Abstract
BACKGROUND
Lung adenocarcinoma is histologically heterogeneous and has 5 distinct histologic growth patterns: lepidic, acinar, papillary, micropapillary, and solid. To date, there is no consensus regarding the clinical utility of these patterns.
METHODS
The authors performed a detailed semiquantitative assessment of histologic patterns of 240 lung adenocarcinomas and determined the association with patients’ clinicopathologic features, including recurrence-free survival (RFS) and overall survival (OS) rates. In a subset of tumors, expression levels of 2 prognostic molecular markers were evaluated: thyroid transcription factor-1 (TTF-1) (n = 218) and a panel of 5 proteins (referred as the FILM signature index) (n = 185).
RESULTS
Four mutually exclusive tumor histology pattern groups were identified: 1) any solid (38%), 2) any papillary but no solid (14%), 3) lepidic and acinar but no solid or papillary (30%), and 4) acinar only (18%). Patients in group 3 had a higher RFS rate than patients in group 1 (hazard ratio [HR], 0.4510; P = .0165) and group 2 (HR, 0.4253; P = .0425). Solid pattern tumors (group 1) were associated with a lower OS rate than nonsolid pattern tumors (all stages: HR; 1.665; P = .0144; stages I and II: HR, 2.157; P = .008). In the patients who had tumors with a nonsolid pattern, high TTF-1 expression was associated significantly with higher RFS (HR, 0.994; P = .0017) and OS (HR, 0.996; P = .0276) rates in all stages, and a high FILM signature index score was associated with lower RFS and OS rates in all stages (RFS: HR, 1.343; P = .0192; OS: HR, 1.371; P = .0156) and in stages I and II (RFS: HR, 1.419; P = .0095; OS: HR, 1.315; P = .0422).
CONCLUSIONS
The presence of a solid histologic pattern was identified as a marker of unfavorable prognosis in patients with primary lung adenocarcinoma. High TTF-1 expression and low FILM signature index scores were associated with a better prognosis for patients who had tumors with a nonsolid pattern.
Keywords: histologic patterns, lung adenocarcinoma, thyroid transcription factor 1, prognostic signature
INTRODUCTION
Lung adenocarcinoma is the most common histologic lung cancer type in the United States.1 It has a wide spectrum of clinical, radiologic, molecular, and morphologic features.2 The 2004 World Health Organization classification of lung tumors includes 4 architectural growth patterns: bronchioloalveolar (also known as lepidic), acinar, papillary, and solid.2,3 More recently, a micropapillary pattern was described.4–7 Most invasive lung adenocarcinomas (>80%) include 2 or more of these patterns2,8; therefore, histologic classification systems of this tumor type and potential clinical applications must account for its histologic heterogeneity.
To date, several studies have attempted to determine the clinical importance of histologic subtypes of lung adenocarcinoma by assessing the presence and extent of histologic growth patterns.9–24 It has been demonstrated that the lepidic growth pattern is associated with a better survival rate in patients with lung adenocarcinoma.9,13,14,19,20,23 In contrast, a solid pattern has been associated with a poor outcome.10,11,17,18,21,22,24 However, most of those studies had had small sample sizes or no rigorous histologic tumor sampling. At present, no consensus exists on the clinical utility of growth patterns in lung adenocarcinoma.
Currently, the use of molecular markers to predict recurrence and survival rates in patients with lung cancer who undergo surgery represents an area of very active investigation.25,26 Recently, our group reported that, in patients with stages I, II, and III lung adenocarcinoma who undergo surgery the immunohistochemical expression of thyroid transcription factor-1 (TTF-1)27 and of a panel of 5 proteins (referred as the FILM signature)28,29 in tumors was correlated with clinical outcome. TTF-1 is a homeodomain-containing transcription factor that is essential for morphogenesis and differentiation of the lungs.30 It has been used commonly as a marker for the diagnosis of primary and metastatic lung adenocarcinomas.31 The results from recent studies suggest that it is a lineage-specific proto-oncogene for lung cancer, and high TTF-1 expression has been associated with better survival by us27 and others.32–36 We recently developed the FILM signature using a risk model based on the protein expression of certain genes (ubiquitin-conjugating enzyme E2C [UBE2C], minichromosome maintenance 2 [MCM2] and 6 [MCM6], flap structure-specific endonuclease 1 [FEN1], and targeting protein for Xklp2 [TPX2]) that were expressed differentially in an in vitro model of lung carcinogenesis.29 We recently reported that an index accounting for the immunohistochemical expression of the proteins included in the FILM signature in archival tumor tissues predicted survival in all stages or stage I only lung adenocarcinomas.28
In the current study, we determined the clinical relevance of histologic growth patterns in primary lung adenocarcinoma by performing a detailed semiquantitative assessment of pattern distribution (lepidic, acinar, papillary, micropapillary, and solid) in 240 surgically resected tumors that were selected by using strict criteria for tumor sampling. We studied the association between tumor growth pattern distribution and clinicopathologic features, including age, sex, stage, and recurrence-free survival (RFS) and overall survival (OS) rates. In addition, we tested whether the use of prognostic molecular markers like TTF-1 and the FILM signature index improved our ability to determine the outcome of patients beyond the histologic subtype assessment.
MATERIALS AND METHODS
Patient Selection
We retrospectively collected surgically resected primary lung adenocarcinoma tissue samples from patients who underwent surgical resection with curative intent between 1997 and 2005 at The University of Texas M. D. Anderson Cancer Center (Houston, Tex). Clinicopathologic information was retrieved from the electronic clinical records for all patients and included age, sex, smoking history and status (current, former, or never), tumor size, tumor stage (according to the Mountain37 and International Association for the Study of Lung Cancer [IASLC]38 classification systems), the number of nodules (single or multiple), receipt of neoadjuvant and adjuvant treatment, and follow-up information for RFS and OS rates. This study was approved by The University of Texas M. D. Anderson Cancer Center institutional review board.
