Abstract
Study Objectives:
Sub-anesthetic doses of NMDA receptor antagonists suppress sleep and elicit continuous high-power gamma oscillations lasting for hours. This effect is subunit-specific, as it was also seen after preferential blockade of the NR2A but not of the NR2B subunit-containing receptors. The objective of this study was to test whether NR2B receptor antagonists that do not induce lasting aberrant gamma elevation affect gamma activity during specific behaviors and states, including REM sleep, when gamma normally occurs.
Design:
Gamma oscillations in cortical EEG were assessed in different vigilance states in rats and were compared before and after injection of nonselective (ketamine, 10 mg/kg, and MK801, 0.2 mg/kg), as well as NR2A-preferring (NVP-AAM077, 20 mg/kg), and NR2B-selective NMDA receptor antagonists (Ro25-6985, 10 mg), and vehicle.
Measurements and Results:
In contrast to nonselective and NR2A-preferring antagonists, Ro25-6985 did not disrupt sleep and had no effect on gamma activity during waking and slow wave sleep. It significantly increased, however, gamma power in the frontal (but not in occipital) cortex during REM sleep (by 37% ± 10%, average in the first 4 h). The effect had a short onset; enhanced gamma activity appeared as early as in the first REM sleep episode post-injection and lasted over 8 hours. Increased gamma power induced by MK-801 (46% ± 5%) and NVP-AAM077 (100% ± 8%) during REM sleep could also be detected several hours after injection when periodic alternation of sleep-wake states returned.
Conclusions:
By acting on gamma oscillations in a state-dependent manner, NMDA receptors might have subunit-specific role in REM sleep-associated cognitive processes.
Citation:
Kocsis B. State-dependent increase of cortical gamma activity during REM sleep after selective blockade of NR2B subunit containing NMDA receptors. SLEEP 2012;35(7):1011–1016.
Keywords: Gamma oscillation, paradoxical sleep, MK-801, ketamine
INTRODUCTION
Gamma-frequency synchronization between neuronal ensembles is critical for a number of different cognitive processes in alert behavioral conditions and during rapid eye movement (REM) sleep. Cortical gamma oscillations are as strong during REM sleep as in the most aroused waking states,1,2 but the role of gamma rhythmicity and the molecular and cellular mechanisms of these oscillations may be different in the 2 states.3–5 Cortical oscillations are generated by fast GABAergic and glutamatergic mechanisms but are also modulated by a large variety of other neurotransmitter-receptor systems. The ionotropic glutamatergic NMDA receptors (NMDA-R) in the cortex are expressed in both pyramidal cells and interneurons, which are the integral components of the cortical networks generating EEG oscillations at different frequencies. The NMDA-R is a hetero-oligomeric complex consisting primarily of 2 NR1 and 2 of several types of NR2 subunits. There are major functional differences between NMDA-Rs containing the NR2A and NR2B subunits, indicating that the 2 receptors may play different roles in network activity, and that selective blockade of these receptors may differently affect gamma synchrony. Sub-anesthetic doses of NMDA receptor antagonists were shown to suppress sleep6 and elicit continuous high-power gamma oscillations lasting for hours.7–9 This effect is subunit-specific, as it was also seen after preferential blockade of the NR2A but not of the NR2B subunit-containing receptors.9 NR2B receptor antagonists, which do not induce lasting aberrant gamma elevation might, however, exert a modulatory influence on functional gamma activity during specific behaviors and states, including REM sleep, when gamma normally occurs. The objective of this study was to test this possibility by analyzing the effect of subunit-specific NMDA antagonists on gamma activity in different vigilant states.
