Abstract
Objective
The relations between acoustic measures and their articulatory bases have rarely been tested in dysarthria but are important for diagnostic and treatment purposes. We tested the association between acoustic measures of F2 range and F2 slope with kinematic measures of tongue movement displacement and speed in individuals with amyotrophic lateral sclerosis (ALS) and healthy controls speaking at normal and slow rates. Relations between acoustic and kinematic measures and speech intelligibility were examined.
Results
As healthy controls reduced their speaking rate, their F2 slopes and movement speeds decreased. In talkers with ALS, acoustic and kinematic variables were associated with changes in speaking rate, characteristic of disease progression. Participants with slow rate had shallower F2 slopes and slower movement speeds than those with normal rate. Relations between F2 range and tongue displacement were weaker. F2 slope, displacement, and duration were correlated with speech intelligibility most consistently.
Conclusion
Findings suggested that F2 slope is a useful marker for tracking disease progression in ALS. F2 slope reflects changes in tongue function with disease progression and is linked to speech intelligibility. Changes in movement speed, however, might be the earliest sign of disease in the tongue.
Key Words: Amyotrophic lateral sclerosis, Tongue kinematics, Speech acoustics, F2 slope, Speech intelligibility, Speaking rate
Introduction
The literature on motor speech disorders is primarily devoted to understanding the acoustic correlates of speech intelligibility. The acoustic measures are particularly clinically meaningful, however, if they are linked to articulatory events. Therapy interventions that target these articulatory events would be expected to lead to changes in the acoustic signal and, subsequently, improvements in speech intelligibility. Establishing direct links between acoustic and articulatory events is not simple. The movements of the articulators (i.e., tongue, lips, jaw) during speech are continuous and multiple articulatory events underlie a single acoustic event [1]. Furthermore, non-linearities exist in the acoustic response of the vocal tract to changes in articulatory positions of speech organs [2].
Despite these challenges, specific articulatory actions are known to generate specific acoustic results. The classic example is the strong link between vowel formant frequencies and tongue positions [3]. Recent studies examining the relationship between the kinematic and acoustic measures associated with variations in speaking rate, intensity, and/or speaking style (i.e., clarity) revealed parallel changes in acoustic and kinematic signals in healthy talkers [4, 5].
A number of neurologic diseases affect speech at the kinematic and acoustic levels. The findings of kinematic studies have suggested that neurologic damage results in the reduction in size and speed of speech-related movements [6, 7, 8]. Parallel findings have been reported in acoustic studies, showing changes in vowel formant frequencies and F2 slopes, reductions in F2 transitions, and increases in segment durations [9, 10, 11, 12, 13]. Although changes in speech kinematics are presumed to underlie changes in speech acoustics, studies that link the two in clinical populations have not, to our knowledge, been completed. This investigation focuses primarily on the slope of the second formant (F2) transition and movement speed as its potential kinematic correlate. F2 slope has been used to represent the rate of change in the vocal tract configuration during a vocalic event (i.e., a vowel or diphthong) and has been viewed as an important correlate of speech intelligibility and speaking rate decline across the severity continuum [11, 14, 15]. Shallow F2 slopes, which are characteristic of dysarthria, have been attributed to reduction in the speed of articulatory movements [14, 16, 17]. In addition, we examined F2 range and displacement as another set of measures that might change in parallel as speech declines due to amyotrophic lateral sclerosis (ALS).
In this study, we aimed to examine changes in kinematic and acoustic measures in a group of talkers with ALS who ranged in severity of dysarthria. We compared their performance to that of healthy controls, speaking at normal and slow rates. Slow speaking rate is a hallmark characteristic of dysarthria in ALS [18, 19]. We hypothesized that slow speech would be associated with decreased average movement speed and F2 slopes as well as reduced F2 range and displacement in talkers with ALS and healthy controls. We also hypothesized that the nature and strength of the relationship between kinematic and acoustic variables across talkers with ALS would be similar to that of the control group due to the fundamental similarity in the mechanism by which individuals slow their speaking rate. Furthermore, we expected that kinematic and acoustic variables would be associated with the decline in both speaking rate and intelligibility across talkers with ALS with varying severity of dysarthria.
Method
Participants
Thirty-one talkers (13 females and 18 males) diagnosed with ALS participated in the study. The mean age of the female group was 57 years (SD = 9.61); the mean age of the male group was 61 years (SD = 11.03). Three talkers presented with predominantly bulbar type ALS at diagnosis; 22 patients were diagnosed with spinal-onset ALS. The ALS classification at the onset was unspecified for the remaining 6 talkers. At the time of the recording, all patients showed signs of bulbar involvement in at least one region of the speech system (e.g., voice, sof t palate, tongue, and/or face). The patients exhibited a wide range of severity scores on the ALS Functional Rating Scale-Revised (ALSFRS-R) [20] at the time of recording (26–44, mean = 34.90; SD = 5.38). The bulbar function scores on the ALSFRS-R were between 5 and 12 (mean = 10.36; SD = 1.81), with a possible range of 0–12. The control group was comprised of 3 males (mean age = 37; SD = 18.50) and 9 females (mean age = 39; SD = 15.54).
Talkers in the ALS group exhibited a range of speech intelligibility and speaking rate scores as measured by the Sentence Intelligibility Test [21]. Eighteen talkers showed intact intelligibility with transcription scores of 99–100%. The remaining 13 talkers with ALS had intelligibility scores ranging between 62.6 and 98.2% (median of 94.5%). Speaking rate varied in the patient sample between 71.6 and 225 words per minute (WPM). The normal speaking rate for the Sentence Intelligibility Test is approximately 200 WPM, with a range of 160–230 WPM [22]. The speaking rate of 16 patients was within the normal range. The remaining 15 patients showed a median rate of 108 WPM, with a range between 72 and 147 WPM. Given that speaking rate typically declines much earlier than speech intelligibility and is considered to be the primary sign of onset of bulbar ALS [19], we divided the ALS group into two subgroups: ALS speaking at normal rate (AN) and ALS speaking at slow rate (AS).
