Abstract
Intranasal exposure to Streptococcus pneumoniae as well as mucosal or parenteral immunization with a recently developed killed pneumococcal whole cell vaccine, confer Th17-mediated protection against subsequent S. pneumoniae colonization in mice. Given our interest in the function of Th17 cells and the ongoing efforts to develop this vaccine for use in infants and children in developing countries, we analyzed Th17 responses to the whole cell antigen (WCA) and individual pneumococcal antigens in healthy individuals and patients with pneumococcal disease and compared responses in children and adults from Sweden and Bangladesh. Peripheral blood mononuclear cells (PBMCs) isolated from Swedish adults produced IL-17A after stimulation with WCA, with the pneumolysoid PdT and with the protein required for cell separation in group B streptococci (PcsB). IL-22 and IFN-γ responses were also detected, but these cytokines originated from separate CD4+ T cell subsets. PBMCs from Swedish children produced lower levels of IL-17A in response to WCA compared to adults, whereas no such difference was noted from the samples from Bangladesh, where responses by children and adults were both significantly higher than those in Sweden. High IL-17A responses to stimulation with WCA were also observed in children with proven or probable pneumococcal pneumonia. Our results thus demonstrate the presence of Th17-type T cells that are specific for pneumococcus in both children and adults. The different levels of Th17 responses to pneumococci in children and adults in developing and developed countries, which may at least partly be due to differences in exposure to pneumococci, are important factors to consider in the evaluation of candidate pneumococcal protein-based vaccines in human trials.
Keywords: T cell, IL-17A, Streptococcus pneumoniae, children, developing country
Introduction
Streptococcus pneumoniae colonizes the nasopharynx as part of the normal flora, but is also an important cause of diseases, including otitis media, pneumonia, sepsis and meningitis [1, 2]. Over 800 000 children under 5 years of age are estimated to die from pneumococcal diseases worldwide each year, with a majority of cases in developing countries [3]. S. pneumoniae colonization precedes development of disease [2]. Children in developing countries have higher colonization rates and earlier acquisition of disease than children in the developed world [4, 5]. Although the introduction of pneumococcal capsular polysaccharide conjugate vaccines has been accompanied by impressive reductions in invasive disease attributable to pneumococcal strains covered by the vaccine, issues of serotype coverage, serotype replacement and cost may limit the applicability of this strategy for the developing world [6, 7]. Therefore, there is a continued search for new pneumococcal vaccines that can give rise to broader protection against pneumococcal colonization and disease, by inducing other arms of immunity than the anticapsular antibody responses elicited by the conjugate vaccines [8].
Intranasal exposure to live S. pneumoniae [9] as well as mucosal [9–11] and parenteral vaccination [12] with killed whole cell antigen (WCA) in combination with adjuvants can protect mice against pneumococcal colonization in the absence of antibodies. This protection is critically dependent on CD4+ IL-17A producing Th17 cells and levels of IL-17A produced by blood cells stimulated with WCA correlate with protection in this vaccination model [13]. Th17-dependent protection can also be induced in mice by mucosal immunization with a combination of purified pneumococcal proteins (a nontoxic derivative of pneumolysin, PdT, pneumococcal surface protein C, PspC, and pneumococcal surface adhesin A, PsaA), administered together with cholera toxin [14].
The broad protection afforded by the whole cell vaccine as well as its low production costs may make it particularly suitable for use in developing countries. This vaccine has been produced under Good Manufacturing Practice (GMP) conditions for use in human trials [12] and a Phase I clinical study of healthy adult volunteers has been initiated. However, little is known about Th17 responses to pneumococci in humans. In particular, it has been suggested, although not proven, that these T cell responses contribute to the age-dependent decrease in pneumococcal colonization and disease in children. In support of this possibility, a recent epidemiological study in Bangladesh suggested serotype-independent protection against S. pneumoniae in infants, indicating that factors other than anticapsular antibodies may be important for early pneumococcal protection [15]. IL-17A responses to WCA have previously been described in studies using whole blood from healthy American adults as well as from mononuclear cells derived from tonsils collected from 2–12 year-old British children [13]. Preliminary data also suggest that production of the Th17-associated cytokine IL-22 in response to WCA stimulation may be inversely correlated to the risk of subsequent pneumococcal colonization in patients with chronic obstructive pulmonary disease (COPD) [16]. However, the cellular source of the IL-17A and IL-22 measured in these studies has not been investigated. Furthermore, it is unclear whether responses differ in children and adults and whether Th17 responses to pneumococci are influenced by the different levels of pneumococcal colonization and disease present in various parts of the world.
In this study, we analysed Th17 and antibody responses to pneumococcal antigens in adults and children in Sweden and Bangladesh. Pneumococcal colonization is established very early in life in Bangladesh, with 50% of infants colonized at least once by 8 weeks of age, 90% by the age of 21 weeks and 100% at 1 year [5]. The colonization rate also remains high in older children and young adults in this population. In contrast, a later onset of pneumococcal acquisition and lower carriage rates have been observed in Swedish infants [17], a pattern that is consistent with several other reports comparing pneumococcal epidemiology in developing and developed countries [18–22].
Here we demonstrate the presence of Th17 type T cells that are specific for pneumococcus in both children and adults. We also show that, in contrast to Swedish children who produce low levels of IL-17A in response to WCA, children from Bangladesh have robust IL-17A responses to these antigens that are comparable to that of adults from the same country.
Materials and methods
Subjects and sample collection
Heparinized venous blood was collected from Swedish and Bangladeshi adults and children (Table 1). Healthy Swedish adults were recruited among students and staff at the Sahlgrenska Academy, University of Gothenburg. A majority of the adults were previously immunized with the Bacillus Calmette-Guérin (BCG) vaccine. Control Swedish children were recruited from patients at Queen Silvia´s Children´s hospital, Gothenburg (Table 2). These healthy children came to the hospital for control visits (n=8) or presented for reasons unrelated to any acute infectious disease (n=3, broken elbow, testicular torsion or dog bite). Children with pneumococcal disease were recruited at the same hospital and were preliminarily identified as possible cases of pneumococcal invasive disease based on medical history and positive blood culture or urine antigen test (Binax NOW) (Table 2). A sample obtained within 10 days after disease onset, while the child had acute disease symptoms, was defined as representing “acute infection”; a sample obtained between the first and third week of disease, when acute disease symptoms had been resolved, was classified as “post infection”; and a sample obtained subsequently, once the child had fully recovered, was defined as a convalescence sample. Because Binax NOW has poor specificity for the diagnosis of pneumococcal disease in children (due to high rates of pneumococcal colonization) [23], we defined pneumococcal pneumonia/invasive disease as an instance where a child had either a blood culture that was positive for S. pneumoniae or a post infection/convalescent antipneumococcal antibody titer that was ≥2-fold higher than the acute sample (Table 2). Healthy Bangladeshi children and adults were recruited in Mirpur, a low-to middle-income community 10 miles west of Dhaka, the capital city of Bangladesh. The Bangladeshi subjects were recruited in a study of immunity to H. pylori [24], including comparative control analyses of immune responses to Gram positive bacteria. Study approval was granted by the Ethical Committee for Human Research in the Gothenburg Region in Sweden and the Ethical Committee at the International center for diarrhoeal disease research, Bangladesh (icddr,b). Informed consent was obtained from subjects or parents, when applicable, before participation.
Table 1.
Subjects included in the study.
| Study group | N | Gender (females/males) |
Age (median, range) |
|---|---|---|---|
| Healthy Swedish adults | 45 | 29/16 | 34 y (21–58) |
| Swedish control children | 11 | 5/6 | 24 m (6–65) |
| Swedish children with pneumonia | 4 | 1/3 | 17 m (8–54) |
| Healthy Bangladeshi adults | 10 | 6/4 | 27 y (19–32) |
| Healthy Bangladeshi children | 23 | 10/13 | 12 m (6–60) |
Table 2.
