Abstract
Study Objectives:
Studies have shown that sleep shelters old verbal memories from associative interference arising from new, more recently acquired memories. Our objective is to extend the forms of interference for which sleep provides a sheltering benefit to non-associative and prospective interference, and to examine experimental conditions and memory strengths for which sleep before or after learning particularly affects verbal memory consolidation.
Design:
Acquiring paired word associates, retention across intervening sleep and wake, training on new, interfering word associates, and test recall of both sets.
Setting:
University laboratory.
Participants:
Healthy volunteers.
Interventions:
N/A.
Measurements and Results:
Comparing recall before and after intervening periods of sleep versus wake, we found that: (i) Sleep preferentially shields weakly encoded verbal memories from retroactive interference. (ii) Sleep immediately following learning helps shelter memory from associative and non-associative forms of retroactive interference. (iii) Sleep protects new verbal memories from prospective interference. (iv) Word associations acquired for the first time in the evening after a day spent in the wake state are encoded more strongly than word associations acquired in the morning following a night of sleep.
Conclusions:
The findings extend the known sleep protection from interference to non-associative as well as prospective interference, and limit the protection to weakly encoded word associations. Combined, our results suggest that sleep immediately after verbal learning isolates newly formed memory traces and renders them inaccessible, except by specific contextual cues. Memory isolation in sleep is a passive mechanism that can reasonably account for several experimental findings.
Citation:
Sheth BR; Varghese R; Truong T. Sleep shelters verbal memory from different kinds of interference. SLEEP 2012;35(7):985-996.
Keywords: Interference, memory consolidation, declarative memory, memory replay, memory reactivation
INTRODUCTION
Studies have shown that there is a modest (of the order of 10% to 20%) benefit of sleep in insulating verbal memories from subsequent associative interference.1,2 The claim is that newly formed verbal memories are vulnerable to interference and rather than providing merely a temporary respite, sleep consolidates memories by stabilizing them, thereby rendering them resistant to subsequently occurring interference.1
The role of sleep in verbal learning and memory was first investigated using the modified modified free recall (MMFR) paradigm by Ekstrand et al. over four decades ago.3,4 Since then, the role of sleep in verbal learning has been intensively elucidated in recent years,1,2,5,6 and a number of interesting findings have resulted. First, newly acquired memories for word associations (stimulus pairs of the form Ai-Ci) were shown to retroactively interfere with memories acquired 12-24 hours earlier (Ai-Bi).2 This form of interference is associative, as the two memories share a common cue word (Ai).
Interference comes in many forms. In the case of associative interference, individual memory traces interfere. Learning a new stimulus pair Ai-Ci interferes with the memory of Ai-Bi learnt earlier, but presumably not that of a non-overlapping pair Aj-Bj. In the case of contextual and task interference, learning new stimulus pairs in a similar context interferes with old memories that were formed the first time the task was run. There is some evidence for a role for sleep in protecting memories from contextual interference. Studies in which both verbal learning and recall during sleep were cued with an odor reported enhanced recall but only if the cue occurred during both learning and sleep, thereby demonstrating that contextual cueing during sleep can trigger memory consolidation.7,8 This view can be extended further by showing that new memories formed in a similar context will interfere with all old memory traces, even if there is no overlap in content between the old and new stimuli. This can be demonstrated experimentally with a verbal memory task as used by Ellenbogen et al.2 using non-overlapping stimulus pairs, i.e. the newly acquired stimulus pairs (C-D) have no words in common with the pairs acquired 12-24 hours earlier (A-B), while task and context remain unchanged from before. Based on the arguments above, one would predict that sleep after A-B learning will protect A-B memories from contextual interference by new C-D memories acquired after sleep. In one such study, A-B and C-D stimulus pairs were acquired back to back within minutes of each other, and it was found that the more recent stimulus pairs (C-D) interfered with the encoding and recall of pairs acquired a few minutes earlier (A-B); A-B and C-D test recall scores declined significantly less if sleep rather than wake followed the acquisition.5 This experiment was designed to examine sleep's influence on interference during memory encoding rather than during consolidation. If a similar outcome, namely a protective effect of sleep, results when C-D stimulus pairs are learnt 12/24 hours after A-B pairs have been acquired and have undergone consolidation, it will go a long way in amplifying and solidifying the insulating role of sleep in a contextual, more generic form of interference.
The forms of interference described thus far are examples of retroactive interference. In contrast, prospective interference (also called proactive interference) is interference from memory traces that were laid down earlier. In the present context, prospective interference means that old A-B memories inhibit the encoding and/or recall of newer A-C memories. The literature thus far shows a curious asymmetry—there are several reports of sleep having sheltered old verbal memories from retroactive interference by new memories, but none, at least to our knowledge, of new memories being sheltered by sleep occurring right before from prospective interference by older memories.
This asymmetry is also reflected in current models for the role of sleep in learning. The preeminent model for the influence of sleep on declarative memory is memory reactivation/replay9,10, which states that memory replay in hippocampal neuronal assemblies during slow wave sleep (SWS) strengthens memories already formed10 and shields them from retroactive interference, but makes no explicit prediction one way or another about prospective interference.
Experimental studies thus far have not been designed to obtain prospective interference, but rather have been designed in such a way that prospective interference is unlikely. Study participants are typically over-trained on new A-C word associates, and we contend that this leads to an A-C memory trace that is strong enough to overcome prospective interference from A-B memories formed earlier (and strong enough to provide strong retroactive interference). The argument implies that if newer A-C memories are encoded less intensely, at least in comparison with older A-B memories, the chances for uncovering prospective interference might enhance. Taking this argument one step further, sleep should have a more pronounced sheltering effect on less intensely encoded new A-C memories from prospective interference than it would on strongly encoded ones.
By the same token, sleep should have a greater sheltering effect on less intensely encoded old A-B memories from retroactive interference (by newer A-C memories), and as memory strength increases, the protection that sleep provides diminishes. A strong prediction of this idea is that when A-B memories are intensely encoded, sleep provides no benefit whatsoever. This prediction and others above are testable.
Our study explores the extent to which sleep protects verbal memories from different forms of interference: associative and non-associative, retroactive and prospective. We vary memory strength and probe the extent to which sleep after encoding protects memory traces of different strength. Finally, the study addresses the extent to which prior sleep influences the strength of verbal memory encoded. Our findings appear to be best explained by a model in which sleep after learning stabilizes the memory by isolating it, renders it less prone to reactivation, and accessible only via specific, select cues.
METHODS
All experiments consisted of two phases: training or acquisition, and testing.
Stimuli
Software for data acquisition and analysis was scripted in MATLAB (Mathworks, Inc.). The initial set of 20 word pairs was obtained from the Harvard group. To generate the remaining word pairs, we used the same methodology as in Ellenbogen et al.2 Briefly, nouns were chosen from the Toronto Word Pool,11 and placed into different groups of 20, matched for imageability, frequency, and concreteness.
Participants
All potential participants completed a screening questionnaire prior to selection. Individuals taking prescription or psychoactive medication were excluded. Participants with known sleep disorders or abnormal sleep patterns, such as habitual sleep onset after 02:00, sleep duration < 6 h, or pathologic sleepiness were excluded. All participants were English speakers. One hundred sixteen volunteers enrolled and successfully completed the study. In brief, there were 4 experimental conditions total. Each experiment typically had 2 (or 3) groups: (i) a Sleep group that acquired the paired associates in the evening (21:00), retained them overnight while they slept, and were tested on their recall the next morning 12 h following the training (09:00); and (ii) a Wake group that learned the paired associates in the morning (09:00), retained them over the course of a typical day during which they were awake, and were tested on their recall of them 12 h following the training (21:00). Each group comprised between 10 and 12 volunteers, depending on the experiment (Table 1 provides demographic details about the participant sample in each experiment). Sleep duration on the nights before and of the experiment were monitored with actigraphy (Actiwatch, Minimitter Inc.) and verified by sleep diaries. Participants were not monetarily compensated for their participation. The study was conducted with the understanding and written consent of each participant and under a protocol approved by the University of Houston Committee for Protection of Human Subjects.
