Abstract
Present theories of visual recognition emphasize the role of interactive processing across populations of neurons within a given network, but the nature of these interactions remains unresolved. In particular, data describing the sufficiency of feed-forward algorithms for conscious vision and studies revealing the functional relevance of feedback connections to the striate cortex seem to offer contradictory accounts of visual information processing. TMS is a good method to experimentally address this issue, given its excellent temporal resolution and its capacity to establish causal relations between brain function and behavior. We studied 20 healthy volunteers in a visual recognition task. Subjects were briefly presented with images of animals (birds or mammals) in natural scenes and were asked to indicate the animal category. MRI-guided stereotaxic single TMS pulses were used to transiently disrupt striate cortex function at different times after image onset (SOA). Visual recognition was significantly impaired when TMS was applied over the occipital pole at SOAs of 100 and 220 msec. The first interval has consistently been described in previous TMS studies and is explained as the interruption of the feedforward volley of activity. Given the late latency and discrete nature of the second peak, we hypothesize that it represents the disruption of a feedback projection to V1, probably from other areas in the visual network. These results provide causal evidence for the necessity of recurrent interactive processing, through feed-forward and feedback connections, in visual recognition of natural complex images.
INTRODUCTION
Our understanding of the functional neuroanatomy of the human visual system (Grill-Spector & Malach, 2004) has not been paralleled by an equally strong neurophysio-logical framework to explain at a systems level how information is processed in these well-described regions. Current theories (Bullier, 2001c; Van Essen, Anderson, & Felleman, 1992) emphasize the role of interactive processing across the different areas that constitute a functional network, yet the nature of these interactions remains unresolved.
A series of elegant studies have described the capacity of the neural system to complete a visual recognition task (e.g., detection of animals in natural scenes) at “ultra-rapid” speeds, eliciting electrophysiological signals correlated with response selection as early as 150 msec after image presentation (Thorpe, Fize, & Marlot, 1996). These results have lead researchers to argue that at least some forms of visual recognition may happen exclusively in a feedforward manner because these short latencies leave no time for information to flow in any direction other than bottom–up (Thorpe & Fabre-Thorpe, 2001).
Still, robust anatomical connections from high-order centers to primary sensory cortices and other low-level areas have been described. The functional relevance of these feedback projections for visual processing has been tested in a variety of paradigms, suggesting that conscious visual recognition requires information to flow back to early stages of the anatomical hierarchy and is the result of recurrent processing through these synaptic loops (Silvanto, Cowey, Lavie, & Walsh, 2005; Lamme, 2003; Bullier, 2001b). Moreover, it has been argued that the well-described fast prefrontal markers of visual detection (Thorpe et al., 1996) may not necessarily impose a limit to the potential for feedback modulation (Michel, Seeck, & Murray, 2004). The anatomical hierarchy of the visual system appears to be inconsistent with the temporal hierarchy described by a number of studies that show how frontal and dorsoparietal areas become active at latencies similar or even earlier than the primary visual cortex (Bullier, 2001a), allowing sufficient time to influence even early responses in V1 via fast feedback pathways (Foxe & Simpson, 2002).
TMS is a technique capable of safely and transiently interfering with normal cortical processing. Early studies demonstrated the capacity of TMS to block visual recognition by applying a single pulse over early visual areas at 80– 100 msec after image presentation (Amassian et al., 1989). Human electrophysiological experiments have classically described these latencies as the time necessary for the electrical signals to travel through the subcortical stages of the retino-thalamo-striate pathway and arrive to the primary visual cortex. In this study, we presented subjects with brief flashes of images containing animals in natural scenes. Animals were either birds or mammals, and we asked participants to respond, as quickly and accurately as possible, to which category the images belonged. As subjects performed this visual task, a TMS coil was positioned over their primary visual cortex (defined on individual MRIs and targeted with a stereotaxic neuronavigation system), and a single TMS pulse was applied at one of various SOAs (range = 20–300 msec in steps of 20 msec). We hypothesized that if the processing of visual information necessary for the recognition of animals was achieved in an exclusively feedforward manner, TMS should impair visual recognition at a single early time window (ca. 80–100 msec after image presentation). On the other hand, if feedback projections to V1 were necessary for the network to process the presented visual stimuli, TMS should be capable of impairing task performance at least at two different times: the early 80- to 100-msec time window and later times representing feedback projections.
Our data show how TMS is capable of significantly disrupting performance at two different times after image presentation: 100 and 220 msec. These results causally link different times of V1 activity with recognition of natural images and argue for the relevance of recurrent interactive mechanisms in conscious vision, operating through feedforward and feedback connections within functional networks.
Also, we present a negative finding (Experiment 1) that describes how allowing subjects to become familiar with the images and learn them by passively exploring them and undertaking a series of psychophysical runs before the TMS experiment resulted in the inability to disrupt behavioral performance with the exact same TMS parameters.