Swedish children included in the study
| Subject | Gender | Age (months) |
Diagnosis | IgG titer (fold rise) |
Pneumococcal infection |
|---|---|---|---|---|---|
| Swedish control children | |||||
| 1 | F | 6 | Healthy, control visit | -- | -- |
| 2 | M | 6 | Healthy, control visit | -- | -- |
| 3 | F | 12 | Healthy, control visit | -- | -- |
| 4 | M | 13 | Healthy, control visit | -- | -- |
| 5 | F | 15 | Healthy, control visit | -- | -- |
| 6 | M | 24 | Healthy, control visit | -- | -- |
| 7 | M | 28 | Healthy, control visit | -- | -- |
| 8 | F | 35 | Healthy, control visit | -- | -- |
| 9 | M | 62 | Broken elbow | -- | -- |
| 10 | M | 65 | Testicular torsion | -- | -- |
| 11 | F | 71 | Dog bite | -- | - |
| Swedish children with pneumonia | |||||
| 12 | M | 15 | Pneumonia with hemolytic uremic syndrome, blood culture pos. |
37 | Definite |
| 13 | M | 18 | Pneumonia with pulmonary abscess, urine test pos. |
3 | Likely |
| 14 | F | 51 | Pneumonia with pleural effusion, urine test pos. |
2 | Likely |
| 15 | M | 8 | Otitis followed by pneumonia, urine test neg. |
1 | Unlikely |
Antigens
WCA was derived from strain Rx1AL-, a capsule and autolysin-negative mutant and prepared as described [10, 11]. A 6xHis-tagged fusion of the pneumococcal toxoid PdT was expressed and purified from pneumococcal strain Rx1, as previously described for the wild type pneumolysin toxin [25] and 6xHis-tagged protein required for cell separation in group B streptococci (PcsB, amino acid 28–278) and PsaA (amino acid 22–309) proteins were expressed in E. coli and purified using standard methods. Level of purity of these proteins was >95% as determined by SDS gel; PdT and PsaA had undetectable levels of LPS contamination (<0.2 ng/mg of protein); the level of LPS contamination in PcsB was measured at 13.5 ng/mg of protein, resulting in stimulation concentrations around 130 pg/ml, levels that do not appear to influence Th17 responses in our studies (data not shown). Purified protein derivative of Mycobacterium tuberculosis (PPD) was purchased from Statens Serum Institut (Denmark), phytohemagglutinin (PHA) from Remel (UK) and staphylococcus enterotoxin B (SEB) from Sigma (Germany).
Cell isolation and stimulation
Peripheral blood mononuclear cells (PBMCs) were isolated by gradient centrifugation on Ficoll-Isopaque (Pharmacia). CD4+ T cells were depleted from the PBMCs using Dynabeads (Invitrogen) and CD45RA+ naïve cells and CD45RO+ effector/memory cells were depleted using MACS beads (Miltenyi) according to the instructions provided by the manufacturers. Depletion resulted in <5% of the depleted cell population remaining among the PBMCs, as determined by flow cytometry.
For analysis of cell proliferation and cytokines in cell culture supernatants, PBMCs (1.5×105 cells/well) were incubated in round-bottomed 96-well culture plates (Nunc). PBMCs were stimulated with WCA (1 µg/ml), PdT, PcsB, and PsaA (all at 10 µg/ml), PPD (5 µg/ml) and PHA (1 µg/ml). In some experiments, anti-HLA-DR, -DP, -DQ antibodies (anti-MHCII antibodies, clone TÜ39) or isotype control antibodies (mouse IgG2a) were added to the cells at the start of the culture (BD Pharmingen, all at 25 µg/ml). After 5 days, 100 µl supernatant were collected from each well and the samples were frozen at −70°C until assayed for cytokines. To analyze cell proliferation, the cells were pulsed with 1 µCi/well of [3H]-thymidine (Amersham) for 8 hours, where after the plates were frozen. The cells were later harvested onto nylon filters (Wallac) and analyzed with a scintillation counter (Trilux 1450 MicroBeta, Wallac).
For analysis of intracellular cytokine production by flow cytometry, PBMCs (1.5×106 cells/tube) were stimulated with WCA (1 µg/ml), PPD (5 µg/ml), SEB (1 µg/ml) or medium alone in the presence of anti-CD28 (1 ug/ml, clone CD28.2, BD) and anti-CD49d antibodies (1 ug/ml, 9F10, BD) for 16 hours. Brefeldin A (10 µg/ml, Sigma) was added to the cultures after 2 hours of incubation.
All cell culture experiments were performed at 37°C in 5% CO2 using DMEM F12 medium (Invitrogen) with 5% human serum (prepared at the blood bank, Sahlgrenska University Hospital, Sweden) and 50 µg/ml gentamicin (Sigma).
Cytokine analyses
The concentration of cytokines in culture supernatants were determined using ELISA (IL-17A; eBiosciences, IL-22; R&D) following manufacturer’s instructions.
Antibody analyses
Plasma and serum anti-WCA IgG and IgA titers were determined by ELISA. Briefly, high binding 96-well plates (Greiner) were coated with 75 µl WCA (100 µg/ml) at +4°C overnight. Plasma samples were tested at an initial dilution of 1:2–1:20 and titrated 3-fold. After incubation for 2 h at 37°C, plates were developed with rabbit anti-human IgA, and IgG antibodies conjugated with horseradish peroxidase (Jackson ImmunoResearch Laboratories, PA) and o-phenylene diamine and H2O2. Endpoint titers were determined as the reciprocal dilutions giving an absorbance of 0.4 above the background levels at 450 nm.
Flow cytometric analysis
For analysis of the purity of PBMCs depleted of CD4+, CD45RA+ and CD45RO+ cells, cells were stained with combinations of the following antibodies diluted in phosphate buffered saline with 0.05% sodium azide and 0.1 % BSA (FACS buffer): anti-CD4 PerCP (clone SK3), anti-CD3 APC (SK7), anti-CD45RO PE (UCHL1) and anti-CD45RA-FITC (L48) (all from BD Pharmingen). Cells were analyzed using FACSCalibur flow cytometer (BD).
For analysis of intracellular cytokine production, stimulated cells were first stained with LIVE/DEAD Fixable Dead Cell Stain Kit (aqua fluorescent reactive dye, Invitrogen) and then labelled with anti-CD3-Pacific Blue (UCHT1) and anti-CD4-Alexa Fluor 700 (RPA-T4) antibodies (both from eBioscience) diluted in FACS buffer. After washing, cells were fixed and permeabilised using cytofix/cytoperm solution (BD). After washing in perm wash solution (BD), the cells were labelled with anti-CD3-Pacific Blue, anti-IFN-γ FITC (25723.11, BD), anti-IL-17A PE (eBio64DEC17, eBioscience) and anti IL-22 Alexa Fluor 645 antibodies (22URTI, eBioscience) diluted in perm wash solution. After washing, the cells were suspended in FACS buffer and immediately analyzed using a LSRII flow cytometer (BD).
All flow cytometric data were analyzed with FlowJo software (Tree Star Inc.). For analysis of intracellular cytokine production, dead cells were identified as AmCyan positive and excluded from the analyses.
Statistical analysis
The Mann-Whitney test was used to evaluate whether differences between two groups were statistically significant. Correlation analyses were performed using the Spearman test. Results were considered statistically significant if P<0.05.
