Abstract
Helicobacter pylori neutrophil-activating protein (NAP) is a major virulence factor and powerful inducer of inflammatory reaction and Th1-polarized immune response. Here, we evaluated the therapeutic efficacy of measles virus (MV) strains engineered to express secretory NAP forms against metastatic breast cancer. Recombinant viruses encoding secretory NAP forms (MV-lambda-NAP and MV-s-NAP) efficiently infect and destroy breast cancer cells by cell-to-cell viral spread and large syncytia formation independently of hormone receptor status. Intrapleural administration of MV-s-NAP doubled the median survival in a pleural effusion xenograft model: 65 days as compared to 29 days in the control group (P < 0.0001). This therapeutic effect correlated with a brisk Th1 type cytokine response in vivo. Secretory NAP was expressed at high levels by infected tumor cells and increased tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and IL-12/23 cytokine concentrations were detected in the pleural effusion. In an aggressive model of lung metastatic breast cancer, MV-lambda-NAP and MV-s-NAP also significantly improved survival of the treated animals (P < 0.05) as compared to the control MV strain. These data suggest that potent immunomodulators of bacterial origin, such as H. pylori NAP, can enhance the antitumor effect of oncolytic viruses and support the feasibility and potential of a combined viroimmunotherapy approach.
Introduction
Breast cancer is the most common malignancy diagnosed in women with >207,000 new cases of invasive cancer and almost 40,000 deaths due to metastatic disease in the United States annually.1 Development of lung and pleural metastases signals incurable disease with poor prognosis. Breast cancer is the second most frequent cause of malignant pleural effusion in cancer patients.2,3 Treatment options for patients with breast cancer metastases to the lung and malignant pleural/pericardial effusions are palliative4,5,6 and development of novel therapeutic approaches is urgently needed.
Attenuated measles virus (MV) represents an attractive oncolytic vector platform in cancer virotherapy.7 MV is an enveloped paramyxovirus with a single strand negative RNA genome.8 One major therapeutic advantage of this oncolytic virus platform is its significant bystander effect mediated through spread of infection via cell-to-cell fusion and formation of large multinucleated syncytia.9 Currently, clinical phase I trials using MV Edmonston vaccine strain derivatives are ongoing for treatment of patients with ovarian cancer, malignant brain tumors and hematological malignancies.7
Recent reports suggest that tumor-specific immune response developed as result of virus-mediated tumor destruction can have synergistic therapeutic effect.10 Induction of nonspecific immune reaction and neutrophil infiltration enhanced the antitumor activity of oncolytic MV.11 Thus, engineering of novel class vectors encoding potent immunomodulators and inducers of robust nonspecific inflammatory response is attractive approach in cancer virotherapy.
Helicobacter pylori neutrophil-activating protein (NAP) is a small 144 amino acid dodecamer-forming, iron-binding protein.12 It is a major virulence factor and protective antigen, involved in pathogenesis of the chronic mucosal inflammation in the course of H. pylori infection.13,14 NAP acts as a Toll-like receptor-2 agonist and potent immunomodulator triggering proinflammatory Th1 cytokine release (including interleukin-12 (IL-12), tumor necrosis factor-α (TNF-α) and interferon-γ) and Th1 type immune response polarization.15,16 We have recently demonstrated that MV vectors expressing high levels of chimeric secretory NAP induce strong, long-lasting humoral and cell-mediated immunity.17 MV-expressed NAP is biologically active and able to stimulate IL-8 production by monocytic cells.17 We therefore hypothesized that NAP transgene being a Toll-like receptor-2 activator can also enhance viral oncolysis by stimulating a strong inflammatory reaction and Th1 cytokine release, thus mobilizing host antitumor immune mechanisms and we tested this hypothesis in refractory metastatic breast cancer models.
Here, we present the evaluation of therapeutic efficacy of oncolytic MV strains expressing H. pylori NAP as a therapeutic transgene for the treatment of metastatic breast cancer and management of malignant pleural effusion. In xenograft lung and intrapleural models of metastatic breast cancer we demonstrated that recombinant MV vectors expressing the secretory NAP forms have enhanced oncolytic potency and significantly prolonged survival as compared to other MV strains, mediated in part by the secretion of proinflammatory cytokines and induction of nonspecific inflammatory reaction in the tumor microenvironment.
Results
MV strains encoding H. pylori NAP have significant antitumor activity against breast cancer lines in vitro
NAP constructs were cloned upstream of the nucleoprotein gene in MV genome (see Figure 1). We have previously demonstrated high levels of NAP expression by two MV strains encoding the chimeric secretory forms of the NAP transgene: MV-lambda-NAP and MV-s-NAP17 and these strains were used for subsequent experiments. Both strains showed potent in vitro antiproliferative activity against estrogen-dependent (MCF-7) and hormone-independent (MDA-MB-231) human breast cancer lines as measured by MTT assay (Figure 2a–c). Viral replication in breast cancer cells and cell-to-cell spread of infection caused large multinucleated syncytia formation within 48 hours postinoculation, preceding massive cell death.