Table 1.
Experimental conditions
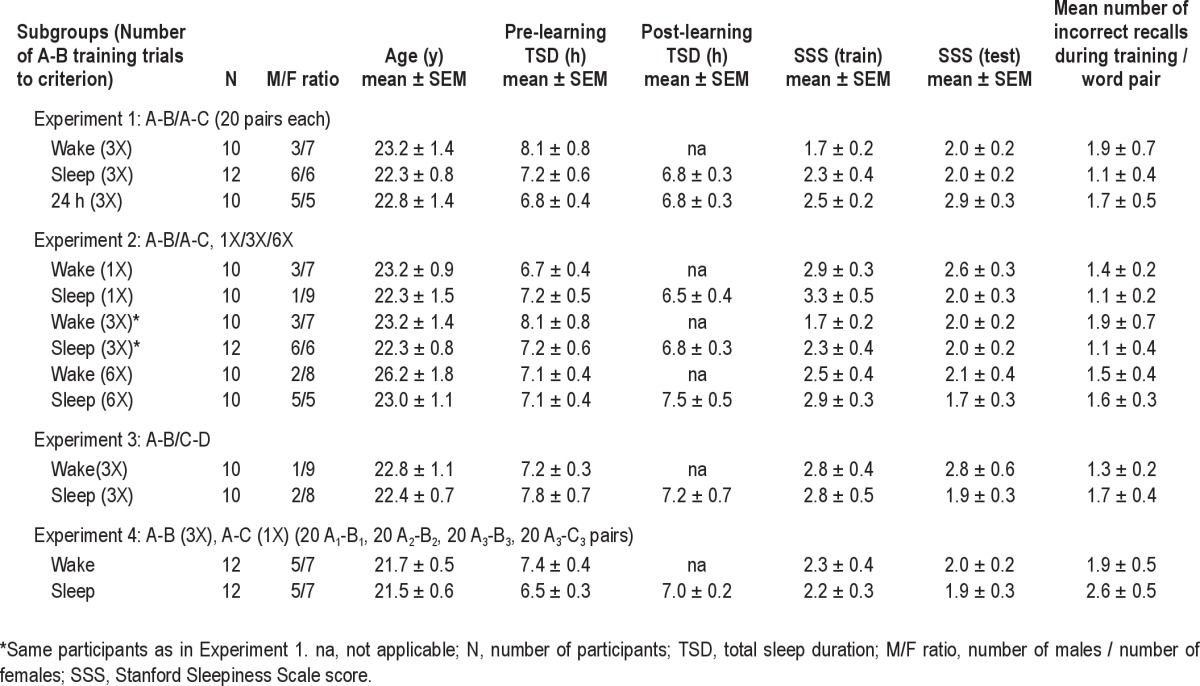
*Same participants as in Experiment 1.
na, not applicable; N, number of participants; TSD, total sleep duration; M/F ratio, number of males / number of females; SSS, Stanford Sleepiness Scale score.
Procedure
The procedures were largely similar to those used in Ellenbogen et al.2 Our experiments typically consisted of 3 phases: study-only training, study-recall training, and testing (Figure 1A). In the study-only training phase, pairs of words (A-B) were presented, one by one, in black at the center of a white screen, in capital letters. Each word pair lasted for 7 s on the screen, and word pairs were presented in the same order for all participants. The second phase of training called the study-recall phase immediately followed the first phase: participants were presented with the first word of each pair (A) and were required to type the second word (B). Following participant response, the correct pairing was displayed on the screen. After an individual pair was correctly recalled X number of times, with X depending on experiment, it was removed from the list. The study-recall phase continued until all word pairs were removed; the learning criterion was thus set to 100% for all participants. After completing this 2-phase training, there was a retention period of 12 h (24 h in one condition), after which participants returned to the laboratory for the testing phase. Participants were provided a list of 20 A words from the A-B pairs, and were instructed to complete the pair (B of A-B). This is known as the cued recall task. The time limit was 6 min. Participants in the interference conditions learned a new list (associative interference: A-C or non-associative interference: C-D) prior to testing; the same training procedure, namely both the study and study-recall phases, was performed as with the original (A-B) list. After training with the new list but before being tested on both the old and new lists, participants performed a 12-min finger-tapping task (FTT) to prevent verbal rehearsal, replicating Ellenbogen's design.2 Following the finger-tapping task, in the associative interference condition, participants were asked to recall the paired words from A-B and A-C lists using the cued recall task as described above (i.e. B of A-B and C of A-C). Morphological errors (e.g., ``fathers'' instead of ``father'') were counted as correct. Only those words that were recalled and identified with the correct cue word (A) and placed in the correct list (B column if learned before the 12-h delay or C column if learned after the delay) were counted as accurate. In the non-associative interference condition, participants were asked to recall the paired words from A-B and C-D lists (i.e., B of A-B and D of C-D). Note that the A-B and C-D lists share no words in common. Only those B and D words that were recalled and identified with the correct cue word—A or C, respectively—were counted as accurate. All training and testing were administered on a computer with the Psychophysics toolbox12,13 utilizing MATLAB software (Mathworks, Inc.).
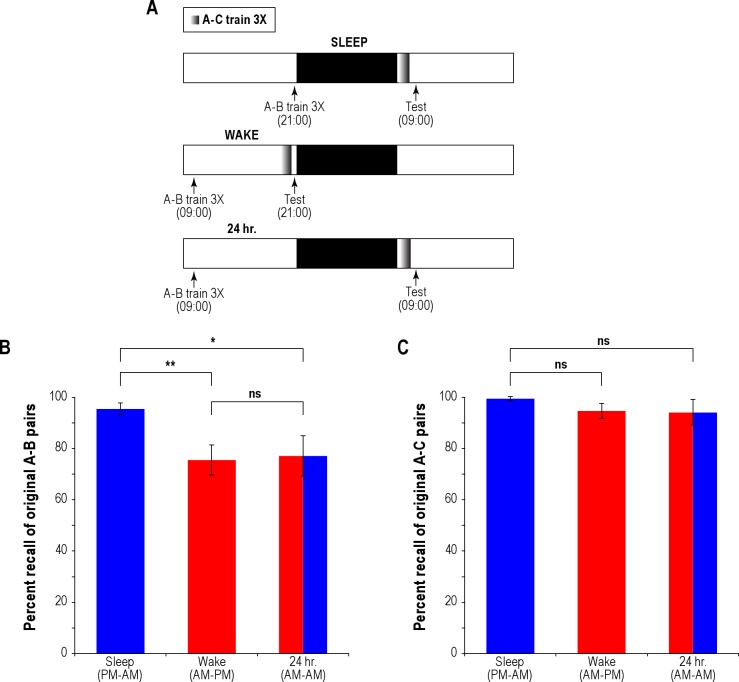
Figure 1.
Experiment 1: Sleep, time of day affect verbal memory. (A) Experimental design is shown for 3 independent groups of participants. All participants learned a list of paired associates (schematically represented as A-B) and following a 12/24-h intervening period, which included either sleep or wakefulness, learned a second, interfering list (A-C) and immediately following, were tested on their memory for both lists in a cued modified modified recall design. (B) Percent test recall of A-B word pairs for the Sleep, Wake, and 24-h groups. (C) Percent recall of A-C word pairs for the Sleep, Wake, and 24-h groups. The bars represent one standard error of the mean (SEM). *P < 0.05, **P < 0.01; ***P < 0.001; ns: not significant. Means, standard errors of the mean, and numerical values are presented in untransformed form in this and all figures.