Results
Circulating cells from Swedish adults produce Th17 type cytokines in response to stimulation with pneumococcal antigens
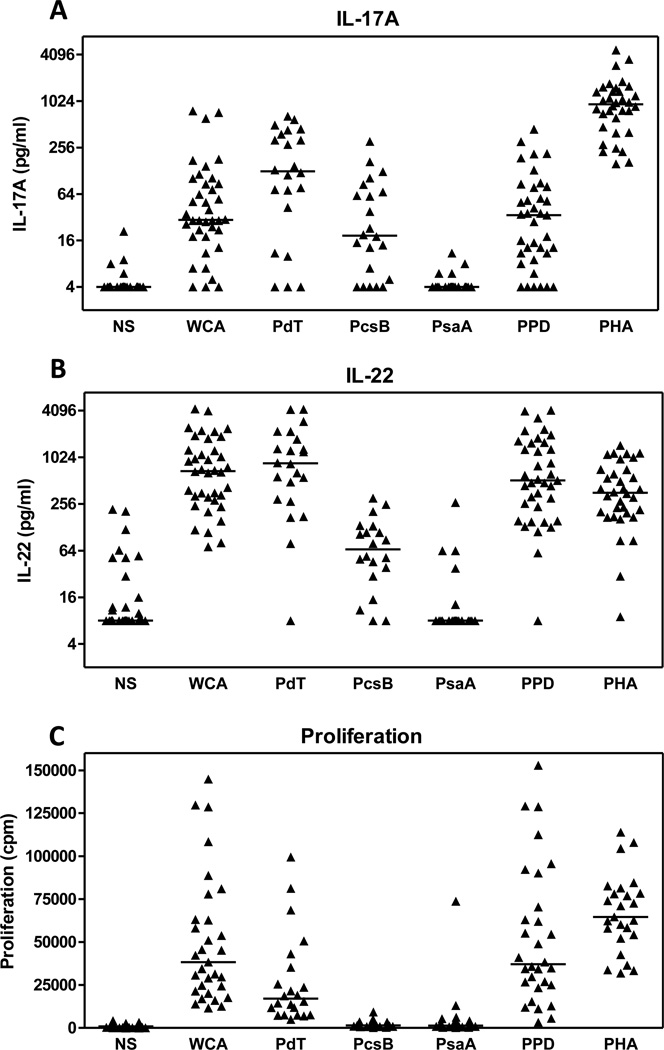
We examined the IL-17A andIL-22 responses from Swedish adults by stimulating their PBMCs with WCA and with the purified pneumococcal proteins PdT, PcsB and PsaA; antigens which have been proposed as components of protein-based pneumococcal vaccines [26, 27]. PBMCs from almost all individuals (32/38) responded to WCA-stimulation with at least 2-fold increased production of both IL-17A over the background of unstimulated cultures (Fig 1A). Stimulation with PdT also gave rise to strong IL-17A responses, whereas PcsB stimulation resulted in somewhat lower, IL-17A concentrations. In contrast, PsaA stimulation resulted in nondetectable IL-17A production (<4 pg/ml) in a majority of the subjects. The levels of IL-17A produced in response to WCA and PdT were significantly correlated (P=0.004, r=0.69). WCA-induced IL-17A production from the majority of individuals was within the same range as responses to the control antigen PPD (Fig 1A), which has previously been reported to give rise to Th17 responses in humans exposed to mycobacteria or immunized with the BCG vaccine [28, 29]. The polyclonal activator PHA always induced higher IL-17A responses than stimulation with pneumococcal antigens, when compared on an individual basis. IL-22 was also readily detectable following stimulation with pneumococcal antigens (Fig 1B), with strong responses induced by WCA and PdT, lower responses to PcsB, and undetectable responses to PsaA in most individuals. PPD and PHA also induced strong IL-22 responses. Stimulation with WCA, PdT and the control antigens, but not PcsB and PsaA, also induced cell proliferation (Fig 1C).
Figure 1.
IL-17A, IL-22 and proliferative responses from PBMCs obtained from healthy Swedish adults in response to stimulation with pneumococcal antigens. PBMCs were stimulated with the pneumococcal antigens WCA, PdT, PcsB, PsaA, the control antigens PPD and PHA or medium alone (NS) as described in the methods section. Five days after stimulation, the concentrations of IL-17A (A) and IL-22 (B) were measured in culture supernatants and cell proliferation was analyzed (C). Each symbol represents the responses from a single individual. Horizontal lines represent median responses.
Collectively, these results demonstrate that cells from adults respond to both WCA and purified pneumococcal antigens by producing the Th17-associated cytokines IL-17A and IL-22.
IL-17A and IL-22 are primarily produced by CD4+ effector/memory T cells
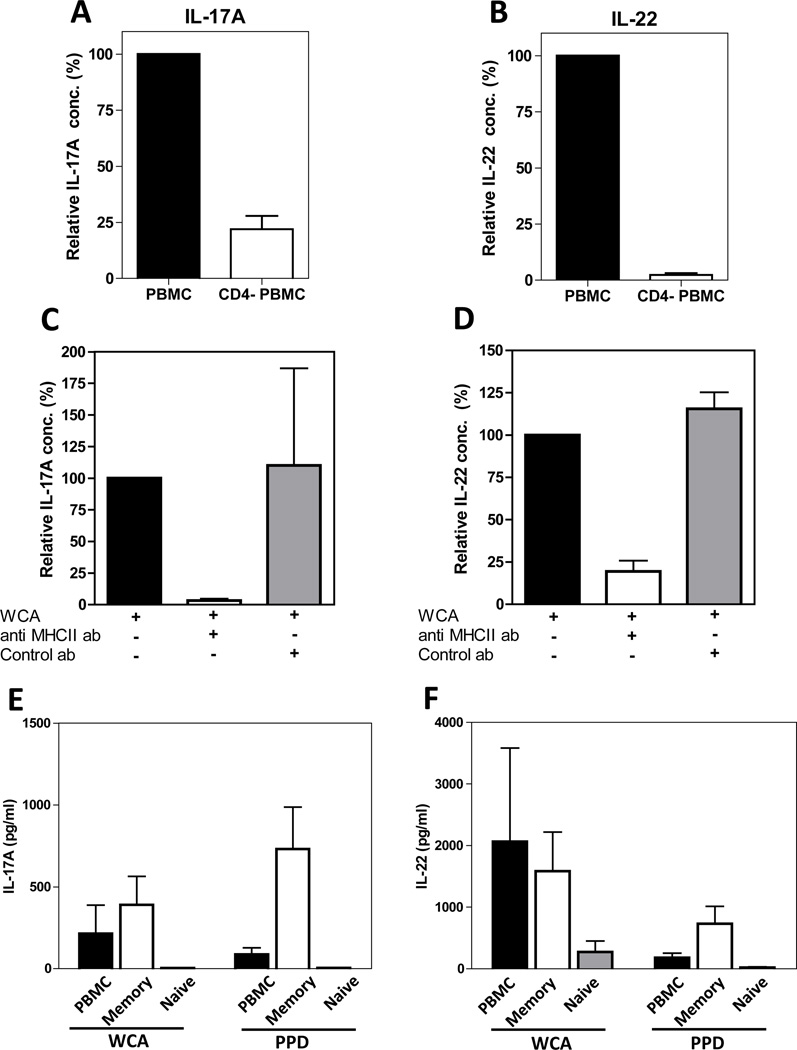
To determine which cells were responsible for IL-17A and IL-22 production, PBMCs from adults were depleted of CD4+ T cells and cytokine production from the depleted cells were compared to the production from the original PBMC preparations. Depletion of CD4+ T cells strongly reduced the production of both IL-17A and IL-22 in response to stimulation with WCA (Fig 2A and B) as well as PHA (data not shown). To further verify that the responses to WCA originate from CD4+ T cells and are dependent on a functional interaction between the T cell receptor and the MHCII-complex, the effect of addition of anti-MHCII antibodies was investigated. Addition of such antibodies strongly reduced the production of both IL-17A and IL-22 , but little effect was seen after addition of isotype control antibodies (Fig 2 C and D), supporting the hypothesis that, in response to pneumococcal antigen stimulation, both cytokines are dependent on MHCII interactions and primarily produced by CD4+ T cells.
Figure 2.
IL-17A and IL-22 are produced by CD4+ memory T cells in a MHCII dependent manner in response to stimulation with pneumococcal antigens. (A and B) PBMCs and PBMCs depleted of CD4+ T cells (CD4- PBMCs) from healthy Swedish adults (n=5) were stimulated with WCA and IL-17A (A) and IL-22 (B) concentrations were measured in culture supernatants. Responses in the absence of depletion were set at 100% in each experiment. (C and D) PBMCs from healthy Swedish adults were stimulated with WCA in the presence or absence of anti-MHCII or isotype control antibodies and the concentrations of IL-17A (C, n=3) and IL-22 (D, n=5) were measured in culture supernatants. Responses in the absence of antibodies were set to 100% in each experiment. (E and F) PBMCs and PBMCs depleted of CD45RA+ naïve T cells (depicted as memory cells in the figure) or CD45RO+ memory cells (depicted as naïve cells) were stimulated with WCA or PPD and the IL-17A (E) and IL-22 (F) concentrations were measured in culture supernatants (n=6). (A–F) Results shown are arithmetic means +SEM.
To further determine the phenotype of the cells producing IL-17A and IL-22 upon stimulation with WCA, we depleted CD45RO+ effector/memory cells and CD45RA+ naive cells from PBMCs. Depletion of effector/memory cells resulted in complete abolishment of IL-17A secretion (Fig 2E) as well as strong reduction in IL-22 production (Fig 2F). In contrast, depletion of naïve cells had only marginal effects on cytokine secretion in response to WCA. Responses to the control antigen PPD were also largely eliminated by the depletion of effector/memory cells (Fig 2 E and F) and CD4+ cells (data not shown).
