Abstract
Nighttime blood pressure (BP) dipping can be quantified as the ratio of mean nighttime (sleep) BP to mean daytime (awake) BP. People whose dipping ratio is 0.90 have been referred to as nondippers, and nondipping is associated with cardiovascular disease events. We examined the relationship between systolic nighttime BP dipping in young adults and presence of coronary artery calcium (CAC) 10-15 years later using data from the ambulatory BP monitoring substudy of the Coronary Artery Risk Development in Young Adults (CARDIA) study. Among 239 participants with adequate measures of both nighttime and daytime readings and coronary artery calcium, the systolic BP dipping ratio ranged from 0.72 to 1.24 (mean 0.88, SD 0.06), and CAC was present 10 to 15 years later in 54 participants (22.6%). Compared to those whose systolic BP dipping ratio ranged from 0.88 to 0.92 (Quartile 3), the 57 participants (23.9%) with less pronounced or absent dipping (ratio 0.92 to 1.24, Quartile 4) had an unadjusted odds ratio of 4.08 (95% CI 1.48-11.2) for presence of CAC. The 60 participants (25.1%) with a more pronounced dipping (ratio 0.72 to 0.85, Quartile 1) also had greater odds for presence of CAC (OR 4.76; 95% CI 1.76-12.9). When modeled as a continuous predictor, a U-shaped relationship between systolic BP dipping ratio and future CAC was apparent, and persisted after adjustment for multiple potential confounders (p<0.001 for quadratic term). Both failure of systolic BP to dip sufficiently and “overdipping” during nighttime may be associated with future subclinical coronary atherosclerosis.
Keywords: ambulatory blood pressure, diurnal blood pressure, blood pressure dipping, coronary artery calcium, subclinical atherosclerosis
Introduction
Ambulatory blood pressure monitoring (ABPM) provides the best noninvasive assessment of a person’s average blood pressure (BP).1 The use of 24-hour ABPM also uniquely permits assessment of a person’s BP during sleep. BP normally decreases (“dips”) during sleep, and people whose BP dips ≤10% from their daytime baseline BP have been referred to as “nondippers”. In patients with hypertension, nondipping is associated with target organ damage and cardiovascular events independent of the overall blood pressure.2-7 Nondipping may be a risk factor for cardiovascular disease even when the 24 hour BP average is not elevated.7 In one study of normotensive individuals, the nondipping pattern was shown to be associated with cardiac hypertrophy and remodeling.8 In a small study of men with angiographically proven CAD, 72% exhibited a nondipping BP pattern compared to 46% of matched controls without known CAD.9 Another study showed that levels of von Willebrand factor, D-dimer, fibrinogen, and P-selectin were significantly higher among patients with documented CAD who were nondippers.10 Whether or not the nighttime BP dipping pattern during young adulthood is associated with development of atherosclerosis later in life is not known.
The Coronary Artery Risk Development in Young Adults (CARDIA) Study presents a unique opportunity to study this association. A substudy conducted at the Year 5 CARDIA examination collected ambulatory BP readings on a subset of CARDIA participants. We used these data to analyze the association between nighttime dipping and presence of coronary artery calcium, an indicator of atherosclerosis, measured 10-15 years later at the Year 15 and Year 20 CARDIA examinations. We hypothesized that BP dipping in young adults would be inversely related to later presence of coronary artery calcium and that the relationship would be maintained after adjustment for traditional atherosclerosis risk factors.
Methods
Overall Design
CARDIA is an ongoing prospective cohort study conducted at four sites throughout the United States.11 The CARDIA study and this substudy were approved by the appropriate Institutional Review Boards, and informed consent was obtained from each study participant.
Study Participants
As previously described,12 ABPM was performed at only one CARDIA study site (Birmingham, AL). A list of people randomly chosen from the four race/gender subgroups was generated at the CARDIA Coordinating Center. After excluding individuals whose jobs would make ambulatory BP monitoring difficult (e.g., truck drivers, delivery people), a total of 316 people (147 men and 169 women; 112 whites and 204 blacks) participated in the substudy.
Ambulatory Blood Pressure Monitoring
Ambulatory BP was monitored over a 24-hour period using a Suntech Accutracker II (Suntech Medical, Morrisville, NC), using an appropriately sized cuff inflated approximately every 20 minutes.13 To reduce anticipation effects, the inflation schedule was variable. If any value outside preset limits (systolic ≥ 220 or ≤80 mm Hg; diastolic ≥130 or ≤40 mm Hg) was detected during a recording, that measurement was rejected and another measurement was immediately made. In addition, a change of 50 mm Hg in systolic pressure, of 40 mm Hg in diastolic pressure, or of 50 mm Hg in pulse pressure also triggered a rejection and a new reading.
Participants were asked to record in a diary their activities and sleep times during the monitoring session. We defined a “daytime sleeper” as someone who, based on the reports in the diary, accumulated 6 hours or more of sleep between the hours of 1000 (10am) and 2200 (10pm), and had no more than 1 hour of accumulated sleep between 0000 (midnight) and 0600 (6am). For everyone else, we defined nighttime as midnight to 0600 and daytime as 1000 to 2200. For a session to be deemed adequate, we required a minimum of 10 daytime measurements and 5 nighttime measurements during these specific intervals. In total, one person was excluded due to being a “daytime sleeper”, and 34 participants were excluded due to inadequate daytime or nighttime measurements, leaving 281 participants in the cohort at baseline. Our primary exposure for this analysis was “BP dipping ratio,” defined as the ratio of mean nighttime systolic BP to mean daytime systolic BP.