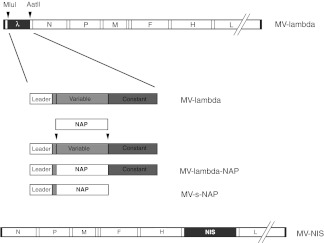
Figure 1.
Schematic representation of the recombinant measles virus (MV) strains used in the experiments: MV-lambda, MV-lambda-NAP, MV-s-NAP and MV-NIS. NAP, neutrophil-activating protein.
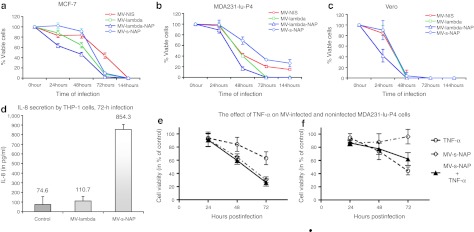
Figure 2.
In vitro antitumor activity of engineered measles virus (MV) strains against breast cancer cells. (a) MCF-7 cell line and the highly tumorigenic MDA231-lu-P4 in vivo derivative of (b) MDA-MB-231 cells were infected with MV strains at an multiplicity of infection (MOI) = 1.0 and cell viability was determined by MTT assay. Both MV-lambda-NAP and MV-lambda viruses propagated rapidly causing complete destruction of the breast cancer monolayers 72 hours postinfection. MV-s-NAP and MV-NIS showed similar tumor cell-killing kinetics with complete eradication of MCF-7 cells or ~80% reduction of cell viability of MDA231-lu-P4 cells. (c) Vero cells were used as control. The data are presented as percent of untreated control cells ± SD. (d) NAP transgene induced significant interleukin (IL)-8 expression in MV vector-infected THP-1 cells. THP-1 monocytic cells were infected with MV-s-NAP or control MV-lambda virus at a MOI = 0.1 and incubated for 72 hours. Supernatants were collected and IL-8 concentration was measured by enzyme-linked immunosorbent assay (ELISA) specific for human IL-8. The effect of TNF-α (one of the known NAP-triggered inflammatory cytokines) on proliferation of MV-infected or uninfected MDA231-lu-P4 cells was examined by MTT assay (e,f). MDA231-lu-P4 cells were plated at 104 cell/well density and inoculated at MOI = 0.5 of MV-s-NAP in the presence or absence of 250 U/ml recombinant human TNF-α (e). The same experiment was repeated with lower cell density (2.5 × 103 per well) and MV-s-NAP at MOI of 0.25 (f).
The presence of a constant lambda domain in the chimeric lambda-NAP protein allowed accurate quantification of transgene expression using a human lambda immunoglobulin-specific enzyme-linked immunosorbent assay (ELISA). Expression of the secretory NAP transgene by MV-lambda-NAP-infected MDA231-lu-P4 breast cancer cells reached ~1 µg/106 cells at 72 hours postinoculation.
In previous experiments, we confirmed that MV-s-NAP-infected Vero cells secreted biologically active NAP by demonstrating stimulation of IL-8 production in THP-1 monocytic cells treated with virus-inactivated supernatants.17 Here, we confirmed that infection with an oncolytic MV strain-encoding NAP transgene also triggers IL-8 expression in THP-1 cells (Figure 2d). Seventy-two hours postinoculation at the low multiplicity of infection (MOI) of 0.1 infection with MV-s-NAP resulted in significantly higher levels of IL-8 release in the supernatant, as compared to the control MV-lambda strain. These in vitro data support that MV-expressed NAP retains its immunomodulatory activity.
Secretory NAP produced by tumor cells in the course of infection could exert its biological effects over the neighboring noninfected cells by recruitment of immune cells and secretion of proinflammatory cytokines. Since NAP is a potent inducer of IL-12 and TNF-α15 and both of them (in contrast to IL-6) are species crossreactive in mouse xenograft models, we tested the in vitro effect of these cytokines on tumor cells. Significant inhibition of breast cancer cell growth was observed following preincubation with 250 U/ml specific activity of recombinant TNF-α. When high cell densities and MV-s-NAP at MOI = 0.5 were employed (Figure 2e), addition of TNF-α did not increase significantly the observed antitumor effect, which was predominated by MV-s-NAP-induced oncolysis. When lower cell densities and a lower MOI = 0.25 were employed, the absence of cell-to-cell contact in the monolayer prevented rapid expansion of MV infection via syncytia formation and the antitumor effect is predominantly TNF-α mediated. TNF-α treatment alone or in combination with MV resulted in > 50% reduction in cell proliferation by 72 hours incubation (Figure 2f). Under these conditions, expansion of MV-s-NAP infection requires multistep viral propagation and 6–7 days to destroy the tumor cells (data not shown). These results suggested that TNF-α released at the earlier stage of oncolytic MV infection could exert significant bystander tumor inhibitory effect. IL-12 did not show significant direct antitumor effect against MDA231-lu-P4 cells in vitro (data not shown).