Taken together, these results suggest that the IL-17A and IL-22 detected after stimulation with WCA are primarily produced by CD4+ effector/memory T cells.
IL-17A and IL-22 are produced by separate CD4+ T cell subsets
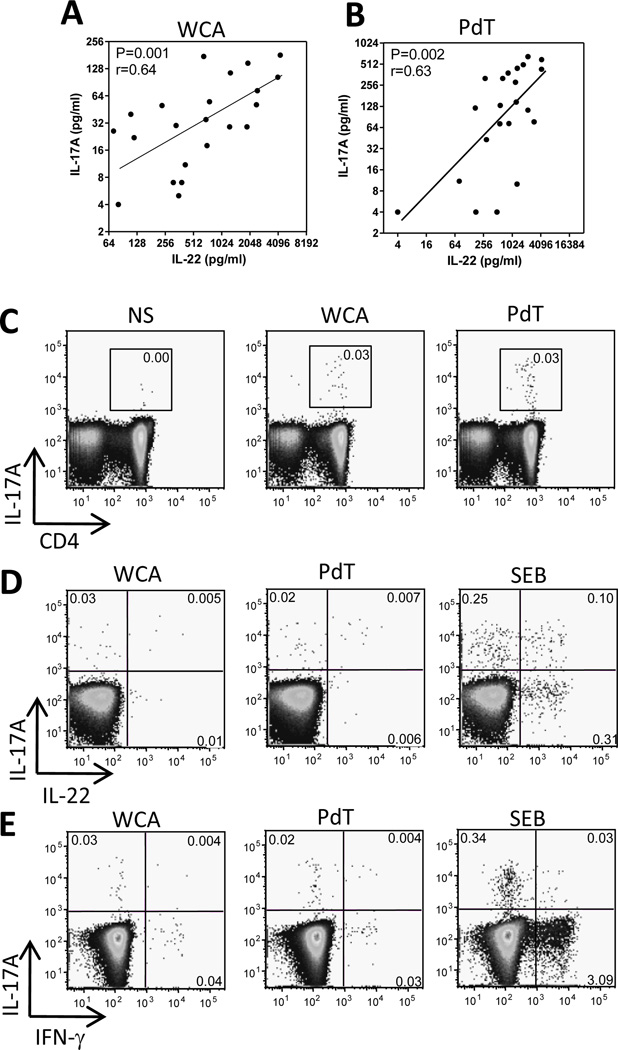
We found a significant correlation between the concentrations of IL-17A and IL-22 in supernatants collected from cells stimulated with WCA (Fig 3A) as well as PdT (Fig 3B). To determine if this correlation is a result of co-expression of the two cytokines by the same subset of T cells, flow cytometric analyses of intracellular cytokine expression were performed. These experiments confirmed that IL-17A was almost exclusively produced by CD4+ T cells (Fig 3C) and further showed that the frequencies of CD4+ cells expressing IL-17A in response to WCA ranged from nondetectable levels (<0.001%) to 0.04%, with a median of 0.03%. IL-17A was co-expressed with IL-22 in small subsets of cells stimulated with WCA or PdT (<0.02%), but the majority of the cells expressed either cytokine alone (Fig 3D). A subset of CD4+ T cells also responded with production of IFN-γ following stimulation with WCA (median 0.03%, range 0–0.3%) and PdT (median 0.1%, range 0.01–0.2%), but this cytokine was rarely co-expressed with IL-17A (Fig 3E) or IL-22 (not shown). A similar pattern of limited co-expression of IL-17A with IL-22 or IFN-γ was found when cells were stimulated with SEB (Fig 3D and E).
Figure 3.
Concentrations of IL-17A and IL-22 secreted in culture supernatants in response to pneumococcal antigens correlate but the cytokines are produced by separate subsets of CD4+ T cells. (A and B) PBMCs were stimulated with WCA or PdT as described in the methods section. The concentrations of IL-17A and IL-22 in culture supernatants were measured by ELISA. (C) PBMCs were stimulated with WCA, PdT, SEB or medium alone (NS) as described in the methods section and the expression of IL-17A in CD3+CD4+ T cells was detected by flow cytometry. The plots show IL-17A and CD4 expression in CD3+ live T cells and the numbers in the gates indicate frequencies (%) of IL-17A+ cells among CD3+CD4+ T cells. (D and E) The plots show expression of IL-17A and IL-22 (D) or IFN-γ (E) in CD3+CD4+ live T cells and the numbers in the quadrants indicate frequencies (%) of positive cells among CD3+CD4+ T cells. (C, D and E) The plots shown are from one representative individual out of 7 (WCA and SEB) and 3 (PdT) tested.
Taken together, our results show that about 0.03% of the CD4+ T cells of adult Swedes respond to WCA with IL-17A production and that, while the levels of IL-17A and IL-22 secreted in response to WCA correlate, these two cytokines are not produced by the same cells.
Cells from children produce Th17 cytokines in response to pneumococcal antigens and the responses are stronger in Bangladeshi than in Swedish children
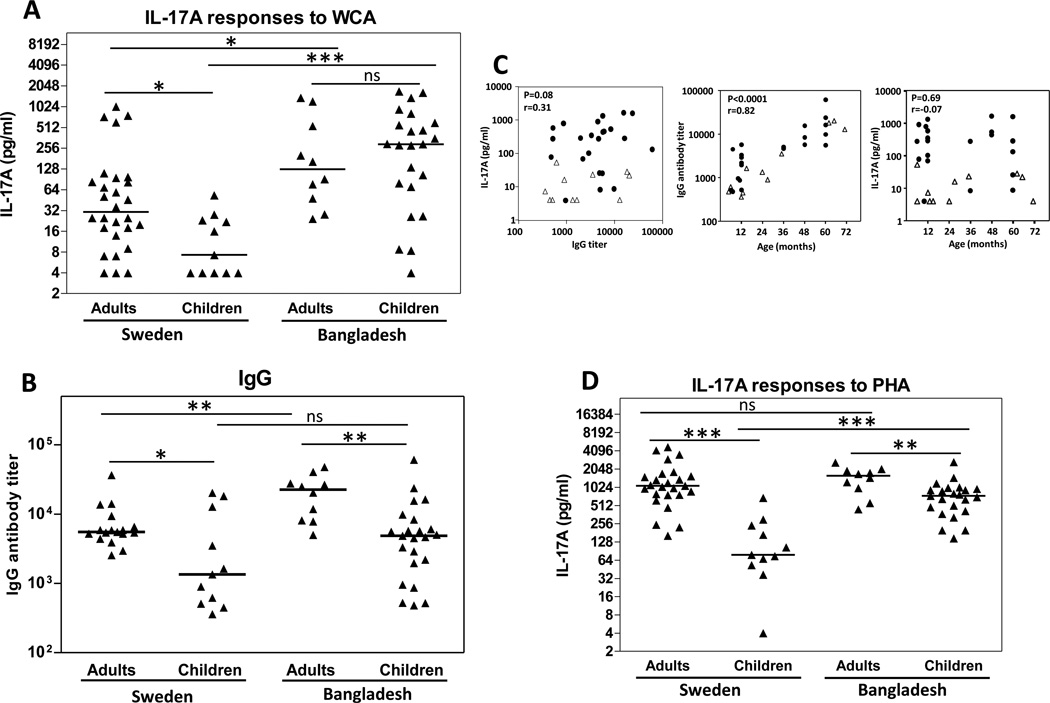
Our data thus indicate that, in response to pneumococcal stimulation, IL-17A and IL-22 are predominantly produced by effector/memory CD4+ T cells, presumably as a consequence of prior nasopharyngeal exposure. We thus hypothesized that PBMCs from young Swedish children would have lower IL-17A and IL-22 responses to pneumococcal antigens compared to adults in Sweden. To evaluate this, PBMCs were collected from healthy Swedish children and stimulated with WCA. Consistent with our hypothesis, only about 50% of the children had detectable (>4 pg/ml) IL-17A production to the stimulation and these responses were significantly lower than those detected in adults (Fig 4A). Cells from children also produced significantly lower levels of IL-22 following stimulation with WCA compared to cells from adults (P=0.002, data not shown).
Figure 4.