We selected 240 patients with a single-nodule, first primary lung adenocarcinoma who had not received neo-adjuvant therapy and had a minimum of 1 hematoxylin and eosin (H&E)-stained histologic slide per centimeter of the greatest tumor dimension available for histologic analysis. In addition, in most patients, formalin-fixed, paraffin-embedded tumor tissue blocks were retrieved for immunohistochemical analysis of molecular prognostic markers. Patients’ clinicopathologic characteristics are listed in Table 1.
Table 1.
Clinical and Pathologic Characteristics of All 240 Primary Lung Adenocarcinoma Tumors Studied and Their Association With 4 Histologic Pattern Groups
| Histologic Pattern Group: No. of Patients (%)a | ||||||
|---|---|---|---|---|---|---|
| Characteristic | All Tumors, N = 240 | Group 1, N = 92 | Group 2, N = 34 | Group 3, N = 72 | Group 4, N = 42 | P |
| Age, yb | .00079 | |||||
| ≤66.8 | 118 | 53 (45) | 20 (17) | 21 (18) | 24 (20) | |
| >66.8 | 122 | 39 (32) | 14 (11) | 51 (42) | 18 (15) | |
| Sex | .09314 | |||||
| Women | 137 | 47 (34) | 16 (12) | 49 (36) | 25 (18) | |
| Men | 103 | 45 (44) | 18 (17) | 23 (22) | 17 (17) | |
| Smoking status | .00010 | |||||
| Current | 97 | 54 (56) | 13 (13) | 15 (15) | 15 (15) | |
| Former | 102 | 30 (29) | 15 (15) | 37 (36) | 20 (20) | |
| Never | 41 | 8 (20) | 6 (15) | 20 (49) | 7 (17) | |
| IASLC stagec | .01377 | |||||
| I | 167 | 54 (32) | 23 (14) | 60 (36) | 30 (18) | |
| II | 42 | 23 (55) | 8 (19) | 7 (17) | 4 (10) | |
| III or IV | 31 | 15 (48) | 3 (10) | 5 (16) | 8 (26) | |
| TNM staged | .00164 | |||||
| I | 173 | 55 (32) | 24 (14) | 63 (36) | 31 (18) | |
| II | 36 | 22 (61) | 7 (19) | 4 (11) | 3 (8) | |
| III or IV | 31 | 15 (48) | 3 (10) | 5 (16) | 8 (26) | |
| Adjuvant therapye | .27792 | |||||
| No | 163 | 57 (35) | 26 (16) | 53 (33) | 27 (17) | |
| Yes | 71 | 33 (47) | 7 (10) | 18 (25) | 13 (18) | |
| Necrosis | <.0001 | |||||
| No | 114 | 26 (23) | 19 (17) | 48 (42) | 21 (18) | |
| Yes | 126 | 66 (52) | 15 (12) | 24 (19) | 21 (17) | |
| Lymphovascular invasion | .1398 | |||||
| No | 200 | 74 (37) | 26 (13) | 66 (33) | 34 (17) | |
| Yes | 40 | 18 (45) | 8 (20) | 6 (15) | 8 (20) | |
Abbreviations: IASLC, International Association for the Study of Lung Cancer; TNM, tumor, lymph node, metastasis.
Histologic pattern groups were defined as follows: group 1, tumors with any solid pattern; group 2, any papillary pattern but no solid pattern; group 3, acinar and lepidic patterns but no solid or papillary patterns; and group 4, acinar pattern only.
The median age of the entire population is indicated.
See Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260–271.38
See Mountain CF. The international system for staging lung cancer. Semin Surg Oncol. 2000;18:106–115.37
Adjuvant treatment information was not available for 6 patients.
Histopathologic Analysis
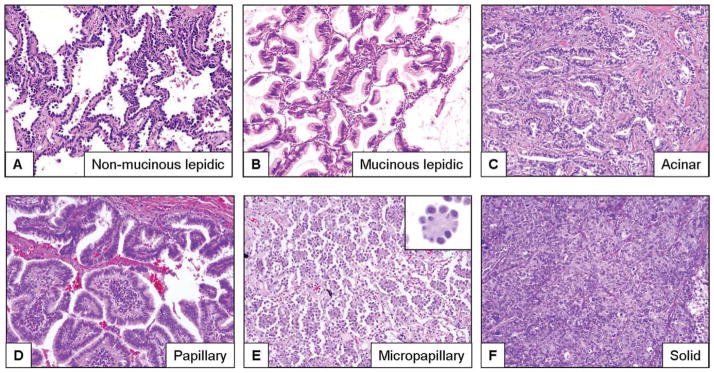
All H&E-stained histologic tumor sections were examined by 3 pathologists (C.A.M., M.G.R., and L.M.S.) for the presence and extent of 5 histologic growth patterns: lepidic, acinar, papillary, micropapillary, and solid. The extent of each pattern was annotated using the percentage present in the entire set of tumor slides per patient in 5% increments. The patterns were defined histologically according to the 2004 World Health Organization classification system2 with slight modifications39,40 and the criteria defined by Amin et al4 for micropapillary growth. Histologic growth patterns were defined as follows: 1) lepidic (mucinous and nonmucinous tumor cells with lepidic growth along alveolar walls and with no evidence of stromal, vascular, or pleural invasion)2 (Fig. 1A,B); 2) acinar (invasive tumor arranged in acini and tubules and composed of cuboidal or columnar cells that resemble bronchial gland or bronchial-lining epithelial cells, including Clara cells)2 (Fig. 1C); 3) papillary (invasive tumor composed of papillae structures with a fibrovascular core and complicated secondary and tertiary branches)2,39,40 (Fig. 1D); 4) micropapillary (small papillary tufts composed of tumor cells with peripheral nuclei and no fibrovascular core)2,4,7,41 (Fig. 1E); and 5) solid (invasive tumor composed of nests or sheets of tumor cells that lack acini, tubules, and papillae with mucin production) (Fig. 1F).2 Tumors with 1 pattern are referred to as pure tumors, and tumors with 2 or more patterns are referred to as mixed tumors. In addition, we also analyzed the presence of lymphovascular invasion and tumor necrosis.
