Foamy viruses (FVs) belong to the Retroviridae family, which includes the g-retrovirus murine leukemia virus (MLV) and lentiviruses (LVs) such as HIV. Whereas vectors derived from HIV and MLV have shown success in the clinic, vectors derived from FVs have yet to be tested clinically. Three articles in this issue of Molecular Therapy clear a path to greater flexibility in the engineering of this promising new class of vectors and bring clinical application of FV vectors a step closer to reality. Nasimuzzaman and Persons1 reveal a long-kept secret of FVs with the identification of an FV receptor that at least partly explains the broad tropism of these viruses and vectors derived from them. Ho et al.2 present a strategy to allow the nearly ubiquitous FV vector tropism to be flexibly modified and restricted. Both studies represent important steps toward facilitating gene therapy applications of FV-based vectors, and in this light Uchiyama et al.3 demonstrate the correction of a preclinical mouse model of Wiskott–Aldrich syndrome (WAS) with a first-generation vector based on FV. The more neutral integration pattern of FV vectors is of particular interest with respect to gene therapy of WAS because the interim analysis of the first two WAS patients treated with conventional g-retroviral vectors has revealed an accumulation of problematic common vector insertion sites that have led to serious adverse events.4
FV vectors have several attributes that could make them valuable alternatives to retroviral vectors currently in use, including their broad tropism and possibly enhanced safety profile, as noted above. A hallmark of FVs is that they are not pathogenic for their natural hosts (nonhuman primates, cats, cattle, and horses), which do not include humans,5 although human cells can be infected zoonotically. As is the case with their retroviral cousins, FVs' proviral DNA integrates into the host genome, giving rise to long-term stable transgene expression. FV vectors might therefore lead to proto-oncogene activation via the interaction of strong enhancers embedded in the vector with a neighboring cellular gene. Indeed, both g-retroviral and LV vectors have resulted in rare cases of insertional mutagenesis leading to clonal expansion, leukemia, or transgene silencing in gene transfer recipients4,6,7,8 In this regard, LV vectors show a preferential integration into actively transcribed genes, whereas vectors based on MLV integrate around transcriptional start sites.9,10 Importantly, previous studies11,12 have shown a more random distribution of FV integrations as compared with that of g-retroviral or LV vectors, and Uchiyama et al.3 present data corroborating these findings. Although FV vectors did not show a strong preference for integration within genes, no integrating vector can be considered completely safe, and prudence remains warranted. Future preclinical and early clinical studies are necessary to provide further insights into the risk of FV-induced genotoxicity.
As noted above, although FVs have an extremely broad host range, the ubiquitously expressed FV receptor(s) have nevertheless remained unidentified.13 Ubiquitously expressed receptors can indeed be difficult to identify; the HTLV-1 receptor (the Glut-1 glucose transporter) was identified only after decades of searching,14 and the hunt for the vesicular stomatitis virus receptor continues. Nasimuzzaman and Persons1 initiated their characterization of the FV receptor with a simple observation: FV vector gene transfer to human CD34+ cells is strongly inhibited by extensive culture on fibronectin. They hypothesized that the heparin-binding domain on fibronectin interacted with heparan sulfate (HS) present on these cells, leading to inhibition of FV infection. HS seemed a plausible candidate receptor, in that it is nearly ubiquitously expressed on different cell types from various species. Several lines of evidence supported the notion that HS is the FV receptor. First, Chinese hamster ovary cells lacking HS surface expression were shown to be resistant to FV attachment and transduction. Second, enzymatic or chemical inhibition of HS expression reduced FV transduction of human, nonhuman primate, and murine cells. Finally, Raji cells, which lack HS, were rendered permissive through ectopic expression of HS-containing syndecan-1. These findings point to HS as an FV receptor, although an FV co-receptor (or receptors) that assists cell entry may yet exist.