Given that our participants run an FTT immediately after learning the second list of word pair associates and just before recall testing, it should be noted that procedural learning right after word-list learning can cause a decrease in word recall across an intervening 12-h period of wake, but not sleep.14 However, a more careful study reveals that these results are not of concern to us, as the relative timings of the 2 tasks in our study preclude motor learning from affecting word recall: If motor learning occurs ≥ 4 h after word acquisition, word recall is unaffected14; here, the FTT occurred 12 h after A-B acquisition. If the recall test occurs soon after the motor learning, recall performance is unaffected14; here, recall occurred right after the FTT. Therefore, it is unlikely for the FTT to have affected A-B or A-C recall in our study.
Data Analysis and Statistics
Statistical analysis for recall performance was conducted following arcsine transformation, which converts a binomial random variable to an approximately normal one with uniform variance independent of probability correct (transformed data = arcsin[sqrt(probability correct)]). Unless otherwise specified, heteroscedastic (unequal sample size, unequal variance) t-tests and random effects ANOVA tests were used to assess statistical significance across group and paired t-tests to assess performance within group. Significance is a P-value ≤ 0.05.
RESULTS
Experiment 1: A-B, A-C Paradigm
Participants first learned a list of word pair associates (A-B) with 100% recall performance in the training phase (3X, i.e., each paired associate was successfully recalled in the study-recall phase 3 times). The Sleep group (n = 12) acquired the A-B word pairs at 21:00 and was tested 12 h later at 09:00. The Wake group (n = 10) acquired the word pairs at 09:00 and was tested the same evening 12 h later (Figure 1A). Thus, the Wake group stayed awake over the 12-h retention period, whereas the Sleep group slept for a large portion of it. Finally, in order to sort out the relative importance of sleep versus time-of-day effects on verbal memory, we ran an additional third group (24 h; n = 10) that learned the word pairs at 09:00, and was tested at the same time (09:00) the next day, following a night of sleep. Participants acquired a second list of word pair associates (A-C) just prior to testing (3X), and memory for both the original (A-B) and new (A-C) word pair lists was tested in a modified modified free recall (MMFR) design.
Pairwise statistical comparisons using heteroscedastic t-tests revealed that mean test recall of the Sleep group (mean ± SD, 95% ± 8%) was significantly higher than that in the Wake group (76% ± 20%, P = 0.009; Figure 1B); mean recall of the 24-h group (77% ± 25%) was nearly identical to that of the Wake group (P > 0.8), and significantly worse than that of the Sleep group (P = 0.026; Figure 1B). The higher level of test recall performance of the Sleep group as compared to that of the Wake group is consistent with the Ellenbogen study.2 The reduced test recall of the 24-h group as compared to that of the Sleep group apparently runs counter to the Ellenbogen study, which found that their 24-h and Sleep groups performed better at test than their Wake group. It is important to note, however, that the 24-h group of the Ellenbogen study acquired the stimulus pairs and recalled them during evenings, whereas our 24-h group acquired the stimulus pairs and recalled them on mornings. Notably, unlike the Sleep group, the 24-h group did not go to sleep right after acquisition (and the Wake group never slept during the retention period); moreover, unlike the Sleep group that acquired the A-B paired associates in the evening, the Wake and 24-h groups acquired the paired associates in the morning. Thus, the results of Experiment 1 could be interpreted to imply that the time of day of acquisition is critical—more specifically, that encoding verbal memories would be more reliable in the evening than in the morning; alternatively, the results imply that sleep must immediately follow verbal memory acquisition in order for it to provide an effective shelter to the labile memory. Both interpretations have been raised earlier to explain previous findings.6
Test recall of the newly acquired list of A-C stimulus pairs showed high, near-ceiling rates of recall in all 3 groups (Figure 1C). Participants comprising the Sleep group successfully recalled 99% ± 3% of all A-C pairs, whereas participants of the Wake group recalled 94% ± 9%, and the difference in A-C test recall between the groups did not reach significance (P = 0.154). The 24-h group successfully recalled 94% ± 16% of all A-C pairs at test, and there was no difference in A-C recall performance between the 24 h and Sleep groups either (P = 0.322). In sum, we did not find a significant influence of immediate, prior sleep on A-C recall, although the near-ceiling rates of recall could have contributed to the lack of significance (see footnote following article).
Experiment 2: 1X, 3X, and 6X
Participants in Experiment 1 were required to successfully each word associate 3 times during the study-recall phase, which means that they had to undergo rather intense memory encoding. One way to further strengthen the emerging memory trace is by additional rehearsal, which will later show up as enhanced recall at the time of testing. This was achieved in Experiment 2 by systematically varying the required number of successful trials to criterion during training. Three groups (n = 62 total) participated and had to correctly recall each word associate 1, 3, or 6 times (1X/3X/6X), respectively, during training (study-recall phase). The amount of required rehearsal sets the level of memory encoding. Participants who had to correctly recall each word associate 6 times during training presumably formed a stronger memory trace than those that had to correctly recall each paired associate only once. One subgroup remained awake throughout the 12-h intervening retention period (Wake subgroup), while the second subgroup slept during a major portion (~7 h; Sleep subgroup) of the intervening period (Figure 2A). Following the intervention, participants acquired an interfering list of new paired associates of the form A-C and recall on both lists—A-B as well as A-C—were investigated (the 3X participants are Sleep and Wake groups from Experiment 1).
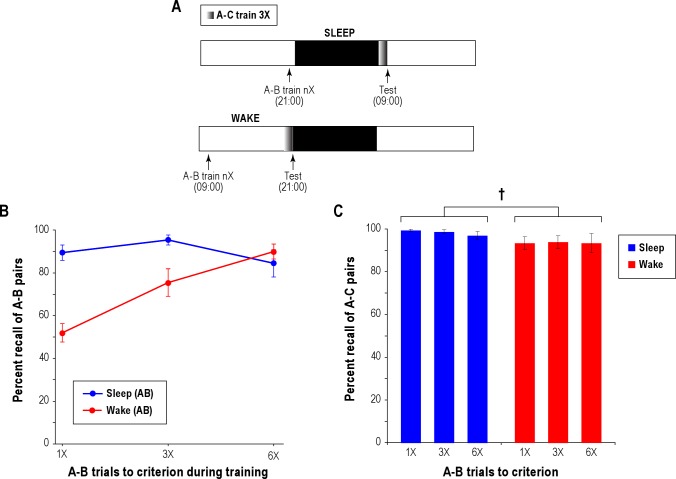
Figure 2.
Experiment 2: Sleep protects weakly encoded memories from interference. (A) The experimental design is similar to that in Experiment 1, with the exception that there were 6 groups of participants, 3 each in the sleep and wake conditions. Subgroups had to correctly recall each associate (A-B) in the second phase of training either n = 1, 3, or 6 times (1X/3X/6X). (B) Percent recall of A-B word associates at test 12 h following the initial training as a function of the number of learning trials to criterion following an intervening period of sleep or wake. The number of trial to criterion at the time of learning is related to the degree of memory encoding. Statistical analysis revealed a highly significant group (Sleep/Wake) × trials to criterion (1X/3X/6X) interaction (see text for details). The bars indicate one standard error of the mean (SEM). (C) Percent recall of newly learned A-C word associates at test in sleep versus wake. The trials to criterion for the A-C stimulus pairs was 3X for all groups. †Indicates marginal significance (0.05 < P < 0.1).