Figure 1.
These are photomicrographs of hematoxylin and eosin-stained sections of lung adenocarcinoma tumors with different growth patterns, including (A) lepidic (nonmucinous), (B) lepidic (mucinous), (C) acinar, (D) papillary, (E) micropapillary, and (F) solid (original magnification, ×100).
Immunohistochemical Analysis
To determine the immunohistochemical expression of TTF-1 and the 5 proteins (UBE2C, MCM2, MCM6, FEN1, and TPX2) of the FILM signature in lung adenocarcinomas, we used formalin-fixed, paraffin-embedded tumor tissues placed in tissue microarray (TMAs) from a subset of patients who were included in this study (TTF-1, n = 218; FILM signature, n = 185). Immunohistochemical staining for TTF-1 was performed using 5-μM-thick sections from TMAs as reported previously27 with slight modifications. For analysis of the FILM signature, we used previously published immunohistochemical data available on the expression of the 5 FILM proteins.28 All staining in malignant cells was nuclear and was quantified by 2 pathologists using a 4-value intensity score (0, 1+, 2+, and 3+) and the percentage (0%-100%) of the extent of reactivity in each core. The final score was then obtained by multiplying the intensity and reactivity extension values (range, 0–300) as reported previously.28,42 To determine the FILM signature index, we used a combined immunoreactivity score for each patient that was computed by simple addition of the individual final scores for each of the 5 biomarkers analyzed as published previously.28
Statistical Analysis
To determine the association between histologic growth pattern and clinicopathologic covariates and time-to-event outcomes (RFS and OS), we classified patients into 4 mutually exclusive groups on the basis of the distribution of histologic growth patterns (Table 2). Chi-square tests or Fisher exact tests were used to determine differences in categorical variables, and the Wilcoxon rank-sum test or the Kruskal-Wallis test was used to detect differences in continuous variables between groups. RFS and OS distributions were estimated using the Kaplan-Meier method. The log-rank test43 was used to determine survival differences between groups. Regression analyses of survival data based on the Cox proportional hazards model43 were conducted for the RFS and OS rates. RFS was calculated from the date of surgery to the date of either recurrence or last contact, and OS was calculated from the date of surgery to the date of either death or last contact. Both RFS and OS were censored at 5 years. Associations between nuclear TTF-1 expression, the FILM signature index, and clinicopathologic variables and tumor growth pattern were calculated using continuous variables and a cutoff value of 160 for TTF-1 and 113.3 for the FILM signature index, which represent the median expression level for adenocarcinomas evaluated using TMAs. The statistical software packages SAS (version 9.1; SAS Institute, Inc., Cary, NC), R (2.80; R Foundation for Statistical Computing, Austria, Vienna), and S-Plus (version 8.0; TIBCO Software, Inc., Palo Alto, Calif) were used to perform the computations for all analyses.
Table 2.
Lung Adenocarcinoma Histologic Groups Identified by Histologic Growth pattern Distributions
| HISTOLOGIC GROWTH PATTERN | ||||||
|---|---|---|---|---|---|---|
| Group | Solida | Papillaryb | Lepidica | Micro-papillarya | Acinara | Frequency, N (%) |
| Group 1, n=92 (38%) | 56 (23.3) | |||||
| 17 (7.1) | ||||||
| 7 (2.9) | ||||||
| 6 (2.5) | ||||||
| 2 (0.8) | ||||||
| 2 (0.8) | ||||||
| 1 (0.4) | ||||||
| 1 (0.4) | ||||||
| Group 2, n= 34 (14%) | 19 (7.9) | |||||
| 7 (2.9) | ||||||
| 3 (1.3) | ||||||
| 1 (0.4) | ||||||
| 1 (0.4) | ||||||
| 1 (0.4) | ||||||
| 1 (0.4) | ||||||
| 1 (0.4) | ||||||
| Group 3, n= 72 (30%) | 68 (28.3) | |||||
| 4(1.6) | ||||||
| Group 4, n= 42 (18%) | 42 (17.5) | |||||
The shaded boxes represent the presence of histological growth patterns in the tumors analyzed.
RESULTS
Growth Pattern Distribution
Most adenocarcinomas that we evaluated (n = 191; 80%) had ≥2 growth patterns (mixed tumors), as expected. Of the 49 adenocarcinomas (20%) that had a single growth pattern (pure tumors), acinar was the most common pattern (n = 42; 18%) followed by solid (n = 6; 3%), and papillary (n = 1; 0.4%). No pure lepidic or micropapillary growth patterns were identified. In all tumors, the most common pattern was acinar (n = 232; 97%), followed by lepidic (n = 116; 48%; 8 mucinous and 108 nonmucinous), solid (n = 92; 38%), papillary (n = 44; 18%), and micropapillary (n = 15; 6%).
To determine the potential clinical implications of histologic pattern distribution in lung adenocarcinomas, we clustered the 240 tumors according to growth pattern distribution (single and combined) (Table 2). We identified 4 mutually exclusive histologic groups: 1) tumors with any solid pattern (n = 92; 38%), 2) tumors with any papillary pattern but no solid pattern (n = 34; 14%); 3) tumors with acinar and lepidic patterns but no solid or papillary pattern (n = 72; 30%); and 4) tumors with an acinar pattern only (n = 42, 18%).