Sleep quality during the ABPM session was also recorded in the diary completed by participants. We defined “poor sleep quality” during the ABPM session as any report of being unable to fall asleep, being awakened more than 5 times, or not being able to sleep at all.
Coronary Artery Calcium
In CARDIA, coronary artery calcium was assessed at year 15 (10 years after the ABPM session) and at year 20 (15 years after the ABPM session) using cardiac computed tomography (CT) scanning. Consenting participants underwent two sequential scans using a multidetector, electrocardiographically-gated CT scanner (General Electric Lightspeed) with a standard phantom for calibration. Total coronary artery calcium score was calculated using a modified Agatston method.14 Readers were masked to participant characteristics as well as the paired scan results. Scans were selectively over-read by an expert in cardiovascular imaging. The validity and reproducibility of these methods have been previously described.15,16 We used CARDIA year 15 results only if year 20 results were not available and adjusted for exam year in our final models.
Clinic Blood Pressure Measurements
We used the BP measured at the CARDIA year 5 visit (same year as the ABPM) as baseline clinic BP. Prior to the study visit, participants were asked to refrain from smoking and heavy physical activity. After five minutes of rest, three seated right arm BP measurements were recorded at one-minute intervals. Using an appropriately sized cuff, the first-phase and fifth-phase Korotkoff sounds were taken as the systolic and diastolic blood pressures, respectively, using a random zero sphygmomanometer. Clinic BP was determined by the average of the second and third readings.
Additional Covariates
Additional covariates included education level, body mass index (BMI), presence of diabetes, family history of hypertension, family history of diabetes, smoking, serum cotinine level, cholesterol levels, and physical activity (please see Methods Supplement at http://hyper.ahajournals.org).
Analysis
We divided BP dipping ratio into quartiles. Characteristics of the participants were analyzed overall and then by quartile of dipping ratio. Coronary artery calcium score was available for 213 participants 15 years following their ABPM session (i.e., at CARDIA year 20). Of the remaining 68 participants, coronary artery calcium was available for 26 of them 10 years following their ABPM session (i.e. at CARDIA year 15). In total, we had coronary artery calcium data on 239 of the 281 participants (85%). Participants without a coronary artery calcium measurement tended to be slightly younger (mean 28.6 vs 30.4 years, p=0.003) and have slightly higher nighttime systolic BP (109.5 mm Hg vs 105.0 mm Hg, p=0.015). To be sure we included important confounders in the models, we examined the associations between baseline participant characteristics and later presence of CAC (please see Supplemental Table 1 at http://hyper.ahajournals.org).
We calculated unadjusted and adjusted odds ratios for presence of coronary artery calcium using the BP dipping quartile centered on 10% lower nocturnal than daytime systolic blood pressure as the reference group. This quartile had the lowest odds of coronary calcium. Logistic regression was used to analyze the association between BP dipping ratio and the probability of coronary artery calcium at year 20 (or year 15 if year 20 was not available), with adjustment for other covariates, and with testing for non-linearity in the dipping ratio association with coronary calcium by adding a quadratic term of the dipping ratio. We assessed the goodness-of-fit of the model by fitting a spline function. Additionally, we stratified our analyses by sex and by race, and tested for interactions in the full model. We also assessed for effect modification by baseline smoking status. As a supplementary analysis we reexamined the relationship of BP dipping with future CAC when defining BP dipping as the difference between the daytime and nighttime average levels rather than the ratio of the two.
To further evaluate for possible confounding, we compared modifiable risk factors of participants across the four quartiles at the time of the coronary artery calcium measurement to assess whether there were significant differences compared to when measured at baseline. Those risk factors with differences at a p-value <0.10 were added to a final multivariable model. Data analyses were performed using SAS version 9.2 (Cary, North Carolina) and R version 2.12.2 (Vienna, Austria).
Results
Characteristics of Sample
The mean age of participants at baseline was 30 years; 131/281 (47%) were male, and 177/281 (63%) were Black (Table 1). Twenty out of 281 (7%) had known hypertension. Few had known diabetes, but more than half were overweight or obese. Among this study sample, 79/281 (28%) were current smokers at baseline. Mean BMI among the participants was 26.6 kg/m2. Mean clinic BP at baseline was 109/73 mm Hg. Mean low density lipoprotein cholesterol (LDL) was 111 mg/dl, and mean high density lipoprotein cholesterol (HDL) was 51 mg/dl.
Table 1. Characteristics of Sample at Baseline (CARDIA Year 5) by Dipping Ratio Quartiles (N=281).