METHODS
Subjects
We tested a total of 20 healthy volunteers, 10 for Experiment 1 and 10 for Experiment 2 (12 women and 8 men, right-handed). All were naive to the study aims, and all met the published safety criteria for TMS (Wassermann, 1998). All gave their written informed consent before entering the study, which had been approved by the local institutional review board. They were compensated for their study participation.
Behavioral Protocol
Subjects were presented with a set of 24 images of animals in natural scenes (12 images containing birds and the other 12 containing big mammals) in variable orientations and vantage points. The images were quadrangular (4.6° × 4.6° in size) and appeared centrally positioned in a PC computer monitor against a gray background. Images were normalized to have identical Fourier amplitude spectra and were degraded by interpolation of visual noise using Fourier techniques described in detail elsewhere (Rainer, Lee, & Logothetis, 2004). This allowed regulating task difficulty by modulating the percentage of added visual noise. Each picture was presented for one refreshment frame (14 msec) and was followed by a 3020- to 3300-msec fixation period in which only a centered red dot was shown until the next image appeared.
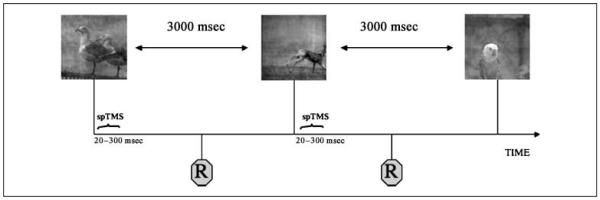
After each picture was presented, our stimulus presentation PC computer sent a TTL pulse to the TMS stimulator unit and triggered a single TMS pulse. Pulses were sent with 1 of 15 different delays after picture presentation (SOA times) ranging from 20 to 300 msec in steps of 20 msec. Every image was presented on 15 different occasions, each associated with one of the different TMS–SOAs, allowing to control for the potential heterogeneity of the images and the inherent levels of difficulty. Images were presented in a randomized fashion and SOAs in a pseudorandomized fashion, so that every 15 trials all SOAs would have been shown in a random order. This combination resulted in 24 trials per SOA category per subject, a total of 360 trials per subject. Subjects were instructed to fixate on a red dot in the center of the computer screen throughout the experiment and to respond as accurately and fast as possible if images belonged to the mammal or to the bird category (Figure 1). The experiment was divided in four blocks of 4.7 min each to allow subjects to rest. The stimulus presentation, sequence randomization, and synchronization with the TMS stimulator were programmed using the software Presentation (Neurobehavioral Systems Inc., Albany, CA).
Figure 1.
Experimental design. Images were flashed for one refreshment rate (14 msec) and subjects were asked to categorize the images with a button click. Following image presentation, a single pulse of TMS was applied at any one of 15 possible SOAs (from 20 to 300 msec). The next image appeared 3000 msec after the TMS pulse.
Both Experiments 1 and 2 used the same protocol and TMS parameters, the only difference being that Experiment 1 was preceded by a learning period and a set of psychophysical tests. Subjects started Experiment 1 with a pure psychophysical test (no TMS), performing a series of runs with all images at different degrees of difficulty as determined by the percentage of superimposed visual noise. This was meant to allow the determination of individualized performance thresholds, attempted to be around 70–80% of correct responses. In addition, before the psychophysical testing, subjects were allowed to see the images for several minutes until they were familiar with them. By doing so, participants were well acquainted with the birds and mammals and learned beforehand which images belonged to each of the two experimental categories. After this learning period, subject performed the TMS experiment.
Because Experiment 1 led to no TMS-induced changes in performance, not even at the expected and well-described 80- to 100-msec SOA, we recruited 10 new subjects for Experiment 2. These were naive to the images, eliminating the learning component previous to the TMS sessions. Participants in Experiment 2 saw the images for the first time during the TMS trial, and a common level of difficulty was selected based on the performance of subjects in Experiment 1.
MRI-guided TMS
We used a Magstim SuperRapid biphasic stimulator and a commercially available, eight-shaped 70-mm coil (MagStim Corporation, Whitland, UK). The stimulation intensity was 80% of the stimulator’s maximum output for all subjects. The coil was placed over the occipital pole (to stimulate primarily V1, our target area of stimulation) and was oriented in the horizontal axis with the handle parallel to the floor pointing to the right. At this intensity and with their eyes open, none of the subjects reported seeing phosphenes during the experiment.
All subjects underwent a structural three-dimensional T1-weighted MRI scan on a GE Signa 1.5-T scanner (General Electric Inc., Waukesha, WI) before the TMS experiment. The high-resolution anatomical brain MRI was used for precise identification of the participant’s striate cortex. We defined V1 anatomically by selecting the most posterior part of the calcarine sulcus in the occipital pole of each subject, using two-dimensional MRI images in the three planes (sagittal, coronal, and axial) and a three-dimensional reconstruction.