MATERIALS AND METHODS
Experimental Procedures
All experiments were performed in accordance with National Institute of Health guidelines and were approved by the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center. The rats were housed in a temperature and humidity-controlled room with 12h/12h light/dark cycle; food and water was available ad libitum both in the home cage and during recordings. The rats were implanted with chronic EEG and EMG electrodes. Stainless steel screws were used to record cortical EEG in the frontal cortex on both sides (1 mm anterior and 2 mm lateral to bregma) and over the occipital cortex (6.5 mm posterior and 3 mm lateral to the bregma), and pairs of twisted wires were implanted in the hippocampus to record field potentials. Two additional screw electrodes were inserted, one ~5 mm anterior to bregma and the other over the cerebellum, for ground and reference. Muscle tone was recorded using multithreaded wires in the neck muscles, on both sides. All electrodes were connected to a miniature connector and the wires and the connector were fixed to the skull with dental acrylic. Electrophysiological recordings started after a 7- to 10-day recovery period. Experiments with drug injections started after several daily control recordings. For recording sessions, the rats were placed in a recording box and connected to a slip-ring commutator. The recordings started early morning and lasted 10-24 h; the drugs were administered after 4-h control recording. Other than the drug injection, the rats were left undisturbed. The injections (in 1 mL/kg volume, subcutaneous) were separated ≥ 4 days to allow time for washout. The following compounds were used: nonselective NMDA-R antagonists ketamine (10 mg/kg, Fort Dodge Animal Health, USA) and MK801 (0.2 mg/kg, Tocris), NR2A-preferring antagonist NVP-AAM077 (20 mg/kg, Novartis), NR2B-selective antagonist Ro25-6985 (10 mg s/c, Tocris). Vehicle: saline.
Electrophysiology and Data Analysis
Cortical field potentials were amplified using an AC differential amplifier (Model 3500, A-M Systems) and filtered below 100 Hz during acquisition (sampling rate: 250 Hz). EEG signals were subjected to fast Fourier transform to generate power spectra for consecutive 16-s windows. Gamma oscillations were assessed in frontal cortex EEG using the average spectral power in the 30-50 Hz and the 65-90 Hz frequency bands. For comparison of the total gamma power between rats, the values were normalized using the average of gamma power during the first hour of control recording that the animals spent waking, i.e., matching the dominant behavior following the injection of nonselective NMDA-R blockers.7–9 For assessment of state-dependent changes, average power in the delta (1-4 Hz) and theta (5-10 Hz) frequency bands were calculated in the hippocampal recordings and the theta/delta ratio along with the root-mean square value of the EMG, calculated in the same 16-s windows, were used to determine the vigilance levels. Two thresholds were determined for the EMG and one for theta/delta ratio (usually between 10 and 20) for each individual rat. Using these thresholds, REM sleep was identified as coincident atonia and large theta/delta ratio. Then, the remaining time was divided into 2 states on the basis of EMG activity, the first included active waking, and the second included episodes of quiet waking and slow wave sleep. Average gamma activity was calculated for each state in 3 consecutive, 4-h periods, one before injection and 2 after the injections (hours 1-4 and 5-8). The results are presented both in absolute values (Figure 1C) and as percent increase relative to control (Table 1 and text). Statistical analysis started with 2-way ANOVA, drug and time being the main factors, with post hoc Bonferroni comparisons of the group means. For more detailed analysis, 2 sets of pair-wise comparisons were performed for each individual compound, using Student's t-test. First, for each vigilance state and for each compound, gamma power in the 2 post-injection test periods was compared with that during control. Then the percent changes after each test compound in the first and second test periods were compared with those after saline injection.