A unique feature of FVs as compared with the other retroviruses is that particle budding is dependent on specific interaction between the viral Gag and Env proteins13,15 (Figure 1a). This dependency on the native interaction of these proteins had prevented modification of FV tropism via pseudotyping with heterologous viral envelope (env) proteins. Given the extremely broad natural tropism of FV, it could be of interest to modify target cell specificity of FV vectors for particular applications. Ho et al.2 describe an approach whereby recombinant heterodimerization domains (Figure 1b) were fused to both the native FV capsid protein and a heterologous viral env protein (Figure 1b). Addition of the dimerizer molecule thereby induced the gag–env interaction, resulting in FV particle egress. In a second approach, a more flexible strategy was employed that left the pseudotyping env protein unmodified. FV gag was modified in the same way as in the first strategy (Figure 1c) and was guided to the membrane by a membrane-targeting domain fused to a second dimerization domain (Figure 1c). This second strategy was more successful and gave rise to infectious FV particles of high titer in a strictly dimerizer-dependent manner using any of four different envelope proteins from heterologous viruses (vesicular stomatitis virus, rabies, Mokola virus, and lymphochoriomeningitis virus). The new study opens the way to redirecting or restricting FV vector gene transfer to specific target cells.
Figure 1.
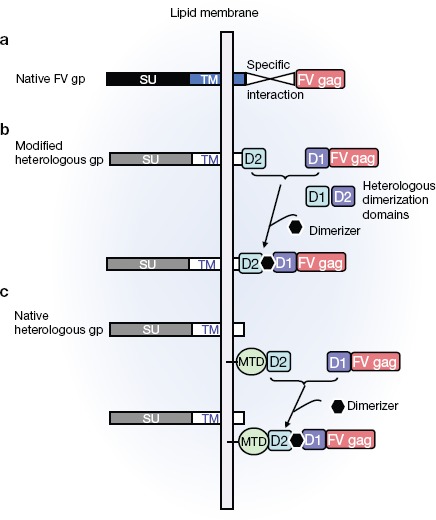
Novel strategy to change foamy virus (FV) vector target cell specificity. (a) Schematic illustration of the interaction between the native FV envelope and FV gag proteins, required for egress of infectious vector particles. (b and c) Schematic representation of strategies that allow the tropism of FV vectors to be modified. (b) In the first strategy, a heterologous dimerization domain (D1) is fused to the FV gag protein and the cognate domain (D2) is fused to an envelope glycoprotein of choice. A dimerizer molecule will bring FV gag to the membrane via binding to the envelope glycoprotein and allow budding of infectious particles. (c) In the second strategy, the heterologous envelope glycoprotein remains unmodified. As in b, a heterologous dimerization domain (D1) is fused to the FV gag protein and the second dimerization domain (D2) is fused to a membrane-targeting domain (MTD). The heterologous unmodified glycoproteins are already naturally expressed at the lipid membrane, while addition of the dimerizer brings FV gag to the lipid membrane as a result of its interaction with the MTD D2 domain. This colocalization of FV gag and envelope glycoprotein results in the efficient release of infectious FV vector particles.
As noted above, FV vectors transduce a broad variety of target cells and allow efficient gene transfer into neuronal progenitors and embryonic stem cells.16,17 However, FV vectors have been applied mainly for gene transfer into hematopoietic stem cells (HSCs).18,19,20 FV vectors are at least as efficient as LV vectors for human HSC transduction, as shown in a NOD/SCID mouse xenograft model. It is important to point out that only a short incubation with FV vectors is required for HSC transduction,20 possibly allowing a better conservation of stem cell properties. However, the FV vectors cannot efficiently transduce quiescent HSCs. Prestimulation of these cells with a cytokine cocktail so as to induce cell cycle entry is required to allow efficient FV-mediated transduction,21 which may be associated with loss of engraftment potential.
