Abstract
Study Objective:
To evaluate the effects of acute sleep deprivation and chronic sleep restriction on vigilance, performance, and self-perception of sleepiness.
Design:
Habitual night followed by 1 night of total sleep loss (acute sleep deprivation) or 5 consecutive nights of 4 hr of sleep (chronic sleep restriction) and recovery night.
Participants:
Eighteen healthy middle-aged male participants (age [(± standard deviation] = 49.7 ± 2.6 yr, range 46-55 yr).
Measurements:
Multiple sleep latency test trials, Karolinska Sleepiness Scale scores, simple reaction time test (lapses and 10% fastest reaction times), and nocturnal polysomnography data were recorded.
Results:
Objective and subjective sleepiness increased immediately in response to sleep restriction. Sleep latencies after the second and third nights of sleep restriction reached levels equivalent to those observed after acute sleep deprivation, whereas Karolinska Sleepiness Scale scores did not reach these levels. Lapse occurrence increased after the second day of sleep restriction and reached levels equivalent to those observed after acute sleep deprivation. A statistical model revealed that sleepiness and lapses did not progressively worsen across days of sleep restriction. Ten percent fastest reaction times (i.e., optimal alertness) were not affected by acute or chronic sleep deprivation. Recovery to baseline levels of alertness and performance occurred after 8-hr recovery night.
Conclusions:
In middle-aged study participants, sleep restriction induced a high increase in sleep propensity but adaptation to chronic sleep restriction occurred beyond day 3 of restriction. This sleepiness attenuation was underestimated by the participants. One recovery night restores daytime sleepiness and cognitive performance deficits induced by acute or chronic sleep deprivation.
Citation:
Philip P; Sagaspe P; Prague M; Tassi P; Capelli A; Bioulac B; Commenges D; Taillard J. Acute versus chronic partial sleep deprivation in middle-aged people: differential effect on performance and sleepiness. SLEEP 2012;35(7):997–1002.
Keywords: Acute and chronic sleep deprivation, cognitive performance, sleepiness
INTRODUCTION
Technologic and societal changes over the past 40 years have significantly increased the number of workers facing sleep deprivation in Western societies.1 In the 1960s, research protocols mainly studied the effects of acute sleep deprivation on performance, but epidemiologic reports2 showed that more and more workers curtail their sleep duration during the week and try to recover during the weekend.
Chronic sleep deprivation protocols have appeared in the literature in the past 10 yr and have confirmed that repeated sleep restriction (i.e., 4 hr of sleep for 5 days) could have deleterious effects not only on daytime alertness but also on cognitive performance.3,4 Van Dongen et al.3 mainly focused on cognitive tasks and subjective measures of somnolence comparing acute and chronic sleep restriction. Using a sustained-attention reaction time task, they found that chronic sleep deprivation increased lapses (reaction time [RT] > 500 ms) in a manner similar to acute sleep deprivation. This protocol also demonstrated the effect of acute and chronic sleep deprivation on subjective measure of sleepiness, but no data were available on a standardized measure of sleepiness such as the multiple sleep latency test. In addition, Belenky et al.4 published a study comparing the effect of chronic sleep restriction on subjective and objective sleep deprivation, but they did not examine the relative effect of acute sleep deprivation versus chronic sleep deprivation. Interestingly, objective measures of sleepiness and lapse occurrence were similarly affected by chronic sleep deprivation, but subjective measures of sleepiness (Stanford Sleepiness Scale) were less sensitive to chronic sleep restriction. This finding was confirmed by Leproult et al.,5 who showed that subjective and objective measures of sleepiness do not systematically evolve in a similar manner during extended wakefulness. Leproult et al.5 also showed that this vulnerability to sleep loss was stable over time among individuals. This makes it possible to reproduce sleep deprivation paradigms and expect trait-type results. Taillard et al.6 and Galliaud et al.7 showed that homeostatic sleep pressure is associated with subjective levels of sleepiness but not with an increase in reaction time during extended wakefulness. Goel et al.8 showed that PER3 polymorphism predicts cumulative sleep homeostatic but not neurobehavioral changes observed during chronic sleep deprivation. These authors drew attention to a very important question on the physiologic basis of performance decrements under sleep deprivation, a finding that suggests that sleep pressure is not the key factor explaining performance loss during sleep deprivation.