First, we report the results of recall tests on the original list A-B (Figure 2B). Overall, rehearsal appeared to enhance recall if the retention period did not include sleep (1X – 52% ± 14%; 3X – 76% ± 20%; 6X – 90% ± 11%), whereas rehearsal had little effect if the retention period did include sleep (1X – 90% ± 11%; 3X – 95% ± 8%; 6X – 85% ± 20%). A statistical comparison of performance using a 2-way random-effects ANOVA did not find a main effect of subgroup (Sleep vs. Wake – F1,56 = 2.73, P = 0.240) or of the number of trials to criterion during training (1X/3X/6X correct recalls – F2,56 = 0.91, P = 0.523). However, the analysis did reveal a highly significant subgroup × criterion interaction (F2,56 = 6.97, P = 0.002). One-way ANOVAs further revealed a significant effect of the number of trials to criterion for the Wake subgroup (F2,27 = 12.49, P = 0.0001) but not for the Sleep subgroup (F2,29 = 1.70, P = 0.200). Post hoc t-tests demonstrated a significant (P < 0.0001) sleep benefit when a single correct recall of each A-B paired associate during training (1X) was required. The sleep benefit persisted, albeit to a reduced degree, when 3 correct recalls (3X) were required at the time of learning (P = 0.009; Figure 1B), but disappeared entirely when 6 correct recalls (6X) were required (P = 0.71; Figure 2B). The convergence in A-B recall of the Sleep and Wake subgroups could not be ascribed to the idea that the Sleep subgroup performed at ceiling and therefore, additional rehearsal during training could not boost memory any further, because test recall of the Sleep subgroup on each of the 3 criterion conditions (1X/3X/6X), while high, was significantly lower than 100% (P < 0.05 in each).
Next, we report the results of recall tests on the new, interfering word list A-C (Figure 2C). The percentages of A-C paired associates successfully recalled by the participants who acquired the A-C paired associates in the morning (Sleep subgroups; 1X – 100% ± 2%; 3X – 99% ± 3%; 6X – 97% ± 6%) were about 5% higher on average than those of their evening (Wake subgroups) counterparts (1X – 94% ± 9%; 3X – 94% ± 9%; 6X – 94% ± 14%). A two-way random-effects ANOVA on A-C paired associate recall confirmed a marginally significant main effect of subgroup (Sleep vs. Wake – F1,56 = 10.55, P = 0.083), an expectedly insignificant effect of trials to criterion of the A-B paired associates (F2,56 = 0.09, P = 0.920), and an insignificant subgroup × criterion interaction (F2,56 = 0.48, P = 0.619).
Summarizing both sets of results, the rehearsal of A-B paired associates during training improved their recall at test if participants remained awake during the intervening period but had negligible effect if the intervening period included a night of sleep; acquiring and remembering new A-C paired associates was slightly more reliable for participants in the morning than in the evening. From these findings, one can conclude that sleep has a greater sheltering effect on weakly encoded rather than strongly encoded memories from retroactive associative interference; furthermore, acquiring new interfering memories is somewhat more reliable early in the morning following sleep than in the evening, which means that sleep probably shields new memories from prospective interference.
Experiment 3: A-B, C-D Paradigm
In past studies and in our experiments thus far, the cue words (Ai) of the original (Ai-Bi) and new (Ai-Ci) lists were the same. Thus, the degree of interference between the 2 lists was high because of the common cue word. The degree of interference can be reduced by the adoption of an A-B, C-D paradigm,15,16 in which the second list of paired associates (C-D) shares no words in common with the original (A-B; Figure 3A). All participants in this experiment underwent the same screening, training and testing procedures as the participants in Experiment 1. In particular, 3 trials for criterion remained the requirement for A-B and C-D paired associates.
Figure 3.
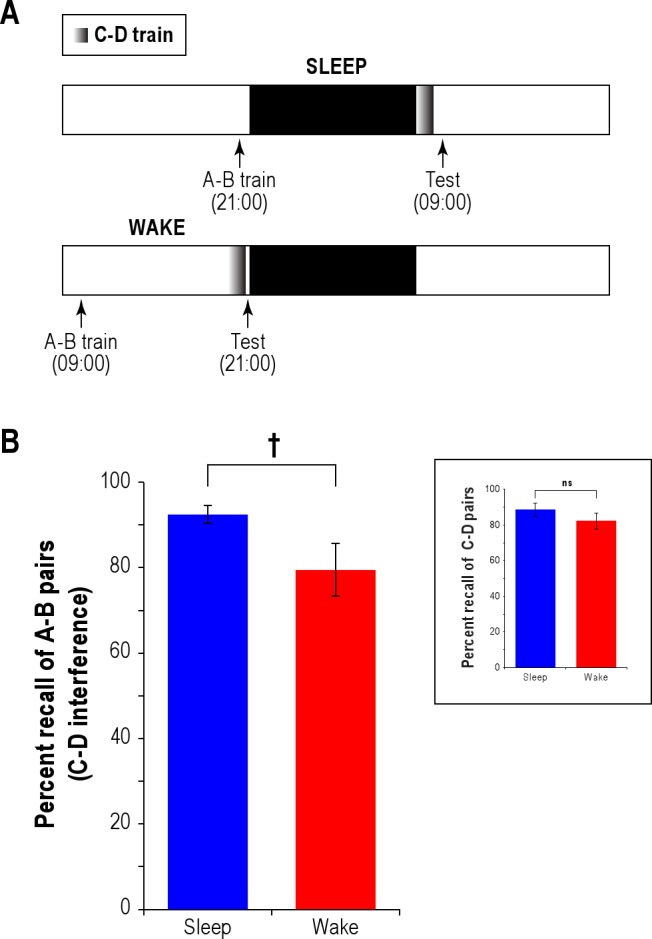
Experiment 3: Sleep protects verbal memory from non-associative interference. (A) Experimental design is nearly identical to Experiment 1 with the following exception: Just before testing, participants acquired a second list (C-D) that had no words in common with the first (A-B). (B) Percent test recall of A-B stimulus pairs for Sleep and Wake groups of participants is shown. Inset shows percent test recall of C-D stimulus pairs for the same 2 groups. †0.05 < P < 0.10; ns, not significant. The bars represent one standard error of the mean (SEM).
The results, illustrated in Figure 3B, were largely similar to those of Experiment 1 as well, in that there was a sleep benefit for memories acquired prior to sleep: the Sleep group (n = 10; 93% ± 7%) still recalled more A-B associates than the Wake group (n = 10; 80% ± 20%; Figure 3B), and the difference was marginally significant (P = 0.076). The magnitudes of the sleep benefit in the associative interference (Figure 1B, A-B→A-C; 19%) and non-associative interference (Figure 3B, A-B→C-D; 13%) conditions were not statistically distinguishable (P > 0.1). On the other hand, the acquisition of new C-D paired associates appeared to be unaffected by prior sleep (Sleep: 96% ± 13%; Wake: 99% ± 3%; P = 0.640; see Figure 3B inset), although the extremely high recall rates of both groups hint at a ceiling effect—perhaps because of overtraining (3X)—that may have precluded a sleep benefit on prospective interference. In sum, the results of Experiment 3 suggest that sleep shelters verbal memories from non-associative retroactive interference in addition to associative interference.
Experiment 4: 60 A-B Stimulus Pairs (3X), 20 A-C Stimulus Pairs (1X)
A powerful way to test the influence of sleep on memory encoding, retroactive and prospective forms of interference in a single experimental design is to adopt a paradigm derived, in part, from Ellenbogen et al.1 Participants initially acquired 60 A-B associate pairs (Figure 4A). Following the training, recall memory of the B word (1) immediately after the training (A1-B1), (2) 12 h after the training (A2-B2), and finally, (3) after acquiring the interfering list A3-C3 (A3-B3), was examined and compared in 2 groups—Sleep and Wake. Notably, participants were required to successfully recall all 60 A-B paired associates thrice (3X) during training, which is likely to lead to moderate to strong encoding. On the other hand, participants were required to successfully recall A-C paired associates only once during training, which is likely to cause a relatively weaker encoding of A-C paired associates and concomitantly somewhat milder degree of retroactive interference of the earlier A-B memories. This manipulation allowed us to study prospective interference and the role of prior sleep.
