-activated kinase 4 regulates ovarian cancer cell proliferation, migration, and invasion and contributes to poor prognosis in patients. Proc Natl Acad Sci USA. 2010;107:18622–18627. doi: 10.1073/pnas.0907481107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons M, Settleman J. Rho family GTPases: more than simple switches. Trends Cell Biol. 2000;10:415–419. doi: 10.1016/s0962-8924(00)01832-8. [DOI] [PubMed] [Google Scholar]
- Trebak M, Begg GE, Chong JM, Kanazireva EV, Herlyn D, Speicher DW. Oligomeric state of the colon carcinoma-associated glycoprotein GA733-2 (Ep-CAM/EGP40) and its role in GA733-mediated homotypic cell-cell adhesion. J Biol Chem. 2001;276:2299–2309. doi: 10.1074/jbc.M004770200. [DOI] [PubMed] [Google Scholar]
- Trerotola M, Cantanelli P, Guerra E, Tripaldi R, Aloisi AL, Bonasera V, La Sorda R, Lattanzio R, de Lange R, Weidle U, Piantelli M, Alberti S. Up-regulation of Trop-2 quantitatively stimulates human cancer growth. Oncogene in press. 2012 doi: 10.1038/onc.2012.36. [DOI] [PubMed] [Google Scholar]
- Trerotola M, Rathore S, Goel HL, Li J, Alberti S, Piantelli M, Adams D, Jiang Z, Languino LR. CD133, Trop-2 and α2β1 integrin surface receptors as markers of putative human prostate cancer stem cells. Am J Transl Res. 2010;2:135–144. [PMC free article] [PubMed] [Google Scholar]
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- Wells CM, Whale AD, Parsons M, Masters JR, Jones GE. PAK4: a pluripotent kinase that regulates prostate cancer cell adhesion. J Cell Sci. 2010;123:1663–1673. doi: 10.1242/jcs.055707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter MJ, Cirulli V, Briaire-de Bruijn IH, Litvinov SV. Cadherins are regulated by Ep-CAM via phosphaditylinositol-3 kinase. Mol Cell Biochem. 2007;302:19–26. doi: 10.1007/s11010-007-9420-y. [DOI] [PubMed] [Google Scholar]
- Wu JD, Haugk K, Woodke L, Nelson P, Coleman I, Plymate SR. Interaction of IGF signaling and the androgen receptor in prostate cancer progression. J Cell Biochem. 2006;99:392–401. doi: 10.1002/jcb.20929. [DOI] [PubMed] [Google Scholar]
- Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- Zanna P, Trerotola M, Vacca G, Bonasera V, Palombo B, Guerra E, Rossi C, Lattanzio R, Piantelli M, Alberti S. Trop-1 are conserved growth stimulatory molecules that mark early stages of tumor progression. Cancer. 2007;110:452–464. doi: 10.1002/cncr.22785. [DOI] [PubMed] [Google Scholar]
- Zheng DQ, Woodard AS, Fornaro M, Tallini G, Languino LR. Prostatic carcinoma cell migration via αvβ3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 1999;59:1655–1664. [PubMed] [Google Scholar]
- Zoubeidi A, Zardan A, Wiedmann RM, Locke J, Beraldi E, Fazli L, Gleave ME. Hsp27 promotes insulin-like growth factor-I survival signaling in prostate cancer via p90Rsk-dependent phosphorylation and inactivation of BAD. Cancer Res. 2010;70:2307–2317. doi: 10.1158/0008-5472.CAN-09-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]