Because subjective and objective measures of sleepiness do not strictly correlate in healthy sleep-deprived humans, it is interesting to compare both measures with performance and compare them with the differential effects of acute and chronic sleep deprivation to better investigate vulnerability to sleep loss.
Here we compared the effects of 1 night of sleep deprivation and 5 consecutive nights of 4 hr of sleep on a cognitive task versus subjective and objective measures of sleepiness.
METHODS
Patient Selection
All participants filled in questionnaires (Self-report symptoms inventory SLC 90, Basic Nordic Sleep Questionnaire, Horne and Osberg and Epworth Sleepiness Scale)9,10 and had a clinical interview with a sleep specialist. They performed 1-wk actimetry and full polysomnography to rule out any sleep disorder.
Study Design
In this study, 18 healthy middle-aged male participants (age (± standard deviation [SD]) = 49.7 ± 2.6 years, range 46-55 yr; body mass index [ ± SD] = 24.5 ± 2.2; Epworth Sleepiness Scale score [ ± SD] = 6.2 ± 3.1) were deprived of sleep during 2 sleep deprivation periods (acute sleep deprivation or chronic sleep restriction). Volunteers spent a total of 9 days in residence in the laboratory (1 day is defined as the time from the beginning of 1 scheduled sleep period to the beginning of the next). Prior to each condition, participants had 3-day actimetry to rule out any sleep deprivation before admission to the laboratory. Participants were submitted in a balanced crossover design first to baseline (8 hr of time in bed from 23:00 to 07:00) followed by acute total sleep deprivation (1 night) or by 5 consecutive nights of chronic sleep restriction (4 hr required time in bed). Each sleep deprivation period was followed by an 8-hr recovery night and was separated by at least 2 wk.
During the night, sleep parameters were recorded by polysomnography. During the day, the levels of sleepiness were objectively measured every 2 hr from 09:00 to 19:00 with a multiple sleep latency test (MSLT) and subjectively measured every 2 hr from 08:00 to 22:00 with the Karolinska Sleepiness Scale (KSS). Cognitive performance was evaluated with a 10-mn simple reaction time test (SRTT) every 2 hr from 08:00 to 22:00.
The study was approved by the local ethics committee (committee for the protection of persons participating in biomedical research, CPP Bordeaux A), and participants gave written informed consent.
Simple Reaction Time Test
A 10-min SRTT on a Palm personal organizer (Palm, Inc.) was performed11. A black square was displayed 100 times on the screen at randomized (2–7 s) intervals over 10 min. The participant was required to respond to the stimulus by pressing a key to turn off the square. The number of lapses (RTs > 500 ms) and the 10% fastest RTs (response speed) during the 10-min task were calculated. The subset of the fastest 10% of responses was not affected by occasional lapses.
Multiple Sleep Latency Test
As recommended by American Academy of Sleep Medicine practice parameters,12 6x20-min MSLT trials were completed. This design was chosen to optimize sleepiness measurements. The room was shielded from external light. An experienced sleep technologist performed the MSLT. Electroencephalogram (C3/A2, O2/A1), electromyogram, and electrooculogram were recorded according to the recommendations of Rechtschaffen and Kales.13 If the participant did not fall asleep, the test was stopped after 20 min; if the participant fell asleep, the test was stopped after the first sleep epoch occurred (i.e., the participant was awake). The sleep latency on the MSLT is the time it takes from turning off the light to the onset of the first scored sleep epoch (Stages 1, 2, 3 of sleep or rapid eye movement). Data were recorded and manually analyzed in 30-sec epochs using the TrackIt device (Deltamed, France). Patients were under video monitoring throughout the entire test. The mean sleep latency of the 6 MSLT trials was then calculated.
Karolinska Sleepiness Scale
Participants were asked to rate their sleepiness on the KSS (a 9-point scale from 1 = “extremely alert” to 9 = “very sleepy, great effort to keep alert, fighting sleep”).