Figure 4.
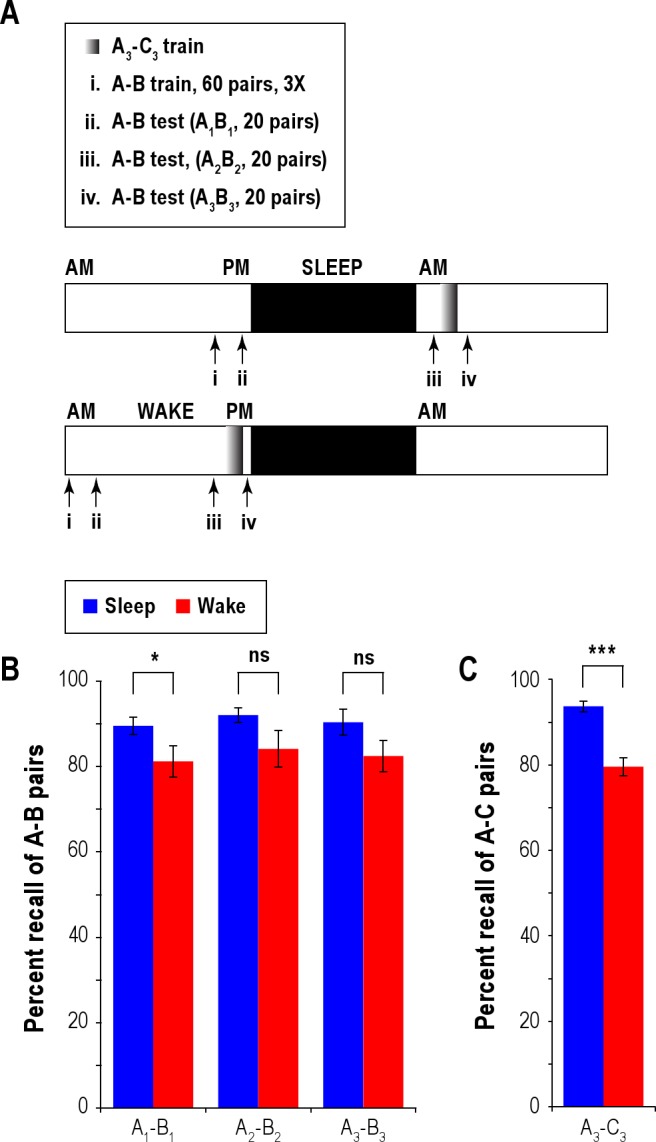
Experiment 4: Time of day of acquisition affects memory encoding; sleep protects memory from prospective interference. (A) Experimental design is shown. Participants learned 60 paired associates (A-B). In the second phase of learning (anticipation-plus-study), participants were required to recall the correct associated B-word of the A-B pair 3 times (3X). Immediately following the training, memory for 20/60 word associates (A1-B1) was tested in a cued recall task identical to previous experiments. Twelve hours later, following an intervening period of wake or sleep, a second recall test was conducted with a different sublist of 20 word associates (A2-B2). Participants next acquired a new list of 20 word associates (A3-C3). Participants were required to correctly recall the associated C-word of the A3-C3 pair once in the second phase of learning. Next, participants were provided a third set of A3-cues and had to recall associates from the old (A3-B3) and the newly learned (A3-C3) lists. (B) Percent test recall of A-B stimulus pairs in Sleep and Wake groups at the 3 different times indicated above is shown. (C) Percent test recall of A-C stimulus pairs for Sleep and Wake groups is shown. Error bars are one SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ns, not significant.
Figure 4B shows the test-retest performance of the Sleep (blue bars) and Wake (red bars) groups. A mixed-model 2-way ANOVA, with test-retest as the within-subject factor and group as the between-subject factor, revealed that the effect of group nearly reached the threshold of statistical significance (F1,22 = 4.210, MSe = 0.072, P = 0.052, partial eta-squared = 0.161); on the other hand, neither the effect of test-retest (initial test/re-test/second re-test) (F2,44 = 1.434, P = 0.249) nor the interaction between the two factors (F2,44 = 0.085, P = 0.919) was significant. The fact that there was a main effect of group but no interaction across the 3 rounds of tests suggests that the gains were present before the sleep/wake manipulation. Indeed, on the initial test (Figure 4B: AB1) right after A-B pairs were acquired and learned but before the sleep/wake manipulation, the Sleep (pm test) group (90% ± 7%) recalled a higher number of the cued associate B-words than the Wake (am test) group (81% ± 12%; P = 0.039, 2-tailed t-test), who had had a night of restful sleep before acquisition. The between-group difference in post-acquisition test performance suggests that verbal memory encoding differs as a function of either the time of day when the stimulus pairs are initially acquired (pm > am), or the timing of sleep relative to the acquisition (sleep before reduces the initial strength of the trace). On the second test conducted 12 h later with a different 20/60 word associate pairs (A2-B2) than those used on the first test (A1-B1), the percentage of cued associate B2-words recalled by the Sleep group (92% ± 6%) remained somewhat greater than the Wake group (84% ± 15%), but the difference was not higher than that before the manipulation. Moreover, test recall after sleep (92% ± 6%) was no better than that before (90% ± 7%, P = 0.32, paired t-test; Figure 4B). On the basis of the above findings, it appears that sleep after learning did not benefit memory retention in this particular experiment. On the final test, which occurred after the interfering A3-C3 list was acquired, the Sleep group recalled 90% ± 10% and the Wake group 83% ± 13% of the A3-B3 paired associates, and the difference in performance (7%) again did not exceed that prior to the sleep/wake manipulation (8%). In summary, the results suggest that verbal memories are better encoded in the evening than in the morning—this is in line with Experiment 1—and that a strongly encoded memory is stable and intervening sleep provides little additional benefit—this is in line with Experiment 2. The findings are in contrast to those of Ellenbogen et al.,1 who did find a sleep benefit in an experimental design similar to ours. It is worth noting, however, that participants in the Ellenbogen study were required to recall, during training, the A-B list only once as opposed to thrice in the present study; it is likely that the shallower memory encoding in the Ellenbogen study contributed to the discrepancy in finding.
The results on A-C paired associate recall revealed a difference between the 2 groups (Figure 4C). The Sleep group (94% ± 4%) recalled a higher number of the cued associate C-words than the Wake group (80% ± 7%), and the difference was highly significant (P < 0.0001). It should be noted that the recall test immediately followed the A-C acquisition, and therefore A-C test recall in effect tests memory encoding. It bears mention that our participants were required to successfully recall the interfering A-C list only once during training, which arguably may not be as strongly interfering as when participants were required to successfully recall the A-C list 3 times, as was the case in our Experiments 1 and 3. In sum, the encoding of the interfering A-C list was superior in the Sleep group compared to the Wake group, and is in accord with a similar finding from Experiment 2. Put another way, sleep protects new verbal memories from prospective interference.