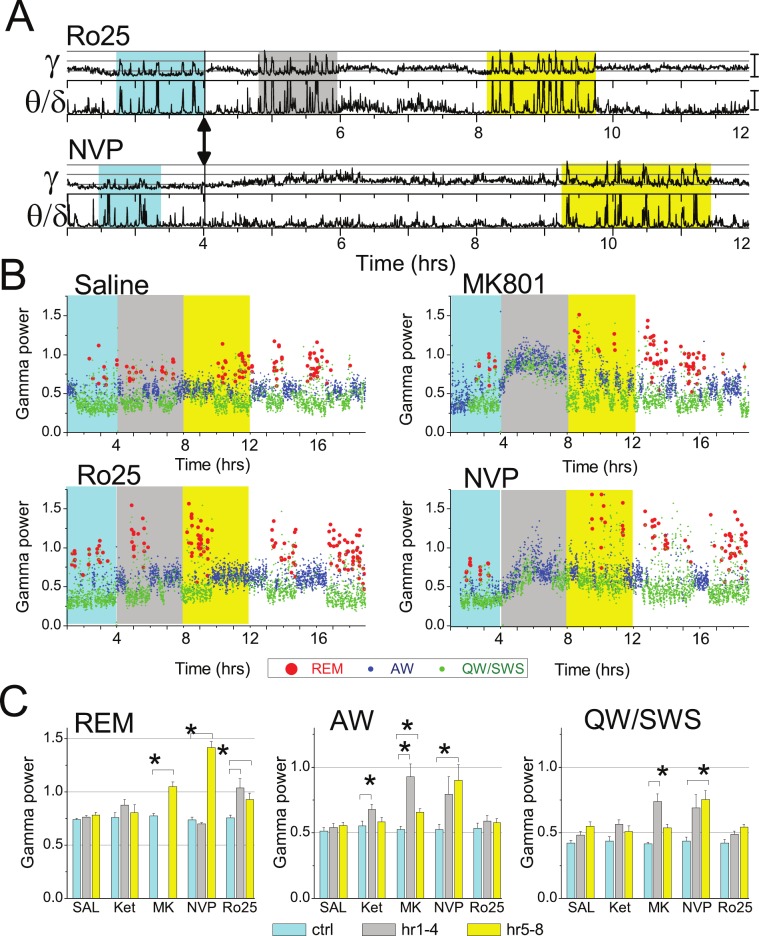
Figure 1.
Enhanced gamma oscillations during REM sleep after NMDA-R blockade. (A) Time course of integrated gamma power in a 12-h recording before and after Ro25-6985 and NVP-AAM077 injection (arrows) in a representative experiment (top traces). Bottom traces show theta/delta ratio to serve as marker of REM sleep episodes. Segments of sleep with REM sleep clusters are highlighted in pre-injection control (cyan), and during the first (gray) and second 4-h segments (yellow), post-injection. (B) Time course of state-dependent gamma power in frontal cortex 3 h before and 15 h after injection of saline and NMDA antagonists (injection at h 4; cyan, gray and yellow backgrounds show pre-injection control and first and second 4-h periods post-injection). Each dot represents one 16-s segment and is colored according to the vigilance state, i.e., active waking (blue), REM sleep (red), and quiet waking/NREM sleep (green) (same experiment as in A). Note stable, state-dependent gamma levels in control (REM sleep > active waking > quiet waking/SWS) and strong increase in REM sleep-related gamma (red dots) after all NMDA antagonists. (C) State-dependent level of gamma power after blockade of NMDA-Rs with different subunit composition. Group averages (n = 6) of gamma power during REM sleep (REM), active waking (AW), and quiet waking/slow wave sleep (QW-SWS) in a 4-h pre-injection control recording (cyan columns), and during the first (gray) and second 4-h post-injection periods (yellow) after administration of saline, ketamine (10 mg/kg), MK-801 (0.2 mg/kg), NVP-AAM077 (20 mg/kg), and Ro25-6985 (20 mg/kg). Calibration in A and C: gamma –1.0E-2mV2, theta/delta: 15.