Statistical Analyses
Number of lapses (RTs > 500 ms) (converted to 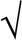 x+
x+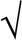 x+1) and 10% fastest RTs
([1/RT]x1000) on the SRTT, KSS scores, and sleep latencies on the
MSLT were analyzed through repeated-measures analyses of variance (rANOVAs) with
“Sleep Deprivation” (baseline (B), acute sleep deprivation (A SD),
recovery night (Ra), 5 consecutive sleep restriction nights (SR1, SR2, SR3, SR4
and SR5) and recovery night (Rc)) and the “Time of Day” (every 2
hr from 8:00 to 22:00 for SRTT and KSS, and every 2 hr from 9:00 to 19:00 for
MSLT) as within-factors. The “Time of Day” factor was included to
help determine whether the sleep deprivation effect was primarily manifested at
particular times of the day. This possibility would be suggested by the
interaction Sleep Deprivation*Time of Day. Therefore, in the results
reported in the following paragraphs, only interactions are reported (main
effect for “Time of Day” is not reported). Nocturnal sleep
parameters assessed with polysomnography were analyzed with nonparametric paired
tests (Wilcoxon test). Results for sleep parameters assessed with
polysomnography are reported as means and standard deviations.
x+1) and 10% fastest RTs
([1/RT]x1000) on the SRTT, KSS scores, and sleep latencies on the
MSLT were analyzed through repeated-measures analyses of variance (rANOVAs) with
“Sleep Deprivation” (baseline (B), acute sleep deprivation (A SD),
recovery night (Ra), 5 consecutive sleep restriction nights (SR1, SR2, SR3, SR4
and SR5) and recovery night (Rc)) and the “Time of Day” (every 2
hr from 8:00 to 22:00 for SRTT and KSS, and every 2 hr from 9:00 to 19:00 for
MSLT) as within-factors. The “Time of Day” factor was included to
help determine whether the sleep deprivation effect was primarily manifested at
particular times of the day. This possibility would be suggested by the
interaction Sleep Deprivation*Time of Day. Therefore, in the results
reported in the following paragraphs, only interactions are reported (main
effect for “Time of Day” is not reported). Nocturnal sleep
parameters assessed with polysomnography were analyzed with nonparametric paired
tests (Wilcoxon test). Results for sleep parameters assessed with
polysomnography are reported as means and standard deviations.
To control any effect of order, supplementary rANOVAs were run with an additional between-factor “order of sleep deprivation period.”
The SPSS statistical package (version 12.0.1, SPSS Inc, Chicago, IL) was used for all analyses.
We applied a mixed-effects regression model for repeated measures data.14 It was thought that middle-aged participants could adapt to chronic sleep restriction. To take this into account, we extended the model by Van Dongen et al.3 for neurobehavioral variables to a model with sleep adaptation. Subjective sleepiness (KSS scores) and SRTT (lapses) data (y), expressed as the mean result obtained during day time, were modeled as:
where t denotes the number of days of chronic sleep restriction ranging from 0 to 5. We modeled β, the baseline value, as a normally distributed random effect. The parameter θ represents curvature in the response profile, and γ represents the adaptability coefficient. The rate of change in subjective sleepiness and cognitive performances, denoted β + γt, varies across days of sleeping restriction (there is adaptation for negative gamma). The model reduces to the one proposed by Van Dongen et al.3 for γ = 0. Statistical significance of γ was tested according to a Wald test at level 5% together with 95% normal confidence intervals. The R package “nlme”15 was used for all analyses.
RESULTS
SRTT Lapses
An overall effect of sleep deprivation was found on the mean number of lapses [F(8, 136) = 2.298, P < 0.05] (Figure 1A). Post hoc tests showed that the number of lapses was higher after acute sleep deprivation than after the baseline night (P < 0.001). Number of lapses did not differ from baseline after the recovery night that followed acute sleep deprivation. Number of lapses was higher after the second night of chronic sleep restriction than after the baseline night (P < 0.05). Number of lapses did not differ from baseline after the recovery night that followed chronic sleep restriction. Interestingly, number of lapses did not significantly differ between acute and chronic sleep restriction (from the second night of sleep restriction).