PBMCs from Bangladeshi children and adults produce more IL-17A in response to pneumococcal antigens than PBMCs from Swedish subjects. (A, D) PBMCs collected from healthy Swedish and Bangladeshi adults and children were stimulated with WCA (A) and PHA (D) and IL-17A concentrations were measured in culture supernatants. Symbols represent responses from different individuals after subtraction of responses to medium alone. (B) Titers of IgG antibodies binding to WCA in plasma samples collected from healthy Swedish and Bangladeshi children and adults. Symbols represent antibody titers in different individuals. (A, B, D) Horizontal lines represent median concentrations. *P<0.05, **P<0.01. ***P<0.001, ns; P>0.05. (C) Associations between WCA-induced IL-17A, anti-WCA IgG plasma antibodies and age in children. Left panel shows IL-17A concentrations in supernatants from PBMCs stimulated with WCA plotted against titers of anti-WCA IgG antibodies in plasma from the same children. Middle panel shows titers of anti-WCA IgG antibodies in plasma, and right panel shows IL-17A concentrations, plotted against the age of each individual child. Data from healthy Swedish and Bangladeshi children are depicted by open triangles and filled circles, respectively.
Because pneumococcal epidemiology in developing countries differs significantly from that in more developed settings [3, 5, 18], we also collected PBMCs from healthy children and adults living in a low-to-middle income area in Bangladesh. Cells from Bangladeshi children produced significantly higher levels of IL-17A than Swedish children (Fig 4A); in fact, the responses were comparable to those detected in Bangladeshi adults. Furthermore, the IL-17A responses in Bangladeshi adults were higher than in Swedish adults.
Collectively, these results suggest that both children and adults can produce Th17-type cytokine responses following stimulation with pneumococcal antigens, but that the responses differ between individuals living in a developed and a developing country, presumably as a consequence of higher pneumococcal exposure, colonization and possibly also mucosal or invasive disease in Bangladesh compared to Sweden [5, 17, 30, 31].
This inference is bolstered by the analysis of pneumococcal antibody titers in the two populations. We found higher median pneumococcal IgG antibodies in plasma from both Bangladeshi children and adults compared to the concentration in plasma from the corresponding groups in Sweden (Fig 4B), but this difference was only statistically significant in adults. Pneumococcal IgA levels followed a similar pattern, with 5-fold median difference in Bangladeshi compared to Swedish children and a 2-fold higher levels in Bangladeshi compared to Swedish adults (Data not shown). The levels of pneumococcal IgG and IgA were significantly higher in adults compared to children in both Sweden and Bangladesh.
No correlation was found between IL-17A response levels and antibody titers in any of the study groups (Fig 4C; Bangladeshi and Swedish children). Although the levels of pneumococcal IgG antibodies in plasma correlated significantly with age of both Bangladeshi and Swedish children, no correlation was found between the IL-17A response levels and age (Fig 4C).
To control for the possibility that general functional differences in T-cell responses between children and adults in the two populations may contribute to the increased IL-17A concentrations detected in the Bangladeshi population, responses to the mitogen PHA were also analyzed. While the PHA-induced responses of children in both settings were significantly lower than those of adults, PBMCs from Bangladeshi children stimulated with PHA produced significantly more IL-17A than Swedish children (Fig 4D).
Pneumococcal disease results in increased IL-17A responses to pneumococci
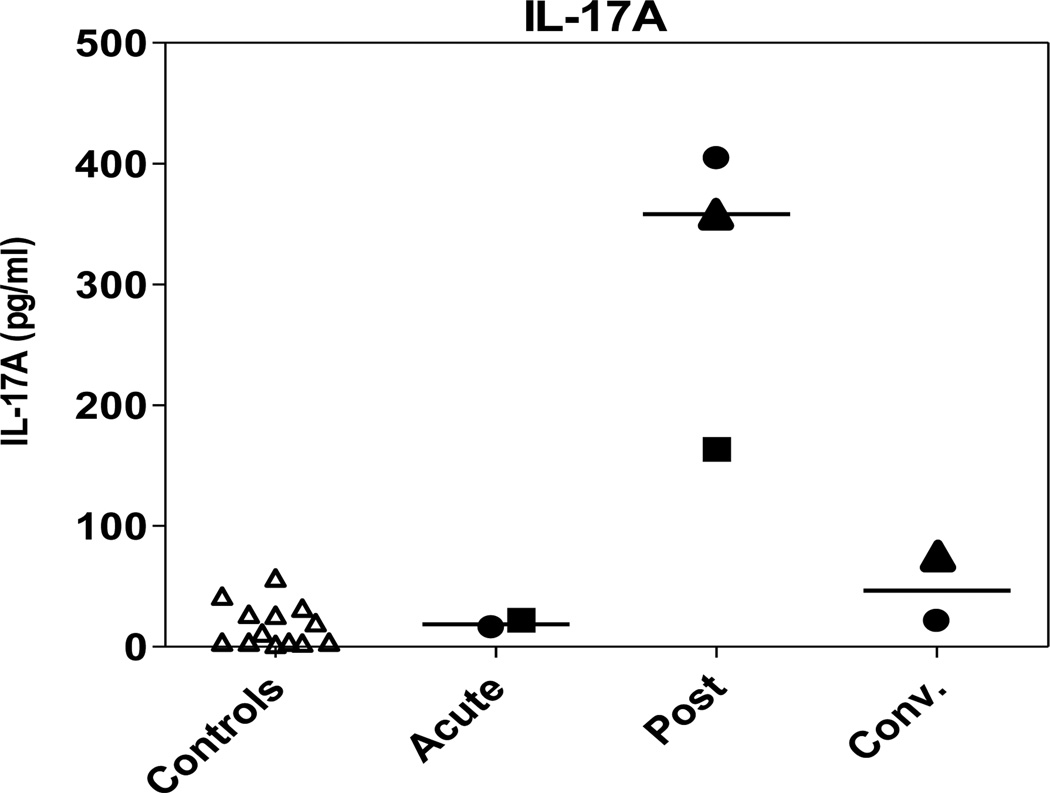
To study the relationship between exposure to pneumococci and the development of T cell immune responses more directly, T cell responses to pneumococci were also analyzed in children with proven or suspected pneumococcal disease. PBMCs were isolated from three Swedish children with pneumonia whose medical history was consistent with pneumococcal infection and who tested positive for pneumococci in blood culture and/or whose post infection/convalescing antibody titers suggested recent exposure to pneumococcus (Table 2). PBMCs collected post-infection produced more IL-17A compared to cells collected at the acute or convalescent phase or compared to Swedish control children without pneumococcal infection (Fig 5). IL-17A responses to PHA were relatively stable at all time points and no major differences were observed between PHA responses in control children and children with pneumococcal disease (Data not shown). As a control, a child with presumed nonpneumococcal pneumonia (negative urine antigen test and no increase in pneumococcal antibody concentration in post infection/convalescent phase) was included, and cells from this child produced undetectable levels of IL-17A (<4 pg/ml) both post-infection and when examined in the convalescent phase (data not shown).
Figure 5.
Pneumococcal disease results in increased IL-17A responses to pneumococci in Swedish children. PBMCs were isolated from children with pneumococcal pneumonia. Samples were collected post infection for all children (n=3). One or two additional samples were collected from each child during the acute (n=2) and/or convalescence period (n=2). IL-17A concentrations were measured in culture supernatants after stimulation with WCA. Responses in each individual child with pneumonia are represented by different symbols (filled circle, triangle or square). Responses in Swedish control children (n=11) are depicted in the first column (open triangles). Horizontal lines represent median responses.
These results suggest that, in response to infection, IL-17A responses to pneumococcal antigens are augmented, at least transiently. Together with our findings of high IL-17A and antibody responses to pneumococci in Bangladeshi individuals, these results also suggest that the range of responses detected in healthy children and adults may be the result of both asymptomatic pneumococcal colonization and repeated episodes of mucosal or invasive pneumococcal disease.