Treatment with NAP-expressing MV strains significantly improved survival in pleural effusion model of advanced breast cancer
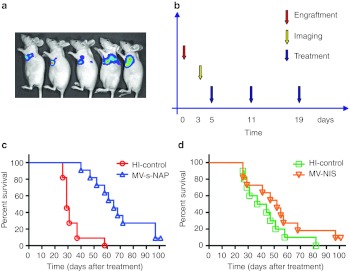
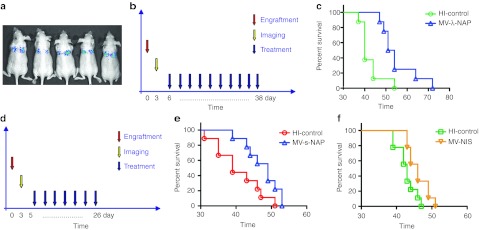
We have previously described the development of a pleural effusion model of metastatic breast cancer in mice.9 Transthoracically injected MDA231-lu-P4 cells grow aggressively in the pleural space and animals develop symptomatic disease with massive accumulation of pleural exudates 5–7 weeks postengraftment. For the therapeutic efficacy experiments, breast cancer cells were implanted by transthoracic (t.t.) injection and 3 days later animals were imaged to verify tumor engraftment (Figure 3). Three repeat t.t. injections of 5 × 105 TCID50 MV-s-NAP prolonged more than twice the survival of the treated group (65 vs. 29 days, respectively, P < 0.0001) in control animals injected with inactivated virus (Figure 3c). In contrast, treatment with MV-NIS (MV strain-encoding human sodium-iodide symporter gene) administered in the same dose and schedule did not significantly impact survival as compared to the corresponding control group treated with heat-inactivated MV-NIS (Figure 3d). Since the vector backbone of MV-NIS is identical to those of MV-s-NAP and MV-lambda-NAP strains, and in vitro data indicate comparable antitumor activity of MV-NIS and MV-s-NAP, it is likely that the expression of NAP is mediating the observed difference in the therapeutic effect in vivo.
Figure 3.
Therapeutic efficacy of attenuated measles virus (MV) engineered to express secretory neutrophil-activating protein (NAP) in the MDA231-lu-P4 pleural effusion breast cancer xenograft model in athymic nude mice. (a) Engraftment of transthoracic (t.t.) implanted tumors was confirmed by bioluminescence imaging. (b) The animals (11 per group) received 3 weekly t.t. injections of 5 × 105 TCID50 of MV-s-NAP or MV-NIS. Viruses were diluted in phosphate-buffered saline (PBS) (MV-s-NAP) or 5% sucrose, Tris-HCl buffer (MV-NIS). Individual control groups for each virus strains (10–11 mice) were injected with heat-inactivated MV-s-NAP or MV-NIS (HI-control), respectively. (c,d) Survival was analyzed using Kaplan–Meyer method and log-rank test. In contrast to to MV-NIS, MV-s-NAP significantly improved median survival of the treated mice from 29 to 65 days (P < 0.0001).
Demonstration of MV replication, NAP expression, and biological activity in a breast cancer pleural effusion model in vivo
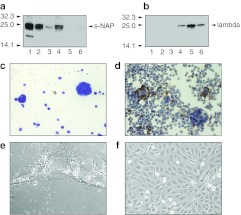
Both secretory NAP and lambda light immunoglobulin chain were detected by immunoblotting in the pleural effusion of the animals treated t.t. with MV-s-NAP (four of four samples tested positive for the chimeric NAP) or MV-lambda (three of four positive for lambda chain), respectively, indicating viral replication (Figure 4a,b). Infection induced large spherical syncytia in tumor cells floating in the pleural fluid and MV was recovered by overlay on Vero cells (Figure 4c–f). Immunohistochemistry staining confirmed a significant neutrophil presence in the pleural fluid (Figure 4d).
Figure 4.