| Quartile: Dipping Ratio* | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Total | Q1: 0.72- 0.85 |
Q2: 85- 0.88 |
Q3: 0.88- 0.92 |
Q4: 0.92- 1.24 |
p- value |
| Age, mean (SD), years | 30.2 (3.7) |
30.8 (3.7) |
29.5 (3.5) |
29.9 (4.2) |
30.5 (3.2) |
0.14 |
| Age categories, % | ||||||
| 23-29 years | 43.4 | 32.9 | 56.3 | 44.3 | 40.0 | 0.04 |
| 30-36 years | 56.6 | 67.1 | 43.7 | 55.7 | 60.0 | |
| Male, % | 46.6 | 47.1 | 40.9 | 44.3 | 54.3 | 0.43 |
| Race categories, % | ||||||
| Black | 63.4 | 44.3 | 60.6 | 70.0 | 78.6 | 0.001 |
| White | 36.7 | 55.7 | 39.4 | 30.0 | 21.4 | |
| Education, mean (SD) | 13.5 | 13.5 | 13.6 | 13.5 | 13.3 | 0.90 |
| years | (2.0) | (2.4) | (1.9) | (1.9) | (1.8) | |
| BMI, mean (SD) kg/ m2 | 26.6 | 25.6 | 26.5 | 26.4 | 27.9 | 0.08 |
| (5.2) | (4.7) | (5.6) | (5.5) | (5.0) | ||
| BMI categories, % | ||||||
| Normal (<25 kg/m2) | 43.0 | 51.4 | 47.9 | 44.3 | 28.6 | 0.08 |
| Overweight (25-29 kg/m2) |
31.0 | 31.4 | 26.8 | 31.4 | 34.3 | |
| Obese (≥ 30 kg/m2) | 26.0 | 17.2 | 25.3 | 24.3 | 37.1 | |
| Current smoker, % | 28.1 | 32.9 | 21.1 | 17.1 | 41.4 | 0.005 |
| Pack-years of tobacco, mean (SD) |
2.6 (5.5) |
3.6 (7.0) |
2.5 (6.6) |
1.7 (3.3) |
2.5 (4.3) |
0.21 |
| Serum cotinine level (ng/ml) at year 0, mean (SD) |
64.8 (129) |
81.5 (157) |
50.0 (121) |
54.5 (113) |
73.4 (119) |
0.41 |
| High density lipoprotein cholesterol (mg/dl), mean (SD) |
50.8 (14) |
51.2 (15) |
52.1 (13) |
52.9 (15) |
46.9 (12) |
0.054 |
| Low density lipoprotein cholesterol (mg/dl), mean (SD) |
111.1 (30) |
117.3 (32) |
111.6 (31) |
106.4 (26) |
108.8 (31) |
0.17 |
| Known diabetes mellitus, % |
2.5 | 0 | 4.2 | 2.9 | 2.9 | 0.43 |
| Known hypertension, % |
7.1 | 5.7 | 9.9 | 4.3 | 8.6 | 0.55 |
| Glucose level (mg/dl) at year 7, mean (SD) |
92.7 (34) |
87.7 (6.9) |
91.1 (32.3) |
92.2 (24.2) |
100.9 (56.7) |
0.19 |
| Baseline clinic SBP (mm Hg), mean (SD) |
109.1 (11) |
108.3 (8.6) |
110.1 (11.8) |
108.5 (10.7) |
109.6 (11.2) |
0.73 |
| Baseline clinic DBP (mm Hg), mean (SD) |
73.1 (9.8) |
71.3 (9.8) |
74.0 (10.9) |
73.5 (8.3) |
73.5 (10.0) |
0.34 |
| Clinic pulse pressure (mm Hg), mean (SD) |
36.0 (8.2) |
37.1 (9.1) |
36.1 (7.8) |
35.0 (7.4) |
36.0 (8.5) |
0.54 |
| Alcohol intake (ML/day), mean (SD) |
11.6 (20.4) |
13.4 (22.9) |
9.6 (15.8) |
10.6 (20.3) |
12.6 (22.2) |
0.68 |
| Physical activity (Kcal/d), mean (SD) |
317.1 (267.2) |
326.4 (275.0) |
282.7 (267.7) |
354.3 (285.5) |
305.3 (239.0) |
0.43 |
| Family history of hypertension, % |
59.1 | 58.6 | 60.6 | 60.0 | 57.1 | 0.98 |
| Family history of diabetes, % |
16.0 | 18.6 | 14.1 | 12.9 | 18.6 | 0.71 |
| Poor sleep quality during ABPM, % |
16.4 | 18.6 | 16.9 | 12.9 | 17.1 | 0.82 |
Quartiles of mean nocturnal systolic BP/mean daytime systolic BP ratio: Q1: 0.7197-0.8454 (n=70); Q2: 0.8455-0.8809 (n=71); Q3: 0.8810-0.9203 (n=70); Q4: 0.9204-1.2358 (n=70)
CARDIA, Coronary Artery Risk Development in Young Adults Study; SBP, systolic blood pressure; DBP, diastolic blood pressure; ABPM, ambulatory blood pressure monitoring
At the time when coronary artery calcium was assessed, the only examined characteristic that differed (at a significance level p<0.10) across the quartiles of year 5 BP dipping ratio was smoking status (please see Supplemental Table 2 at http://hyper.ahajournals.org). The actual rate of smoking cessation was greatest among those in quartile 1, although the differences across quartiles were not significant (p=0.52).
Blood Pressure Dipping
Nighttime BP dipping ranged from 28% (i.e., average nighttime BP was 28% less than average daytime BP; BP dipping ratio = 0.72) to −24% (i.e., BP rose during nighttime; dipping ratio = 1.24). As shown in Table 1, there was a significantly greater percentage of Blacks in the higher ratio quartiles (i.e., those with less dipping, including risers). BMI also was greater in the higher ratio quartiles, but the difference across quartiles was not statistically significant. There was a greater proportion of smokers in the lowest and highest quartiles. During the ABPM session, poor sleep quality was greatest among those in the first quartile, although the difference was not statistically significant across the quartiles.
The ambulatory BP averages within each BP dipping ratio quartile are shown in Table 2. The mean (±SD) 24-hour ambulatory BP average was 116/68 (±10/7) mm Hg and did not differ significantly between the groups.