DISCUSSION
Our study examined the role(s) of sleep in the consolidation of verbal memory, namely memory for novel paired word associates using a modified modified free recall design (MMFR),3,4 by comparing test recall of cued associates before and after intervening periods of sleep versus wake. Our study replicates previous works on sleep and verbal memory1,2,5,6 and extends them by examining the extent to which sleep shields verbal memories from different kinds of interference and for differing strengths of the memory trace. Our findings confirm past reports that sleep protects memory from associative interference, but also demonstrate that sleep protects against interference in general, not just associative. In particular, we show that sleep insulates old memories from non-associative interference arising from newly acquired memories that share no words in common with the old, and sleep shelters newly formed, labile memories from prospective interference arising from strong, established memories acquired earlier. In addition, we demonstrated that the sheltering effect of sleep from retroactive interference decreases with increasing strength of encoded memory and vanishes for very high memory strengths. Finally, we found a modest but significant effect of prior sleep and/or of the time of day when verbal memories are acquired: Paired associates acquired for the first time in the evening are encoded somewhat more strongly than those acquired in the morning following a night of sleep.
In the remainder of the discussion, we will discuss each of our findings, namely circadian effects, effect of sleep on weak and strong memories, the effect of sleep in sheltering memories from non-associative (retroactive) interference, and the effect of sleep in sheltering memories from prospective interference. Finally, we will critically evaluate evidence to date on memory rehearsal/replay of declarative memories in humans and offer a simple framework of memory isolation to account for findings.
Circadian Effects?
Our experiments revealed a modest effect of either the time of day or prior sleep on the level of memory encoding. Participants in Experiment 1 who acquired A-B stimulus pairs in the morning following a night of sleep, and had to recall them either 12 or 24 hours later (which would include a night of sleep) recalled similar numbers of A-B stimulus pairs but significantly fewer numbers of pairs than participants who acquired them in the evening. The result could be interpreted as indicating either that the encoding of novel word associations is affected by the time of day when they are acquired, or that the encoding of new memory is weaker following sleep. Even more directly to this point, participants in Experiment 4 that acquired the original A-B list in the evening recalled significantly greater paired associates immediately afterwards than those who acquired and recalled them after a night of sleep in the morning. Here, there was no interval between acquisition and recall and therefore no intervening manipulation. Therefore, the time of day (evening vs. morning) is an attractive variable to account for the differing amount of memory. However, it must be pointed out that our morning participants acquired stimulus pairs immediately following a night of sleep, whereas those in the evening had been awake throughout the day before acquiring the new memories. This fact holds true across the studies that have found an inkling of a circadian effect: Gais et al.6 have also found a similar effect of time of day and in the same direction (forgetting is reduced if learning occurs in the evening); however, unlike our study, theirs did not assess memory right after learning, allowing other factors to possibly influence performance. To our knowledge, there have been two other studies that have data on circadian effects on verbal memory: Drosopoulos et al.5 studied immediate recall in groups who learned A-B stimulus pairs in the morning or evening and found a small but insignificant effect of learning time on recall 10 minutes later (P = 0.20) and, of importance, in the same direction as us (see page 175 and Figure 2 of the citation). Ellenbogen et al.1 studied immediate recall in both the evening and morning learning groups and stated that the P-value of the circadian effect on initial testing at 10 minutes was greater than 0.2 (page 3 of the citation), although the direction of the effect (evening > morning, as we expect) is not provided. In sum, the time of day at which the original A-B stimulus pairs are acquired could play a role, although our finding (and the marginal findings of others) can also be explained by positing that the encoding of new verbal memories following a night of sleep is somehow weaker and less reliable. Indeed, we do not claim that all (or even most) findings of sleep benefit on verbal memory recall are accounted for by circadian variation at the time of learning—Gais et al. have shown that the sleep benefit remains even when groups acquire stimulus pairs at the same time of day but differ in whether they sleep or stay awake after the learning. The alternative possibility, namely that memory encoding following sleep is less reliable than following a period of wake, is an equally surprising one: future experiments must resolve this issue.
Sleep Protects Weakly Encoded Memories—Evidence of an Active Sleep-Dependent Mechanism?
Experiment 2 explicitly tested the idea of whether weakly encoded memories are selectively stabilized during sleep and obtained an affirmative answer. This issue was examined earlier in a somewhat less rigorous way by Drosopoulos et al.5 using an A-B, C-D paradigm (A-B and C-D stimulus pairs were learned within minutes of each other in their study, which places demands on immediate short-term memory and not on the reconsolidation of memory from long-term stores). Weak encoding was ensured by enforcing a learning criterion of only 60% correct responses and shortening the duration of feedback presentation of the stimulus pairs. Test recall of weakly encoded A-B and C-D stimulus pairs was found to be higher following a night of sleep than after a similar period of wake, and the sleep benefit was reduced (but not eliminated) for strongly encoded stimulus pairs. Our results, and theirs to some extent, suggest that sleep benefits weak verbal memories, regardless of whether the weak memory trace was achieved because of strong associative interference or owing to a less stringent learning criterion.
How does sleep preferentially preserve weakly encoded memories? One possibility is via an active mechanism: Weakly encoded memories are preferentially reactivated in sleep.5 In order to establish this mechanism, a reasonable account of how the sleeping brain recognizes memory strength is required. Even though studies have shown some reactivation of hippocampus based memories in sleep in humans,8,10 there is no evidence as yet, at least to our knowledge, that weakly encoded memories are selectively reactivated in sleep, as behavioral studies appear to suggest. In theory at least, there is a passive alternative mechanism to account for the behavioral findings. One can reasonably posit a threshold of memory strength below which memories are not readily accessible at retrieval and above which, they are. Over time during wake, memories—weak and strong—deteriorate. Weakly encoded memories were just above the putative retrieval threshold after learning, but the inevitable deterioration over a prolonged period of wakefulness causes the weak memories to fall below threshold, rendering them inaccessible. Strongly encoded memories deteriorate in wake as well, but still remain above retrieval threshold. Thus, following wake, the recall of weakly encoded memories declines, while that of strongly encoded memories does not; in sleep by contrast, memories do not deteriorate, perhaps due to memory reactivation in sleep, memory isolation in sleep (which we will look at below), or via some other as yet unknown candidate mechanism. Thus, this line of reasoning can explain a more pronounced sleep benefit for weakly rather than strongly encoded memories without having to posit an active selection mechanism. Clearly, questions about the neural basis for the threshold, neural substrates unique to wake that cause memory deterioration and others remain. Nonetheless, we hope that a serious consideration of alternative theories may trigger research into alternative brain mechanisms for the stabilization of weak memories in sleep.
Sleep Shelters Verbal Memory from Non-Associative and Associative Interference
Unlike Experiments 1, 2, and 4, there were no words in common between old (Ai-Bi) and new (Cj-Dj) stimulus pairs in Experiment 3, and therefore, no direct cues to trigger interference. However, associative and non-associative experiments are similar at some level—the tasks (MMFR) are identical, and the stimuli are words (not letters, numbers, or combinations of vowels and consonants, which have been used in past studies15,16). Past studies have shown that intrusions are likely in an AB-CD paradigm mainly when the response classes in AB and CD are similar16 (words in our experiments). Thus, the task and materials (non-associated word pairs in the two cases) are perhaps similar enough that a new C-D triggered trace may reactivate an older one simply due to the common context of the task. In other words, even if the interference is non-associative, it is interference nevertheless, and one that presumably reactivates the older trace at a lower intensity. Thus, specific cues are not required in wake to trigger interference. Contextual cues will suffice instead. In this context, our finding of a sleep benefit in the AB-CD paradigm (and to a similar level as that in the AB-AC paradigm) generalizes the role of sleep in verbal memory: the insulation that sleep provides from interference is not naturally at the level of individual memory traces but rather to the set of memories as a whole (but can be specific to individual memories when cued during sleep in laboratory conditions17). Put another way, the natural shelter that sleep affords to memories appears to be somewhat general (An example of generic protection is preserved access to the set of retrieval cues to the entire memory set in sleep, but lost or decaying access across wake).