Procedures
Two sentences, ‘Say doily again’ and ‘I love Seattle in the spring’, were repeated 5 times by each talker. Tongue movements and formant frequencies were analysed during the opening gesture in /doI/ and /jæ/ as well as the closing gesture in /oI/. Talkers with ALS produced these sentences at their normal speaking rate, while talkers in the control group read them at their normal (CN) and slow (CS, ‘half of their normal’) speaking rates [22].
Tongue movements were collected using JT-3D. This electromagnetic system is designed to track movements of a small magnet attached to the tongue, relative to the coordinate space defined by the frame of the helmet [23, 24]. During the experiment, the magnet was attached to the tongue blade, an average of 20.18 mm from the tongue tip (SD = 2.36). The system acquires positional data in three dimensions (vertical, anteroposterior, and lateral) at a sampling rate of 1,000 Hz. During acquisition, the signals were expressed relative to the positions at the first collection frame, low-pass-filtered at 50 Hz, and then oversampled at 1,000 Hz. Because the system is only able to record a single magnet, the jaw was stabilized with a 5-mm bite block placed between the molars. Thus tongue movements were obtained independent from jaw movements. Acoustic signals were recorded simultaneously with movements directly onto a computer at the sampling rate of 22 kHz and 16 bit resolution. A head-mounted, professional quality microphone (Countryman B3P4FF05B) was positioned at approximately 5 cm from the mouth during the recordings.
Measurements
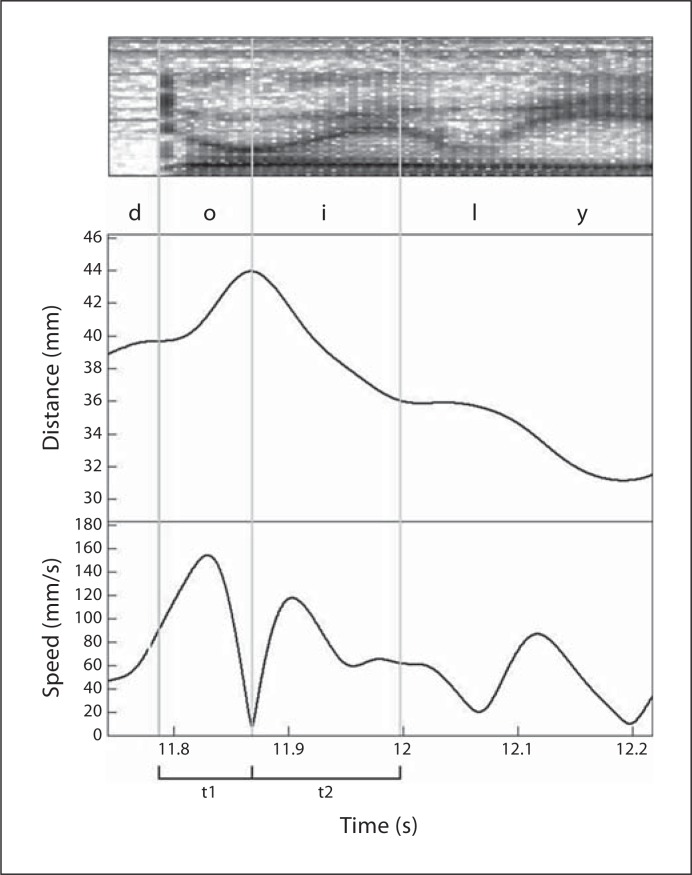
A typical acoustic and kinematic record with measurements is shown in figure 1 for the word ‘doily’. The typical course of the F2 in this word is characterized by a decreasing transition between the consonant and the first vowel followed by the rapidly rising transition that is more typically associated with the /oI/ diphthong. These transitions were identified on hand-edited, F2 LPC tracks on a wide-band spectrogram (300 Hz analysis bandwidth; number of coefficients = 26) [25]. The 20 × 20 rule, which specified the change in frequencies of 20 Hz in 20 ms at the onset and offset of the transition, was applied [11]. F2 slopes (F2 range/time), ranges (F2 max – F2 min), and durations were measured for each transition. The three-dimensional kinematic time series were reduced into a single Euclidean distance time series. Euclidian speed (i.e., the absolute value of the derivative of the Euclidean distance) and displacement were computed using custom-made algorithms. Average movement speed and three-dimensional displacement were calculated for each acoustically defined transition interval. All acoustic measures were conducted by a single measurer. Approximately 10% of the F2 transitions were re-tracked and transition durations as well as F2 slopes were re-measured to obtain intra-judge reliability. The average error was 9.83 ms (SD = 16.68) for duration and 23.60 Hz (SD = 39.95) for F2 slope.
Fig. 1.
‘Doily’ in ‘Say doily again’ produced by a control talker at a comfortable speaking rate and loudness. The interval initiated by the release of ‘d’ and terminated by the end of the downward F2 transition is identified as t1; the interval enclosing a rising transition during the diphthong /oI/ is identified as t2. F2 slope and F2 range were measured for each transition. Path distance and average speed functions associated with these intervals were measured as well.