Discussion
An analysis of epidemiologic data suggests that the age-dependent development of resistance to pneumococcal disease may be derived from factors other than anticapsular antibodies [32, 33]. While the gradual acquisition of such resistance is almost certainly multifactorial, the recent discovery from our group and others that, in mice, IL-17A producing Th17 CD4+ T cells are important effector cells against S. pneumoniae colonization have indicated the existence of one possible alternative mechanism [13, 34]. Exposure to live pneumococci or to a killed whole cell pneumococcal vaccine in mice reduces subsequent pneumococcal colonization in an antibody-independent, CD4+ IL-17A dependent manner [10, 13, 35]. This Th17 mechanism also mediates protection against colonization after immunization with more well-defined vaccine preparations, including the cell wall polysaccharide and purified pneumococcal proteins [14, 36, 37]. The preclinical work on the whole cell antigen combined with efforts to develop inexpensive vaccines with broad serotype coverage suitable for use in developing countries has led to the development of a whole cell pneumococcal vaccine, which has recently been produced under GMP conditions for clinical trials [12]. This vaccine, in contrast to conjugate vaccines, would theoretically provide serotype-independent protection, via a dual mechanism that is dependent on IL-17A producing T cells for prevention of colonization as well as noncapsular antibodies for prevention of sepsis and death [12].
It is important to note, however, that to date and with a few exceptions [13, 16, 38–40], the demonstration of IL-17A responses to pneumococci has been mostly based on murine studies. It remains to be seen whether such an immune protective mechanism is operative in humans. The work presented here confirms the existence of this type of immunological response to pneumococcus in children and in adults and characterizes the IL-17A and IL-22 responses to pneumococcal antigens in humans. Thus, we found that PBMCs collected from healthy Swedish adults respond to stimulation with WCA by producing IL-17A. Cell depletion experiments and flow cytometric analysis confirmed that the detected IL-17A originated from CD4+ effector/memory T cells, suggesting that natural exposure to pneumococci gives rise to a Th17-type immune response in humans. IL-17A responses were also observed following stimulation with PdT and, albeit to a lower extent, also with PcsB. IL-17A responses to these purified pneumococcal antigens correlated with the responses to WCA, supporting the hypothesis that the observed responses originated from S. pneumoniae-specific T cells. IL-17A responses to PcsB and PsaA were recently evaluated in PBMCs collected from healthy adult or elderly Austrian volunteers [38]. Consistent with our results, relatively stronger responses to PcsB and weaker responses to PsaA were observed. However, we detected even stronger responses to stimulation with PdT than PcsB or any other antigen tested, a finding which may be related to the Toll-like receptor (TLR) 4 agonistic activity of the toxin [41, 42]. However, our preliminary data indicate that T cell responses to PdT can be strongly inhibited by addition of anti-MHCII antibodies to the cultures (Data not shown). This suggests that the observed responses to PdT are dependent on regular antigen processing and presentation of the PdT protein to the T cells, but that the TLR activating capacity of the protein may contribute to and enhance the responses. IL-17A responses to pneumolysin have also been reported in Gambian adults and British children [39, 42]. The strong IL-17A responses detected to WCA, PdT and PcsB in our study as well as in several previous studies demonstrate that these antigens are immunogenic in humans.
We further showed that IL-17A responses to WCA as well as PdT and PcsB are associated with production of relatively high levels of IL-22 and that the concentrations of IL-17A and IL-22 in culture supernatants correlate significantly. These data strongly suggest the possibility that the cellular mechanisms whereby CD4+ T cells may reduce duration of colonization are not solely dependent on IL-17A, or even Th17 cells alone. Indeed, although initial studies suggested that IL-22 is primarily co-produced with IL-17A in Th17 cells [43, 44], recent studies demonstrate that IL-22 can also be produced by separate subsets of T cells [28, 45–47]. Consistent with these findings, flow cytometric analysis demonstrated that these cytokines were primarily expressed by separate subsets of CD4+ T cells specific for PdT or WCA. In contrast to IL-17A, which has a clear proinflammarory function, the role of IL-22 is dependent on the microenvironment in which it is secreted [45]. In the context of pneumococcal colonization and disease, recruitment and/or activation of neutrophils is likely to be an important protective mechanism of IL-17A, since WCA immunization does not protect mice depleted of neutrophils, and recombinant IL-17A can enhance both antibody-dependent and -independent killing of pneumococci in vitro [13]. IL-17A is also essential for the recruitment of monocytes/macrophages to the site of pneumococcal colonization in naïve mice [34]. IL-22 can induce production of antimicrobial peptides, including beta defensin 2 [43, 48], which can inhibit growth of pneumococcal bacteria [49], and is important for epithelial regeneration and barrier function [45]. In addition, IL-22 is important in protection against several different infections, including pneumonia caused by Gram-negative bacteria [45, 48]. Furthermore, in mice immunized with WCA, in vitro IL-22 responses following stimulation with WCA correlate both with IL-17A responses and protection from subsequent pneumococcal colonization (Lu YJ and Malley R., unpublished).
In addition to IL-17A and IL-22, IFN-γ was also expressed by stimulation with WCA and PdT, primarily by a subset of cells distinct from both IL-17- and IL-22-producing cells. Although IFN-γ is clearly dispensable in mice for protection against pneumococcal colonization after immunization with WCA [13], IFN-γ may enhance many immune functions, including killing and presentation of pneumococcal antigens by macrophages.
Overall, we believe that the data presented here as well as those obtained from various mouse models commend further studies to ascertain the relative importance of IL-17A and IL-22, as well as other CD4+ T cell cytokines, in natural protection against pneumococcal colonization and possibly also a role for the measurement of these cytokines in the evaluation of potential protein-based vaccine candidates. Since children are the main target group for such vaccines, the ontogeny of these responses is a particularly important area of study. Indeed, when we compared IL-17A responses in supernatants from PBMCs collected from Swedish children and adults after stimulation with WCA, we found that PBMCs from children produce lower levels of IL-17A and IL-22 than cells from adults. Since we have previously observed comparable frequencies of CD4+ T cells in PBMCs from children and adults (Lundgren A. et al, unpublished data) the IL-17A production per CD4+ T cell is thus lower in children, supporting the hypothesis that limited T cell immunity to pneumococci may contribute to increased colonization in the younger age groups. However, when we compared the IL-17A responses in Swedish individuals to those in adults or children living in Bangladesh, a resource-poor country where pneumococcal colonization and disease begin at an early age [5, 30, 31], we found that children from Bangladesh produced significantly higher levels of IL-17A in response to WCA compared to Swedish children.
Our studies thus suggest a more complex view of the potential role of Th17 cells in protection against pneumococcal colonization. While the results in Sweden are consistent with our hypothesis that lower IL-17A responses in children are a major determinant of their greater propensity to colonization compared to adults, the results from Bangladesh are at odds with this view. It is likely that increased exposure to pneumococci in Bangladeshi subjects may, at least in part, contribute to differences in T cell responses between individuals in Bangladesh and Sweden. Furthermore, the T cell responses to pneumococcal antigens are likely to be influenced by multiple factors, including not only the frequency and intensity of previous asymptomatic colonization of pneumococcal bacteria, but also genetic factors and the use of antibiotics that may play a role in reducing carriage duration. The crowded living circumstances in Mirpur, where the Bangladeshi subjects were recruited, are also likely to result in more intense and frequent pneumococcal exposure in the Bangladeshi compared to the Swedish adults. Furthermore, clinically apparent (or even asymptomatic) episodes of pneumococcal disease in some individuals may also affect T cell responses, as supported by the increased IL-17 responses observed in children with pneumonia and clinical findings consistent with pneumococcal infection.
Levels of S. pneumoniae-specific antibodies in serum were also higher in Bangladeshi compared to Swedish adults, supporting increased pneumococcal exposure in this population. However, although the levels of IL-17A responses were also higher in Bangladeshi compared to Swedish children, the pneumococcal antibody levels were not significantly different between these two groups. Antibody responses in young children are weak and of short duration [50]. Although Th1 type responses are often also impaired during early life, Th17 type responses may be more easily triggered in young children, possibly as a result of preferential production of Th17 inducing cytokines by antigen presenting cells [51]. Furthermore, maternal antibodies can limit induction of antibody responses, while having little or instead enhancing effects on T cell responses [50]. It is therefore tempting to speculate that pneumococcal antibody responses may develop more slowly than Th17 type responses to the same antigens. However, over time, the increased exposure to pneumococci in Bangladesh may eventually give rise to higher levels of both IL-17A and antibodies, as observed in the adult populations.
The differences in T cell and antibody responses may also be partly explained by different kinetics of the two types of immune responses; one could hypothesize that antibody levels in serum may more closely reflect the cumulative exposure to pneumococcus, while the numbers of T cells specific for pneumococcus circulating in peripheral blood at a specific time point may be more strongly influenced by very recent exposure and therefore show greater variation than the antibody levels. These potential differences in kinetics and persistence of responses may further explain the lack of correlation between IL-17A and antibody responses in our study.