Figure 1.
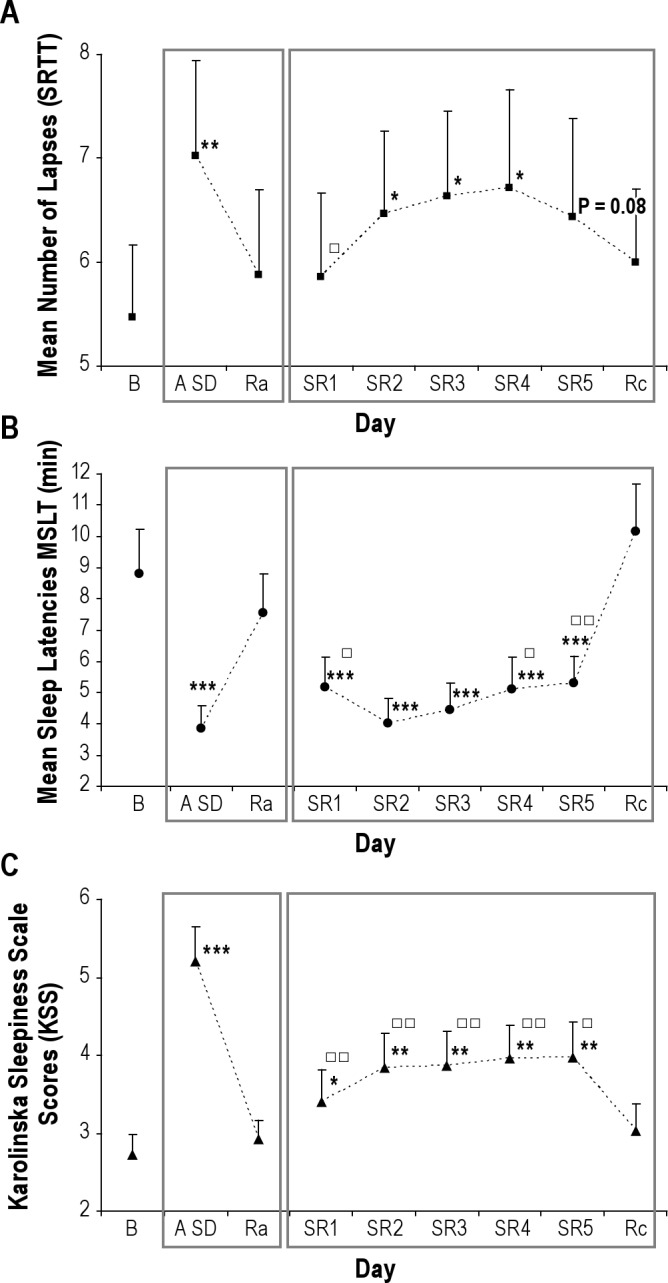
Mean number of lapses (A), mean sleep latencies on the multiple sleep latency test (MSLT) (B), and mean Karolinska Sleepiness Scale (KSS) scores (C) (mean ± standard error of the mean) across days after Baseline night (B), Acute Sleep Deprivation (A SD), Recovery night after acute sleep deprivation (Ra), 5 consecutive Sleep Restriction nights (SR1-SR5) and Recovery night after the 5 sleep restriction nights (Rc). Baseline night (B) was the first night of the protocol followed either by acute sleep deprivation or chronic sleep restriction. Asterisks indicate significant differences with Baseline. *P < 0.05, **P < 0.01, ***P < 0.001. Squares indicate significant differences with acute sleep deprivation (only for the 5 sleep restriction nights). ☐P < 0.01, ☐☐P < 0.001.
We did not find any interaction Sleep deprivation*Time of day [F(56, 952) = 0.908, not significant NS].
No effect of order of sleep deprivation period was found [F(1, 16) = 1.393, NS].