Measles virus (MV) infection and neutrophil-activating protein (NAP) transgene expression in the malignant pleural effusion of mice-bearing MDA231-lu-P4 pleural xenografts. Mice were treated by a single transthoracic (t.t.). injection of MV-s-NAP or MV-lambda. NAP transgene expression in pleural fluid was demonstrated by immunoblotting using (a) NAP-specific monoclonal antibody (MAb) 16F4 or (b) lambda chain-specific antibody. The secretory form of the NAP transgene was detected in the pleural fluid of four of four MV-s-NAP-treated mice (lanes 1–4) but not in MV-lambda-injected (lanes 5, 6) control mice (a). Human lambda light chain was detected in three of four samples from MV-lambda-injected (lanes 3–6) mice but not in samples from MV-s-NAP-treated (lanes 1, 2) animals (b) using a lambda chain-specific detection antibody. MV-s-NAP (c) induced large multinucleated syncytia in infected tumor cells (Giemsa staining) in the pleural fluid of mice with MDA231-lu-P4 xenografts. Immunohistochemistry (IHC) staining for neutrophils in the pleural fluid of MV-s-NAP is shown in (d). MV-s-NAP was isolated from the pleural fluid by overlay on Vero cells. MV-s-NAP induced giant syncytia formation 24 hours after overlay (e). Uninfected control Vero cells (f).
To determine the role of NAP expression for induction of inflammatory reaction that can contribute to enhancement of the MV oncolytic activity, advanced intrapleural MDA231-lu-P4 tumors were treated by a single t.t. injection of MV-s-NAP or the control MV-lambda virus. Pleural fluid and serum were collected and analyzed for inflammatory Th1 cytokines. MV-lambda was used as the control virus in these experiments because it encodes a full-length human lambda light immunoglobulin chain18 and was used as the backbone for engineering the secretory NAP transgene forms (see Figure 1). Mice were sacrificed (2–6 per time point) on days 1, 2, 4, and 9 post-treatment and cytokine levels in pleural fluid and serum were determined by ELISA. Increase of Th1 type cytokine concentrations in the pleural fluid samples from MV-s-NAP-injected mice was observed at 48 hours post-treatment (Table 1). The levels of IL-6 in MV-s-NAP-treated mice were more than fourfold higher (group mean value >11,000 pg/ml) than in the animals that received control MV-lambda virus. Higher levels of IL-6 were also detected in serum samples from the same animals indicating increased systemic inflammatory cytokine response. Pleural fluid concentration of IL-12/23 p40 and TNF-α were also elevated in MV-s-NAP-injected mice as compared to the MV-lambda-treated controls (Table 1). The cells isolated from pleural fluid and cultured in vitro from mice treated with MV-s-NAP also produced detectable TNF-α in four of six samples in the range of 35.4–378.0 pg/ml after 24 hours of incubation. In contrast, none of the cell samples isolated from control MV-lambda-treated group was positive for TNF-α. Pleural cells from MV-s-NAP-injected mice also secreted higher levels of IL-6 (range 193.9–1,104.3 pg/ml at 24 hours) as compared to pleural cells from control MV-lambda-treated mice (range 46.2–96.9 pg/ml at 24 hours).
Table 1. Inflammatory cytokine levels in biological fluids of mice with MDA231-lu-P4 pleural tumors 48 hours after injection with MV-s-NAP or the MV-lambda control strain.