Table 2. Mean Blood Pressures (mm Hg) of Sample at Baseline (CARDIA Year 5) by Dipping Ratio Quartiles (N=281).
| Quartile: Dipping Ratio | ||||||
|---|---|---|---|---|---|---|
| Blood Pressure | Total | Q1: 0.72- 0.85 |
Q2: 0.85 - 0.88 |
Q3: 0.88- 0.92 |
Q4: 0.92- 1.24 |
p-value |
| 24-hour systolic ABPM average, mean (SD) |
115.5 (10.0) |
114.7 (9.4) |
117.1 (11.0) |
113.7 (9.7) |
116.6 (9.7) |
0.16 |
| 24-hour diastolic ABPM average, mean (SD) |
68.0 (6.6) |
67.9 (6.2) |
69.1 (7.5) |
66.7 (5.8) |
68.3 (6.8) |
0.18 |
| Daytime systolic ABPM average, mean (SD) |
119.2 (10.6) |
121.0 (10.4) |
121.7 (11.5) |
117.1 (10.1) |
117.2 (9.7) |
0.01 |
| Nighttime systolic ABPM average, mean (SD) |
105.7 (11.1) |
98.6 (8.8) |
105.0 (9.8) |
105.3 (9.0) |
113.9 (11.2) |
<0.001 |
| Daytime diastolic ABPM average, mean (SD) |
71.1 (7.2) |
72.7 (7.0) |
72.8 (8.0) |
69.6 (6.1) |
69.5 (7.1) |
0.003 |
| Nighttime diastolic ABPM average, mean (SD) |
58.9 (7.6) |
55.0 (5.8) |
58.3 (7.4) |
58.3 (6.5) |
64.1 (7.9) |
<0.001 |
Quartiles of mean nocturnal systolic BP/mean daytime systolic BP ratio: Q1: 0.7197-0.8454 (n=70); Q2: 0.8455-0.8809 (n=71); Q3: 0.8810-0.9203 (n=70); Q4: 0.9204-1.2358 (n=70)
ABPM, ambulatory blood pressure monitoring
Later Prevalence of Coronary Artery Calcium
A total of 54 participants (22.6%) had a non-zero Agatston coronary artery calcium score. Compared to those in the quartile of nighttime to daytime systolic BP dipping ratio of 0.88 to 0.92, those whose nighttime to daytime systolic BP ratio was 0.92 to 1.24 (less dipping) had 4 times the odds (OR 4.08; 95% CI 1.48-11.2) of having coronary artery calcium. Additionally, those in the quartile of BP dipping ratio of 0.72 to 0.85 (more dipping) also had significantly higher odds (OR 4.76; 95% CI 1.76-12.9) of having coronary artery calcium (Table 3). While only 6/59 (10%) of participants in the third quartile and 9/63 (14%) in the second quartile had coronary artery calcium, 21/60 (35%) in the first quartile and 18/57 (32%) in the fourth quartile did so. This U-shaped relationship persisted after adjustment for covariates (Table 3 and Figure 1). In the final adjusted analysis, compared to those in the third quartile (dipping approximately 8% to 12%), those in the fourth quartile (dipping <8% to −24%) had 5.7 (95% CI 1.27 to 25.8) times the odds of having coronary artery calcium 10 to 15 year later, and those in the first quartile had 5.3 times the odds (95% CI 1.41 to 20.2) (Table 3).
Table 3. Presence of Coronary Artery Calcification (CARDIA Year 20 or Year 15) by Quartile of Baseline Mean Nighttime to Daytime Systolic BP Ratio (N=239).
| Quartile | % with CAC |
OR | 95% confidence interval |
p-value | Nonlinearity (quadratic) p-value |
|---|---|---|---|---|---|
| Crude (n/N)* | <0.001 | ||||
| Q1 (21/60) | 35.0 | 4.76 | 1.76-12.9 | 0.002 | |
| Q2 (9/63) | 14.3 | 1.47 | 0.49-4.42 | 0.49 | |
| Q3 (6/59) | 10.2 | 1.00 (ref) | |||
| Q4 (18/57) | 31.6 | 4.08 | 1.48-11.2 | 0.007 | |
|
Adjusted for age, sex, race, baseline smoker, BMI, and HDL-C |
0.038 | ||||
| Q1 | 4.49 | 1.48-13.6 | 0.008 | ||
| Q2 | 1.50 | 0.45-5.08 | 0.51 | ||
| Q3 | 1.00 (ref) | ||||
| Q4 | 2.81 | 0.90-8.72 | 0.07 | ||
|
Adjusted for Year 5 ASBP, ADBP, SDBP |
0.001 | ||||
| Q1 | 4.21 | 1.44-12.3 | 0.009 | ||
| Q2 | 1.23 | 0.39-3.83 | 0.72 | ||
| Q3 | 1.00 (ref) | ||||
| Q4 | 3.92 | 1.31-11.8 | 0.015 | ||
|
Adjusted for additional year 5 covariates† and CAC exam year |
0.012 | ||||
| Q1 | 5.76 | 1.53-21.8 | 0.01 | ||
| Q2 | 1.37 | 0.33-5.74 | 0.67 | ||
| Q3 | 1.00 (ref) | ||||
| Q4 | 6.19 | 1.38-27.8 | 0.017 | ||
|
Additionally-adjusted for smoking status in CAC year |
0.015 | ||||
| Q1 | 5.34 | 1.41-20.2 | 0.014 | ||
| Q2 | 1.21 | 0.29-5.11 | 0.80 | ||
| Q3 | 1.00 (ref) | ||||
| Q4 | 5.73 | 1.27-25.8 | 0.023 |
OR, odds ratio; ref, referent; ASBP, awake systolic blood pressure average; ADBP, awake diastolic blood pressure average; SDBP, sleep diastolic blood pressure average; CAC, coronary artery calcium.