Statistical Analyses
Prior to the analyses, the shape of the distribution was evaluated for each variable. A number of variables appeared to have positively skewed distributions. To normalize these distributions, displacement (all), F2 range (for /oI/ and /jæ/), F2 slope (/jæ/), and average speed (/d /) were square-root transformed. Group comparisons were conducted using linear mixed model (LMM) procedures in SPSS. The analyses involved comparing the data from four sets: talkers with ALS with normal speaking rate (AN), talkers with ALS with slower than normal speaking rate (AS), and control talkers speaking at a normal rate (CN) and slow rate (CS). The group variable was treated as a fixed effect in this analysis. Although the study was designed in such a way that the control group was used in two different conditions (normal and slow rate), the analytical technique selected for the analyses accounted for the interdependencies and repeated nature of the data by treating individuals as a random effect. LMM pairwise comparisons were performed between groups using t tests, accounting for data interdependencies and adjusting for multiple comparisons using the Bonferroni method.
/) were square-root transformed. Group comparisons were conducted using linear mixed model (LMM) procedures in SPSS. The analyses involved comparing the data from four sets: talkers with ALS with normal speaking rate (AN), talkers with ALS with slower than normal speaking rate (AS), and control talkers speaking at a normal rate (CN) and slow rate (CS). The group variable was treated as a fixed effect in this analysis. Although the study was designed in such a way that the control group was used in two different conditions (normal and slow rate), the analytical technique selected for the analyses accounted for the interdependencies and repeated nature of the data by treating individuals as a random effect. LMM pairwise comparisons were performed between groups using t tests, accounting for data interdependencies and adjusting for multiple comparisons using the Bonferroni method.
LMM analysis was also used to predict acoustic variables from their kinematic counterparts. Average speed was used as a predictor of F2 slope, and displacement was used as a predictor of F2 range. Because duration was highly correlated with all of the variables, it was added to each of these models as a covariate. A procedure described in Roberts and Monaco [26] was used to calculate fit statistics. A separate R2 statistic was computed to show the fit relative to the null model as measured by R2 on each level of partitioned variance (within and between individuals). The relationships between speech intelligibility and speaking rate and the dependent variables, computed only across ALS talkers, were examined using Pearson correlations.
Results
Acoustic and Kinematic Measures: Group Comparisons
Group means and standard deviations for each dependent variable are presented in table 1. The results of the LMM analysis revealed significant main effects of group on F2 slope for /d /, /oI/, and /jæ/ transitions [F(3, 53) = 30.58, p < 0.001; F(3, 52) = 17.51, p < 0.001; and F(3, 50) = 121.14, p < 0.001, respectively]. The pairwise comparisons between groups showed that F2 slope was significantly shallower in both the volitionally slowed speech of the control participants and the disease-related slowed speech of participants with ALS across all three transitions (see p values in table 2). Control talkers who spoke slowly differed from the less impaired ALS talkers in /jæ/ and the slow ALS talkers in /oI/.
/, /oI/, and /jæ/ transitions [F(3, 53) = 30.58, p < 0.001; F(3, 52) = 17.51, p < 0.001; and F(3, 50) = 121.14, p < 0.001, respectively]. The pairwise comparisons between groups showed that F2 slope was significantly shallower in both the volitionally slowed speech of the control participants and the disease-related slowed speech of participants with ALS across all three transitions (see p values in table 2). Control talkers who spoke slowly differed from the less impaired ALS talkers in /jæ/ and the slow ALS talkers in /oI/.
Table 1.
Raw means ± SD of each dependent variable computed for four groups of participants
| Transition | Group | F2 slope, Hz/ms | F2 range, Hz | Duration, ms | SPD, mm/s | DISP, mm |
|---|---|---|---|---|---|---|
/d / / |
CN | 8.44 ± 1.40 | 613.03 ± 92.50 | 72.74 ± 9.20 | 81.75 ± 25.40 | 3.03 ± 1.39 |
| CS | 6.69 ± 1.20 | 756.18 ± 160.00 | 114.73 ± 17.90 | 67.23 ± 16.33 | 4.63 ± 2.01 | |
| AN | 7.14 ± 1.60 | 568.19 ± 171.70 | 86.35 ± 19.10 | 99.06 ± 22.40 | 5.15 ± 2.40 | |
| AS | 4.98 ± 2.10 | 605.09 ± 156.00 | 119.60 ± 43.60 | 68.03 ± 29.80 | 4.08 ± 2.25 | |
| /oI/ | CN | 6.01 ± 1.30 | 613.13 ± 131.70 | 102.78 ± 13.80 | 80.59 ± 22.49 | 4.84 ± 2.90 |
| CS | 5.03 ± 1.30 | 1,127.59 ± 273.30 | 237.94 ± 70.60 | 51.94 ± 12.62 | 5.35 ± 2.50 | |
| AN | 4.89 ± 1.30 | 491.05 ± 143.70 | 101.91 ± 20.40 | 83.78 ± 16.70 | 4.54 ± 3.00 | |
| AS | 3.63 ± 1.50 | 511.56 ± 218.10 | 146.36 ± 5.80 | 60.00 ± 32.24 | 4.44 ± 2.70 | |
| /jæ/ | CN | 6.86 ± 2.20 | 873.07 ± 101.50 | 172.67 ± 50.80 | 99.88 ± 16.50 | 10.35 ± 3.90 |
| CS | 2.84 ± 1.40 | 953.83 ± 119.70 | 567.32 ± 314.2 | 45.76 ± 12.50 | 10.01 ± 3.40 | |
| AN | 6.01 ± 2.30 | 707.02 ± 194.60 | 117.60 ± 85.60 | 76.28 ± 17.70 | 8.31 ± 2.80 | |
| AS | 4.10 ± 2.40 | 731.88 ± 219.20 | 279.33 ± 124.80 | 61.94 ± 27.20 | 7.05 ± 4.30 | |
SPD = Average speed; DISP = displacement.