We determined the associations among the 4 pattern groups and patients’ clinical and pathologic characteristics. Table 1 indicates that the histologic pattern groups differed significantly in a comparison according to the presence of tumor necrosis (P < .0001), age (median, 66.8 years; P = .00079), smoking status (P = .0001), and tumor classification (IASLC, P = .01377; Mountain et al, P = .00164). It is noteworthy that the tumors with solid growth patterns (group 1) had more tumor necrosis and were more common in younger patients (aged ≤66.8 years), current smokers, and patients with stage II through IV disease (according to Mountain et al and IASLC). Tumors with nonsolid or papillary patterns but with acinar and lepidic growth patterns (group 3) had less tumor necrosis and were more common in older patients (aged >66.8 years), never smokers, and patients with stage I disease (Table 1).
Association Between Growth Patterns and Outcome
We determined the association between histologic growth pattern and RFS and OS rates. The median follow-up was 4.21 years. In multivariate survival analysis, as expected, patients with stage III and IV disease (IASLC staging) had lower RFS and OS rates than patients with stage I and II disease. In addition, patients who were older than the median age (>66.8 years) and men had lower OS rates than younger patients and women, respectively (Table 3). Adjuvant chemotherapy was not associated significantly with RFS or OS when all tumor stages were examined.
Table 3.
Multivariate Cox Model of 5-Year Recurrence-Free and Overall Survival in All Patients (n = 240) According to Solid (Group 1) Versus Nonsolid (Groups 2–4) Growth Patterns
| RFS | OS | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | P | HR (95% CI) | P |
| Solid growth pattern: Solid vs nonsolid | 1.722 (1.060–2.796) | .0280 | 1.665 (1.036–2.675) | .0144 |
| Final IASLC stage: III or IV vs I or IIa | 4.087 (2.179–7.665) | <.0001 | 2.791 (1.404–5.549) | .0034 |
| Adjuvant treatment: yes vs no | 0.945 (0.536–1.667) | .8459 | 0.551 (0.289–1.051) | .0705 |
| Age: >66.8 y vs ≤66.8 yb | — | — | 1.769 (1.064–2.943) | .0280 |
| Sex: Men vs women | — | — | 1.974 (1.192–3.271) | .0083 |
Abbreviations: CI, confidence interval; HR, hazard ratio; IASLC, International Association for the Study of Lung Cancer; OS, overall survival; RFS, recurrence-free survival.
See Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260–271.38
The median age of the entire population is indicated.
Overall, on multivariate analysis, we observed that lung adenocarcinoma pattern groups were associated significantly with RFS (P = .0418). Specifically, patients in group 3 had significantly higher RFS rates than patients in group 1 (hazard ratio [HR], 0.4510; 95% confidence interval [CI], 0.235–0.865; P = .0165) and group 2 (HR, 0.4253; 95% CI, 0.1862–0.9713; P = .0425) after adjusting for IASLC stage and adjuvant treatment. Although multivariate analysis revealed no significant association between all histologic groups and OS (P = .0738), patients in group 3 had significantly higher OS rates (HR, 0.437; 95% CI, 0.225–0.847; P = .0143) than patients in group 1 after adjusting for IASLC stage, adjuvant treatment, age, and sex. No other differences in the RFS or OS rates were observed between the 4 groups.
In patients with stage I or II disease, lung adenocarcinoma pattern groups were correlated significantly with OS (P = .0278) in multivariate analysis. Specifically, patients in group 3 had significantly higher OS rates than patients in group 1 (HR, 0.317; 95% CI, 0.148–0.679; P = .0031) after adjusting for IASLC stage, adjuvant treatment, age, and sex. Although we did not observe a significant association between pattern groups and RFS in multivariate analysis (P = .1149), patients in group 3 had significantly higher RFS rates than patients in group 1 (HR, 0.400; 95% CI, 0.183–0.875; P = .0217) after adjusting for IASLC stage and adjuvant treatment. In addition, we determined the survival rates of all patients according to the presence of a lepidic (vs nonlepidic), papillary (vs nonpapillary), and micropapillary (vs nonmicropapillary) growth pattern component and observed no statistically significant association among these patterns and RFS or OS (data not shown).
Association Between Solid Growth Pattern and Outcome
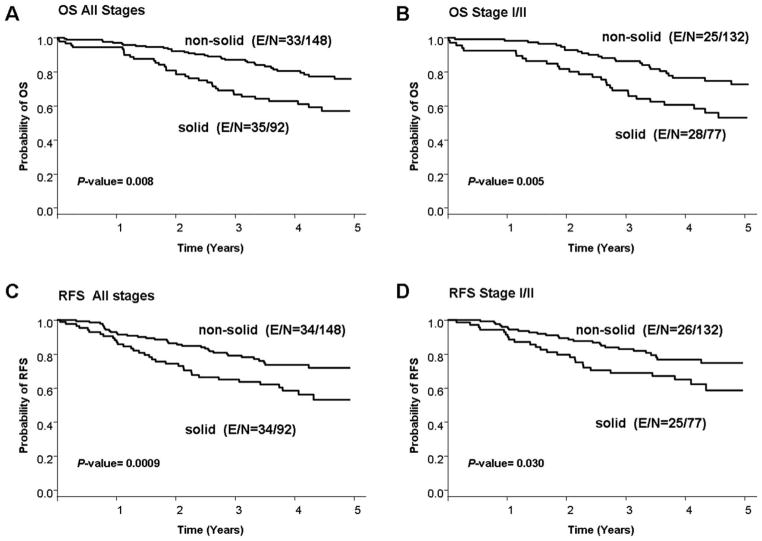
Because patients who had tumors with solid patterns (group 1) had a trend toward lower RFS and OS rates than patients who had tumors with other patterns, we performed a survival analysis of patients with solid tumors (group 1) versus nonsolid tumors (groups 2–4). Patients who had tumors with a solid pattern had lower OS and RFS rates than patients who had tumors with nonsolid patterns at all stages (Fig. 2A,C). These associations were statistically significant on univariate and multivariate analyses for both RFS (HR, 1.722; 95% CI, 1.060–2.796; P = .0280) and OS (HR, 1.665; 95% CI, 1.036–2.675; P = .0144) after adjusting for stage and adjuvant therapy for RFS and additionally for age and sex for OS (Table 3). In patients with stage I or II disease, tumors with a solid pattern were associated with a lower OS rate on univariate and multivariate analyses (HR, 2.157; 95% CI, 1.222–3.808; P = .008) after adjusting for stage (I vs II) and adjuvant therapy. The presence of a solid pattern was associated with a lower RFS rate on univariate analysis (HR, 1.820; 95% CI, 1.050–3.152; P = .0327), but this association was not significant on multivariate analysis after adjusting for stage (II vs I) and adjuvant therapy (HR, 1.543; 95% CI, 0.872–2.733; P = .1367) (Fig. 2B,D).