Bangladeshi children also seemed to have a more general propensity to produce IL-17A than Swedish children, since stimulation with PHA also resulted in higher levels of IL-17A production in Bangladeshi children. Since our depletion experiments showed that IL-17A is primarily produced by memory/effector cells in response to PHA, the higher IL-17A levels observed in Bangladeshi children may be explained by a higher proportion of memory/effector T cells in this population as a result of frequent infections during early life. This hypothesis is supported by a comparative study performed in Malawi and the UK, where significantly higher proportions of memory cells were found in Malawian compared to age matched UK adolescents [52]. Many of the infections frequently encountered by Bangladeshi children during childhood may also specifically promote Th17 responses, including gastrointestinal infections, such as H. pylori, Vibrio cholerae and Shigella, as well as respiratory infections, including not only pneumococci, but also mycobacteria and Bordetella pertussis [53].
We believe that a prospective and longitudinal assessment of pneumococcal Th17 and antibody responses and pneumococcal colonization in children will help to further clarify the questions raised in this study, including the ontogeny and kinetics of T and B cell responses following repeated episodes of colonization. We are aware of at least one major ongoing effort in this direction in a developing country (Adam Finn, personal communication); the results of such a study will be most informative.
We conclude that both pneumococcal colonization and disease may result in Th17 responses to pneumococci in humans and that pneumococcal antigens are immunogenic and can activate T cells even in infants and children with a relatively immature immune system. Our observations of higher IL-17A as well as antibody responses in adults compared to children and in Bangladeshi subjects compared to Swedes illustrate the difficulty in extrapolating data between different populations and age groups. These factors will become especially important to consider when moving the development of new pneumococcal vaccines from mice to humans and from trials in adults from higher income countries to children in developing countries.
Highlights.
Memory IL-17A and IL-22 responses to pneumococcal antigens demonstrated in humans
Separate subsets of CD4+ T cells produce IL-17A and IL-22
Stronger Th17 responses in adults compared to children in Sweden
Strong and comparable Th17 responses in children and adults in Bangladesh
Exposure likely determines IL-17A responses, important for future vaccine research
Acknowledgements
We gratefully acknowledge PATH for their support of this work. This work was also supported by the Mucosal Immunobiology and Vaccine Center (MIVAC), funded by the Swedish Foundation for Strategic Research (to AL) and by NIH/NIAID grant AI066013 (to RM). RM gratefully acknowledges support from the Translational Research Program at Children’s Hospital Boston. We thank Mark Alderson, Porter Anderson and Jan Holmgren for helpful suggestions regarding the work and the manuscript. We thank Netanya Sandler for technical advice. RM is a member of the scientific advisory board of Genocea Biosciences, Cambridge MA and Arsanis Biosciences, Vienna, Austria.
Abbreviations
- BCG
Bacillus Calmette-Guérin vaccine
- COPD
chronic obstructive pulmonary disease
- PBMCs
peripheral blood mononuclear cells
- PcsB
protein required for cell separation in group B streptococci
- PdT
pneumolysoid
- PHA
phytohemagglutinin
- PPD
Mycobacterium tuberculosis purified protein derivative
- PsaA
pneumococcal surface adhesin A
- PspC
pneumococcal surface protein C
- SEB
staphylococcus enterotoxin B
- TLR
toll like receptor
- WCA
whole cell antigen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
A. Lundgren, Email: anna.lundgren@microbio.gu.se.
T. Bhuiyan, Email: taufiqur@icddrb.org.
D. Novak, Email: daniel.novak@vgregion.se.
J. Kaim, Email: joanna.kaim@microbio.gu.se.
A. Reske, Email: adi.reske@ childrens.harvard.edu.
Y. J. Lu, Email: yingjie.lu @childrens.harvard.edu.
F. Qadri, Email: fqadri@icddrb.org.
R. Malley, Email: richard.malley@childrens.harvard.edu.
References
- 1.Johnson HL, Deloria-Knoll M, Levine OS, Stoszek SK, Freimanis Hance L, Reithinger R, et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 2010;7(10) doi: 10.1371/journal.pmed.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Austrian R. Some aspects of the pneumococcal carrier state. J Antimicrob Chemother. 1986;18(Suppl A):35–45. doi: 10.1093/jac/18.supplement_a.35. [DOI] [PubMed] [Google Scholar]
- 3.O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374(9693):893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 4.Plotkin SA, Orenstein W, Offit PA. Vaccines. 5th ed. Saunders; 2008. [Google Scholar]
- 5.Granat SM, Mia Z, Ollgren J, Herva E, Das M, Piirainen L, et al. Longitudinal study on pneumococcal carriage during the first year of life in Bangladesh. Pediatr Infect Dis J. 2007;26(4):319–324. doi: 10.1097/01.inf.0000257425.24492.11. [DOI] [PubMed] [Google Scholar]
- 6.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011 doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rozenbaum MH, Boersma C, Postma MJ, Hak E. Observed differences in invasive pneumococcal disease epidemiology after routine infant vaccination. Expert Rev Vaccines. 2011;10(2):187–199. doi: 10.1586/erv.10.163. [DOI] [PubMed] [Google Scholar]
- 8.Moffitt KL, Malley R. Next generation pneumococcal vaccines. Curr Opin Immunol. 2011;23(3):407–413. doi: 10.1016/j.coi.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci U S A. 2005;102(13):4848–4853. doi: 10.1073/pnas.0501254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malley R, Lipsitch M, Stack A, Saladino R, Fleisher G, Pelton S, et al. Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by capsulated pneumococci. Infect Immun. 2001;69(8):4870–4873. doi: 10.1128/IAI.69.8.4870-4873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu YJ, Yadav P, Clements JD, Forte S, Srivastava A, Thompson CM, et al. Options for inactivation, adjuvant, and route of topical administration of a killed, unencapsulated pneumococcal whole-cell vaccine. Clin Vaccine Immunol. 2010;17(6):1005–1012. doi: 10.1128/CVI.00036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu YJ, Leite L, Goncalves VM, Dias Wde O, Liberman C, Fratelli F, et al. GMP-grade pneumococcal whole-cell vaccine injected subcutaneously protects mice from nasopharyngeal colonization and fatal aspiration-sepsis. Vaccine. 2010;28(47):7468–7475. doi: 10.1016/j.vaccine.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu YJ, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q, et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 2008;4(9):e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basset A, Thompson CM, Hollingshead SK, Briles DE, Ades EW, Lipsitch M, et al. Antibody-independent, CD4+ T-cell-dependent protection against pneumococcal colonization elicited by intranasal immunization with purified pneumococcal proteins. Infect Immun. 2007;75(11):5460–5464. doi: 10.1128/IAI.00773-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granat SM, Ollgren J, Herva E, Mia Z, Auranen K, Makela PH. Epidemiological evidence for serotype-independent acquired immunity to pneumococcal carriage. J Infect Dis. 2009;200(1):99–106. doi: 10.1086/599364. [DOI] [PubMed] [Google Scholar]
- 16.Gross JE, Lu YJ, Forte S, Tekwe C, Sethis S, Murphy TF, et al. IL-22 predicts resistance to bacterial colonization in adults with chronic obstructive pulmonary disease. Tel Aviv, Israel: ISPPD7; 2010. [Google Scholar]
- 17.Aniansson G, Alm B, Andersson B, Larsson P, Nylen O, Peterson H, et al. Nasopharyngeal colonization during the first year of life. J Infect Dis. 1992;165(Suppl 1):S38–S42. doi: 10.1093/infdis/165-supplement_1-s38. [DOI] [PubMed] [Google Scholar]