However, the effect of HSC cycle entry on stem cell–ness did not prevent the correction of several monogenetic diseases. Indeed, FV vectors have been used to correct a canine model for leukocyte deficiency. In this model, mutation of the leukocyte integrin gene (CD18) predisposes animals to severe infections. Four out of five dogs transplanted with autologous HSCs corrected by FV vectors coding for CD18 showed complete reversal of the disease.11 In a mouse model of Fanconi anemia, FV vectors expressing the Fancc gene corrected the HSC repopulation defect associated with this disease, even in secondary-transplant recipients.20 Another study showed that FV vectors can provide therapeutic levels of hemoglobin in a b-thalassemia mouse model, similar to what has been shown with lentiviral vectors.22 Impressive results from another group demonstrated that transplantation of FV-corrected HSCs in a murine model of X-linked granulomatous disease restored the oxidase expression in neutrophils.23
In this issue, Uchiyama et al.3 add Wiskott–Aldrich syndrome (WAS), an X-linked immunodeficiency, to the list of diseases that can be corrected by FV vectors. Was knockout mice were transplanted with HSCs transduced with FV vectors encoding for the human WAS gene. T-cell receptor–driven responses and B-cell migration were restored upon gene correction. Platelet adhesion was also restored, as was podosome formation in dendritic cells. Secondary transplantations confirmed that bona fide HSCs were successfully transduced by WAS protein–expressing FV vectors.
In summary, the relatively new FV vector system could become a viable alternative to LV and MLV vectors for HSC-based gene therapy of diseases with very diverse phenotypes. FV vectors will potentially further increase the efficacy of HSC-based gene therapy and decrease the risk of genotoxicity. Hence, the first clinical trial using FV vectors is probably close at hand. Moreover, the discovery of the FV receptor will lead to a greater understanding of FV biology and its suitability as a vector system for transduction of certain target cells such as lymphocytes, which are important gene therapy targets that cannot be modified easily with native FV vectors. Interestingly, the new strategy for FV vector pseudotyping could allow modification of the target cell specificity of FV vectors by pseudotyping with wild-type or targeted measles env proteins, which show a lymphocyte tropism.24,25 In the future, a side-by-side comparison of FV vectors with their MLV and LV vector counterparts will permit the identification of the best vector system for gene transfer into a specific target cell and disease indication.
REFERENCES
- Nasimuzzaman Md., and, Persons DA. Cell membrane–associated heparan sulfate is a receptor for prototype foamy virus in human, monkey, and rodent cells. Mol Ther. 2012;20:1158–1166. doi: 10.1038/mt.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y-P, Schnabel V, Swiersy A, Stirnnagel K., and, Lindemann D. A small-molecule-controlled system for efficient pseudotyping of prototype foamy virus vectors. Mol Ther. 2012;20:1167–1176. doi: 10.1038/mt.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T, Adriani M, Jagadeesh GJ, Paine A., and, Candotti F. Foamy virus vector-mediated gene correction of a mouse model of Wiskott–Aldrich syndrome. Mol Ther. 2012;20:1270–1279. doi: 10.1038/mt.2011.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boztug K, Schmidt M, Schwarzer A, Banerjee PP, Díez IA, Dewey RA.et al. (2010Stem-cell gene therapy for the Wiskott-Aldrich syndrome N Engl J Med 3631918–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer WM, Salemi M, Shanmugam V, Gao F, Cong M, Kuiken C.et al. (2005Ancient co-speciation of simian foamy viruses and primates Nature 434376–380. [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F.et al. (2010Transfusion independence and HMGA2 activation after gene therapy of human b-thalassaemia Nature 467318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E.et al. (2008Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1 J Clin Invest 1183132–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Kowhl U.et al. (2006Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1 Nat Med 12401–409. [DOI] [PubMed] [Google Scholar]