For the TMS experiment, participants sat in front of the stimulus presentation personal computer. Using the Brainsight software and a stereotaxic infrared system (Rogue Industries, Montreal, Canada), we monitored in real time the brain region targeted by each TMS stimulus throughout the experiment, thus assuring that every TMS stimulus was indeed delivered to the same cortical location (Gugino et al., 2001).
Analysis
We analyzed RT and performance (proportion of correct responses) for both Experiments 1 and 2 using a mixed effects regression model to account for the correlations between repeated measures from the same subject. The 15 SOA times were modeled via a fixed time factor with 15 levels, one level for each SOA time. The first time window (SOA = 20 msec) served as the baseline. Using this internal baseline allowed to control for nonspecific confounders and to unmask the effects specifically induced by TMS electromagnetic disruption of task-relevant neural activity. The choice of this SOA as baseline assumes that at that time visual information is still flowing through the retino-geniculate-striate pathway (Schmolesky et al., 1998) and does not reach V1 for at least another 40–60 msec (Amassian et al., 1989). Using slew-rate limiting amplifiers and a subtraction procedure, Thut et al. (2003) used EEG and TMS simultaneously to study the effects that single pulses of TMS over the occipital pole had on the visual-evoked potentials generated by a checkerboard visual stimulus. The authors described how a TMS pulse applied concomitantly with the images did not induce any changes in the visual-evoked potential, whereas the same pulse ca. 100 msec after the checkerboard did. The exact times around 100 msec were determined on an individual basis to coincide with the build-up period and peak of theP1component that fMRI–EEG studies have described to be generated in V1 with the possible contribution of V2/ V3 (Di Russo, Martinez, Sereno, Pitzalis, & Hillyard, 2002; Bonmassar et al., 2001). These results and the previous TMS literature studying visual suppression (Kammer, Puls, Strasburger, Hill, & Wichmann, 2005) support the idea that in the initial moments after the presentation of a visual stimulus, a pulse of TMS over striate and peristriate areas will not affect information processing (or its behavioral and electrophysiological correlates) because there is no information being processed yet. Despite the fact that the effects of a single pulse can be observed reverberating in the functional network for more than 100 msec (Moliadze, Zhao, Eysel, & Funke, 2003; Thut et al., 2003; Ilmoniemi et al., 1997), their effects on cognition, although task dependent, are estimated to be approximately 5 to 10 msec (Sack, 2006). Therefore, a single TMS pulse with an SOA of 20 msec was unlikely to disrupt visual processing but induced all the nonspecific effects such as the somatosensory and the auditory stimulations due to the light tapping sensation, the loud clicking noise, etc.
We estimated the differences in the outcomes between 20 msec and each of the other SOA times and obtained the associated unadjusted p values for these pairwise comparisons, each testing whether the outcome at the corresponding SOA was the same as that at 20 msec. Note that this analysis is similar to performing a priori pairwise comparisons using multiple paired t tests, but it estimates the common variability based on the entire sample and not just the two individual pairs. We adjusted for multiple comparisons using the method of false discovery rate (Benjamini & Hochberg, 1995) to ensure at most 5% false discovery rate in the 14 comparisons.
To test whether the overall effects of TMS were different under the learning condition of Experiment 1 and the naive condition of Experiment 2, we used a repeated measures analysis via the mixed effects model to obtain a global F value and its associated p value for each one of the two experiments, and we also tested the interaction of SOA by experiment (familiarity). Because our design was balanced, this approach is identical to a repeated measures ANOVA.
All statistical analyses were performed using the software SAS v.9 (SAS Institute Inc., Cary, NC). Two-sided p values were used to determine statistical significance.
RESULTS
All subjects tolerated the stimulation without complications, and none experienced any adverse effects.
The mean RT (across subjects and SOA times) in Experiment 1 was 775.74 msec (SD = 129.96 msec) and in Experiment 2 was 769.28 msec (SD =106.54 msec). We did not detect any significant changes from baseline in RT due to TMS–SOA.
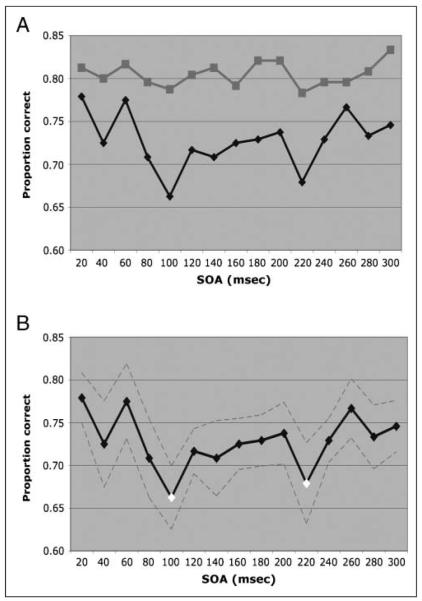
For Experiment 1 (Figure 2A), the TMS pulse did not induce a significant change in performance (proportion of correct responses) from baseline in any of the SOAs. In Experiment 2 (Figure 2B), the TMS pulse lead to a significant reduction in performance with respect to baseline for SOA = 100 msec (mean difference = −0.1165, p = .0005) and SOA = 220 msec (mean difference = −0.1000, p = .0028).