Ten Percent Fastest RTs
No effect of sleep deprivation on 10% fastest RTs was found [F(8, 136) = 1.736, NS].
We did not find any interaction Sleep deprivation*Time of day [F(56, 952) = 1.312, NS].
No effect of order of sleep deprivation period was found [F(1, 16) = 0.083, NS].
Objective Sleepiness (MSLT)
We found an effect of condition on mean sleep latencies measured by the MSLT [F(8, 136) = 25.345, P < 0.001] (Figure 1B). Post hoc tests indicated that sleep latencies were both affected by acute sleep deprivation (P < 0.001) and chronic sleep restriction (P < 0.001). Moreover, mean sleep latencies did not significantly differ after acute sleep deprivation and after the second and third nights of chronic sleep restriction. Sleep latencies did not differ from baseline after the recovery night that followed acute sleep deprivation and chronic sleep restriction.
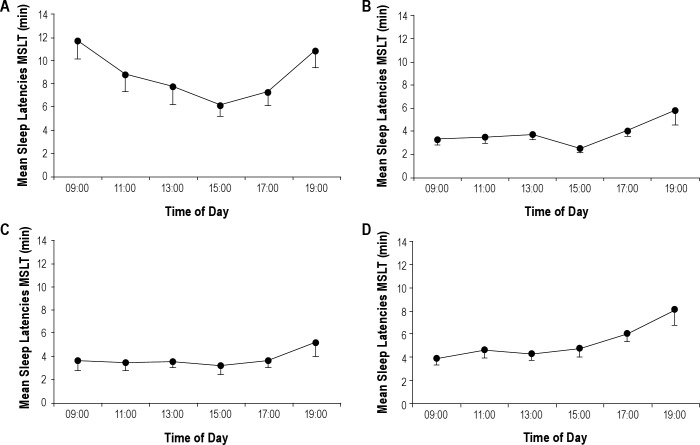
We found an interaction Sleep deprivation*Time of day [F(40, 680) = 2.645, P < 0.001]. We observed that sleep latencies decreased from 9:00 to 15:00 (P < 0.01) and increased from 15:00 to 19:00 (P < 0.05) (U curve) after the baseline night and after the 2 recovery nights, but not after acute sleep deprivation or the 5 sleep restriction nights (Figure 2).
Figure 2.
Sleep latencies during the 6 naps on the multiple sleep latency test (MSLT) across daytime after baseline night (A), acute sleep deprivation (B), second sleep restriction night (C), and fifth sleep restriction night (D).
No effect of order of sleep deprivation period was found [F(1, 16) = 0.598, NS].
Subjective Sleepiness (KSS)
We found an effect of condition on KSS scores [F(8, 136) = 15.611, P < 0.001] (Figure 1C). Post hoc tests showed that KSS scores were affected by acute sleep deprivation (P < 0.001) and chronic sleep restriction (P < 0.01), and that acute sleep deprivation induced higher levels of subjective sleepiness than chronic sleep restriction (P < 0.001). KSS scores did not differ from baseline after the recovery night that followed acute sleep deprivation and chronic sleep restriction.
Moreover, we found an interaction Sleep deprivation*Time of day [F(56, 952) = 1.390, P < 0.05]. We observed a variation in KSS scores during daytime (peak of arousal at 16:00 and 20:00) after acute sleep deprivation but not after the baseline night or the 5 sleep restriction nights (P < 0.01). No effect of order of sleep deprivation period was found [F(1, 16) = 0.347, NS].