Figure 2.
The rates of (A,B) 5-year overall survival (OS) and (C,D) 5-year recurrence-free survival (RFS) are illustrated in patients with lung adenocarcinoma according to tumor growth pattern (solid vs nonsolid) both (A,C) for all stages and (B,D) for stages I and II only. E indicates events; N, total number of patients.
Prognostic Value of TTF-1 Expression and FILM Signature Index In Tumors With Nonsolid Patterns
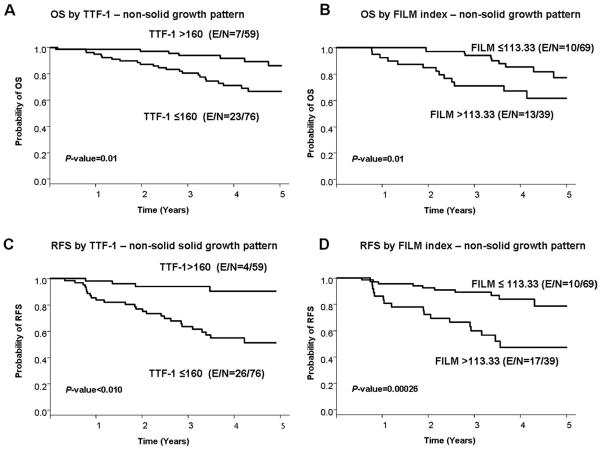
To test whether the use of prognostic molecular markers improves our ability to determine the outcome of patients who have tumors with nonsolid patterns, we compared tumor immunohistochemical expression of TTF-1 and the FILM signature index with RFS and OS rates in all patients and in patients with stage I and II tumors. First, we determined that both TTF-1 and the FILM signature index correlated significantly with tumor histology patterns. TTF-1 expression was significantly lower (P = .015) in solid pattern tumors (group 1) compared the other groups; in contrast, the FILM signature index was significantly higher (P < .001) in solid pattern tumors compared with nonsolid pattern tumors.
In all tumor stages, in tumors with nonsolid patterns, higher TTF-1 expression (as a continuous variable) was correlated significantly with higher RFS and OS rates (RFS: HR, 0.994; P = .0017; OS: HR, 0.996; P = .0276) on multivariate analysis (Table 4, PFig. 3A,C). We did not observe any significant association between TTF-1 expression and outcome in patients with stage I and II disease. Conversely, we observed that, in all patients and in patients with stage I and II disease only, a higher FILM signature index (as a continuous variable) was with lower RFS and OS rates at all stages (RFS: HR, 1.343; = .0192; OS: HR, 1.371; P = .0156;) and for stages I and II only (RFS: HR, 1.419; P = .0095; OS: HR, 1.315; P = .0422) on multivariate analysis (Table 4, PFig. 3B,D). It is noteworthy that, in tumors with a solid pattern, TTF-1 expression and the FILM signature index were not associated with RFS or OS rates in all patients (TTF1: RFS; = .6838; OS; P = .4088; FILM index: RFS; P = .5127; OS; P = .6560) or in patients with stage I and II disease only (TTF1: RFS; P = .9776; OS; P = .6752; FILM index: RFS; P = .3906; OS; P = .5231).
Table 4.
Multivariate Cox Model of 5-Year Recurrence-Free and Overall Survival Only in Patients With Nonsolid Growth Patterns According to Thyroid Transcription Factor 1 Expression and FILM Signature Index
| RFS | OS | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | P | HR (95% CI) | P |
| Nonsolid tumors according to TTF-1 expression, n = 135 | ||||
| Nuclear TTF-1 expression: per unit increase | 0.994 (0.992–0.998) | .0017 | 0.996 (0.992–1.000) | .0276 |
| Final IASLC stage: III and IV vs I and IIa | 4.555 (1.934–10.729) | .0005 | 4.959 (2.092–11.752) | .0003 |
| Adjuvant treatment: Yes vs no | 1.205 (0.550–2.638) | .6409 | 0.558 (0.206–1.513) | .2514 |
| Age: >66.8 y vs ≤66.8 yb | — | — | 1.620 (0.703–3.735) | .2574 |
| Sex: Men vs women | — | — | 1.510 (0.715–3.187) | .2810 |
| Nonsolid tumors by FILM signature index, n = 108c | ||||
| FILM signature index:1-Fold increase | 1.343 (1.049–1.719) | .0192 | 1.371 (1.062–1.770) | .0156 |
| Final IASLC stage: III and IV vs I and IIa | 2.290 (0.775–6.765) | .1338 | - | — |
| Adjuvant treatment: Yes vs no | 1.124 (0.455–2.776) | .8008 | 0.733 (0.286–1.878) | .5176 |
| Age: >66.8 y vs ≤66.8 yb | — | — | — | — |
| Sex: Men vs women | — | — | 1.810 (0.771–4.247) | .1730 |
Abbreviations: CI, confidence interval; HR, hazard ratio; IASLC, International Association for the Study of Lung Cancer; OS, overall survival; RFS, recurrence-free survival; TTF-1, thyroid transcription factor 1.
See Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260–271.38
The median age of the entire population is indicated.
The FILM signature index is a panel of 5 proteins that was developed using a risk model based on the protein expression of certain genes.
Figure 3.
The rates of (A,B) 5-year overall survival (OS) and (C,D) 5-year recurrence-free survival (RFS) are illustrated in patients with lung adenocarcinoma who had nonsolid tumor growth patterns according to (A,C) thyroid transcription factor 1 (TTF-1) expression and (B,D) scores on a panel of 5 proteins (referred as the FILM signature index). E indicates events; N, total number of patients.
DISCUSSION
In this study, we determined the clinical relevance of growth pattern quantification in 240 primary lung adenocarcinomas that had been surgically resected with curative intent. The patients were selected using strict tumor-sampling inclusion criteria and were subjected to a detailed semiquantitative histologic assessment. Most adenocarcinomas (80%) had mixed patterns. By analyzing the pattern distributions, we identified 4 mutually exclusive groups of tumors, including 1 in which any solid pattern was present (38%). On multivariate analysis, we observed that patients who had tumors with any solid pattern had lower OS rates than patients from all other histologic groups combined (nonsolid), both for all tumor stages and for stages I and II. To improve our ability to determine the outcome of patients who had tumors with nonsolid patterns, we compared tumor immunohistochemical expression of TTF-1 and the FILM signature index with RFS and OS rates. In these patients, higher TTF-1 expression and a lower FILM signature index were correlated significantly with higher RFS and OS rates on multivariate analysis at all stages. In addition, a lower FILM signature index correlated with higher RFS and OS rates in patients with stage I and II disease. It is noteworthy that these associations were not detected in patients who had solid pattern tumors. We demonstrated that a histologic solid pattern is a marker of a poor prognosis in surgically resected lung adenocarcinomas and that high immunohistochemical TTF-1 expression and a low FILM signature index are associated with a better prognosis in patients with nonsolid histologic patterns.
We observed a correlation between the presence of a solid histologic pattern and poor outcome in surgically resected lung adenocarcinoma, which highlights the clinical importance of a detailed histologic assessment of this tumor type by pathologists examining at least 1 slide per centimeter of the tumor’s greatest dimension. The association between a solid histologic component and patient prognosis using a multivariate analysis in stages I through III lung adenocarcinoma has been reported previously in 7 studies.10,11,17,18,21,24,44 However, only 1 study provided complete outcome data for patients at all stages and those with stage I and II disease,17 and only 2 reported RFS or disease-free survival data.21,24
To our knowledge, the current study, which was based on a large patient series, is the first to report the use of rigorous selection criteria for histologic examination and detailed semiquantitative assessment of histologic growth patterns. We also assessed RFS and OS and reported outcome data both for all stages and for stages I and II. Therefore, we believe our results provide important and novel insights into the clinical relevance of detailed histologic assessments of lung adenocarcinoma growth patterns as suggested by the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society for the Classification of Lung Adenocarcinoma.45 Our results indicate that a simple semiquantitative assessment to determine the presence of a solid pattern is sufficient to predict RFS and OS in patients with lung adenocarcinoma who undergo curative surgery.
In this study we have improved our ability to determine the outcome of patients who have tumors with non-solid patterns by comparing tumor immunohistochemical expression of TTF-1 and the FILM signature index with RFS and OS rates in patients with disease in all stages and with stage I and II disease. We observed that high TTF-1 immunohistochemical expression levels were associated significantly with prognosis in patients with lung adenocarcinoma. Although we examined TTF-1 expression in TMA specimens, our findings are in agreement with previously published data using either TMAs33,46 or whole-section samples.32,35 However, to our knowledge, ours is the first study to demonstrate that this marker is predictive of prognosis in patients who have tumors with nonsolid patterns. We also observed that the FILM signature index was able to predict RFS and OS outcomes in our patients with adenocarcinoma who had nonsolid histologic patterns at all stages and at stages I and II. The robustness of the gene-derived and protein-derived protein signature has been tested before and was predictive of a poor outcome in patients with lung adenocarcinoma rather than in patients with squamous cell carcinoma.28 It is noteworthy that in this study, we also demonstrated that the expression of TTF-1 and the FILM signature index were not predictive of outcome for patients who had tumors with any solid growth pattern.
In conclusion, our findings demonstrate that the presence of a histologic solid pattern in primary, single-nodule lung adenocarcinoma is a marker of unfavorable prognosis and that high immunohistochemical TTF-1 expression and a low FILM signature index are associated with a better prognosis in patients who have tumors non-solid patterns. Here, we demonstrate that the integration of molecular markers and the semiquantitative assessment of histologic growth patterns improve the prognostic stratification of patients with lung adenocarcinomas.