Figure 2.
Changes in visual categorization induced by TMS. (A) Contrasts of the results obtained with 10 subjects in Experiment 1 (red) and 10 different subjects in Experiment 2 (blue). Experiment 1 involved extensive learning of the visual stimuli and practice in the task. TMS was unable to induce any significant changes in performance. In Experiment 2, subjects had never seen the images or practiced the task. These results reflect the changes in performance induced by single pulses of TMS at different SOAs for novel images. (B) The results of Experiment 2 in detail. The SEM is highlighted in dotted lines, and the significant SOAs are marked in orange. TMS was capable of significantly impairing recognition at two different time points: 100 and 220 msec.
When analyzing the overall effects of TMS over the familiar or novel images independently, the repeated measures ANOVA for Experiment 1 (familiar images) was not significant, F(14, 126) = 0.64, p = .8310, whereas for Experiment 2 (novel images) it was significant, F(14, 126) = 1.91 p = .0312. However, the interaction for SOA Time × Familiarity was not significant, F(14, 252) = 0.62, p =.6242.
DISCUSSION
The present study demonstrates two different times after image presentation when TMS over the striate cortex is capable of disrupting recognition of animals in natural scenes: 100 and 220 msec.
The first SOA has been consistently described in different TMS experiments using various visual and stimulation parameters such as letters (Corthout, Uttl, Walsh, Hallett, & Cowey, 1999; Masur, Papke, & Oberwittler, 1993; Beckers & Homberg, 1991; Amassian et al., 1989), numbers (Miller, Fendrich, Eliassen, Demirel, & Gazzaniga, 1996), or flashes of light (Kamitani & Shimojo, 1999; Kastner, Demmer, & Ziemann, 1998). Electrophysiological studies using TMS, EEG, and MEG have classically defined this latency as the delay necessary for the feedforward volley of visual information to travel through the early subcortical stages of the retino-geniculo-striate pathway and arrive to V1. Given the later latency of the second time point and its separation from the first, we hypothesize that it reflects a cortico-cortical feedback of activity to V1.
These findings seem to be in conflict with pure feed-forward models of object recognition. Using a very similar task (detection of animals in natural scenes), Thorpe et al. (1996) described median RTs of 445 msec and a prefrontal EEG signal correlated with response selection at 150 msec after stimulus presentation. The timing of this decision-related electrophysiological marker seems to be irreducible even after intense learning (Fabre-Thorpe, Delorme, Marlot, & Thorpe, 2001) or experimental design modifications leading to 100 msec shorter RTs ( VanRullen & Thorpe, 2001). Based on the short RTs, the fast latency of the EEG signal, and the number of processing stages in the hierarchy of the visual network, the authors argued that information processing occurred in a feedforward manner, leaving no time for feedback and recurrent processing to affect perceptual processes (Thorpe & Fabre-Thorpe, 2001). This argument has been questioned by others (Michel et al., 2004) based on a series of findings that challenge the classical anatomical models of cortical organization (Felleman & Van Essen, 1991). The anatomical models are inconsistent with electrophysiological data showing activation latencies in high-order regions, such as the dorsoparietal and prefrontal cortices, at similar if not earlier times than V1. Given the well-characterized and rich pattern of fast feedback connections from these high-order regions back to low-level areas such as the striate cortex, it has been argued that even the early phase of V1 activation could indeed be already modulated in atop–down manner by these frontal and parietal areas through feedback priming (Michel et al., 2004; Foxe & Simpson, 2002).
One should also consider that the electrophysiological signal described by Thorpe et al. (1996) implies that the system has processed information to a point where it can begin encoding a correct response, even if conscious perception may be lacking or incomplete (VanRullen & Thorpe, 2001). Blindsight patients process information and select appropriate stimulus-based responses without having conscious access to the visual percept (Stoerig, 1996). Also interestingly, Libet, Gleason, Wright, and Pearl (1983) published a series of motor studies with results replicated later by others (Haggard, 2005) in which the initial parts of the readiness potential, supposed to reflect motor planning computations, were shown to precede the subjective conscious decision to perform a voluntary movement. These results imply that neuronal processes encoding motor decisions and plans can be preconscious and are illustrative of the unconscious nature of certain decision-making neural computations. Therefore, even if information may have reached the prefrontal cortex in an exclusive feedforward manner and the nervous system may have analyzed it to a point where it is ready to prepare a correct response, further processing may still be necessary in order for the stimuli to reach awareness. In such a scenario, the information computed in prefrontal areas at times around 150 msec could directly or indirectly be projected back to the occipital cortex and arrive to V1 in the ~220-msec latencies we describe. Such feedback input to V1 may in fact be critical for awareness (Silvanto et al., 2005; Pascual-Leone & Walsh, 2001).