Sleep Parameters (Nocturnal Recordings)
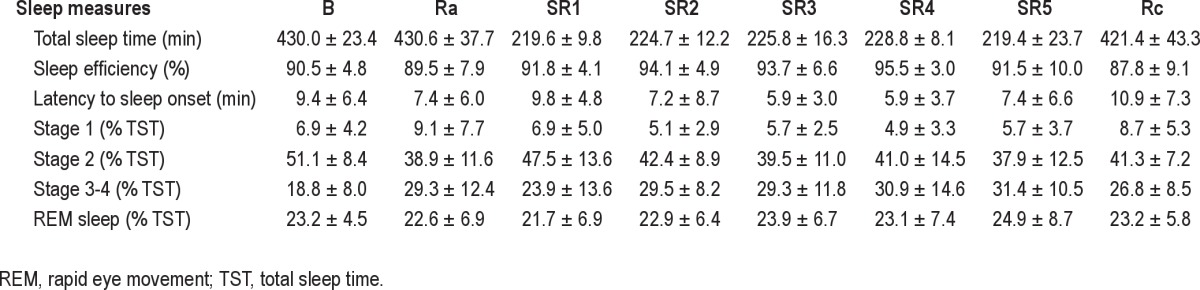
There was no significant difference between total sleep time during the baseline night versus the 2 recovery nights (Wilcoxon test, Z = −1.189, NS and Z = −0.632, NS, respectively). From the second sleep restriction night, sleep efficiency was increased in comparison with the baseline night (Wilcoxon test, Z = −2.330, P < 0.05, Z = −2.012, P < 0.05, Z = −3.549, P < 0.001, Z = −1.764, P = 0.07, respectively). Regarding sleep architecture, percentage of stage 2 sleep decreased during the 2 recovery nights compared to the baseline night (Wilcoxon test, Z = −3.724, P < 0.001 and Z = −3.375, P < 0.001, respectively) and from the second sleep restriction night compared to the baseline night (Wilcoxon test, Z = −3.070, P < 0.01, Z = −2.959, P < 0.01, Z = −2.744, P < 0.01, Z = −2.940, P < 0.01, respectively). By contrast, the percentage of sleep slow waves increased during the 2 recovery nights in comparison with the baseline night (Wilcoxon test, Z = −3.419, P < 0.001 and Z = −2.983, P < 0.01, respectively) and from the second sleep restriction night in comparsion with the baseline night (Wilcoxon test, Z = −3.375, P < 0.001, Z = −3.053, P < 0.01, Z = −3.245, P < 0.001, Z = −3.288, P < 0.001, respectively) (Table 1).
Table 1.
Polysomnographic sleep measures during baseline night (B), recovery night after acute sleep deprivation (Ra), 5 consecutive sleep restriction nights (SR1-SR5), and recovery night after the 5 sleep restriction nights (Rc) (mean ± SD)
Mixed-Effects Regression Models
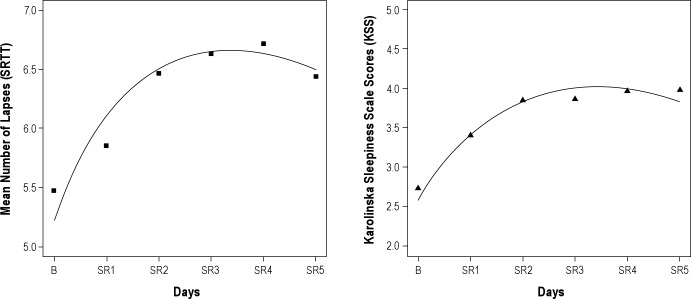
Concerning the mean number of lapses, the parameter estimators were = 5.23 (IC95% = [4.18; 6.29]), = 0.31 (IC95% = [0.21; 0.41]) and = −0.30 (IC95% = [-0.44; −0.17]). Results for KSS scores were = 2.58 (IC95% = [2.18; 2.97]), = 0.53 (IC95% = [0.41; 0.65]) and = −0.22 (IC95% = [-0.27, −0.16]). Significant adaptations (γ) for lapses and KSS were found: improvements in the mean number of lapses and KSS scores were noticeable after a few days of chronic sleep restriction (Figure 3).
Figure 3.
Mean number of lapses (left) and mean Karolinska Sleepiness Scale (KSS) scores (right) fitted by the mixed-effects regression model. B, baseline; SR1-SR5, 5 consecutive sleep restriction nights.