Acknowledgments
FUNDING SOURCES
This study was supported in part by grants from the Department of Defense (W81XWH-04-1-0142), Uniting Against Lung Cancer, Specialized Program of Research Excellence in Lung Cancer Grant P50CA70907, and Cancer Center Support Grant CA-16672 from the National Cancer Institute.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
References
- 1.Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117:294–299. doi: 10.1002/ijc.21183. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E. In: World Health Organization Classification of Tumours. Pathology and Genetics of Tumous of the Lung, Pleura, Thymus and Heart. Muller–Hemelink HK, Harris CC, editors. Lyon, France: IARC Press; 2004. [Google Scholar]
- 3.Travis WD, Garg K, Franklin WA, et al. Bronchioloalveolar carcinoma and lung adenocarcinoma: the clinical importance and research relevance of the. 2004 World Health Organization pathologic criteria. J Thorac Oncol. 2006;1(9 suppl):S13–S19. [PubMed] [Google Scholar]
- 4.Amin MB, Tamboli P, Merchant SH, et al. Micropapillary component in lung adenocarcinoma: a distinctive histologic feature with possible prognostic significance. Am J Surg Pathol. 2002;26:358–364. doi: 10.1097/00000478-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Makimoto Y, Nabeshima K, Iwasaki H, et al. Micropapillary pattern: a distinct pathological marker to subclassify tumours with a significantly poor prognosis within small peripheral lung adenocarcinoma (≤20 mm) with mixed bronchioloalveolar and invasive subtypes (Noguchi’s type C tumours) Histopathology. 2005;46:677–684. doi: 10.1111/j.1365-2559.2005.02126.x. [DOI] [PubMed] [Google Scholar]
- 6.Miyoshi T, Satoh Y, Okumura S, et al. Early stage lung adenocarcinomas with a micropapillary pattern, a distinct pathologic marker for a significantly poor prognosis. Am J Surg Pathol. 2003;27:101–109. doi: 10.1097/00000478-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Mora N, Presmanes MC, Monroy V, et al. Micropapillary lung adenocarcinoma: a distinctive histologic subtype with prognostic significance. Case series. Hum Pathol. 2008;39:324–330. doi: 10.1016/j.humpath.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 8.Motoi N, Szoke J, Riely GJ, et al. Lung adenocarcinoma: modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am J Surg Pathol. 2008;32:810–827. doi: 10.1097/PAS.0b013e31815cb162. [DOI] [PubMed] [Google Scholar]
- 9.Anami Y, Iijima T, Suzuki K, et al. Bronchioloalveolar carcinoma (lepidic growth) component is a more useful prognostic factor than lymph node metastasis. J Thorac Oncol. 2009;4:951–958. doi: 10.1097/JTO.0b013e3181ad8631. [DOI] [PubMed] [Google Scholar]
- 10.Barletta JA, Yeap BY, Chirieac LR. Prognostic significance of grading in lung adenocarcinoma. Cancer. 2010;116:659–669. doi: 10.1002/cncr.24831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryant CM, Albertus DL, Kim S, et al. Clinically relevant characterization of lung adenocarcinoma subtypes based on cellular pathways: an international validation study [serial online] PLoS One. 2010;5:e11712. doi: 10.1371/journal.pone.0011712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurokawa T, Matsuno Y, Noguchi M, Mizuno S, Shimosato Y. Surgically curable “early” adenocarcinoma in the periphery of the lung. Am J Surg Pathol. 1994;18:431–438. doi: 10.1097/00000478-199405000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Maeshima AM, Tochigi N, Yoshida A, Asamura H, Tsuta K, Tsuda H. Histological scoring for small lung adenocarcinomas 2 cm or less in diameter: a reliable prognostic indicator. J Thorac Oncol. 2010;5:333–339. doi: 10.1097/JTO.0b013e3181c8cb95. [DOI] [PubMed] [Google Scholar]
- 14.Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer. 1995;75:2844–2852. doi: 10.1002/1097-0142(19950615)75:12<2844::aid-cncr2820751209>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Okudela K, Woo T, Mitsui H, et al. Proposal of an improved histological subtyping system for lung adenocarcinoma—significant prognostic values for stage I disease. Int J Clin Exp Pathol. 2010;3:348–366. [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen I, Kotb WF, Friedrich KH, Schluns K, Bocking A, Dietel M. Core classification of lung cancer: correlating nuclear size and mitoses with ploidy and clinicopathological parameters. Lung Cancer. 2009;65:312–318. doi: 10.1016/j.lungcan.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Riquet M, Foucault C, Berna P, Assouad J, Dujon A, Danel C. Prognostic value of histology in resected lung cancer with emphasis on the relevance of the adenocarcinoma subtyping. Ann Thorac Surg. 2006;81:1988–1995. doi: 10.1016/j.athoracsur.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Russell PA, Wainer Z, Wright GM, Daniels M, Conron M, Williams RA. Does lung adenocarcinoma subtype predict patient survival? A clinicopathologic study based on the new International Association for the Study of Lung Cancer/ American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol. 2011;6:1496–1504. doi: 10.1097/JTO.0b013e318221f701. [DOI] [PubMed] [Google Scholar]
- 19.Sakurai H, Dobashi Y, Mizutani E, et al. Bronchioloalveolar carcinoma of the lung 3 centimeters or less in diameter: a prognostic assessment. Ann Thorac Surg. 2004;78:1728–1733. doi: 10.1016/j.athoracsur.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Sakurai H, Maeshima A, Watanabe S, et al. Grade of stromal invasion in small adenocarcinoma of the lung: histopathological minimal invasion and prognosis. Am J Surg Pathol. 2004;28:198–206. doi: 10.1097/00000478-200402000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Sica G, Yoshizawa A, Sima CS, et al. A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am J Surg Pathol. 2010;34:1155–1162. doi: 10.1097/PAS.0b013e3181e4ee32. [DOI] [PubMed] [Google Scholar]