Another framework to bring our results and feedforward theories together may come from a closer analysis of the methodological differences between different experiments. Although Thorpe et al. (1996) presented images of natural scenes that could contain animals or not and asked subjects to detect those with animals, we presented images that always contained animals and asked subject to identify birds and mammals. Although similar, the two tasks challenged subjects with different cognitive loads, ours requiring a higher level of detail analysis. Hochstein and Ahissar’s (2002) Reverse Hierarchy Theory differentiates between global-feature identification (“vision at a glance”) and detail-oriented analysis (“vision with scrutiny”) and proposes that the nervous system may use a flexible strategy, solving the first problem with a faster feedforward approach but making use of feedback projections and recurrent processing for the latter more complex analysis. The RTs we report are substantially longer than those measured by Thorpe et al., which is indicative of increased processing demands potentially requiring alternative computational strategies. According to the Reverse Hierarchy Theory, we could explain the difference in results arguing that one task requires a lower level of detail analysis (“vision at a glance”) and therefore presents with faster RTs and electrophysiological markers suggestive of feedforward processing. On the other hand, our task challenges subjects with a more demanding and detailed visual analysis (“vision with scrutiny”) and results in longer RTs and markers of both feedforward and feedback processing in V1. This approach may suffer from being too simplistic given that the division between the two modes of vision is abstract and that feedback and recurrences can happen in parallel at different local and global levels (Lamme, 2003) and with variable number of iterations. Nonetheless, it helps consider our data in the context of previous reports.
Studies using a variety of techniques, from MEG in humans (Liu, Harris, & Kanwisher, 2002) to single unit recordings in nonhuman primates (Tsao, Freiwald, Tootell, & Livingstone, 2006; Sugase, Yamane, Ueno, & Kawano, 1999), have shown different discrete times of activity in extrastriate regions that correlate with different stages of cognitive processing. The different periods of neural activity seem to be organized in such a way that global analyses (e.g., face categorization) correspond to the earlier discrete peaks whereas more refined and detailed levels of analyses (e.g., face identification) correlate with later periods of neural activity (Liu et al., 2002; Sugase et al., 1999). Within V1 proper, single-cell studies in nonhuman primates have also described different phases of activity correlated with different perceptual subfunctions such as feature identification, texture boundary detection, figure-ground segregation, etc., with increasing complexity being computed in later periods of neural processing ( Lamme, Rodriguez-Rodriguez, & Spekreijse, 1999). These results and ours seem to be indicative of the involvement of cortical regions at multiple discrete times, suggestive of an interactive dialogue among neuronal populations within a functional network. The computational strategy used for visual recognition may have a certain degree of flexibility, as Hochstein and Ahissar (2002) suggest, using the rich pattern of feedforward and feedback connections to engage in a pattern of connectivity and even a number of iterations, determined by the difficulty of the task.
In another TMS study, Heinen, Jolij, and Lamme (2005) investigated in a similar experimental design the chronometry of occipital pole cortical regions in the recognition of abstract figure-ground patterns. Interestingly, they described a bimodal distribution similar to ours but with later latencies (130–160 msec for the first time window and 250–280 msec for the second). The authors hypothesized these periods might correlate with basic perceptual operations such as boundary detection and figure-ground segregation that have already been described to occur in a sequential order in V1 (Lamme et al., 1999). Our data cannot attribute concrete low- or high-level operations to the times of V1 disruption because such TMS designs are limited in their capacity to assign very concrete perceptual or cognitive subfunctions to the times of disruption. This is, among other reasons, because each recurrent loop may in fact not be so specifically related to a certain function but be part of a more general and flexible computational algorithm of information processing. Therefore, our data cannot define concrete functional roles to these periods of V1 activation, but from a neurophysiological perspective, these results describe the causal need for different discrete periods of V1 activation in explicit visual categorization of natural images. More generally, we argue for the relevance of recurrent interactive mechanisms in conscious vision, operating through feedforward and feedback connections within functional networks.
Finally, we report the negative result of our first attempt to perform this experiment (Experiment 1). Initially, we had the intention to individually titrate task difficulty by doing careful psychophysics and establishing the optimal level of visual noise for every image and every subject. It is generally assumed that TMS can disrupt a behavior when the system is at threshold conditions, but that when a task is too easy, the effects of TMS may not be sufficient to overcome the different compensation strategies the nervous system may use (Robertson, Theoret, & Pascual-Leone, 2003; Walsh & Cowey, 2000). This required having subjects be extensively acquainted with all images, observing them passively first and performing several sessions of pure behavioral measurements. Under these circumstances, we were unable to elicit any significant effects of TMS, not even the consistently described peri-100-msec dip. Thinking of possible caveats in our design, we decided to eliminate the practice component and allow a new group of participants to sit in front of the computer and do the TMS experiment with no practice and a common difficulty level for all images and subjects. This a priori less careful approach lead to the results of Experiment 2 and the biphasic distribution.