Sleep Shelters Verbal Memory from Prospective as well as Retroactive Interference
Studies thus far have reported evidence for retroactive interference, i.e., new A-C pairs in memory inhibit memory for old A-B pairs. However, in our study and of others before, A-C stimulus pairs are typically learned 12-24 hours after the original A-B pairs, and the common cue word ensures the potential for mutual interference. Given that retroactive interference of verbal memories has been observed several times, prospective interference is at least possible, namely that A-B memories will interfere with the new A-C memories. Experiment 2 to some extent and Experiment 4 in particular show that sleep shelters new memories from prospective interference and old memories from retroactive interference.
At this juncture, it is important to ask what the underlying cause is for the effect of sleep on prospective interference in Experiment 4. One possible cause could be differential sleep pressure. A second one is sleep inertia: participants who acquired new A-C paired associates in the morning were not fully alert and functional, and hence were not good learners. There are several points that run counter to both possibilities, however. First, the self-reported Stanford Sleepiness Scale scores of the participants who acquired the A-C pairs in the morning (1.9 ± 0.3) versus in the evening (2.0 ± 0.2) did not indicate greater sleepiness (lower scores indicate self-reported more alertness; P = 0.79). Second, the mean number of A-C trials to criterion, namely the amount of training it took, for morning (1.3 ± 0.2) and evening learners (1.0 ± 0.3) was statistically indistinguishable (P = 0.51) as well. Third, our participants had been awake for > 1 hour before running (and some had even driven themselves to the test site just prior); therefore, sleep inertia ought to have been minimal, if at all. In brief, neither sleep pressure nor sleep inertia can account for why sleep shelters memory from prospective interference in Experiment 4.
However, a positive finding of shelter from prospective interference in our hands begs the question: why no past reports in the literature? Prospective interference, while found to be highly significant in our hands, has not yet been reliably replicated, and we need to find the conditions under which prospective interference occurs reliably. Note that even we ourselves did not find any signs of prospective interference in Experiment 3 while we did for the retroactive form; it is safe to suggest that retroactive interference is the more common of the two forms. This hints at the possibility that there is some key difference between Experiment 4 on the one hand and Experiment 1-3 on the other (and past studies as well). It is worth mentioning that the experimental procedures, instructions and conditions in Experiment 4 did not differ from those in the other experiments. However, there is one difference between Experiment 4 and Experiments 1-3 that could be critical: the encoding strengths of A-B and A-C stimulus pairs. In this regard, we contend that the somewhat unusual learning criteria of Experiment 4 that may have helped uncover prospective interference. First, A-B memories were strong: 60 A-B pairs were rehearsed to 100% criterion three times during training and recalled twice afterwards, and sleep following training did not benefit A-B recall, which suggests that A-B memories were strongly encoded; Second, A-C memories were weak: A-C stimulus pairs were rehearsed to criterion only once. Thus, strong, stable A-B memories competed with relatively weak, labile A-C memories in Experiment 4—a design that is a distinct departure from our own Experiments 1-3, as well as from previous studies. Moreover, if one were to extend the idea of a sleep shelter from memories formed before sleep to memories that will form soon after sleep, it would account for a sheltering role from prospective interference for sleep in Experiment 4 (it could also be that the two forms of interference are mutually exclusive, i.e., cannot both occur in the same design for the reason that old and new memories cannot both be strong or weak at the same time). In the next section, we provide a speculative but plausible framework for findings in this exciting area, and in so doing, offer an account for how sleep before learning could protect de novo memories from prospective as well as retroactive interference.
Memory Replay and Isolation in Sleep
Memory reactivation in sleep is the preeminent model of memory consolidation in sleep. According to this model, memories are reactivated, at least partially, in hippocampal structures during sleep and these memories get progressively transferred to neocortical structures in sleep.9,10,18 The model can account for at least some of our findings, e.g., that lists that do not share any stimulus pairs in common interfere as well, and that sleep insulates against this non-associative form of interference. The model explains this result by positing that memory replay in sleep strengthens the memory, thereby overcoming decay from both specific (associative) and generic (non-associative) forms of retroactive interference. The model can also explain our finding that sleep shields weakly encoded memories from retroactive interference by positing that weak memories are preferentially reactivated in sleep, although one would then have to additionally posit a sleep-dependent mechanism that actively chooses only the weak memories for reactivation. Specifically, it needs to be shown that weaker verbal memory traces are reactivated more during sleep than stronger ones in the same individual. To our knowledge this has not been shown as yet; rather, a positron emission tomography (PET) imaging study of motor learning showed a strong correlation across individuals between learning level prior to sleep and reactivation of the right cuneus during REM sleep.19 That is to say, stronger memory traces are reactivated more in sleep than weaker ones, contrary to prediction. Also discussed above, a passive threshold model can parsimoniously explain this finding without having to assume the existence of active mechanisms in sleep. Moreover, memory reactivation explains how sleep stabilizes old memories, not how sleep protects new memories; therefore, memory reactivation is somewhat silent when it comes to explaining our finding that prior sleep shields newly acquired, labile memories from prospective interference.
Next, we will examine the evidence for memory reactivation in human sleep. A recent PET study of spatial navigation learning in a sample of six healthy humans found a significant correlation between increase in regional cerebral blood flow in the right hippocampus and parahippocampal gyrus during SWS (versus wake) and overnight enhancement in spatial navigation.10 The result is in line with the idea that (spatial) memories are reactivated in SWS, but it could also reflect residual activity in hippocampal neuronal assemblies used previously for encoding, especially given that early sleep is rich in SWS (the authors did not look for a correlation between overnight improvement in spatial navigation and hippocampal activity while participants initially learnt the task, but see Peigneux et al.20). Unfortunately, a similar study has not been conducted, at least to our knowledge, on verbal learning. In this regard, it is worth noting that overnight enhancement in paired associate recall has not been observed by us or by others; therefore, the putative correlation between brain and overnight verbal memory improvement may be harder to find. Recent studies that used odor7,8 and sound17 cues in human SWS to trigger spatial memories that were acquired earlier during wake in the presence of the identical cue found an improvement in post-sleep memory performance. These elegantly designed studies suggest that if spatial memories are reactivated in (slow wave) sleep, memory will strengthen. It is worth noting in this regard that there was no sleep benefit when memories were cued during learning but not during SWS or vice-versa. Thus, in order for these outcomes to be extended to the real world, it must be shown that similar cueing occurs as a matter of course during both learning and SWS outside of the laboratory. Again, it is important to note that these were studies of spatial memory and the results have not been replicated for verbal learning.
Nevertheless, there is some evidence from human studies supporting the idea that SWS is crucial for the consolidation of verbal memory: low levels of acetylcholine in the brain, which are a hallmark of SWS, have been demonstrated to be critical for verbal memory consolidation in humans21; The degree of EEG coherence that is phase-locked to the depolarizing phase of slow oscillations in human SWS has been found to increase following verbal learning.22 If participants are informed after the learning of word pairs is complete that they will be tested on their memory of them later, recall improves, but only if they sleep during the intervening period.23 The last study's findings are in line with the idea of memory reactivation in sleep, and with the idea that memories just acquired are residually present for some time after (which could be why cueing is effective after the end of learning) and get increasingly isolated from memories, old and new, during the course of sleep. In summary, although experimental human data obtained thus far are consistent with memory replay in sleep, the memory replay model has not yet been pitted against other viable models of verbal learning in human sleep in rigorous empirical studies; other candidate mechanisms need to be tested in conjunction with memory replay.