- 22.Sorensen JB, Hirsch FR, Olsen J. The prognostic implication of histopathologic subtyping of pulmonary adenocarcinoma according to the classification of the World Health Organization. An analysis of 259 consecutive patients with advanced disease. Cancer. 1988;62:361–367. doi: 10.1002/1097-0142(19880715)62:2<361::aid-cncr2820620222>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 23.Terasaki H, Niki T, Matsuno Y, et al. Lung adenocarcinoma with mixed bronchioloalveolar and invasive components: clinicopathological features, subclassification by extent of invasive foci, and immunohistochemical characterization. Am J Surg Pathol. 2003;27:937–951. doi: 10.1097/00000478-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24:653–664. doi: 10.1038/modpathol.2010.232. [DOI] [PubMed] [Google Scholar]
- 25.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sudhindra A, Ochoa R, Santos ES. Biomarkers, prediction, and prognosis in nonsmall-cell lung cancer: a platform for personalized treatment [published online ahead of print May 8, 2011] Clin Lung Cancer. 2011 doi: 10.1016/j.cllc.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Tang X, Kadara H, Behrens C, et al. Abnormalities of the TITF-1 lineage-specific oncogene in NSCLC: implications in lung cancer pathogenesis and prognosis. Clin Cancer Res. 2011;17:2434–2443. doi: 10.1158/1078-0432.CCR-10-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kadara H, Behrens C, Yuan P, et al. A 5-gene and corresponding protein signature for stage-I lung adenocarcinoma prognosis. Clin Cancer Res. 2010;17:1490–1501. doi: 10.1158/1078-0432.CCR-10-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kadara H, Lacroix L, Behrens C, et al. Identification of gene signatures and molecular markers for human lung cancer prognosis using an in vitro lung carcinogenesis system. Cancer Prev Res (Phila) 2009;2:702–711. doi: 10.1158/1940-6207.CAPR-09-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bingle CD. Thyroid transcription factor-1. Int J Biochem Cell Biol. 1997;29:1471–1473. doi: 10.1016/s1357-2725(97)00007-1. [DOI] [PubMed] [Google Scholar]
- 31.Moldvay J, Jackel M, Bogos K, et al. The role of TTF-1 in differentiating primary and metastatic lung adenocarcinomas. Pathol Oncol Res. 2004;10:85–88. doi: 10.1007/BF02893461. [DOI] [PubMed] [Google Scholar]
- 32.Barlesi F, Pinot D, Legoffic A, et al. Positive thyroid transcription factor 1 staining strongly correlates with survival of patients with adenocarcinoma of the lung. Br J Cancer. 2005;93:450–452. doi: 10.1038/sj.bjc.6602717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barletta JA, Perner S, Iafrate AJ, et al. Clinical significance of TTF-1 protein expression and TTF-1 gene amplification in lung adenocarcinoma. J Cell Mol Med. 2009;13(8B):1977–1986. doi: 10.1111/j.1582-4934.2008.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berghmans T, Mascaux C, Haller A, Meert AP, Van Houtte P, Sculier JP. EGFR, TTF-1 and Mdm2 expression in stage III nonsmall cell lung cancer: a positive association. Lung Cancer. 2008;62:35–44. doi: 10.1016/j.lungcan.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Berghmans T, Mascaux C, Martin B, Ninane V, Sculier JP. Prognostic role of thyroid transcription factor-1 in stage III nonsmall cell lung cancer. Lung Cancer. 2006;52:219–224. doi: 10.1016/j.lungcan.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 36.Kwei KA, Kim YH, Girard L, et al. Genomic profiling identifies TITF1 as a lineage-specific oncogene amplified in lung cancer. Oncogene. 2008;27:3635–3640. doi: 10.1038/sj.onc.1211012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mountain CF. The international system for staging lung cancer. Semin Surg Oncol. 2000;18:106–115. doi: 10.1002/(sici)1098-2388(200003)18:2<106::aid-ssu4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 38.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260–271. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- 39.Silver SA, Askin FB. True papillary carcinoma of the lung: a distinct clinicopathologic entity. Am J Surg Pathol. 1997;21:43–51. doi: 10.1097/00000478-199701000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Moran CA. Pulmonary adenocarcinoma: the expanding spectrum of histologic variants. Arch Pathol Lab Med. 2006;130:958–962. doi: 10.5858/2006-130-958-PATESO. [DOI] [PubMed] [Google Scholar]
- 41.Borczuk AC. Micropapillary histology: a frequent morphology of mutation-associated lung adenocarcinoma? Am J Clin Pathol. 2009;131:615–617. doi: 10.1309/AJCP9NA3YQSWDYUN. [DOI] [PubMed] [Google Scholar]
- 42.Solis LM, Behrens C, Dong W, et al. Nrf2 and Keap1 abnormalities in nonsmall cell lung carcinoma and association with clinicopathologic features. Clin Cancer Res. 2010;16:3743–3753. doi: 10.1158/1078-0432.CCR-09-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalbfleischl JD, Prentice RL. The Statistical Analysis of Failure Time Data (Wiley Series in Probability and Statistics) 2. New York: John Wiley & Sons, Inc; 2002. [Google Scholar]
- 44.Haruki T, Shomori K, Shiomi T, Taniguchi Y, Nakamura H, Ito H. The morphological diversity of small lung adenocarcinoma with mixed subtypes is associated with local invasiveness and prognosis. Eur J Cardiothorac Surg. 2011;39:763–768. doi: 10.1016/j.ejcts.2010.07.047. [DOI] [PubMed] [Google Scholar]
- 45.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anagnostou VK, Syrigos KN, Bepler G, Homer RJ, Rimm DL. Thyroid transcription factor 1 is an independent prognostic factor for patients with stage I lung adenocarcinoma. J Clin Oncol. 2009;27:271–278. doi: 10.1200/JCO.2008.17.0043. [DOI] [PubMed] [Google Scholar]