Although the overall effects of TMS SOA over performance are shown to be not significant when images are highly familiar, Experiment 1, F(14, 126) = 0.64, p = .8310, but significant when they are novel, Experiment 2, F(14, 126) = 1.91 p = .0312, the repeated measures ANOVA testing the interaction does not allow the statistical confirmation of the impact of learning over the capacity of TMS to disrupt visual recognition, F(14, 252) = 0.62, p = .6242. This could be expected given the high number of degrees of freedom and the fact that only 2 of the 15 SOA showed a differential effect, making the study seriously underpowered to test this hypothesis. The primary aim of the present work was not to analyze such learning effects and was therefore not designed to test this question, but we found very suggestive that TMS had such a different impact on a cognitive task after modifying the familiarity of images. We therefore decided to report the negative result of Experiment 1 and describe the trend highlighted by the fact that the individual ANOVAs for familiar and novel images are respectively nonsignificant and significant.
Neary, Anand, and Hotson (2005) conducted an experiment specifically designed to answer the question of the influence of learning in the effects of TMS. In a line orientation discrimination task, the authors reported how perceptual learning progressively reduced the capacity of TMS to disrupt recognition. Our trend supports these data, which provide empirical evidence for the role of learning and task difficulty in the capacity of TMS to induce cognitive changes and point to the relevance of behavioral variables in TMS designs.
The difference in the design of Experiments 1 and 2 is the effect of practice and learning that preceded the TMS trial in Experiment 1 but not in Experiment 2. However, the effects of learning may be confounded by a third variable, task difficulty, as practice may cause the task to be easier and the cognitive load to decrease. Subjects are therefore likely to start the TMS trial at different points on the psychometric curve, being further to the right in Experiment 1 and potentially starting at ceiling conditions. The effects of TMS are actually known to be minimal or unobservable under ceiling conditions, as the “neural noise” that TMS introduces in the circuit can be easily overcome by the system, resulting in no behavioral change. Furthermore, the neural mechanisms by which learning or difficulty affect performance and potentially influence the effects of TMS are likely to be different. We did not control for task difficulty between Experiments 1 and 2, and baseline performance is in fact higher in Experiment 1 as shown in Figure 2A. Still, the difference is small and statistically nonsignificant ( p = .46), making task difficulty less likely to be a real confounder. Further experiments aiming to specifically answer this question should take special care in controlling for the differential effects of these two variables and potentially reveal their likely related but different neural mechanism.
We should also note that previous reports of experiments to study visual performance by using TMS to disrupt V1 activity did observe significant effects even after allowing subjects to see the images and to train to a certain degree. It seems that the capacity of learning to affect the disruption of TMS is not an all-or-nothing binary effect and must have its influence at different neurocognitive levels. As mentioned above, learning implies a change in cognitive load and level of difficulty, shifting subjects’ performance to the right of the psychometric curve. Depending on the baseline difficulty level for a given task and the slope of its psychometric curve, pushing subjects further to the right may or may not place them at the ceiling performance range. Also, complex images such as the ones used in our experiment will likely recruit a more complex network of regions than simple visual stimuli, and the physiological effects of learning and those of TMS over V1 may interact differently in this expanded network. Along this line, learning low-level or high-level features such as line orientation or animal category will engage memory systems differently, affecting the interactions of these circuits with the pulses of TMS over V1.
Acknowledgments
The authors would like to thank Gregor Rainer for providing the visual stimuli. Supported in part by grants from La Caixa Foundation to J. A. C., McDonnell foundation (grant #220020046) to E. Z., a US–Israel Binational Foundation to E. Z. and A. P.-L., and the National Institutes of Health (K24 RR018875 and RO1-EY12091) to A. P.-L. The authors would like to thank Mark Thivierge for the invaluable administrative support.