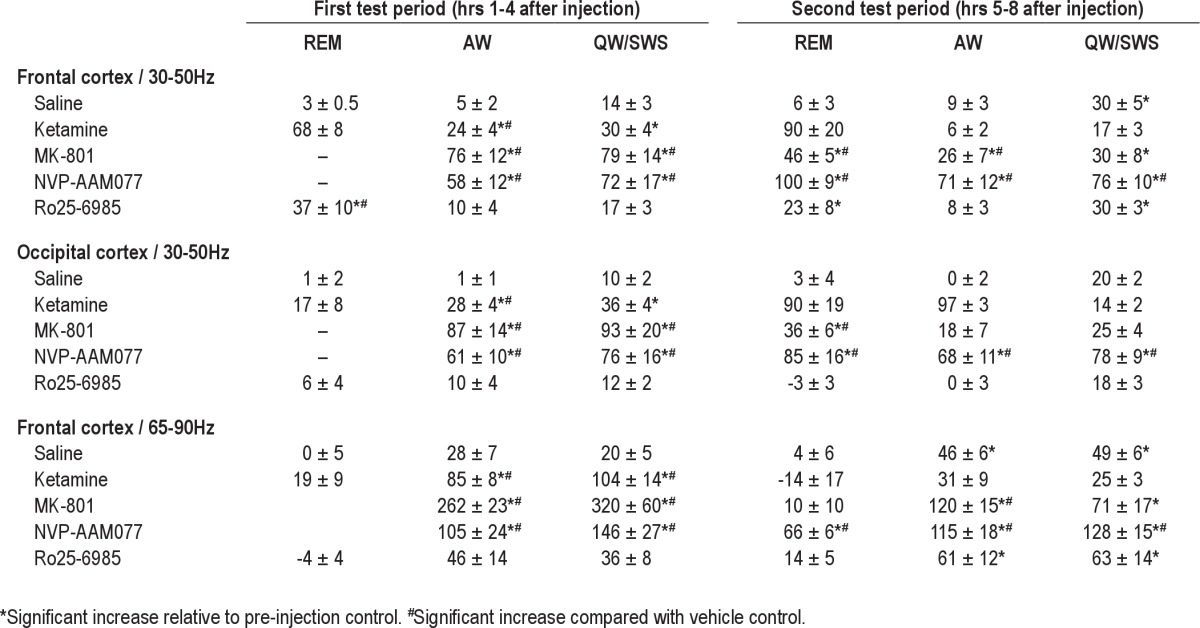
Table 1.
Changes in gamma power after nonselective and subunit-selective NMDA-R blockade (percent of pre-injection control)
RESULTS
As shown earlier,7–9 nonselective NMDA-R antagonists ketamine and MK801, injected in sub-anesthetic doses, induced gamma oscillations; 30-50 Hz power increased by 89% ± 7% (P < 0.001, t-test comparison with saline) and 197% ± 50% (P = 0.014), respectively. The effect developed with a latency of 5.5+1.1 min after ketamine and 16.2+1.1 min after MK-801, and lasted for 1 h after ketamine and for ~4 h after MK-801, during which time gamma was continuously at an elevated level while the animals were mostly awake and showed characteristic behavior, i.e. increased motor activity, ataxia, and stereotypic head movements.10 The effect of the NR2A-preferring antagonist NVP-AAM077 was similar to that after MK-801, although the increase in gamma power was lower (147% ± 21%, P = 0.003), appeared with longer latency (38.8+2.4 min), and had longer duration (Figure 1B). The NR2B-selective antagonist Ro25-6985 failed to replicate the effects of nonselective antagonists; it was ineffective in altering total 30-50 Hz power (14% ± 8%, P = 0.12).9
The lack of steady gamma hyperactivity, induced by NR2B-selective NMDA antagonist, however, did not rule out a positive effect, which could be limited to short events, states, or behaviors when gamma normally occurs. To test this possibility, active waking (AW) and REM sleep, i.e., the natural gamma states, were separated from the rest of the recording, which thus included periods of quiet waking and slow wave sleep (QW/SWS), and gamma power was calculated separately in each state. For statistical analysis, state-dependent gamma power was averaged in 3 time periods, each 4-h: control before injection, and the first and second test periods (i.e., hours 1-4 and 5-8 post-injection, respectively), as shown in Figure 1B. For ketamine, the length of test periods was set to 1 hour.
In pre-injection control recordings and after saline injection, the highest power of gamma activity in the frontal cortex was observed during REM sleep; gamma was of lower amplitude during AW, and even lower in QW/SWS (Figure 1B). After NR2B receptor blockade, 30-50 Hz power, averaged over the first 4 h increased during REM sleep by 37% ± 10% (significantly different from vehicle, P = 0.01) but was unaltered during other states (AW: 10% ± 3% P = 0.35, QW/SWS: 17% ± 3%, P = 0.83) (Figure 1C). The effect had a short onset; enhanced gamma activity already appeared during the first REM sleep episode post-injection, which occurred at a latency (74 ± 29 min; range: 25-59 min, except one outsider 202 min) not significantly different (P = 0.31) from that after vehicle (46 ± 6 min). This pattern of state-dependent gamma increase lasted for 12-16 hours. REM sleep-associated gamma power remained high over the second test period (23% ± 8%, P = 0.054), whereas changes in gamma power during AW (8 ± 3%, P = 0.60) and QW/SWS (30% ± 3%, P = 0.87) were not different from vehicle control.