DISCUSSION
Our findings confirm that chronic sleep restriction in middle-aged individuals significantly decreases MSLT sleep latencies, as described previously.4,16 This study demonstrates that sleep propensity is affected after only 1 night of 4 hr of sleep. Sleepiness continues to accumulate until the third day of sleep restriction (after the second and third night of sleep restriction, the level of sleepiness is comparable to that observed after acute sleep deprivation). Then, sleepiness improves after the fourth night of sleep restriction (the latencies are longer than those observed after acute sleep deprivation). The effect of chronic sleep restriction is evident throughout the waking day: the typical “U” pattern of the distribution of sleep latency during the waking day disappears after chronic sleep restriction and acute sleep deprivation.
Like objective sleepiness, subjective sleepiness increases immediately in response to sleep restriction and remains stable throughout sleep restriction, as described previously.3,4,16 The level of sleepiness is not comparable to a full night of sleep loss. Using a mixed-effects regression model, we confirm that the habituation effect, which has been described by Van Dongen et al.3 in a younger population of healthy individuals, is also present in older individuals. Interestingly, our study shows that this differential effect cannot be explained by lower sleep pressure because MLST scores decreased dramatically after day 3 of restriction. These results confirm that individuals underestimate the impact of sleep restriction.
With regard to SRTT (lapses) in middle-aged individuals, chronic sleep deprivation induced neurobehavioral deficits equivalent to those observed after acute sleep deprivation. Unlike sleepiness, the number of lapses increased only after the second day of sleep restriction. The significant adaptation after a few days of chronic sleep restriction found for the mean number of lapses indicated that there was no cumulative effect across sleep restriction, contrary to what is observed in populations consisting essentially of young individuals.3,4,8,16,17 Unfortunately we had no group of young participants with which we could compare the effect of chronic sleep restriction on sleepiness and performances, although middle-aged individuals seem to achieve a stabilized response to sleep restriction. Optimal performance capacity (i.e., fastest 10% of responses) was preserved, suggesting that the performance degradation that occurs during sleep deprivation in middle-aged individuals is explained solely by an increased incidence of lapses (microsleeps). Again, we had no group of young participants to confirm that middle-aged individuals preserve their fastest performances under sleep loss whereas younger individuals do not, but there is evidence in the literature4,18 to show that young individuals are affected in both slowest and fastest performances under sleep loss.
In effect, SRTT (i.e., lapses) significantly decreases after a single night of total sleep deprivation but also after the second night of partial sleep resturction (4 hr/night of sleep) in middle-aged individuals. This decrement in performance was of the same level between acute deprivation and the 2 to 5 consecutive nights of chronic sleep restriction. Chee et al.19 have shown that the fastest responses after normal sleep and after sleep deprivation elicited comparable frontoparietal activation contrary to the slowest responses, suggesting that performing a task while sleep deprived involves periods of apparently normal neural activation interleaved with periods of depressed cognitive control, visual perceptual functions and arousal. SRTT is a basic component of cognitive performance, so more tests (e.g., Stroop, memory, divided attention) should be used under similar conditions in the future.
Full recovery to baseline level after 5 nights of sleep restriction to 4 hr of sleep was obtained on performance and objective level of alertness after a single night of 8 hr of sleep for both types of sleep deprivation. This demonstrates the beneficial effect of sleep on recovery in our population, as young individuals require several nights of recovery.4,18
On the one hand, our findings confirm that waking brain impairment from chronic sleep restriction in middle-aged individuals rapidly becomes as severe as that resulting from total sleep deprivation. On the other hand, complete recovery from chronic sleep restriction in this population is obtained after a single night of recovery, and optimal performance capacity (10% fastest RT) is preserved in middle-aged individuals. Rapid recovery to baseline is typical of performance following acute total sleep deprivation and is not observed in young people.
Our findings suggest that some adaptation to chronic sleep restriction in middle-aged individuals occurs during consecutive nights of moderate sleep restriction (i.e., 4 hr/night of sleep). Further studies are needed to compare age-related profiles of waking neurobehavioral deficits and recovery to acute sleep deprivation versus chronic sleep restriction. Crucial implications are expected for at-risk sleep-restricted workers.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Andrew Vakulin, PhD, from the Adelaide Institute for Sleep Health, Repatriation General Hospital, Australia, for his comments on an early version of the manuscript. This research was supported by a grant from the French National Agency for Research (ANR PRIVASOM). The funding organization had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
A commentary on this article appears in this issue on page 901.