REFERENCES
- Amassian VE, Cracco RQ, Maccabee PJ, Cracco JB, Rudell A, Eberle L. Suppression of visual perception by magnetic coil stimulation of human occipital cortex. Electroencephalography and Clinical Neurophysiology. 1989;74:458–462. doi: 10.1016/0168-5597(89)90036-1. [DOI] [PubMed] [Google Scholar]
- Beckers G, Homberg V. Impairment of visual perception and visual short term memory scanning by transcranial magnetic stimulation of occipital cortex. Experimental Brain Research. 1991;87:421–432. doi: 10.1007/BF00231859. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Correcting the false discovery rate—A practical and powerful approach to multiple testing. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 1995;57:289–300. [Google Scholar]
- Bonmassar G, Schwartz DP, Liu AK, Kwong KK, Dale AM, Belliveau JW. Spatiotemporal brain imaging of visual-evoked activity using interleaved EEG and fMRI recordings. Neuroimage. 2001;13:1035–1043. doi: 10.1006/nimg.2001.0754. [DOI] [PubMed] [Google Scholar]
- Bullier J. Cortical connections and functional interactions between visual cortical areas. In: Fahle M, editor. Neuropsychology of vision. Oxford University Press; Oxford: 2001a. [Google Scholar]
- Bullier J. Feedback connections and conscious vision. Trends in Cognitive Sciences. 2001b;5:369–370. doi: 10.1016/s1364-6613(00)01730-7. [DOI] [PubMed] [Google Scholar]
- Bullier J. Integrated model of visual processing. Brain Research, Brain Research Reviews. 2001c;36:96–107. doi: 10.1016/s0165-0173(01)00085-6. [DOI] [PubMed] [Google Scholar]
- Corthout E, Uttl B, Walsh V, Hallett M, Cowey A. Timing of activity in early visual cortex as revealed by transcranial magnetic stimulation. NeuroReport. 1999;10:2631–2634. doi: 10.1097/00001756-199908200-00035. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Martinez A, Sereno MI, Pitzalis S, Hillyard SA. Cortical sources of the early components of the visual evoked potential. Human Brain Mapping. 2002;15:95–111. doi: 10.1002/hbm.10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre-Thorpe M, Delorme A, Marlot C, Thorpe S. A limit to the speed of processing in ultra-rapid visual categorization of novel natural scenes. Journal of Cognitive Neuroscience. 2001;13:171–180. doi: 10.1162/089892901564234. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cerebral Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV. Flow of activation from V1 to frontal cortex in humans. A framework for defining “early” visual processing. Experimental Brain Research. 2002;142:139–150. doi: 10.1007/s00221-001-0906-7. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. The human visual cortex. Annual Review of Neuroscience. 2004;27:649–677. doi: 10.1146/annurev.neuro.27.070203.144220. [DOI] [PubMed] [Google Scholar]
- Gugino LD, Romero JR, Aglio L, Titone D, Ramirez M, Pascual-Leone A, et al. Transcranial magnetic stimulation coregistered with MRI: A comparison of a guided versus blind stimulation technique and its effect on evoked compound muscle action potentials. Clinical Neurophysiology. 2001;112:1781–1792. doi: 10.1016/s1388-2457(01)00633-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggard P. Conscious intention and motor cognition. Trends in Cognitive Sciences. 2005;9:290–295. doi: 10.1016/j.tics.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Heinen K, Jolij J, Lamme VA. Figure-ground segregation requires two distinct periods of activity in V1: A transcranial magnetic stimulation study. NeuroReport. 2005;16:1483–1487. doi: 10.1097/01.wnr.0000175611.26485.c8. [DOI] [PubMed] [Google Scholar]
- Hochstein S, Ahissar M. View from the top: Hierarchies and reverse hierarchies in the visual system. Neuron. 2002;36:791–804. doi: 10.1016/s0896-6273(02)01091-7. [DOI] [PubMed] [Google Scholar]
- Ilmoniemi RJ, Virtanen J, Ruohonen J, Karhu J, Aronen HJ, Naatanen R, et al. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. NeuroReport. 1997;8:3537–3540. doi: 10.1097/00001756-199711100-00024. [DOI] [PubMed] [Google Scholar]
- Kamitani Y, Shimojo S. Manifestation of scotomas created by transcranial magnetic stimulation of human visual cortex. Nature Neuroscience. 1999;2:767–771. doi: 10.1038/11245. [DOI] [PubMed] [Google Scholar]
- Kammer T, Puls K, Strasburger H, Hill NJ, Wichmann FA. Transcranial magnetic stimulation in the visual system. I. The psychophysics of visual suppression. Experimental Brain Research. 2005;160:118–128. doi: 10.1007/s00221-004-1991-1. [DOI] [PubMed] [Google Scholar]
- Kastner S, Demmer I, Ziemann U. Transient visual field defects induced by transcranial magnetic stimulation over human occipital pole. Experimental Brain Research. 1998;118:19–26. doi: 10.1007/s002210050251. [DOI] [PubMed] [Google Scholar]
- Lamme VA. Why visual attention and awareness are different. Trends in Cognitive Sciences. 2003;7:12–18. doi: 10.1016/s1364-6613(02)00013-x. [DOI] [PubMed] [Google Scholar]
- Lamme VA, Rodriguez-Rodriguez V, Spekreijse H. Separate processing dynamics for texture elements, boundaries and surfaces in primary visual cortex of the macaque monkey. Cerebral Cortex. 1999;9:406–413. doi: 10.1093/cercor/9.4.406. [DOI] [PubMed] [Google Scholar]