Nonselective NMDA-R blockade suppressed REM sleep for the duration of the first test period; REM sleep latency was 130 ± 12 min after ketamine and 292 ± 30 min after MK-801 injections (both significantly different from saline, P < 0.001). In the states of AW and QW/SWS, which remained detectable, gamma was continuously increased (76% ± 12%, P = 0.003; and 79% ± 14%, P = 0.002). Similarly, the NR2A receptor-preferring antagonist, NVP-AAM077 also suppressed REM sleep during the first test period (REM sleep latency: 354 ± 60 min, P = 0.003) and increased gamma activity both in AW (58% ± 12%, P = 0.044) and QW/SWS (72% ± 17%, P = 0.048). In the second test period, when the primary gamma elevation started to decline and the periodic alternation of sleep-wake states returned (Figure 1A-B), enhanced gamma activity also appeared during REM sleep. In fact, increase in 30-50 Hz power during this state (100% ± 8%; P < 0.001, after NVP-AAM077 and 46% ± 5%; P < 0.001, after MK-801) exceeded the increase in gamma power in other states (see Table 1). Ketamine did not have such a delayed effect on gamma level during REM sleep (P = 0.07).
Two-way ANOVA using the entire data set revealed highly significant (P < 0.0001, power > 0.99) effects of the 2 main factors (drug: MK-801, NVP-AAM077 and Ro25-6985 and time: control, 1st and 2nd test periods) as well as their interactions, underlining the robustness of the reactions. Post hoc Bonferroni test showed significant differences in gamma power increases after injection of saline vs. MK-801 and NVP but not Ro25-6895 during AW and after Ro25-6895 vs. saline (1st test period) and after MK-801 and NVP-AAM077 vs. saline (2nd test period) in REM sleep.
REM sleep-associated gamma increase in the frontal cortex was limited to the 30-50 Hz frequency range. At higher gamma frequencies, analyzed within the 65-90 Hz frequency band, there was no change during REM sleep after injection of any of the test compounds. During AW and QW/SWS, spectral power in 65-90 Hz showed similar alterations to those in the 30-50 Hz range, i.e., significantly increased after ketamine during the first and after MK-801 and NVP-AAM077 during both the first and second test periods. On the other hand, the variations in 65-90 Hz power after Ro25-6895 were not significantly different from those after saline in any state, either during the first or the second test periods (see Table 1).
In the occipital cortex, NR2B subunit selective antagonist did not alter 30-50 Hz gamma rhythm, whereas the general increase in gamma power after nonselective NMDA-R blockade was similar to that in the frontal cortex,9,11 i.e., ketamine increased occipital cortex gamma activity during AW, whereas MK801 and NVP-AAM077 increased gamma power in all vigilance states (see Table 1).
DISCUSSION
It has been shown previously that sub-anesthetic doses of NMDA-R antagonists elicit a lasting increase in gamma power,7,8 due primarily to blockade of a specific subtype of NMDA-Rs that contains the NR2A subunit.9 The present study demonstrates a second type of NMDA-R dependent gamma enhancement which appears in a state-dependent manner during REM sleep and can be elicited by selective blockade of the NR2B subunit-containing receptor. Enhanced gamma activity during REM sleep also appeared after full NMDA-R blockade, but only at a later stage when the aberrant gamma oscillations and other psychotic-like symptoms ceased and the periodic alternation of sleep-wake states returned.