Sleep-dependent memory isolation is a candidate framework. According to this scheme, sleep isolates memories encoded earlier and renders them inaccessible unless specific contextual cues are provided. In other words, sleep renders it more difficult for the memory to be reactivated when interfering material is presented later. This keeps the memory pristine, which naturally ends up benefitting weak, fragile memories more than strong, stable ones and also explains how sleep would almost equally shield the old, isolated memory from associative and non-associative forms of retroactive interference. Moreover, if an old memory has been isolated by sleep, it cannot interfere with the formation and stabilization of more recent memories acquired during a subsequent wake period, which explains why prior sleep protects from prospective, and not just retroactive, interference. Note that SWS plays a critical role under the memory isolation framework as well and the various studies showing the significance of SWS in verbal memory are consistent with it as well as with memory reactivation.
There is indirect support for memory isolation and resistance to reactivation following sleep. During the course of learning, the region of the brain that is activated changes in spatial extent from a wide swath of weakly activated tract to a small but intensely activated area at the end.24,25 In fact, the shrinkage of brain area during learning may well be a neural signature of successful learning. These findings are consistent with (but do not prove) the idea that the extensive rehearsal of a large number of word associations creates a new memory that is neurally isolated from other memories. Further, it may be that during and following sleep, the memories formed are more isolated and are not as readily reactivated. Studies using transcranial magnetic stimulation combined with high-density electroencephalography have found that long-range cortical effective connectivity breaks down during sleep,26 indicating that brain areas become more isolated from one another in sleep, which could persist in subsequent wake. Reports of net synaptic depression occurring in sleep27 and of local learning-driven increase in slow wave activity28 are also in line with the idea that sleep drives memories to become segregated from one another and become accessible only via specific cues. Nevertheless, the account is speculative but does go to show that experimental findings thus far can be accounted for under more than one model.
CONCLUSIONS
The present study suggests a more complex and nuanced role for sleep in verbal learning and memory than previously thought: our findings extend the known sleep benefit to protection from non-associative and prospective forms of interference, and limit it to gains for weakly encoded word associations. Combined, our results suggest that sleep immediately after verbal learning isolates the newly formed memory traces and renders them inaccessible, unless specific contextual cues are provided: Isolating weak memories is more beneficial; isolated memories cannot interfere or be interfered with. Experiments designed to test the memory isolation model and other models of sleep-dependent gains in verbal memory are required.
FOOTNOTE
The slightly lower mean performance of the 24hr. group is attributable entirely to a single outlier who only recalled 50% of A-C pairs. Discarding the outlier, the 24hr. group recalled 98 ± 4 % of all A-C pairs at testing, which was comparable to the recall rates of the Sleep group and somewhat higher than those of the Wake group.
DIISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are indebted to the volunteers in our study. The authors owe a special debt of gratitude to Saumil Patel for reviewing several earlier versions of the manuscript and for innumerable valuable suggestions. The authors thank the two anonymous reviewers for their careful and insightful criticisms.
REFERENCES
- 1.Ellenbogen JM, Hulbert JC, Jiang Y, Stickgold R. The sleeping brain's influence on verbal memory: boosting resistance to interference. PLoS One. 2009;4:e4117. doi: 10.1371/journal.pone.0004117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellenbogen JM, Hulbert JC, Stickgold R, Dinges DF, Thompson-Schill SL. Interfering with theories of sleep and memory: sleep, declarative memory, and associative interference. Curr Biol. 2006;16:1290–4. doi: 10.1016/j.cub.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Ekstrand BR. Effect of sleep on memory. J Exp Psychol. 1967;75:64–72. doi: 10.1037/h0024907. [DOI] [PubMed] [Google Scholar]
- 4.Ekstrand BR, Sullivan MJ, Parker DF, West JN. Spontaneous recovery and sleep. J Exp Psychol. 1971;88:142–4. doi: 10.1037/h0030642. [DOI] [PubMed] [Google Scholar]
- 5.Drosopoulos S, Schulze C, Fischer S, Born J. Sleep's function in the spontaneous recovery and consolidation of memories. J Exp Psychol Gen. 2007;136:169–83. doi: 10.1037/0096-3445.136.2.169. [DOI] [PubMed] [Google Scholar]
- 6.Gais S, Lucas B, Born J. Sleep after learning aids memory recall. Learn Mem. 2006;13:259–62. doi: 10.1101/lm.132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diekelmann S, Buchel C, Born J, Rasch B. Labile or stable: opposing consequences for memory when reactivated during waking and sleep. Nat Neurosci. 2011;14:381–6. doi: 10.1038/nn.2744. [DOI] [PubMed] [Google Scholar]
- 8.Rasch B, Buchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–9. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- 9.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–26. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 10.Peigneux P, Laureys S, Fuchs S, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–45. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Friendly M, Franklin PE, Hoffman D, Rubin DC. The Toronto word pool: Norms for imagery, concreteness, orthographic variables, and grammatical usage for 1,080 words. Behav Res Methods Instrum Comput. 1982;14:375–99. [Google Scholar]
- 12.Brainard DH. The psychophysics toolbox. Spat Vis. 1997;10:433–6. [PubMed] [Google Scholar]
- 13.Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10:437–42. [PubMed] [Google Scholar]
- 14.Brown RM, Robertson EM. Off-line processing: reciprocal interactions between declarative and procedural memories. J Neurosci. 2007;27:10468–75. doi: 10.1523/JNEUROSCI.2799-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forrester WE. Retroactive inhibition and spontaneous recovery in the A-B, D-C paradigm. Journal of Verbal Learning and Verbal Behavior. 1970;9:525–8. [Google Scholar]
- 16.Delprato DJ, Garskof BE. Specific associative unlearning in the AB-CD paradigm. Can J Psychol. 1969;23:402–9. [Google Scholar]
- 17.Rudoy JD, Voss JL, Westerberg CE, Paller KA. Strengthening individual memories by reactivating them during sleep. Science. 2009;326:1079. doi: 10.1126/science.1179013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takashima A, Petersson KM, Rutters F, et al. Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc Natl Acad Sci U S A. 2006;103:756–61. doi: 10.1073/pnas.0507774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peigneux P, Laureys S, Fuchs S, et al. Learned material content and acquisition level modulate cerebral reactivation during posttraining rapid-eye-movements sleep. Neuroimage. 2003;20:125–34. doi: 10.1016/s1053-8119(03)00278-7. [DOI] [PubMed] [Google Scholar]
- 20.Peigneux P, Orban P, Balteau E, et al. Offline persistence of memory-related cerebral activity during active wakefulness. PLoS Biol. 2006;4:e100. doi: 10.1371/journal.pbio.0040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gais S, Born J. Low acetylcholine during slow-wave sleep is critical for declarative memory consolidation. Proc Natl Acad Sci U S A. 2004;101:2140–4. doi: 10.1073/pnas.0305404101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molle M, Marshall L, Gais S, Born J. Learning increases human electroencephalographic coherence during subsequent slow sleep oscillations. Proc Natl Acad Sci U S A. 2004;101:13963–8. doi: 10.1073/pnas.0402820101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilhelm I, Diekelmann S, Molzow I, Ayoub A, Molle M, Born J. Sleep selectively enhances memory expected to be of future relevance. J Neurosci. 2011;31:1563–9. doi: 10.1523/JNEUROSCI.3575-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pigarev IN, Nothdurft HC, Kastner S. Evidence for asynchronous development of sleep in cortical areas. Neuroreport. 1997;8:2557–60. doi: 10.1097/00001756-199707280-00027. [DOI] [PubMed] [Google Scholar]
- 25.Petersen SE, van Mier H, Fiez JA, Raichle ME. The effects of practice on the functional anatomy of task performance. Proc Natl Acad Sci U S A. 1998;95:853–60. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–32. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- 27.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–8. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 28.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]