Table 2.
p values for each pairwise comparison between specified groups for each dependent variable
| F2 slope | F2 range | Duration | SPD | DISP | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
/d / / |
/oI/ | /j / / |
/d / / |
/oI/ | /j / / |
/d / / |
/oI/ | /j / / |
/d / / |
/oI/ | /j / / |
/d / / |
/oI/ | /j / / |
|
| CN-CS | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.002 | |
| CN-AN | 0.048 | ||||||||||||||
| CN-AS | 0.001 | 0.001 | 0.008 | 0.001 | 0.023 | 0.001 | |||||||||
| CS-AN | 0.001 | 0.001 | 0.008 | 0.014 | 0.001 | 0.001 | 0.017 | 0.008 | 0.011 | ||||||
| CS-AS | 0.028 | 0.002 | 0.001 | 0.014 | 0.001 | 0.048 | |||||||||
| AN-AS | 0.008 | 0.041 | 0.003 | 0.007 | 0.030 | 0.005 | 0.046 | ||||||||
Only p values <0.05 (statistically significant) are indicated. SPD = Average speed; DISP = displacement.
The main effect of group was significant for all of the remaining four variables as well. For F2 range, the results were F(3, 52) = 24.48, p < 0.001; F(3, 50) = 165.46, p < 0.001; and F(3, 47) = 8.32, p < 0.001 for /d /, /oI/ and /jæ/, respectively. Pairwise comparisons showed an expanded F2 range in the CS as compared to CN condition across contexts, when F2 range in talkers with ALS was most consistently reduced relative to the CS performance. For duration, the main effects of group showed F(3, 54) = 104.12, p < 0.001; F(3, 55) = 221.23, p < 0.001; and F(3, 51) = 190.39, p < 0.001 for /d
/, /oI/ and /jæ/, respectively. Pairwise comparisons showed an expanded F2 range in the CS as compared to CN condition across contexts, when F2 range in talkers with ALS was most consistently reduced relative to the CS performance. For duration, the main effects of group showed F(3, 54) = 104.12, p < 0.001; F(3, 55) = 221.23, p < 0.001; and F(3, 51) = 190.39, p < 0.001 for /d /, /oI/ and /jæ/, respectively. The CS data was consistently slower than CN, AN and AS productions, when AS was significantly slower than AN. For the average speed measure, they were F(3, 53) = 16.58, p < 0.001; F(3, 53) = 53.67, p < 0.001, and F(3, 50) = 260.07, p < 0.001 for /d
/, /oI/ and /jæ/, respectively. The CS data was consistently slower than CN, AN and AS productions, when AS was significantly slower than AN. For the average speed measure, they were F(3, 53) = 16.58, p < 0.001; F(3, 53) = 53.67, p < 0.001, and F(3, 50) = 260.07, p < 0.001 for /d /, /oI/ and /jæ/, respectively. Pairwise comparisons revealed slowing of the movement speed in the CS condition relative to the CN condition and AN performance. The AS group showed slower speed than the AN group in /d
/, /oI/ and /jæ/, respectively. Pairwise comparisons revealed slowing of the movement speed in the CS condition relative to the CN condition and AN performance. The AS group showed slower speed than the AN group in /d / and /oI/ contexts. Finally, for displacement, the main effect of group revealed F(3, 54) = 20.03, p < 0.001; F(3, 54) = 4.63, p < 0.006, for /d
/ and /oI/ contexts. Finally, for displacement, the main effect of group revealed F(3, 54) = 20.03, p < 0.001; F(3, 54) = 4.63, p < 0.006, for /d / and /oI/ but not /jæ/, with significant differences detected between CN and CS conditions only.
/ and /oI/ but not /jæ/, with significant differences detected between CN and CS conditions only.
Predicting Acoustics from Kinematics
Predicting F2 Slope
For the /d / transition, when average speed was used as a predictor of F2 slope in LMM and while controlling for group but allowing the speaking rate to vary within and across talkers, the effect of average speed on the acoustic measure was significant [F(1, 234) = 38.16, p < 0.001]; the group-by-average speed interaction was not significant. Average speed explained (R2) 22% of variance in F2 slope across repetitions for the within-talker model and 26% of variance for the across-talker model. When duration was added to the model, the effects of average speed remained significant across groups, with duration explaining an additional 12% of variance at both levels of the model [F(1, 241) = 16.72, p < 0.001]. This model revealed that group and average speed contributed uniquely to variation in F2 slope above and beyond duration.
/ transition, when average speed was used as a predictor of F2 slope in LMM and while controlling for group but allowing the speaking rate to vary within and across talkers, the effect of average speed on the acoustic measure was significant [F(1, 234) = 38.16, p < 0.001]; the group-by-average speed interaction was not significant. Average speed explained (R2) 22% of variance in F2 slope across repetitions for the within-talker model and 26% of variance for the across-talker model. When duration was added to the model, the effects of average speed remained significant across groups, with duration explaining an additional 12% of variance at both levels of the model [F(1, 241) = 16.72, p < 0.001]. This model revealed that group and average speed contributed uniquely to variation in F2 slope above and beyond duration.
The F2 slope prediction models in /oI/ were similar to the ones in /d /, with significant effects of group [F(1, 39) = 10.44, p < 0.003] and average speed [F(1, 265) = 158.33, p < 0.001] and no interaction. Variation in average speed explained 26% of variance in the within-talker models and 22% of variance in the across-talker models in each group. Adding duration to the model resulted in a significant group-by-duration interaction [F(1, 267) = 4.08, p < 0.045]. Adding duration accounted for only an additional 3% of the model's variance, explained at each level.
/, with significant effects of group [F(1, 39) = 10.44, p < 0.003] and average speed [F(1, 265) = 158.33, p < 0.001] and no interaction. Variation in average speed explained 26% of variance in the within-talker models and 22% of variance in the across-talker models in each group. Adding duration to the model resulted in a significant group-by-duration interaction [F(1, 267) = 4.08, p < 0.045]. Adding duration accounted for only an additional 3% of the model's variance, explained at each level.