REFERENCES
- 1.Luckhaupt SE, Tak S, Calvert GM. The prevalence of short sleep duration by industry and occupation in the National Health Interview Survey. Sleep. 2010;33:149–59. doi: 10.1093/sleep/33.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Sleep Foundation. Sleep in America Poll: Summary of Findings. 2008. Available at http://www.sleepfoundation.org/sites/default/files/2008%20POLL%20SOF.PDF. [Google Scholar]
- 3.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 4.Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 5.Leproult R, Colecchia EF, Berardi AM, Stickgold R, Kosslyn SM, Van Cauter E. Individual differences in subjective and objective alertness during sleep deprivation are stable and unrelated. Am J Physiol Regul Integr Comp Physiol. 2003;284:R280–90. doi: 10.1152/ajpregu.00197.2002. [DOI] [PubMed] [Google Scholar]
- 6.Taillard J, Moore N, Claustrat B, Coste O, Bioulac B, Philip P. Nocturnal sustained attention during sleep deprivation can be predicted by specific periods of subjective daytime alertness in normal young humans. J Sleep Res. 2006;15:41–5. doi: 10.1111/j.1365-2869.2006.00505.x. [DOI] [PubMed] [Google Scholar]
- 7.Galliaud E, Taillard J, Sagaspe P, Valtat C, Bioulac B, Philip P. Sharp and sleepy: evidence for dissociation between sleep pressure and nocturnal performance. J Sleep Res. 2008;17:11–5. doi: 10.1111/j.1365-2869.2008.00629.x. [DOI] [PubMed] [Google Scholar]
- 8.Goel N, Banks S, Mignot E, Dinges DF. PER3 polymorphism predicts cumulative sleep homeostatic but not neurobehavioral changes to chronic partial sleep deprivation. PLoS One. 2009;4:e5874. doi: 10.1371/journal.pone.0005874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 10.Partinen M, Gislason T. Basic Nordic Sleep Questionnaire (BNSQ): a quantitated measure of subjective sleep complaints. J Sleep Res. 1995;4:150–55. doi: 10.1111/j.1365-2869.1995.tb00205.x. [DOI] [PubMed] [Google Scholar]
- 11.Gillberg M, Kecklund G, Akerstedt T. Relations between performance and subjective ratings of sleepiness during a night awake. Sleep. 1994;17:236–41. doi: 10.1093/sleep/17.3.236. [DOI] [PubMed] [Google Scholar]
- 12.Littner MR, Kushida C, Wise M, Davila DG, Morgenthaler T, Lee-Chiong T, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–21. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 13.Rechtschaffen A, Kales A, editors. A manual of standardized terminology, techniques and scoring system of sleep stages in human subjects. Los Angeles: Brain Information Service/Brain Research Institute, University of California; 1968. [Google Scholar]
- 14.Lindstrom ML, Bates DM. Nonlinear mixed effects models for repeated measures data. Biometrics. 1990;46:673–87. [PubMed] [Google Scholar]
- 15.Pinheiro J, Bates D, DebRoy S, Sarkar D. 2009. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1. p. 97. [Google Scholar]
- 16.Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 17.Goel N, Banks S, Mignot E, Dinges DF. DQB1*0602 predicts interindividual differences in physiologic sleep, sleepiness, and fatigue. Neurology. 2010;75:1509–19. doi: 10.1212/WNL.0b013e3181f9615d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banks S, Van Dongen HP, Maislin G, Dinges DF. Neurobehavioral dynamics following chronic sleep restriction: Dose-response effects of one night for recovery. Sleep. 2010;33:1013–26. doi: 10.1093/sleep/33.8.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chee MW, Tan JC, Zheng H, Parimal S, Weissman DH, Zagorodnov V, et al. Lapsing during sleep deprivation is associated with distributed changes in brain activation. J Neurosci. 2008;28:5519–28. doi: 10.1523/JNEUROSCI.0733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]