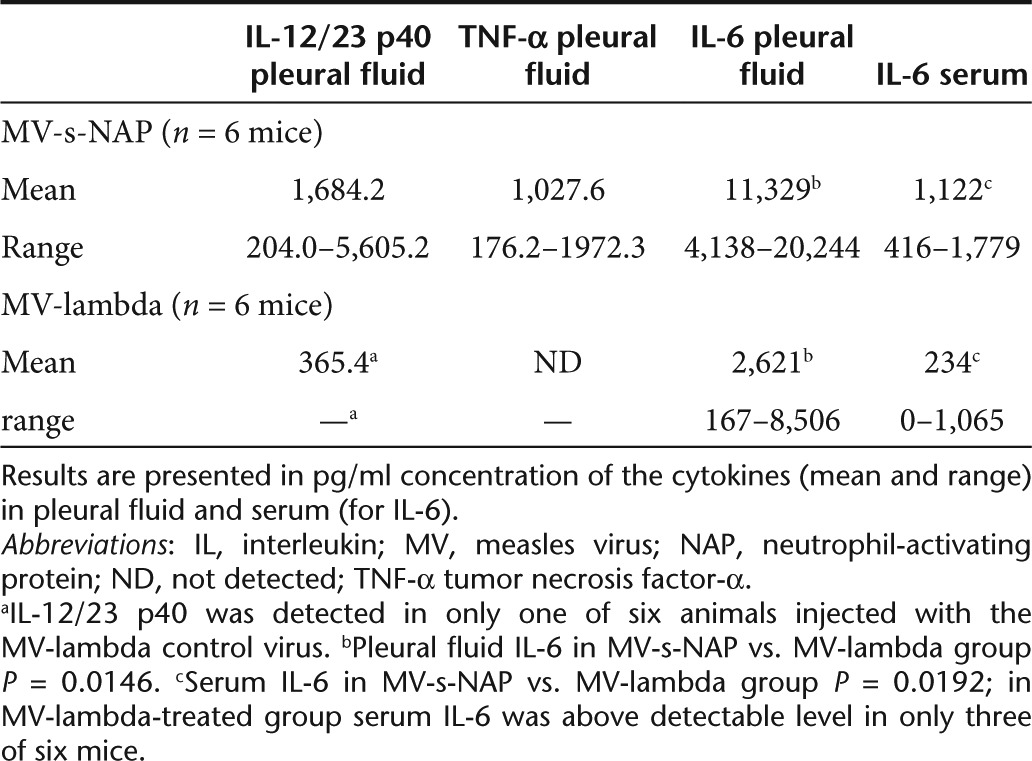
These results demonstrate that MV-s-NAP-infected tumor cells produce high levels of the NAP transgene in vivo. The elevated pleural fluid concentration of Th1 cytokine IL-12/23 as well as local and systemic IL-6 levels correlated with better oncolytic effect in MV-s-NAP-treated mice and suggest that the NAP transgene could significantly increase the therapeutic efficacy of MV in management of malignant effusions by enhancement of local inflammatory reaction in the pleural space. In vitro experiments (described above) confirmed that at least one of the NAP-induced proinflammatory cytokines, TNF-α, can also contribute to the MV-s-NAP antitumor effect by direct inhibition of tumor cell growth. Although IL-12 has no direct inhibitory effect in vitro on MDA231-lu-P4, as a key Th1 cytokine NAP-induced IL-12 expression can also play a crucial role indirectly by activation of nonspecific inflammatory or tumor-specific immune mechanisms.
Therapeutic activity of NAP-expressing MV strains against a disseminated breast cancer mode
We have previously shown that in vivo passaging in the mouse lungs can significantly increase the tumorigenicity of MDA-MB-231 cells, forming disseminated lung metastases after tail vein injection. The MDA231-lu-P4 derivative line, isolated after four in vivo passages demonstrates a gene expression profile that is consistent with the gene expression profiling observed in lung metastases of breast cancer patients.9 Mice were injected with 1–2 × 106 MDA231-lu-P4 cells via the tail vein. Tumor implantation was confirmed by bioluminescence imaging (Figure 5a) and animals were assigned into groups to ensure comparable tumor burden. In two separate experiments mice received either: (i) MV-lambda-NAP (10 repeated intravenous (i.v.) injections of 2 × 106 TCID50/injection) or (ii) MV-NIS or MV-s-NAP (7 i.v. injections of 106 TCID50/injection) via systemic i.v. route of administration (Figure 5b and d). Control groups were injected with the corresponding heat-inactivated viruses in a similar administration schedule. Treatment with either MV-lambda-NAP or MV-s-NAP significantly prolonged median survival: P = 0.0055 and P = 0.0378, respectively (Figure 5c and e). In contrast, MV-NIS treatment (7 therapeutic injections of 106 TCID50) did not significantly affect survival in this model (Figure 5f).
Figure 5.
Therapeutic effect of neutrophil-activating protein (NAP)-expressing measles virus (MV) strains against lung metastatic breast cancer compared to control MV-NIS. (a) Engraftment of the systemically injected MDA231-lu-P3 or P4 cells was confirmed by bioluminescence imaging. (b,c) The animals with MDA231-lu-P3 lung tumors (8 per group) were treated with 10 repeat intravenous (i.v.) injections of 2 × 106 TCID50 of MV-lambda-NAP or heat-inactivated (HI-control) control. In a separate in vivo experiment MDA231-lu-P4 lung metastatic xenografts (9 mice per group) were treated by 7 i.v. injections of 106 TCID50 of MV-s-NAP, MV-NIS or the corresponding heat-inactivated controls (d–f). Both MV-lambda-NAP and MV-s-NAP improved the median survival with 10–12 days (P < 0.05) in this aggressive model of breast cancer metastatic to the lungs.