In contrast with /d / and /oI/, a significant group by average speed interaction was observed in /jæ/ [F(1, 152) = 13.34, p < 0.001], suggesting that the relationship between F2 slope and average speed differed by group for this transition and was larger for the control group. After controlling for duration in the model, the effect of speed remained significant [F(1, 161) = 14.04, p < 0.001]. However, by adding duration, the strength of model fit increased from 37 to 75% for within-talker levels and from 19 to 74% for between-talker levels.
/ and /oI/, a significant group by average speed interaction was observed in /jæ/ [F(1, 152) = 13.34, p < 0.001], suggesting that the relationship between F2 slope and average speed differed by group for this transition and was larger for the control group. After controlling for duration in the model, the effect of speed remained significant [F(1, 161) = 14.04, p < 0.001]. However, by adding duration, the strength of model fit increased from 37 to 75% for within-talker levels and from 19 to 74% for between-talker levels.
Predicting F2 Range
Examination of the models that predicted F2 range based on displacement, revealed a significant displacement-by-group interaction [F(1, 238) = 6.2, p < 0.01 for /d /, F(1, 149) = 6.33, p < 0.01 for /oI/, and F(1, 154) = 4.72, p < 0.03 for /jæ/]. For the ALS group, displacement decreased in parallel with F2 range for all three transitions. Controls showed this effect only for the /jæ/ transition. Displacement minimally explained variation in F2 range in /d
/, F(1, 149) = 6.33, p < 0.01 for /oI/, and F(1, 154) = 4.72, p < 0.03 for /jæ/]. For the ALS group, displacement decreased in parallel with F2 range for all three transitions. Controls showed this effect only for the /jæ/ transition. Displacement minimally explained variation in F2 range in /d / in the control group (9% in the within- and 11% in the between-subject models). Adding duration did not change the strength of this weak prediction. For /oI/, the original model explained a significant portion of the variance (23 and 30%), yet adding duration eliminated the significant effect, suggesting the primary role of duration on the relation between displacement and F2 range in /oI/. In /jæ/, adding duration did not change the significant interaction between group and displacement [F(1, 157) = 5.07, p < 0.03]. For the /jæ/ transition, displacement explained 25 and 33% of variance in the F2 range within and across talkers (primarily due to the ALS group); adding duration improved the overall model only by 9 and 6%, respectively.
/ in the control group (9% in the within- and 11% in the between-subject models). Adding duration did not change the strength of this weak prediction. For /oI/, the original model explained a significant portion of the variance (23 and 30%), yet adding duration eliminated the significant effect, suggesting the primary role of duration on the relation between displacement and F2 range in /oI/. In /jæ/, adding duration did not change the significant interaction between group and displacement [F(1, 157) = 5.07, p < 0.03]. For the /jæ/ transition, displacement explained 25 and 33% of variance in the F2 range within and across talkers (primarily due to the ALS group); adding duration improved the overall model only by 9 and 6%, respectively.
Relating the Severity of Speech Impairment to Physiologic Measures
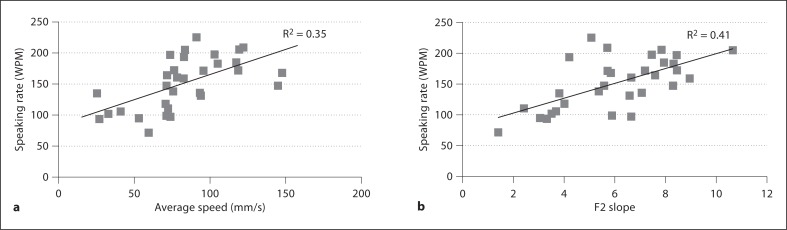
Pearson correlations were computed between speaking rate and acoustic and kinematic measures, and speech intelligibility and all acoustic and kinematic measures for talkers with ALS. Figure 2 shows the relations between measures of average speed and speaking rate (a) and F2 slope and speaking rate (b) in /d /. Across all contexts, correlations between these variables were of similar magnitude (r = 0.41–0.64). Figure 3 shows an association between F2 slope in /d
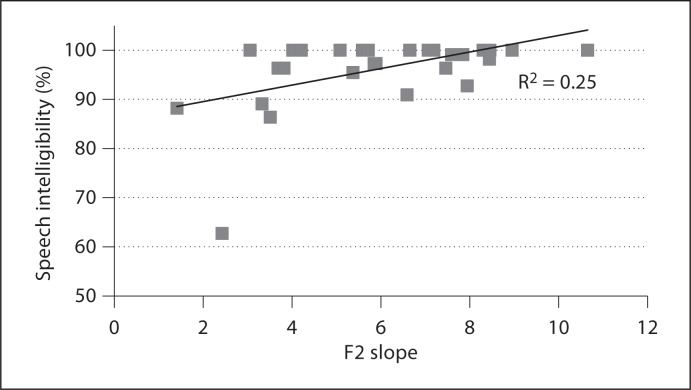
/. Across all contexts, correlations between these variables were of similar magnitude (r = 0.41–0.64). Figure 3 shows an association between F2 slope in /d / and speech intelligibility (r = −0.50; correlation between speed and intelligibility was r = 0.27 in the same context). Relatively weak relationships were observed in /jæ/ and /oI/ for F2 slope (r = 0.28 and r = 0.34, respectively). Speech intelligibility was moderately correlated with average speed in /oI/ (r = 0.51) and F2 range in /jæ/ (r = 0.49).