n/N: No. of cases/Total number of subjects with known non-zero Agatston score
Adjusted for age, sex, race, education, BMI, baseline smoker, ASBP, ADBP, SDBP, serum cotinine level, LDL-C, HDL-C, known diabetes, known hypertension, baseline clinic systolic BP, baseline clinic diastolic BP, pack-years of tobacco, alcohol intake, physical activity, family history of hypertension, family history of diabetes, poor sleep quality during ABPM.
Figure 1. Dipping Ratio and Probability of Coronary Artery Calcium 10 to 15 Years Later.
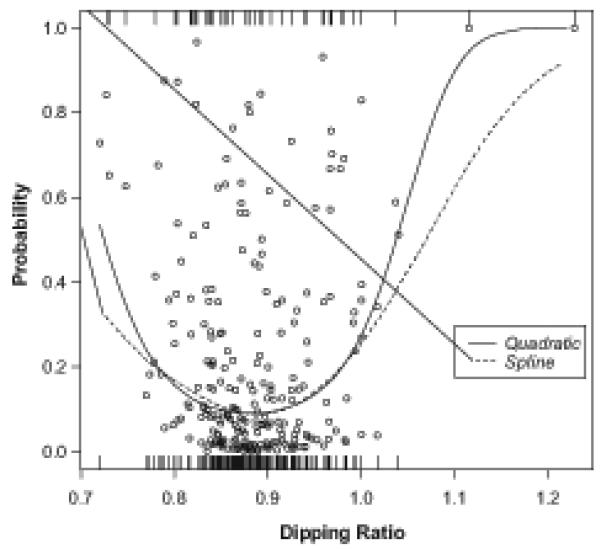
The figure shows the individual predicted probability of having coronary artery calcium, indicated by circles, in adjusted logistic regression analysis with quadratic dipping ratio as the predictor of interest and covariates in the final model in Table 3. The rug at the bottom of the graph indicates individuals in whom coronary artery calcium was absent while the rug at the top of the graph indicates those in whom coronary artery calcium was present. Goodness-of-fit is shown by a spline function that does not specify the relation between the ratio and the likelihood of the occurrence. Even after adjustment for possible confounding factors, the lowest risk of having the Agatston score greater than 0 is still around the 0.9 ratio, and both non-dipper (high ratio) and over-dipper (low ratio) are at a higher risk of having coronary artery calcification.
A spline function did not improve the fit of the predictive model. In fact, the spline and the quadratic functions overlap almost perfectly where there are data (Figure 1). The performance of the spline indicates that there is more ambiguity at the extremes, where there is little data.
In the full model, there was an interaction between sex and race (p=0.03). While the U-shaped relationship (adjusted) between baseline dipping quartile and future presence of coronary artery calcium was evident in each sex-race subgroup, it was weakest in white women (Figure 2). There was no interaction with smoking status (p=0.22), and the U-shaped relationship persisted (though not statistically significant in most quartile comparisons) among both smokers and non-smokers (please see Supplemental Table 3 at http://hyper.ahajournals.org).
Figure 2. Dipping Ratio and Probability of Coronary Artery Calcium 10 to 15 Years Later, Stratified by Sex and Race Subgroups.
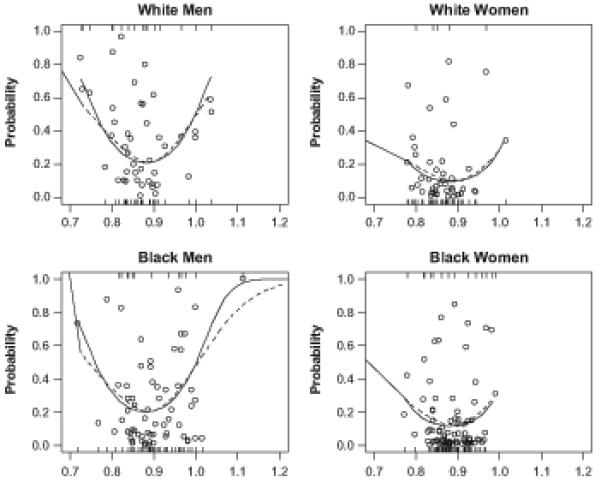
The figure shows the adjusted analysis results across each of the four sex-race subgroups. The U-shaped relationship between nighttime systolic BP dipping (and its quadratic term as a covariate) and presence of future coronary artery calcium is present in all four groups. Circles indicated the predicted probability. The top rug represents the coronary artery calcium cases and the bottom represents the non-cases.
Discussion
We found that among a sample of adults with a mean age of 45 years, the presence of coronary artery calcium was lowest in those whose nighttime BP exhibited a dip of approximately 10% on ambulatory BP monitoring performed 10-15 years earlier. The presence of coronary artery calcium was greater not only among those participants whose BP dipped less than around 8%, but also among those whose BP dipped more than around 15%. These results persisted after adjustment for multiple potential confounders, including the average ambulatory and clinic BP levels. Our findings suggest that nondipping and over-dipping are potential risk factors for subclinical atherosclerosis beginning in young adulthood, even in the absence of hypertension.