Assessment of toxicity of MV-s-NAP in the measles susceptible transgenic mouse model Ifnarko-CD46Ge
MV Edmonston strain derivatives do not infect rodent cells because of the lack of expression of MV receptors and intracellular restriction mechanisms impairing viral replication. Consequently, our xenograft efficacy models in athymic nude mice cannot be used for assessment of MV-s-NAP toxicity. We therefore employed a measles infection-susceptible transgenic mouse model, the type I interferon receptor knockout/human CD46 transgenic (Ifnarko-CD46Ge) mice, for toxicity assessment.19 These mice express the MV receptor CD46 with a tissue distribution and levels of expression that parallel human CD46 expression.19 Although breast cancer cell lines are not tumorigenic in Ifnarko-CD46Ge mice and as a result this model cannot be used for assessment of efficacy, it is still an excellent system for comparative evaluation of measles toxicity and has been accepted by the United States Food and Drug Administration as a model of measles toxicity in toxicology studies, prior to initiation of three ongoing clinical trials of engineered MV Edmonston strains.20,21
A preliminary analysis of potential toxicity of i.v. or intraperitoneal (i.p.) administration of the NAP-expressing MV strains was performed in 3–5-week-old female Ifnarko-CD46Ge mice. The animals were injected either with 106 TCID50 (i.p. route of administration, n = 11 animals) or with 2 × 106 TCID50 (i.v. group, n = 9 animals) of MV-s-NAP and blood samples were collected on day 1, 2, 3, 7, and 14 for complete blood count. Prior to start of study, age-matched female Ifnarko-CD46Ge mice (n = 6 total) were bled retro-orbitally and their hematology parameters served as controls. As shown in Supplementary Table S1, there were transient changes in some hematologic parameters at different time points postvirus injection, although the corresponding values still remained within the normal range for mice. A transient decrease in the white blood cell count was observed in the i.v. injected mice on day 1 (mean = 3.99 × 109/l, P = 0.0251). Although a transient increase in the platelet count was detected on day 7 for i.v. injected mice (mean = 784.4 × 109/l, P = 0.041 vs. controls) and on day 14 for i.p. injected mice (mean 696.1 × 109/l, P = 0.0012), the values were within the normal range for mice: 100–1,000 × 109/l.22 Slight decrease in hematocrit (mean = 46.03%, P = 0.034 vs. control) and erythrocyte count (red blood cells) (mean = 8.39 × 1012/l, P = 0.0026) observed on day 7 in the i.p injected mice (Supplementary Table S1), but with normal hemoglobin.22 The effect was transient and the mean red blood cells values of the i.p. treated mice on day 14 returned to the normal range (9.78 × 1012/l) with higher than control hemoglobin (16.46 g/dl, P = 0.026) and hematocrit (mean = 52.9%, P = 0.0018) (Supplementary Table S1). Low levels of serum IL-6 was detected in one animal on day 1 after i.p. administration (92 pg/ml/group) and one animal on day 2 after i.v. administration (116 pg/ml). Both animals were asymptomatic. Low levels of serum TNF-α were detected in two of the i.p. injected mice on day 2 (82 pg/ml) and day 3 (147 pg/ml), postinjection, again without associated symptoms. MV-s-NAP-injected animals remained alive and healthy without signs of toxicity of oncolytic agent until the end of the study—more than 3 months post-treatment. These results from the pilot toxicology study support the safety of MV-s-NAP administration in a measles replication susceptible transgenic mouse model: a larger study is expected to be required prior to clinical translation.
Discussion
MV strains of Edmonston vaccine lineage demonstrated potent antitumor activity against different human tumor types in vitro and in preclinical in vivo studies.23,24,25,26,27,28 It has been reported that granulocyte macrophage colony-stimulating factor-mediated neutrophil attraction could additionally increase the MV antitumor effect against lymphoma xenografts in immunocompromised mice.11 Insertion of foreign genes in the MV genome is tolerated without significant impact on virus propagation and recombinant strains such as MV-CEA (MV encoding carcinoembryonic antigen as reporter) and MV-NIS are currently being tested in clinical trials for cancer patient therapy.7,29
Our main hypothesis is that expression of bacteria-derived potent immunostimulatory factors can significantly enhance the therapeutic activity of oncolytic viruses. We have recently characterized and tested the immunogenicity of a panel of NAP-expressing MV constructs.17 The NAP transgene was cloned upstream of the N gene in the MV genome. To allow extracellular secretion, the NAP transgene was inserted in the human lambda light immunoglobulin chain gene by replacing the variable domain while maintaining (MV-lambda-NAP) or deleting (MV-s-NAP) the constant lambda chain domain. Our data showed that secretory NAP forms encoded by recombinant MV strains were expressed at high levels by infected virus producer Vero cells. NAP inserts did not affect viral propagation and growth kinetics in Vero cells as compared to the control strains not expressing the transgene. Both MV-lambda-NAP and MV-s-NAP replicated efficiently in vivo inducing strong and long-lasting immunity in measles infection permissive transgenic animals.17 H. pylori NAP is a potent immunomodulator, stimulating robust inflammatory reaction and Th1 polarization of the immune response.30 Systemic NAP administration can reverse the Th2-biased immune response against allergens, stimulating IL-12 production and reduction of plasma IgE levels.31,32
We tested this concept using the NAP-expressing MV constructs in two aggressive metastatic breast cancer models (lung and pleural metastases) corresponding to challenging clinical scenarios frequently encountered in breast cancer patients. Malignant pleural effusions in cancer patients are an indication of advanced disseminated disease. Therapy is limited to treatments such as thoracocentesis, drainage and therapeutic pleurodesis33 plus systemic therapy, all of which are palliative. Induction of strong local inflammation and obliteration of the pleural space is the main current palliative approach in management of malignant pleural effusions in cancer patients.34,35 Activation of immune cells and secretion of proinflammatory cytokines such as TNF-α, IL-1, IL-6, and IL-8 have been shown to play a key role in successful treatment outcome.36,37
The rationale for developing MV as an oncolytic platform is based on clinically observed remission of hematological malignancies following natural measles infection.7 Documented reports on spontaneous tumor regression in patients are frequently related to infections accompanied by high fever as a clinical manifestation of a robust immune reaction and proinflammatory cytokine release.38 These findings led to development of therapeutic approaches using bacterial extracts or components in cancer immunotherapy. Bacterial preparations from Streptococcus pyogenes, staphylococcal superantigens and muramyl dipeptide derivatives have been successfully tested in the treatment of pleural effusions in patients with lung cancer and metastatic breast cancer.37,39,40,41 Thus, oncolytic vectors engineered to express bacterial factors with potent immunostimulatory activity are promising alternatives to the conventional pleurodesis approach combining the antitumor effect of viral oncolysis with the therapeutic impact of proinflammatory cytokine release.