/ and speech intelligibility (r = −0.50; correlation between speed and intelligibility was r = 0.27 in the same context). Relatively weak relationships were observed in /jæ/ and /oI/ for F2 slope (r = 0.28 and r = 0.34, respectively). Speech intelligibility was moderately correlated with average speed in /oI/ (r = 0.51) and F2 range in /jæ/ (r = 0.49).
Fig. 2.
a Average speed versus speaking rate across individuals with ALS producing the transition in /d /. b F2 slope versus speaking rate across individuals with ALS producing the transition in /d
/. b F2 slope versus speaking rate across individuals with ALS producing the transition in /d /.
/.
Fig. 3.
F2 slope transition in /d / versus speech intelligibility as measured by Sentence Intelligibility Test.
/ versus speech intelligibility as measured by Sentence Intelligibility Test.
Discussion
Summary
The goal of our investigation was to test the strength of association between the acoustic measures of average F2 slope and F2 range with their parallel measures in the kinematic domain, tongue movement speed and displacement. We found moderately strong associations between F2 slope and speed across the group of talkers with ALS of varying severity, and in controls, who were asked to volitionally slow their speaking rate. Under the same conditions, the relations between F2 range and displacement were much weaker than those of F2 slope and speed and bound to a single transition only. Speaking rate was consistently related to duration, F2 slope, and movement speed. Speech intelligibility was most consistently associated with the acoustic measures of F2 slope but not the movement measures.
Slow Speech in Healthy Talkers and Patients with ALS
Speaking slowly had a significant effect on speech movements in control talkers. As these talkers slowed their speech to approximately 50% of their typical rate, they also reduced their movement speed by the same proportion and increased displacements by approximately 10%. These findings agree with other studies showing that slowing speaking rate is associated with significant decreases in movement velocities and smaller increases in movement displacements in healthy talkers [27, 28, 29, 30]. In the acoustic domain, both F2 slope and F2 range changed notably as rate slowed. The F2 range increased as speaking rate decreased in the control talkers. Previous literature on gesture overlap has shown that F2 frequencies at vocalic onsets and offsets undergo significant adjustments with changes in speaking rate in the direction closer to the preceding consonant loci [31]. Previously, the largest F2 changes due to rate were reported for female speakers [10]. In our sample, the F2 range changes might have been particularly prominent due to the mostly female composition of our control group. Although F2 slope was proposed to be an invariant characteristic of diphthongs during rate variations [32], in more recent studies, the slope of F2 has been shown to be sensitive to rate adjustments [10, 33]. The F2 slope has been observed to change from shallow to steep as speaking rate changed from slow to fast, although these changes have been diphthong-specific [10]. In this study, we observed relatively large rate-induced changes in F2 range and slope in the control group in all three contexts.
Slow rate is a characteristic feature of dysarthria due to ALS and is one of the earliest indicators of an impending decline in speech intelligibility [19, 34]. In this study, a speaking rate of 160 WPM was used to categorize the participants into two groups. On all of the acoustic and kinematic measures, talkers in the normal-rate ALS group performed similarly to control talkers speaking at normal rates. Talkers with slower than normal speaking rate, however, differed from controls speaking at normal speaking rates, primarily in the F2 slope measure in all three contexts and average movement speed of /jæ/. At the same time, talkers in this group and control talkers speaking slowly differed with respect to their F2 range values. Interestingly, talkers in the slow ALS group showed a significantly reduced F2 range as compared to the controls, who showed an increased F2 range when speaking slowly. This suggests that the mechanism of rate control in the healthy system is different from a slow rate induced by disease. Healthy talkers, when speaking slowly, increased their F2 range, which might have allowed them to reach intended acoustic targets more precisely. In contrast, talkers with ALS who spoke slowly showed a reduced F2 range, indicative of performance decline due to disease progression. Modelling the relation between transition durations and F2 ranges for a group of patients with PD and controls, Weismer [17] suggested that timing abnormalities alone (i.e., longer than normal durations) are not able to fully explain the spatial abnormalities in F2 ranges. Our data agreed with this suggestion and showed that, instead of the larger F2 ranges predicted by increased transition durations seen in healthy talkers, slower speech in talkers with ALS was associated with smaller formant excursions.
Are Acoustic and Kinematic Measures Related?
Our findings add support to prior suggestions that F2 slope can serve as a potential proxy for characterizing some aspects of aberrant lingual performance during speech [15, 35]. The association between acoustic and kinematic variables is complicated by the fact that they both respond to speaking rate variations. The relation between these two variables in patients with ALS was in the direction predicted by the normal model (i.e., longer durations led to slower speed and shallower slopes) in our data, therefore, it is possible that these changes are driven solely by increased durations. If changes in movement speed during disease progression are driven purely by changes in speaking rate reflected in segment duration, then we cannot single out the tongue function from the acoustic measures. Speaking rate is a variable that is ‘systemic’ in nature, reflecting changes across multiple speech subsystems (i.e., from respiratory to velopharyngeal). However, the significant effect of movement speed on F2 slope remained after statistically controlling for duration. This finding suggests that F2 slope provides information about tongue movements, even in the presence of significant durational effects.
Hartelius et al. [16] also suggested that the slope of F2 can be used to estimate underlying articulatory speed if the duration differences across productions are equated using time normalization. In our study, however, the variation in movement speed alone accounted only for a modest portion of the variance in F2 slope (at best 26%) when the durations were controlled for statistically. Other factors must be involved (e.g., abnormalities in articulators other than tongue blade) and should be investigated for their effect on the acoustic characteristics of speech and lingual kinematics. However, it is also possible that stronger relations would have been observed in our movement variables if we used other tongue regions [36]. Additionally, the method we chose for parsing the signals may have contributed noise to the data and different results would have been obtained if intervals were defined based on movement signals.