Our finding that coronary artery calcium was more common among those who would be categorized as nondippers (including risers) is consistent with most other literature on the adverse associations with this abnormal diurnal BP pattern, 2-8 although the only other study of which we are aware of that examined coronary artery calcium and BP dipping found no association.17 That study included 298 white subjects with a mean age of 40 years, defined dipping as the difference between daytime and nighttime BP levels rather than as a ratio. As a supplementary analysis, we therefore reanalyzed our data using the difference instead of the ratio and found that the U-shaped curve persisted (please see Supplementary Table 4 at http://hyper.ahajournals.org).
While average nighttime to daytime BP ratio <0.80 has been defined as “extreme dipping,” and in at least a couple of studies it has been associated with an increased risk of cerebrovascular disease in older adults, a large meta-analysis examining the prognostic significance of nighttime BP patterns found no difference between dippers and extreme dippers in the rate of cardiovascular events.6,18-20 The median age of the 3468 patients included in the meta-analysis was 63 years, and 61% were under antihypertensive treatment, making the populations in that analysis quite different than the cohort examined in our study.
Studies of biomarkers and other intermediate outcomes also suggest that extreme dipping is not benign. Among 98 hypertensive patients whose mean age was 57 years, plasma B-type natriuretic peptide levels were higher among the extreme dippers than the dippers, and higher yet among the risers (also called reverse dippers).21 Another study of 314 untreated hypertensives (mean age 48 years) showed a J-shaped relationship between nighttime-to-daytime BP ratio and pulse wave velocity, with a nadir around a ratio of 0.90- 0.94.22 Finally, among treated hypertensive patients with CAD, nocturnal ischemia was shown to be significantly more frequent in over-dippers than in dippers and nondippers.23
It has been suggested that an alternative way of looking at extreme dipping is that it represents an excessive daytime rise above a basal nighttime level. This has been described as “extreme blipping.”24 However, our inclusion of daytime ambulatory BP averages would control for this possibility. Some investigators postulate that it is the extreme amplitude in the circadian BP variation (“overswinging”) rather than the extreme dipping per se that is a risk factor for CVD. In a study of 297 adults, those whose circadian BP amplitude exceeded the 90th percentile based on sex- and age-matched peers had a higher rate of ischemic strokes, nephropathy, angina, and retinopathy.25 The differences in strokes and nephropathy between those with and without abnormal circadian amplitude were statistically significant among both hypertensives and normotensives. Dipping status alone however, was not discriminatory.
Our study is unique in that we examined a cohort of adults who were young and largely healthy at baseline. Nevertheless, abnormal night-to-day BP patterns despite otherwise optimal BP, seem to be related to the presence of subclinical coronary atherosclerosis over a decade later. Mechanistically, it seems most plausible that alterations in sympathetic nervous system activity (e.g., baroreceptor sensitivity) would explain an association with altered diurnal BP pattern and cardiovascular sequelae. However, the pathophysiologies between nondippers and extreme dippers (or overswingers) may be different.
Limitations
Our findings need to be interpreted in the context of some limitations. We do not know the reproducibility of dipping ratio among these participants, which may be worse at the extremes (i.e., extreme dipping and rising).26 We did not have measurements of coronary artery calcium at baseline, so we do not know with certainty that it developed over the years subsequent to the measurement of ambulatory BP. However, coronary artery calcium is very rare in young adults27, and was present in only 4.5% (17/379) at year 10 in the CARDIA cohort28, suggesting that the altered nocturnal BP dipping observed in our cohort preceded the development of subclinical coronary atherosclerosis. We were unable to find differences in known predictors such as cholesterol, clinic BP, glucose, or smoking either at baseline or follow-up examinations to the extent that they would provide an alternative explanation for our findings, though the possibility of residual confounding cannot be ruled out. For example, although tobacco use and cotinine level were included in the final models, it remains possible that tobacco use continues to operate as a confounder due to its influence on BP variability.29 Our analyses stratified by baseline smoking status suffered from relatively small sample sizes, which may explain the instability in the significance of the comparisons. Given these limitations, our findings ought to be replicated in larger samples before drawing a more robust conclusion.
Nocturnal BP may be affected by disruption of sleep by the ambulatory BP monitor itself.30 By our definition, however, only 16% of participants with adequate ambulatory BP data had poor sleep quality, and our results were unchanged when this factor was included in multivariable analysis. While a strength of our study is the biracial cohort, the CARDIA ambulatory BP monitoring substudy took place in one region of the country within the “stroke belt”. While BP patterns may vary across regions of the country, it seems unlikely that the association itself between those BP patterns and coronary calcium would vary.
Perspectives
Our analysis suggests that coronary artery calcium may be a potential mediator of the relationship between abnormal nighttime BP pattern and CVD. As suggested by previous literature, the residual CVD risk associated with abnormal nighttime BP among patients with hypertension warrants rigorous testing of interventions (e.g., chronotherapy) in clinical trials.31 However, what do we make of the possible risk associated with abnormal ambulatory BP patterns in people who do not have hypertension, as suggested in this study and others?7,8,31 First, the findings may be of prognostic importance, implying that when abnormal BP patterns are detected, they can be a harbinger of greater cardiovascular disease risk in years to come. Those at increased risk may warrant more aggressive control of risk factors and closer monitoring for development of hypertension.32 Second, if future research demonstrates that abnormal diurnal BP patterns in youth are associated with adverse clinical outcomes, evaluation of interventions (either pharmacologic or nonpharmacologic) to restore a healthier BP pattern may also be warranted in this group. The challenges of conducting such research are formidable.