Virotherapy with locally administered MV encoding secretory forms of H. pylori NAP was well tolerated and showed significant activity against metastatic breast cancer in a pleural effusion xenograft model in mice. Treatment prolonged more than twice the median survival of the animals. MV exerted its oncolytic activity via massive infection of malignant cells and large syncytia formation in the pleural tumor deposits. Although athymic nude mice are T-cell response-deficient, they still have intact activity of the innate immune mechanisms, such as the complement system, macrophages, and NK cells. As a Toll-like receptor-2 agonist and potent attractant of immune cells,16,42,43 the NAP transgene can contribute to stimulation of local inflammatory reaction preventing reaccumulation of malignant pleural effusion. Analysis of pleural fluid samples within 48 hours after treatment revealed high levels of secretory NAP in the pleural fluid and increase of the local and systemic proinflammatory cytokines in MV-s-NAP-injected mice, including IL-12 as an activator of NK cells and marker of Th1 shift of the immune response. Elevated levels of TNF-α, IL-12, and IL-6 indicated induction of a strong local inflammatory reaction in MV-s-NAP-treated groups. Our in vitro results (see Figure 2) indicate that NAP transgene-mediated induction of TNF-α can contribute to enhancement of MV oncolytic activity via its bystander antiproliferative effect on noninfected cancer cells. The effect of elevated TNF-α secretion was not observed in animals treated with MV-lambda, the control virus encoding the full-length human lambda immunoglobulin chain, which was used as the backbone for secretory NAP engineering. In addition to the direct inhibition of tumor cell growth, TNF-α can act synergistically with chemotherapeutic agents against breast cancer cells.44,45
These data support the potential use of NAP as an immunoadjuvant for stimulation of specific cell-mediated and humoral antitumor immunity. While the virus-mediated tumor destruction requires massive infection of tumor tissue, stimulation of the antitumor immune mechanisms and enhancement of the virotherapy efficacy by potent vector-encoded immunomodulators, such as NAP, could be achieved by infection of a fraction of the cancer cells. Our results confirmed that MV-encoded NAP is synthesized and secreted in biologically active form, capable of stimulate cytokine production by immune cells. Additional experiments comparing replication competent to nonreplicating NAP gene delivery systems are in progress to help us characterize the individual contribution of NAP-induced inflammatory reaction versus productive virus infection as mediators of tumor regression and therapeutic outcome. We have previously demonstrated that infected cells were efficient carriers for systemic or local delivery of oncolytic agents to metastatic tumors46 and that dendritic cells were capable of delivering oncolytic MV to the pleural tumor deposits and significantly improved survival in a pleural effusion xenograft model.9 Thus, professional antigen-presenting cells, such as monocyte/macrophages and dendritic cells, could be used in a dual purpose—both transfer of MV infection to the tumor site and NAP-mediated augmentation of the immune reaction against tumor cells. Experiments using NAP-expressing MV strains in this context are ongoing.
In conclusion, clinical translation of MV vectors encoding NAP could lead to development of a novel therapeutic strategy for treatment of metastatic solid tumors in patients by combining the benefits of oncolytic virotherapy and immunotherapy.
Materials and Methods
Cell lines and cell culture. Vero cells, human THP-1 monocytic line and human breast cancer cell lines MCF-7 and MDA-MB-231 were purchased from American Type Culture Collection (Manassas VA). Vero and breast cancer cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics (Invitrogen, Carlsbad, CA). THP-1 monocytic cells were grown in RPMI 1640 medium with 10% fetal bovine serum and supplements according to American Type Culture Collection recommendations (American Type Culture Collection). We have previously reported on development of an aggressive model of metastatic breast cancer with spread to the lungs and pleural cavity. MDA231-lu-P3 and MDA231-lu-P4 cells are derivatives of the MDA-MB-231 line following three or four in vivo passages in athymic nude mice and transduced to express firefly luciferase (F-lu) as reporter gene.9 MDA231-lu-P3 and MDA231-lu-P4 cells form metastatic tumor deposits after i.v. injection or t.t. implantation in mice.