- Schroder AR, Shinn P, Chen H, Berry C, Ecker JR., and, Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521–529. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- Hematti P, Hong B-K, Ferguson C, Adler R, Hanawa H, Sellers S.et al. (2004Distinct genomic integration of MLV and SIV vectors in primate hematopoietic stem and progenitor cells PLoS Biol 2e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer TR, Jr, Allen JM, Hai M, Tuschong LM, Khan IF, Olson EM.et al. (2008Successful treatment of canine leukocyte adhesion deficiency by foamy virus vectors Nat Med 1493–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trobridge GD, Miller DG, Jacobs MA, Allen JM, Kiem H-P, Kaul R.et al. (2006Foamy virus vector integration sites in normal human cells Proc Natl Acad Sci USA 1031498–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CL, Bieniasz PD., and, McClure MO. Properties of human foamy virus relevant to its development as a vector for gene therapy. J Gen Virol. 1999;80:2003–2009. doi: 10.1099/0022-1317-80-8-2003. [DOI] [PubMed] [Google Scholar]
- Manel N, Kim FJ, Kinet S, Taylor N, Sitbon M., and, Battini JL. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell. 2003;115:449–459. doi: 10.1016/s0092-8674(03)00881-x. [DOI] [PubMed] [Google Scholar]
- Pietschmann T, Heinkelein M, Heldmann M, Zentgraf H, Rethwilm A., and, Lindemann D. Foamy virus capsids require the cognate envelope protein for particle export. J Virol. 1999;73:2613–2621. doi: 10.1128/jvi.73.4.2613-2621.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenaigner I, Kramer S, Meggendorfer M, Rethwilm A., and, Brack-Werner R. Transduction of human neural progenitor cells with foamy virus vectors for differentiation-dependent gene expression. Gene Ther. 2009;16:349–358. doi: 10.1038/gt.2008.173. [DOI] [PubMed] [Google Scholar]
- Gharwan H, Hirata RK, Wang P, Richard RE, Wang L, Olson E.et al. (2007Transduction of human embryonic stem cells by foamy virus vectors Mol Ther 151827–1833. [DOI] [PubMed] [Google Scholar]
- Leurs C, Jansen M, Pollok KE, Heinkelein M, Schmidt M, Wissler M.et al. (2003Comparison of three retroviral vector systems for transduction of nonobese diabetic/severe combined immunodeficiency mice repopulating human CD34+ cord blood cells Hum Gene Ther 14509–519. [DOI] [PubMed] [Google Scholar]
- Kiem HP, Allen J, Trobridge G, Olson E, Keyser K, Peterson L.et al. (2007Foamy virus–mediated gene transfer to canine repopulating cells Blood 10965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si Y, Pulliam AC, Linka Y, Ciccone S, Leurs C, Yuan J.et al. (2008Overnight transduction with foamyviral vectors restores the long-term repopulating activity of Fancc–/– stem cells Blood 1124458–4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann-Che J, Renault N, Giron ML, Roingeard P, Clave E, Tobaly-Tapiero J.et al. (2007Centrosomal latency of incoming foamy viruses in resting cells PLoS Pathog 3e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morianos I, Siapati EK, Pongas G., and, Vassilopoulos G. Comparative analysis of FV vectors with human alpha- or beta-globin gene regulatory elements for the correction of beta-thalassemia. Gene Ther. 2012;19:303–311. doi: 10.1038/gt.2011.98. [DOI] [PubMed] [Google Scholar]
- Chatziandreou I, Siapati EK., and, Vassilopoulos G. Genetic correction of X-linked chronic granulomatous disease with novel foamy virus vectors. Exp Hematol. 2011;39:643–652. doi: 10.1016/j.exphem.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Frecha C, Costa C, Lévy C, Nègre D, Russell SJ, Maisner A.et al. (2009Efficient and stable transduction of resting B lymphocytes and primary chronic lymphocyte leukemia cells using measles virus gp displaying lentiviral vectors Blood 1143173–3180. [DOI] [PubMed] [Google Scholar]
- Funke S, Maisner A, Mühlebach MD, Koehl U, Grez M, Cattaneo R.et al. (2008Targeted cell entry of lentiviral vectors Mol Ther 161427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]