The NMDA antagonist, Ro25-6985 has a high selectivity for the NR2B subunit containing receptors (IC50 values are 0.009 and 52 μM for cloned receptor subunit combinations NR1C/NR2B and NR1C/NR2A, respectively12), providing strong support for the conclusion that an NR2B-dependent mechanism is sufficient to elicit enhanced state-dependent gamma activity during REM sleep. The effect had a short latency, i.e., gamma power was already at an elevated level during the first REM sleep episode after the injection. Enhanced gamma during REM sleep also occurred after MK-801 or NVP-AAM077 injection but the > 4h latency of the effect makes the role of the different NMDA-R subtypes in these reactions less clear. Specifically, a critical involvement of NR2B subunit containing receptors activated by these compounds cannot be excluded, indicating that REM sleep-dependent gamma enhancement that appeared early after NR2B blockade but only developed hours after injection of nonselective or NR2A preferring antagonists could, in fact, have been NR2B-dependent in both experiments. First, since these compounds suppressed REM sleep for several hours, one can only speculate whether the long latency was due to a delayed effect or due to masking of any possible state-dependent effect by the overall increase in gamma activity. Importantly, REM sleep-associated gamma enhancement induced by selective NR2B blockade also had a long duration, i.e., it was still present > 4 hours after injection. Thus, the action of nonselective NMDA antagonist on NR2B receptors might just have outlasted the shorter NR2A-dependent general gamma activation. Second, the long latency may indicate the involvement of secondary, downstream, or compensatory changes in the NMDA-R expression. Indeed, NMDA-Rs are regulated by receptor activity in a subtype-specific manner, which can change the NR2A/NR2B ratio in less than 8 hours.13 Compensatory up-regulation of NR2A receptors was shown to take place 4 hours after MK801 application, with no significant change in the expression of NMDA-Rs containing NR2B or other subunits.14 Prolonged wakefulness (4h sleep deprivation) was also shown to induce a similar shift in the NR2B/NR2A ratio, which was directly related to changes in synaptic function and was fully reversed after recovery sleep.15 The EEG characteristics also showed remarkable similarities 8 to 20 h after MK-801 injection with those after sleep deprivation.6 The importance of prolonged NMDA-R activation for changes in NR2B/NR2A ratio can also explain the lack of a delayed effect of the short-acting ketamine, which may not have provided the necessary exposure to initiate regulatory processes.
The NR2B/NR2A ratio has strong effects on the processes of long-term potentiation and depression16 and cognitive performance.17 Gamma synchrony in REM sleep is known to be involved in normal neuronal processing.3 Gamma oscillations are as strong during REM sleep as in the most aroused waking states,1,2 but the origin of gamma rhythmicity may be different in the two states. REM sleep is characterized by cholinergic dominance,18 which may facilitate cortical gamma oscillations.19,20 REM sleep is not homogeneous, however. The state of “tonic REM sleep” is periodically interrupted by short episodes of “phasic REM sleep,” associated not only with phasic events, e.g., eye movements, muscle twitches, and pontine waves, but also with augmented theta and gamma oscillations, which are atropine resistant.3,18 Gamma generating circuits driven by cholinergic and glutamatergic mechanisms coexist in REM sleep networks22,23 and in the cortex,21 where selective suppression of one of these circuits was shown to lead to enhanced activity in the other.24 NMDA and cholinergic mechanisms are also known to interact to modulate cortical arousal.25 Although NMDA antagonists do not induce gamma in vitro by themselves, they enhance gamma oscillations induced by the glutamatergic receptor agonist kainate.26,27 Importantly, this type of glutamatergic gamma oscillation was also potentiated in cortical slices by the selective blockade of the NR2B subunit containing NMDA-Rs.27 It is possible therefore that the increase in gamma power during REM sleep after NR2B blockade reflects a shift from cholinergic to non-cholinergic oscillations involving kainate or metabotropic glutamate receptor mechanisms.
Phasic REM sleep plays an important role in REM sleep-associated cognitive processes.28 Montgomery et al.3 found increased theta and gamma synchrony in the hippocampal trisynaptic circuit during short episodes occurring in REM sleep, and hypothesized that increased output to cortical targets during such events promote memory consolidation and/or the generation of dream content. A similar idea was advanced by Llinas and Ribary.4 They found that the central difference between wake and REM sleep-related gamma oscillations in human was the lack of sensory reset of REM sleep 40-Hz activity, which allowed increased attentiveness to intrinsic activity of the brain in the dream state by excluding external stimuli. They theorized further that similar mechanism may also be found in waking conditions when hallucinations are evoked and proposed a thalamocortical circuit model that involves near 40-Hz resonant circuits in the nonspecific intralaminar thalamic complex, where expression of NR2B (but not NR2A) subunit containing NMDA-Rs were found decreased in schizophrenia patients.29 Functional differences between waking and REM sleep gamma activity were emphasized in a recent human EEG/MEG study, which demonstrated that increased gamma power was associated with uncoupling of fast oscillations between the left frontal executive areas and posterior sensory association regions during REM sleep, specifically during phasic REM sleep episodes.5
In summary, the present findings suggest that by acting on gamma oscillations in a state-dependent manner, NMDA-Rs might have an NR2B subunit-specific role in REM sleep-associated cognitive processes. Alteration of gamma oscillations due to subtype-specific changes in NMDA-R function may have serious implications for cognitive processes and for the development and treatment of cognitive impairment.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Kocsis has received research support from Pfizer Inc.