Tongue displacements were relatively weakly associated with F2 range in our data, particularly when duration was controlled. Their relationships were comparable to F2 slope results for the /jæ/ transition only. Interestingly, Mefferd and Green [4] also observed a significant association between movement displacements and formant frequencies in response to changes in speaking rate in healthy talkers producing sequence /ja/ (in ‘Mia’). Obviously, there is a strong effect of context on the F2 range and displacement relations. Additionally, comparing the two studies suggests that the effect of the jaw (the jaw was not decoupled in [4]) and the correspondence between acoustic and kinematic measures must be considered when studying the nature of the relations across acoustic and kinematic domains.
Correlates of Speaking Rate and Intelligibility Decline in ALS
One important clinical issue in managing patients with ALS is to predict when an individual will lose functional oral communication [19, 37]. Several investigators have advocated for monitoring speaking rate for this purpose [19, 34]. The speed of jaw movement emerged in one previous study as another potential predictor [37] with a transient increase in jaw speed occurring prior to changes in speech intelligibility. We have previously suggested that this change in jaw movement might be compensatory in response to the onset of tongue function decline. Interestingly, tongue movement speed during /jæ/ was the only measure that distinguished mild ALS from control talkers speaking at normal rates, showing a decrease of approximately 40% in the mildly impaired group. This measure might be a sensitive indicator of early disease-related changes. The relationship between jaw and tongue speed as the disease progresses, however, needs to be explored further in the future.
Another important clinically motivated goal of this research was to identify the aspects of impaired speech that may be related to speech intelligibility. F2 slope emerged as one of those aspects in several earlier studies [9, 11]. In our study, the correlations between F2 slopes and speech intelligibility were moderate at best and varied for different contexts, in agreement with previous research. Kim et al. [15] also found that the relationship between F2 slope and intelligibility was moderate and context-specific. Their findings and ours underscore the importance of word selection when testing speech motor impairment [11].
Kinematic variables were not consistently correlated with speech intelligibility; one moderate correlation between average speed and intelligibility was observed in /oI/. This finding is in contrast to those of Weismer et al. [35], who observed significant correlations between articulatory working space and movement speed across groups of talkers with ALS and PD and speech intelligibility. In contrast to the current study which used segment-specific movement measures, their kinematic measures were based on calculations across a relatively large phonetically balanced passage.
In conclusion, the findings of this study suggested that F2 slope is a useful clinical marker to track disease progression in patients with ALS when kinematic techniques are not available. The advantage of F2 slope is that it is related, to a degree, to movement speed, sensitive to disease progression, and is linked to decline in speech intelligibility. Movement measures, however, might be more useful to diagnose early changes in the tongue due to disease. Furthermore, they are more specific by nature and reflect the function of a single organ, whereas acoustic measures are a product of interactions between multiple speech organs. Future work should continue to identify measurements other than average speed (e.g., peak speed) that might be more sensitive in detecting the presence of disease.
Acknowledgements
This work has been supported by the National Institute of Health, National Institute on Deafness and Other Communication Disorders, grant R01DC009890, Canadian Foundation for Innovation (CFI-LOF No. 15704), and Connaught Foundation, University of Toronto. The authors would like to thank Michelle Falikowski, Sonya Spalding, Cynthia Didion, and Lori Synhorst for assistance with data collection and analysis.
References
- 1.Lindblom BEF, Sundberg JEF. Acoustical consequences of lip, jaw, tongue, and larynx movement. J Acoust Soc Am. 1971;50:1166–1179. doi: 10.1121/1.1912750. [DOI] [PubMed] [Google Scholar]
- 2.Stevens KN. On the quantal nature of speech. J Phonet. 1989;17:3–45. [Google Scholar]
- 3.Stevens KN, House AS. Development of a quantitative description of vowel articulation. J Acoust Soc Am. 1955;27:484–493. [Google Scholar]
- 4.Mefferd AS, Green JR. Articulatory-to-acoustic relations in response to speaking rate and loudness manipulations. J Speech Lang Hear Res. 2010;53:1206–1219. doi: 10.1044/1092-4388(2010/09-0083). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tasko SM, Greilick K. Acoustic and articulatory features of diphthong production: a speech clarity study. J Speech Lang Hear Res. 2010;53:84–99. doi: 10.1044/1092-4388(2009/08-0124). [DOI] [PubMed] [Google Scholar]
- 6.Hirose H, Kiritani S, Sawashima M. Velocity of articulatory movements in normal and dysarthric subjects. Folia Phoniatr. 1982;34:210–215. doi: 10.1159/000265651. [DOI] [PubMed] [Google Scholar]
- 7.Kent RD, Netsell R, Bauer LL. Cineradiography assessment of articulatory mobility in the dysarthrias. J Speech Hear Disord. 1975;40:467–480. doi: 10.1044/jshd.4004.467. [DOI] [PubMed] [Google Scholar]