- 18.Greenwood B. The epidemiology of pneumococcal infection in children in the developing world. Philos Trans R Soc Lond B Biol Sci. 1999;354(1384):777–785. doi: 10.1098/rstb.1999.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leino T, Auranen K, Jokinen J, Leinonen M, Tervonen P, Takala AK. Pneumococcal carriage in children during their first two years: important role of family exposure. Pediatr Infect Dis J. 2001;20(11):1022–1027. doi: 10.1097/00006454-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Montgomery JM, Lehmann D, Smith T, Michael A, Joseph B, Lupiwa T, et al. Bacterial colonization of the upper respiratory tract and its association with acute lower respiratory tract infections in Highland children of Papua New Guinea. Rev Infect Dis. 1990;12(Suppl 8):S1006–S1016. doi: 10.1093/clinids/12.supplement_8.s1006. [DOI] [PubMed] [Google Scholar]
- 21.Woolfson A, Huebner R, Wasas A, Chola S, Godfrey-Faussett P, Klugman K. Nasopharyngeal carriage of community-acquired, antibiotic-resistant Streptococcus pneumoniae in a Zambian paediatric population. Bull World Health Organ. 1997;75(5):453–462. [PMC free article] [PubMed] [Google Scholar]
- 22.Lloyd-Evans N, O'Dempsey TJ, Baldeh I, Secka O, Demba E, Todd JE, et al. Nasopharyngeal carriage of pneumococci in Gambian children and in their families. Pediatr Infect Dis J. 1996;15(10):866–871. doi: 10.1097/00006454-199610000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Dowell SF, Garman RL, Liu G, Levine OS, Yang YH. Evaluation of Binax NOW, an assay for the detection of pneumococcal antigen in urine samples, performed among pediatric patients. Clin Infect Dis. 2001;32(5):824–825. doi: 10.1086/319205. [DOI] [PubMed] [Google Scholar]
- 24.Bhuiyan TR, Qadri F, Janzon A, Chowdhury MI, Lundin SB, Lundgren A. Th17 and Th1 responses to Helicobacter pylori in Bangladeshi children and adults. 9th International Workshop on Pathogenesis and Host Response in Helicobacter Infections; Helsingör, Denmark. 2010. [Google Scholar]
- 25.Srivastava A, Casey H, Johnson N, Levy O, Malley R. Recombinant bactericidal/permeability-increasing protein rBPI21 protects against pneumococcal disease. Infect Immun. 2007;75(1):342–349. doi: 10.1128/IAI.01089-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Briles DE, Hollingshead S, Brooks-Walter A, Nabors GS, Ferguson L, Schilling M, et al. The potential to use PspA and other pneumococcal proteins to elicit protection against pneumococcal infection. Vaccine. 2000;18(16):1707–1711. doi: 10.1016/s0264-410x(99)00511-3. [DOI] [PubMed] [Google Scholar]
- 27.Giefing C, Meinke AL, Hanner M, Henics T, Bui MD, Gelbmann D, et al. Discovery of a novel class of highly conserved vaccine antigens using genomic scale antigenic fingerprinting of pneumococcus with human antibodies. J Exp Med. 2008;205(1):117–131. doi: 10.1084/jem.20071168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scriba TJ, Kalsdorf B, Abrahams DA, Isaacs F, Hofmeister J, Black G, et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol. 2008;180(3):1962–1970. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith SG, Lalor MK, Gorak-Stolinska P, Blitz R, Beveridge NE, Worth A, et al. Mycobacterium tuberculosis PPD-induced immune biomarkers measurable in vitro following BCG vaccination of UK adolescents by multiplex bead array and intracellular cytokine staining. BMC Immunol. 2010;11:35. doi: 10.1186/1471-2172-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flannery B, Whitney CG. Uncovering pneumococcal disease burden in Bangladesh. Am J Trop Med Hyg. 2007;77(5):793–794. [PubMed] [Google Scholar]
- 31.Brooks WA, Breiman RF, Goswami D, Hossain A, Alam K, Saha SK, et al. Invasive pneumococcal disease burden and implications for vaccine policy in urban Bangladesh. Am J Trop Med Hyg. 2007;77(5):795–801. [PubMed] [Google Scholar]
- 32.Malley R. Antibody and cell-mediated immunity to Streptococcus pneumoniae: implications for vaccine development. J Mol Med. 2010;88(2):135–142. doi: 10.1007/s00109-009-0579-4. [DOI] [PubMed] [Google Scholar]
- 33.Lipsitch M, Whitney CG, Zell E, Kaijalainen T, Dagan R, Malley R. Are anticapsular antibodies the primary mechanism of protection against invasive pneumococcal disease? PLoS Med. 2005;2(1):e15. doi: 10.1371/journal.pmed.0020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest. 2009;119(7):1899–1909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trzcinski K, Thompson C, Malley R, Lipsitch M. Antibodies to conserved pneumococcal antigens correlate with, but are not required for, protection against pneumococcal colonization induced by prior exposure in a mouse model. Infect Immun. 2005;73(10):7043–7046. doi: 10.1128/IAI.73.10.7043-7046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moffitt KL, Gierahn TM, Lu YJ, Gouveia P, Alderson M, Flechtner JB, et al. T(H)17-based vaccine design for prevention of Streptococcus pneumoniae colonization. Cell Host Microbe. 2011;9(2):158–165. doi: 10.1016/j.chom.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malley R, Srivastava A, Lipsitch M, Thompson CM, Watkins C, Tzianabos A, et al. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect Immun. 2006;74(4):2187–2195. doi: 10.1128/IAI.74.4.2187-2195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmid P, Selak S, Keller M, Luhan B, Magyarics Z, Seidel S, et al. Th17/Th1 biased immunity to the pneumococcal proteins PcsB, StkP and PsaA in adults of different age. Vaccine. 2011;29(23):3982–3989. doi: 10.1016/j.vaccine.2011.03.081. [DOI] [PubMed] [Google Scholar]
- 39.Mureithi MW, Finn A, Ota MO, Zhang Q, Davenport V, Mitchell TJ, et al. T cell memory response to pneumococcal protein antigens in an area of high pneumococcal carriage and disease. J Infect Dis. 2009;200(5):783–793. doi: 10.1086/605023. [DOI] [PubMed] [Google Scholar]
- 40.Sharma SK, Casey JR, Pichichero ME. Reduced Memory CD4+ T-Cell Generation in the Circulation of Young Children May Contribute to the Otitis-Prone Condition. J Infect Dis. 2011;204(4):645–653. doi: 10.1093/infdis/jir340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malley R, Henneke P, Morse SC, Cieslewicz MJ, Lipsitch M, Thompson CM, et al. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc Natl Acad Sci U S A. 2003;100(4):1966–1971. doi: 10.1073/pnas.0435928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Q, Bagrade L, Bernatoniene J, Clarke E, Paton JC, Mitchell TJ, et al. Low CD4 T cell immunity to pneumolysin is associated with nasopharyngeal carriage of pneumococci in children. J Infect Dis. 2007;195(8):1194–1202. doi: 10.1086/512617. [DOI] [PubMed] [Google Scholar]
- 43.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203(10):2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8(9):942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 45.Eyerich S, Eyerich K, Cavani A, Schmidt-Weber C. IL-17 and IL-22: siblings, not twins. Trends Immunol. 2010;31(9):354–361. doi: 10.1016/j.it.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 46.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10(8):857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 47.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10(8):864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 48.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14(3):275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee HY, Andalibi A, Webster P, Moon SK, Teufert K, Kang SH, et al. Antimicrobial activity of innate immune molecules against Streptococcus pneumoniae, Moraxella catarrhalis and nontypeable Haemophilus influenzae. BMC Infect Dis. 2004;4:12. doi: 10.1186/1471-2334-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siegrist CA. The challenges of vaccine responses in early life: selected examples. J Comp Pathol. 2007;137(Suppl 1):S4–S9. doi: 10.1016/j.jcpa.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Vanden Eijnden S, Goriely S, De Wit D, Goldman M, Willems F. Preferential production of the IL-12(p40)/IL-23(p19) heterodimer by dendritic cells from human newborns. Eur J Immunol. 2006;36(1):21–26. doi: 10.1002/eji.200535467. [DOI] [PubMed] [Google Scholar]
- 52.Ben-Smith A, Gorak-Stolinska P, Floyd S, Weir RE, Lalor MK, Mvula H, et al. Differences between naive and memory T cell phenotype in Malawian and UK adolescents: a role for Cytomegalovirus? BMC Infect Dis. 2008;8:139. doi: 10.1186/1471-2334-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van de Veerdonk FL, Gresnigt MS, Kullberg BJ, van der Meer JW, Joosten LA, Netea MG. Th17 responses and host defense against microorganisms: an overview. BMB Rep. 2009;42(12):776–787. doi: 10.5483/bmbrep.2009.42.12.776. [DOI] [PubMed] [Google Scholar]