- Libet B, Gleason CA, Wright EW, Pearl DK. Time of conscious intention to act in relation to onset of cerebral activity (readiness-potential). The unconscious initiation of a freely voluntary act. Brain. 1983;106:623–642. doi: 10.1093/brain/106.3.623. [DOI] [PubMed] [Google Scholar]
- Liu J, Harris A, Kanwisher N. Stages of processing in face perception: An MEG study. Nature Neuroscience. 2002;5:910–916. doi: 10.1038/nn909. [DOI] [PubMed] [Google Scholar]
- Masur H, Papke K, Oberwittler C. Suppression of visual perception by transcranial magnetic stimulation-experimental findings in healthy subjects and patients with optic neuritis. Electroencephalography and Clinical Neurophysiology. 1993;86:259–267. doi: 10.1016/0013-4694(93)90107-7. [DOI] [PubMed] [Google Scholar]
- Michel CM, Seeck M, Murray MM. The speed of visual cognition. Supplements to Clinical Neurophysiology. 2004;57:617–627. doi: 10.1016/s1567-424x(09)70401-5. [DOI] [PubMed] [Google Scholar]
- Miller MB, Fendrich R, Eliassen JC, Demirel S, Gazzaniga MS. Transcranial magnetic stimulation: Delays in visual suppression due to luminance changes. NeuroReport. 1996;7:1740–1744. [PubMed] [Google Scholar]
- Moliadze V, Zhao Y, Eysel U, Funke K. Effect of transcranial magnetic stimulation on single-unit activity in the cat primary visual cortex. Journal of Physiology. 2003;553:665–679. doi: 10.1113/jphysiol.2003.050153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary K, Anand S, Hotson JR. Perceptual learning of line orientation modifies the effects of transcranial magnetic stimulation of visual cortex. Experimental Brain Research. 2005;162:23–34. doi: 10.1007/s00221-004-2117-5. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V. Fast backprojections from the motion to the primary visual area necessary for visual awareness. Science. 2001;292:510–512. doi: 10.1126/science.1057099. [DOI] [PubMed] [Google Scholar]
- Rainer G, Lee H, Logothetis NK. The effect of learning on the function of monkey extrastriate visual cortex. PLoS Biology. 2004;2:E44. doi: 10.1371/journal.pbio.0020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EM, Theoret H, Pascual-Leone A. Studies in cognition: The problems solved and created by transcranial magnetic stimulation. Journal of Cognitive Neuroscience. 2003;15:948–960. doi: 10.1162/089892903770007344. [DOI] [PubMed] [Google Scholar]
- Sack AT. Transcranial magnetic stimulation, causal structure-function mapping and networks of functional relevance. Current Opinion in Neurobiology. 2006;16:593–599. doi: 10.1016/j.conb.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Hanes DP, Thompson KG, Leutgeb S, Schall JD, et al. Signal timing across the macaque visual system. Journal of Neurophysiology. 1998;79:3272–3278. doi: 10.1152/jn.1998.79.6.3272. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Cowey A, Lavie N, Walsh V. Striate cortex (V1) activity gates awareness of motion. Nature Neuroscience. 2005;8:143–144. doi: 10.1038/nn1379. [DOI] [PubMed] [Google Scholar]
- Stoerig P. Varieties of vision: From blind responses to conscious recognition. Trends in Neurosciences. 1996;19:401–406. doi: 10.1016/S0166-2236(96)10051-5. [DOI] [PubMed] [Google Scholar]
- Sugase Y, Yamane S, Ueno S, Kawano K. Global and fine information coded by single neurons in the temporal visual cortex. Nature. 1999;400:869–873. doi: 10.1038/23703. [DOI] [PubMed] [Google Scholar]
- Thorpe S, Fize D, Marlot C. Speed of processing in the human visual system. Nature. 1996;381:520–522. doi: 10.1038/381520a0. [DOI] [PubMed] [Google Scholar]
- Thorpe SJ, Fabre-Thorpe M. Neuroscience. Seeking categories in the brain. Science. 2001;291:260–263. doi: 10.1126/science.1058249. [DOI] [PubMed] [Google Scholar]
- Thut G, Northoff G, Ives JR, Kamitani Y, Pfennig A, Kampmann F, et al. Effects of single-pulse transcranial magnetic stimulation (TMS) on functional brain activity: A combined event-related TMS and evoked potential study. Clinical Neurophysiology. 2003;114:2071–2080. doi: 10.1016/s1388-2457(03)00205-0. [DOI] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Tootell RB, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–674. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Anderson CH, Felleman DJ. Information processing in the primate visual system: An integrated systems perspective. Science. 1992;255:419–423. doi: 10.1126/science.1734518. [DOI] [PubMed] [Google Scholar]
- VanRullen R, Thorpe SJ. The time course of visual processing: From early perception to decision-making. Journal of Cognitive Neuroscience. 2001;13:454–461. doi: 10.1162/08989290152001880. [DOI] [PubMed] [Google Scholar]
- Walsh V, Cowey A. Transcranial magnetic stimulation and cognitive neuroscience. Nature Reviews Neuroscience. 2000;1:73–79. doi: 10.1038/35036239. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: Report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalography and Clinical Neurophysiology. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]