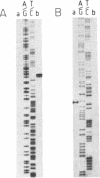
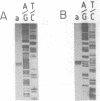
Abstract
A DNA fragment of Streptomyces fradiae is able to activate the antibiotic actinorhodin biosynthetic pathway when cloned in Streptomyces lividans. The activator DNA region has been sequenced and its transcription initiation and termination sites accurately mapped in vivo. This DNA encodes a 132 nucleotides long transcript which is apparently responsible for the actinorhodin production phenotype, possibly acting as an antisense RNA. The sequence of the activator gene revealed no homology with any other known Streptomyces coelicolor genes concerned with actinorhodin biosynthesis or its pleiotropic regulation.
Full text
PDF


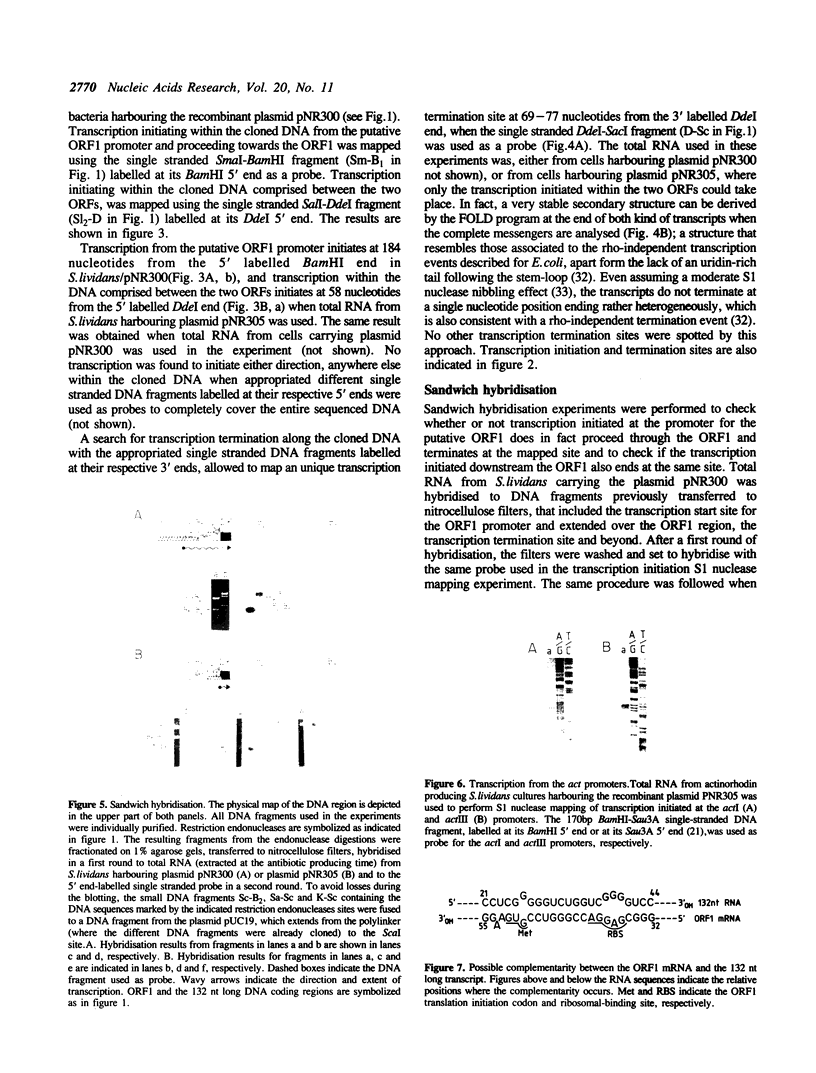


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamidis T., Riggle P., Champness W. Mutations in a new Streptomyces coelicolor locus which globally block antibiotic biosynthesis but not sporulation. J Bacteriol. 1990 Jun;172(6):2962–2969. doi: 10.1128/jb.172.6.2962-2969.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthelemy I., Salas M., Mellado R. P. In vivo transcription of bacteriophage phi 29 DNA: transcription initiation sites. J Virol. 1986 Dec;60(3):874–879. doi: 10.1128/jvi.60.3.874-879.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthelemy I., Salas M., Mellado R. P. In vivo transcription of bacteriophage phi 29 DNA: transcription termination. J Virol. 1987 May;61(5):1751–1755. doi: 10.1128/jvi.61.5.1751-1755.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champness W. C. New loci required for Streptomyces coelicolor morphological and physiological differentiation. J Bacteriol. 1988 Mar;170(3):1168–1174. doi: 10.1128/jb.170.3.1168-1174.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater K. F. Multilevel regulation of Streptomyces differentiation. Trends Genet. 1989 Nov;5(11):372–377. doi: 10.1016/0168-9525(89)90172-8. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn A. R., Sambrook J. Mapping viral mRNAs by sandwich hybridization. Methods Enzymol. 1980;65(1):468–478. doi: 10.1016/s0076-6879(80)65056-3. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Fernández-Moreno M. A., Caballero J. L., Hopwood D. A., Malpartida F. The act cluster contains regulatory and antibiotic export genes, direct targets for translational control by the bldA tRNA gene of Streptomyces. Cell. 1991 Aug 23;66(4):769–780. doi: 10.1016/0092-8674(91)90120-n. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hara O., Horinouchi S., Uozumi T., Beppu T. Genetic analysis of A-factor synthesis in Streptomyces coelicolor A3(2) and Streptomyces griseus. J Gen Microbiol. 1983 Sep;129(9):2939–2944. doi: 10.1099/00221287-129-9-2939. [DOI] [PubMed] [Google Scholar]
- Holmes W. M., Platt T., Rosenberg M. Termination of transcription in E. coli. Cell. 1983 Apr;32(4):1029–1032. doi: 10.1016/0092-8674(83)90287-8. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A. The Leeuwenhoek lecture, 1987. Towards an understanding of gene switching in Streptomyces, the basis of sporulation and antibiotic production. Proc R Soc Lond B Biol Sci. 1988 Nov 22;235(1279):121–138. doi: 10.1098/rspb.1988.0067. [DOI] [PubMed] [Google Scholar]