Supplementary Material
Novelty and Significance.
This study uniquely showed that in a cohort of adults who were young and largely healthy at baseline, abnormal night-to-day BP patterns (either not dipping enough or dipping too much) despite otherwise optimal BP, seem to be associated with the presence of subclinical coronary atherosclerosis over a decade later. These findings may be of prognostic importance, implying that when abnormal BP patterns are detected, they can be a harbinger of greater cardiovascular disease risk in years to come. Those at increased risk may warrant more aggressive control of risk factors and closer monitoring for development of hypertension.
Acknowledgments
Sources of Funding
The CARDIA study is funded by the CARDIA contract with NHLBI (N01-HC-48047 – N01-HC-48050 and N01-HC-95095).
Anthony Viera has a research grant from the National Heart Lung and Blood Institute (R01 HL098604) to study ambulatory blood pressure monitoring. He has also served on the Medical Advisory Board of Suntech Medical, manufacturer of a brand of ambulatory blood pressure monitor.
Alan L Hinderliter receives research support from the National Heart Lung and Blood Institute (R01 HL098604-01) to study ambulatory blood pressure monitoring.
Sharina D Person receives research support from the National Institutes of Health (N01HC95095-62-0-1, CARDIA Study).
Footnotes
Disclosures
Feng-Chang Lin: None
Daichi Shimbo: None
Mark J Pletcher: None
David R Jacobs, Jr: None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pickering TG, Shimbo D, Haas D. Ambulatory blood pressure monitoring. N Engl J Med. 2006;354:2368–2374. doi: 10.1056/NEJMra060433. [DOI] [PubMed] [Google Scholar]
- 2.Verdecchia P, Schillaci G, Guerrieri M, Gatteschi C, Benemio G, Boldrini F, Porcellati C. Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation. 1990;81:528–536. doi: 10.1161/01.cir.81.2.528. [DOI] [PubMed] [Google Scholar]
- 3.Shimada K, Kawamoto A, Matsubayashi K, Nishinaga M, Kimura S, Ozawa T. Diurnal blood pressure variations and silent cerebrovascular damage in elderly patients with hypertension. J Hypertens. 1992;10:875–878. [PubMed] [Google Scholar]
- 4.Bianchi S, Bigazzi R, Baldari G, Sgherri G, Campese VM. Diurnal variations of blood pressure and microalbuminuria in essential hypertension. Am J Hypertens. 1994;7:23–29. doi: 10.1093/ajh/7.1.23. [DOI] [PubMed] [Google Scholar]
- 5.Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Guerrieri M, Gatteschi C, Zampi I, Santucci A, Reboldi G. Ambulatory blood pressure: an independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793–801. doi: 10.1161/01.hyp.24.6.793. [DOI] [PubMed] [Google Scholar]
- 6.Fagard R, Celis H, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008;51:55–61. doi: 10.1161/HYPERTENSIONAHA.107.100727. [DOI] [PubMed] [Google Scholar]
- 7.Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, Matsubara M, Hashimoto J, Hoshi H, Araki T, Tsuji I, Satoh H, Hisamichi S, Imai Y. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens. 2002;20:2183–89. doi: 10.1097/00004872-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Hoshide S, Kario K, Hoshide Y, Umeda Y, Hashimoto T, Kunii O, Ojima T, Shimada K. Associations between nondipping of nocturnal blood pressure decrease and cardiovascular target organ damage in strictly selected community-dwelling normotensives. Am J Hypertens. 2003;16:434–38. doi: 10.1016/s0895-7061(03)00567-3. [DOI] [PubMed] [Google Scholar]
- 9.Mousa T, El-Sayed MA, Motawea AK, Salama MA, Elhendy A. Association of blunted nighttime blood pressure dipping with coronary artery stenosis in men. Am J Hypertens. 2004;17:977–980. doi: 10.1016/j.amjhyper.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Lee KW, Blann AD, Lip GY. High pulse pressure and nondipping circardian blood pressure in patients with coronary artery disease: relationship to thrombogenesis and endothelial damage/dysfunction. Am J Hypertens. 2005;18:104–115. doi: 10.1016/j.amjhyper.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 12.Knox SS, Hausdorff J, Markovitz JH. Reactivity as a predictor of subsequent blood pressure racial differences in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Hypertension. 2002;40:914–919. doi: 10.1161/01.hyp.0000041417.94797.57. [DOI] [PubMed] [Google Scholar]
- 13.White WB, Lund-Johansen P, McCabe EJ, Omvik P. Clinical evaluation of the Accutracker II ambulatory blood pressure monitor: assessment of performance in two countries and comparison with sphygmomanometry and intra-arterial blood pressure at rest and during exercise. J Hypertens. 1989;7:967–75. doi: 10.1097/00004872-198912000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 15.Carr JJ, Crouse JR, 3rd, Goff DC, Jr, D’Agostino RB, Jr, Peterson NP, Burke GL. Evaluation of subsecond gated helical CT for quantification of coronary artery calcium and comparison with electron beam CT. AJR Am J Roentgenol. 2000;174:915–921. doi: 10.2214/ajr.174.4.1740915. [DOI] [PubMed] [Google Scholar]