ACKNOWLEDGMENTS
This study was supported by National Institute of Health Grants MH83199, MH87777 and HL095491. NVP-AAM077 was provided by Dr. Yves P. Auberson (Novartis Institute of BioMedical Research, Basel, Switzerland).
REFERENCES
- 1.Maloney KJ, Cape EG, Gotman J, Jones BE. High-frequency gamma electroencephalogram activity in association with sleep-wake states and spontaneous behaviors in the rat. Neuroscience. 1997;76:541–55. doi: 10.1016/s0306-4522(96)00298-9. [DOI] [PubMed] [Google Scholar]
- 2.Franken P, Dijk DJ, Tobler I, Borbely AA. High-frequency components of the rat electrocorticogram are modulated by the vigilance states. Neurosci Lett. 1994;167:89–92. doi: 10.1016/0304-3940(94)91034-0. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery SM, Sirota A, Buzsaki G. Theta and gamma coordination of hippocampal networks during waking and rapid eye movement sleep. J Neurosci. 2008;28:6731–41. doi: 10.1523/JNEUROSCI.1227-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llinas R, Ribary U. Coherent 40-Hz oscillation characterizes dream state in humans. Proc Natl Acad Sci U S A. 1993;90:2078–81. doi: 10.1073/pnas.90.5.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corsi-Cabrera M, Guevara MA, del Rio-Portilla Y. Brain activity and temporal coupling related to eye movements during REM sleep: EEG and MEG results. Brain Res. 2008;1235:82–91. doi: 10.1016/j.brainres.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 6.Campbell IG, Feinberg I. Comparison of MK-801 and sleep deprivation effects on NREM, REM, and waking spectra in the rat. Sleep. 1999;22:423–32. doi: 10.1093/sleep/22.4.423. [DOI] [PubMed] [Google Scholar]
- 7.Pinault D. N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry. 2008;63:730–5. doi: 10.1016/j.biopsych.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Kittelberger K, Hur EE, Sazegar S, Keshavan V, Kocsis B. Comparison of the effects of acute and chronic administration of ketamine on hippocampal oscillations: relevance for the NMDA receptor hypofunction model of schizophrenia. Brain Struct Funct. 2012;217:395–409. doi: 10.1007/s00429-011-0351-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kocsis B. Differential role of NR2A and NR2B subunits in N-Methyl-D-Aspartate receptor antagonist-induced aberrant cortical gamma oscillations. Biol Psychiatry. 2012;71:987–95. doi: 10.1016/j.biopsych.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manahan-Vaughan D, von Haebler D, Winter C, Juckel G, Heinemann U. A single application of MK801 causes symptoms of acute psychosis, deficits in spatial memory, and impairment of synaptic plasticity in rats. Hippocampus. 2008;18:125–34. doi: 10.1002/hipo.20367. [DOI] [PubMed] [Google Scholar]
- 11.Hakami T, Jones NC, Tolmacheva EA, et al. NMDA receptor hypofunction leads to generalized and persistent aberrant gamma oscillations independent of hyperlocomotion and the state of consciousness. PLoS One. 2009;4:e6755. doi: 10.1371/journal.pone.0006755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer G, Mutel V, Trube G, et al. Ro 25-6981, a highly potent and selective blocker of N-methyl-D-aspartate receptors containing the NR2B subunit. Characterization in vitro. J Pharmacol Exp Ther. 1997;283:1285–92. [PubMed] [Google Scholar]
- 13.von Engelhardt J, Doganci B, Seeburg PH, Monyer H. Synaptic NR2A- but not NR2B-containing NMDA receptors increase with blockade of ionotropic glutamate receptors. Front Mol Neurosci. 2009;2:19. doi: 10.3389/neuro.02.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson MA, Kinsman SL, Johnston MV. Expression of NMDA receptor subunit mRNA after MK-801 treatment in neonatal rats. Brain Res Dev Brain Res. 1998;109:211–20. doi: 10.1016/s0165-3806(98)00084-4. [DOI] [PubMed] [Google Scholar]