- 8.Yunusova Y, Weismer G, Westbury JR, Lindstrom MJ. Articulatory movements during vowels in speakers with dysarthria and healthy controls. J Speech Lang Hear Res. 2008;51:596–611. doi: 10.1044/1092-4388(2008/043). [DOI] [PubMed] [Google Scholar]
- 9.Rosen KM, Goozée JV, Murdoch BE. Examining the effects of multiple sclerosis on speech production: does phonetic structure matter? J Commun Disord. 2008;41:49–69. doi: 10.1016/j.jcomdis.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Tjaden K, Wilding GE. Rate and loudness manipulations in dysarthria: acoustic and perceptual findings. J Speech Lang Hear Res. 2004;47:766–783. doi: 10.1044/1092-4388(2004/058). [DOI] [PubMed] [Google Scholar]
- 11.Weismer G, Kent R, Hodge M, Martin R. The acoustic signature for intelligibility test words. J Acoust Soc Am. 1988;84:1281–1291. doi: 10.1121/1.396627. [DOI] [PubMed] [Google Scholar]
- 12.Liss JM, Weismer G. Selected acoustic characteristics of contrastive stress production in control geriatric, apraxic, and ataxic dysarthric speakers. Clin Linguist Phon. 1994;8:45–66. [Google Scholar]
- 13.Ziegler W, von Cramon D. Vowel distortion in traumatic dysarthria: a formant study. Phonetica. 1983;40:63–78. doi: 10.1159/000261681. [DOI] [PubMed] [Google Scholar]
- 14.Kent RD, Kent JF, Weismer G, Martin RE. Relationships between speech intelligibility and the slope of second-formant transitions in dysarthric subjects. Clin Linguist Phon. 1989;3:347–358. [Google Scholar]
- 15.Kim Y, Weismer G, Kent RD, Duffy JR. Statistical models of F2 slope in relation to severity of dysarthria. Folia Phoniatr Logop. 2009;61:329–335. doi: 10.1159/000252849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartelius L, Schalling E, Krull D, Lindblom B. Formant transitions in ataxic speech: the shape and speed of formant trajectories in individuals with multiple sclerosis and control speakers. J Med Speech-Lang Pathol. 2010;18:54–60. [Google Scholar]
- 17.Weismer G. Assessment of articulatory timing. In: Cooper JA, editor. Assessment of Speech and Voice Production. Research and Clinical Applications. Vol. 1. Bethesda: National Institutes of Health; 1991. pp. 84–95. [Google Scholar]
- 18.Duffy JR. Motor Speech Disorders: Substrates, Differential Diagnosis, and Management. Philadelphia: Elsevier; 2005. [Google Scholar]
- 19.Yorkston KM, Strand E, Miller R, Hillel A, Smith K. Speech deterioration in amyotrophic lateral sclerosis: implications for the timing of intervention. J Med Speech-Lang Pathol. 1993;1:35–46. [Google Scholar]
- 20.Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A, BDNF ALS Study Group The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 21.Beukelman D, Yorkston K, Hakel M, Dorsey M. Speech Intelligibility Test, Computer software. Lincoln: Madonna Rehabilitation Hospital; 2007. [Google Scholar]
- 22.Turner GS, Tjaden K, Weismer G. The influence of speaking rate on vowel space and speech intelligibility for individuals with amyotrophic lateral sclerosis. J Speech Lang Hear Res. 1995;38:1001–1013. doi: 10.1044/jshr.3805.1001. [DOI] [PubMed] [Google Scholar]
- 23.Dromey C, Nissen S, Nohr P, Fletcher SG. Measuring tongue movements during speech: adaptation of a magnetic jaw tracking system. Speech Commun. 2006;48:463–473. [Google Scholar]
- 24.Yunusova Y, Giannakeas V, Green J.Using JT-3D (jaw tracker) for the acquisition of tongue movement. Department of Speech-Language Pathology, University of Toronto, 2010, Tech Rep 1.
- 25.Milenkovic PH. TF32, Computer software. Madison: University of Wisconsin; 2001. [Google Scholar]
- 26.Roberts KJ, Monaco JP. Effect size measures for the two-level linear multilevel model. Annu Meet Am Educ Res Assoc: Mississippi; 2006. [Google Scholar]
- 27.Kuehn DP, Moll KL. A cineradiographic study of VC and CV velocities. J Phon. 1976;4:303–320. [Google Scholar]
- 28.Perkell JS, Zandipour M, Matthies ML, Lane H. Economy of effort in different speaking conditions. I. A preliminary study of intersubject differences and modeling issues. J Acoust Soc Am. 2002;112:1627–1641. doi: 10.1121/1.1506369. [DOI] [PubMed] [Google Scholar]
- 29.Smith A, Kleinow J. Kinematic correlates of speaking rate changes in stuttering and normally fluent adults. J Speech Lang Hear Res. 2000;43:521–536. doi: 10.1044/jslhr.4302.521. [DOI] [PubMed] [Google Scholar]
- 30.Tasko SM, McClean MD. Variations in articulatory movement with changes in speech task. J Speech Lang Hear Res. 2004;47:85–100. doi: 10.1044/1092-4388(2004/008). [DOI] [PubMed] [Google Scholar]
- 31.Tjaden K, Weismer G. Speaking-rate-induced variability in F2 trajectories. Speech Lang Hear Res. 1998;41:976–989. doi: 10.1044/jslhr.4105.976. [DOI] [PubMed] [Google Scholar]
- 32.Gay T. Effect of speaking rate on diphthong formant movements. J Acoust Soc Am. 1968;44:1570–1573. doi: 10.1121/1.1911298. [DOI] [PubMed] [Google Scholar]
- 33.Dolan WB, Mimori Y. Rate-dependent variability in English and Japanese complex vowel F2 transitions. UCLA Working Papers in Phonetics. 1986;63:125–153. [Google Scholar]
- 34.Ball LJ, Beukelman DR, Pattee GL. Communication effectiveness of individuals with amyotrophic lateral sclerosis. J Commun Disord. 2004;37:197–215. doi: 10.1016/j.jcomdis.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Weismer G, Yunusova Y, Bunton K.Measures to evaluate the effects of DBS on speech production. J Neurolinguist, in press. [DOI] [PMC free article] [PubMed]
- 36.Stevens K. Current Studies in Linguistics. Cambridge: MIT Press; 2000. Acoustic Phonetics. [Google Scholar]
- 37.Yunusova Y, Green JR, Lindstrom MJ, Ball LJ, Pattee GL, Zinman L. Kinematics in disease progression in bulbar ALS. J Commun Disord. 2010;43:6–20. doi: 10.1016/j.jcomdis.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]