- 16.Detrano RC, Anderson M, Nelson J, Wond ND, Carr JJ, McNitt-Gray M, Bild DE. Coronary calcium measurements: effect of CT scanner type and calcium measure on rescan reproducibility—MESA study. Radiology. 2005;236:477–484. doi: 10.1148/radiol.2362040513. [DOI] [PubMed] [Google Scholar]
- 17.Turner ST, Bielak LF, Narayana AK, Sheedy PF, Schwartz GL, Peyser PA. Ambulatory blood pressure and coronary artery calcification in middle-aged and younger adults. Am J Hypertens. 2002;15:518–524. doi: 10.1016/s0895-7061(02)02271-9. [DOI] [PubMed] [Google Scholar]
- 18.Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K. Stroke prognosis and abnormal nocturnal fall in older hypertensives. Hypertension. 2001;38:852–857. doi: 10.1161/hy1001.092640. [DOI] [PubMed] [Google Scholar]
- 19.Kario K, Matsuo T, Kobayashi H, Imiya M, Matsuo M, Simada K. Nocturnal fall of blood pressure and silent cerebrovascular damage in elderly hypertensive patients. Advanced silent cerebrovascular damage in extreme dippers. Hypertension. 1996;27:130–135. doi: 10.1161/01.hyp.27.1.130. [DOI] [PubMed] [Google Scholar]
- 20.Fagard R. Dipping pattern of nocturnal blood pressure in patients with hypertension. Exp Rev Cardiovasc Ther. 2009;7:599–605. doi: 10.1586/erc.09.35. [DOI] [PubMed] [Google Scholar]
- 21.Nakatsu T, Shinohata R, Mashima K, Yuki Y, Nishitani A, Toyonaga S, Ogawa H, Hiorhata S, Usui S, Kusachi S. Use of plasma B-type natriuretic peptide level to identify asymptomatic hypertensive patients with abnormal diurnal blood pressure variation profiles: nondippers, extreme dippers, and risers. Hypertens Res. 2007;30:651–658. doi: 10.1291/hypres.30.651. [DOI] [PubMed] [Google Scholar]
- 22.Jerrard-Dunne P, Mahmud A, Feely J. Circadian blood pressure variation: relationship between dipper status and measures of arterial stiffness. J Hypertens. 2007;25:1233–1239. doi: 10.1097/HJH.0b013e3280eec79f. [DOI] [PubMed] [Google Scholar]
- 23.Pierdomenico SD, Bucci A, Costantani F, Lapenna D, Cuccurullo F, Mezzetti A. Circadian blood pressure changes and myocardial ischemia in hypertensive patients with coronary artery disease. J Am Coll Cardiol. 1998;31:1627–1634. doi: 10.1016/s0735-1097(98)00163-6. [DOI] [PubMed] [Google Scholar]
- 24.Stanton AV. The clinical relevance of extreme dipping. Blood Press Monit. 1998;3:163–166. [PubMed] [Google Scholar]
- 25.Otsuka K, Cornelissen G, Halberg F, Oehlerts G. Excessive circadian amplitude of blood pressure increases risk of ischemic stroke and nephropathy. J Med Eng Technol. 1997;21:23–30. doi: 10.3109/03091909709030299. [DOI] [PubMed] [Google Scholar]
- 26.Cuspidi C, Meani S, Salerno M, Valerio C, Fusi V, Severgnini B, Lonati L, Magrini F, Zanchetti A. Reproducibility of nocturnal blood pressure fall in early phases of untreated essential hypertension: a prospective observational study. J Hum Hypertens. 2004;18:503–509. doi: 10.1038/sj.jhh.1001681. [DOI] [PubMed] [Google Scholar]
- 27.Hoff JA, Chomka EV, Krainik AJ, Daviglus M, Rich S, Kondos GT. Age and gender distributions of coronary artery calcium detected by electron beam tomography in 35,246 adults. Am J Cardiol. 2001;87:1335–1339. doi: 10.1016/s0002-9149(01)01548-x. [DOI] [PubMed] [Google Scholar]
- 28.Gross M, Steffes M, Jacobs DR, Jr, Yu X, Lewis L, Lewis CE, Loria CM. Plasma F2-isoprostanes and coronary artery calcification: the CARDIA Study. Clin Chem. 2005;51:125–131. doi: 10.1373/clinchem.2004.037630. [DOI] [PubMed] [Google Scholar]
- 29.Kawano Y. Diurnal blood pressure variation and related behavioral factors. Hypertens Res. 2011;34:281–285. doi: 10.1038/hr.2010.241. [DOI] [PubMed] [Google Scholar]
- 30.Manning G, Rushton L, Donnelly R, Millar-Craig MW. Variability of diurnal changes in ambulatory blood pressure and nocturnal dipping status in untreated hypertensive and normotensive subjects. Am J Hypertens. 2000;13:1035–1038. doi: 10.1016/s0895-7061(00)00261-2. [DOI] [PubMed] [Google Scholar]
- 31.Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension. 2011;57:3–10. doi: 10.1161/HYPERTENSIONAHA.109.133900. [DOI] [PubMed] [Google Scholar]
- 32.Viera AJ, Zhu S, Hinderliter AL, Shimbo D, Person SD, Jacobs DR., Jr. Diurnal blood pressure pattern and development of prehypertension or hypertension in young adults: the CARDIA study. J Am Soc Hypertens. 2011;5:48–55. doi: 10.1016/j.jash.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