- 15.Kopp C, Longordo F, Nicholson JR, Luthi A. Insufficient sleep reversibly alters bidirectional synaptic plasticity and NMDA receptor function. J Neurosci. 2006;26:12456–65. doi: 10.1523/JNEUROSCI.2702-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Z, Chen RQ, Gu QH, et al. Metaplastic regulation of long-term potentiation/long-term depression threshold by activity-dependent changes of NR2A/NR2B ratio. J Neurosci. 2009;29:8764–73. doi: 10.1523/JNEUROSCI.1014-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Engelhardt J, Doganci B, Jensen V, et al. Contribution of hippocampal and extra-hippocampal NR2B-containing NMDA receptors to performance on spatial learning tasks. Neuron. 2008;60:846–60. doi: 10.1016/j.neuron.2008.09.039. [DOI] [PubMed] [Google Scholar]
- 18.Pace-Schott EF, Hobson JA. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat Neurosci. 2002;3:591–605. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- 19.Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–9. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- 20.Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci. 1992;12:4701–11. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopell N, Kramer MA, Malerba P, Whittington MA. Are different rhythms good for different functions? Front Hum Neurosci. 2010;4:187. doi: 10.3389/fnhum.2010.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kezunovic N, Urbano FJ, Simon C, Hyde J, Smith K, Garcia-Rill E. Mechanism behind gamma band activity in the pedunculopontine nucleus. Eur J Neurosci. 2011;34:404–15. doi: 10.1111/j.1460-9568.2011.07766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon C, Kezunovic N, Williams DK, Urbano FJ, Garcia-Rill E. Cholinergic and glutamatergic agonists induce gamma frequency activity in dorsal subcoeruleus nucleus neurons. Am J Physiol Cell Physiol. 2011;301:C327–35. doi: 10.1152/ajpcell.00093.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palhalmi J, Paulsen O, Freund TF, Hajos N. Distinct properties of carbachol- and DHPG-induced network oscillations in hippocampal slices. Neuropharmacology. 2004;47:381–9. doi: 10.1016/j.neuropharm.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Lydic R, Baghdoyan HA. Ketamine and MK-801 decrease acetylcholine release in the pontine reticular formation, slow breathing, and disrupt sleep. Sleep. 2002;25:617–22. [PubMed] [Google Scholar]
- 26.Middleton S, Jalics J, Kispersky T, et al. NMDA receptor-dependent switching between different gamma rhythm-generating microcircuits in entorhinal cortex. Proc Natl Acad Sci U S A. 2008;105:18572–7. doi: 10.1073/pnas.0809302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNally JM, McCarley RW, McKenna JT, Yanagawa Y, Brown RE. Complex receptor mediation of acute ketamine application on in vitro gamma oscillations in mouse prefrontal cortex: modeling gamma band oscillation abnormalities in schizophrenia. Neuroscience. 2011;199:51–63. doi: 10.1016/j.neuroscience.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mavanji V, Datta S. Activation of the phasic pontine-wave generator enhances improvement of learning performance: a mechanism for sleep-dependent plasticity. Eur J Neurosci. 2003;17:359–70. doi: 10.1046/j.1460-9568.2003.02460.x. [DOI] [PubMed] [Google Scholar]
- 29.Ibrahim HM, Hogg AJ, Jr., Healy DJ, Haroutunian V, Davis KL, Meador-Woodruff JH. Ionotropic glutamate receptor binding and subunit mRNA expression in thalamic nuclei in schizophrenia. Am J Psychiatry. 2000;157:1811–23. doi: 10.1176/appi.ajp.157.11.1811. [DOI] [PubMed] [Google Scholar]