- Kasir J., Aksamit R. R., Backlund P. S., Jr, Cantoni G. L. Amino acid sequence of S-adenosyl-L-homocysteine hydrolase from Dictyostelium discoideum as deduced from the cDNA sequence. Biochem Biophys Res Commun. 1988 May 31;153(1):359–364. doi: 10.1016/s0006-291x(88)81231-2. [DOI] [PubMed] [Google Scholar]
- Lawlor E. J., Baylis H. A., Chater K. F. Pleiotropic morphological and antibiotic deficiencies result from mutations in a gene encoding a tRNA-like product in Streptomyces coelicolor A3(2). Genes Dev. 1987 Dec;1(10):1305–1310. doi: 10.1101/gad.1.10.1305. [DOI] [PubMed] [Google Scholar]
- Leskiw B. K., Lawlor E. J., Fernandez-Abalos J. M., Chater K. F. TTA codons in some genes prevent their expression in a class of developmental, antibiotic-negative, Streptomyces mutants. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2461–2465. doi: 10.1073/pnas.88.6.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. F., Demain A. L. Control of antibiotic biosynthesis. Microbiol Rev. 1980 Jun;44(2):230–251. doi: 10.1128/mr.44.2.230-251.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mellado R. P., Delius H., Klein B., Murray K. Transcription of sea urchin histone genes in Escherichia coli. Nucleic Acids Res. 1981 Aug 25;9(16):3889–3906. doi: 10.1093/nar/9.16.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick M. J. A morphological and genetic mapping study of bald colony mutants of Streptomyces coelicolor. J Gen Microbiol. 1976 Oct;96(2):299–315. doi: 10.1099/00221287-96-2-299. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA). Proc Natl Acad Sci U S A. 1984 Apr;81(7):1966–1970. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Ochi K. Metabolic initiation of differentiation and secondary metabolism by Streptomyces griseus: significance of the stringent response (ppGpp) and GTP content in relation to A factor. J Bacteriol. 1987 Aug;169(8):3608–3616. doi: 10.1128/jb.169.8.3608-3616.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parro V., Hopwood D. A., Malpartida F., Mellado R. P. Transcription of genes involved in the earliest steps of actinorhodin biosynthesis in Streptomyces coelicolor. Nucleic Acids Res. 1991 May 25;19(10):2623–2627. doi: 10.1093/nar/19.10.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido D., Jiménez A., Salas M., Mellado R. P. A Bacillus subtilis phage phi 29 transcription terminator is efficiently recognized in Streptomyces lividans. Gene. 1987;56(2-3):277–282. doi: 10.1016/0378-1119(87)90144-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Kleckner N. Biological regulation by antisense RNA in prokaryotes. Annu Rev Genet. 1988;22:567–600. doi: 10.1146/annurev.ge.22.120188.003031. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Reeder R. H. The nucleotide sequence of the initiation and termination sites for ribosomal RNA transcription in X. laevis. Cell. 1979 Oct;18(2):485–499. doi: 10.1016/0092-8674(79)90066-7. [DOI] [PubMed] [Google Scholar]
- Staden R. A computer program to search for tRNA genes. Nucleic Acids Res. 1980 Feb 25;8(4):817–825. [PMC free article] [PubMed] [Google Scholar]
- Ward J. M., Janssen G. R., Kieser T., Bibb M. J., Buttner M. J., Bibb M. J. Construction and characterisation of a series of multi-copy promoter-probe plasmid vectors for Streptomyces using the aminoglycoside phosphotransferase gene from Tn5 as indicator. Mol Gen Genet. 1986 Jun;203(3):468–478. doi: 10.1007/BF00422072. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]