MV strains, virus propagation, and titration. Attenuated MV Edmonston vaccine strain has been proposed as a platform for generation of recombinant MV constructs.47 We have recently reported on construction of MV strains encoding H. pylori NAP.17 MV-lambda-NAP and MV-s-NAP were designed to express secretory chimeric NAP replacing the main part of variable domain of human lambda light immunoglobulin chain with (MV-lambda-NAP) or without (MV-s-NAP) the constant part of the molecule (Figure 1). Recombinant MVs encoding either the full lambda immunoglobulin chain (MV-lambda) as a reporter18 or the human sodium-iodide symporter (MV-NIS) gene48 were used as control strains in the experiments. Recombinant strains were propagated on Vero cells as described previously and the viral stocks were stored at −80 °C.49 Viral titer was determined in plaque-forming units and tissue culture infectious doses 50% (TCID50) per ml.18 MN-NIS stocks were produced and purified as previously described.50
In vitro antitumor activity of recombinant MV strains and cytokines. MCF-7, MDA-MB-231, and MDA231-lu-P4 breast cancer lines were plated at a density of 104 cells/well in 96-well plates. The monolayers were inoculated at a MOI of 1.0 with viral strains diluted in Opti-MEM (Invitrogen). In vitro tumor-killing effect was determined at 24, 48, 72 hours, and 6–7 days postinfection using MTT proliferation assay (American Type Culture Collection) as previously described.9
In another experiment we tested the direct effect of NAP-inducible proinflammatory cytokines on breast cancer cells. Briefly, MDA231- lu-P4 cells were plated at higher (104 cells/well) or lower density (2.5 × 103 cells/well) in 96-well plates (Falcon; Becton Dickinson Labware, Franklin Lakes, NJ). The cells were treated with 100 ng/ml recombinant mouse IL-12 p70 (BioLegend, San Diego, CA) corresponding to 500 U/ml specific activity or 100 ng/ml of human TNF-α (Novus Biologicals, Littleton, CO) with 250 U/ml specific activity. The cells were infected with MV-s-NAP at MOI of 0.25–0.5 in the presence or absence of cytokines and cell viability at different time points was measured by MTT assay as described above.
ELISA and immunoblotting for detection of NAP transgene expression by infected cells. Chimeric lambda-NAP and lambda light immunoglobulin chain expression by MV-lambda-NAP and control MV-lambda-infected cells in cell culture supernatants in vitro and biological fluids in vivo was quantified using a human lambda immunoglobulin-specific ELISA kit, following the manufacturer's recommendations (Bethyl Laboratories, Montgomery, TX). Secretory NAP produced by MV-s-NAP-infected cells was detected by immunoblotting using the NAP-specific monoclonal antibody clone 16F4, which we have recently generated and characterized.17 Expression of chimeric lambda-NAP and lambda chain transgene was confirmed by immunoblotting using an antihuman lambda immunoglobulin secondary antibody (Bethyl Laboratories) as previously described.18
Human IL-8 expression analysis. Human THP-1 monocytic cells were inoculated with MV strains at MOI = 0.1. Supernatants were collected at different time intervals during the infection course and IL-8 concentration was measured by ELISA kits according to the manufacturer's protocol (eBioscience, San Diego, CA).
Immunohistochemistry. Microscopy slides were prepared by smearing of the pleural fluid samples. The slides were dried at room temperature and fixed in ice-cold methanol for 5–10 minutes. Endogenous peroxidase was inactivated by incubation of the slides in 0.2% H2O2 for 5 minutes. Samples were blocked with 5% normal goat serum in phosphate-buffered saline (PBS), washed and incubated for 1 hour with neutrophil marker specific rat monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA). After washing in PBS the slides were incubated with biotin-conjugated goat anti-rat immunoglobulin secondary antibody (Santa Cruz Biotechnology) for 30 minutes. The immunohistochemistry reaction was visualized using Vectastain Elite ABC kit and DAB peroxidase substrate according to the manufacturer's protocol (Vector Laboratories, Burlingame, CA). Hematoxylin (Sigma, St Louis, CA) was used for counterstaining. Parallel slides were stained using Giemsa's staining procedure.
Animal models and MV therapy experiments. For the in vivo studies 4–5-week-old female athymic nude mice were purchased from Harlan, Indianapolis IN. The animal experiments were reviewed and approved by the Mayo Foundation Institutional Animal Care and Use Committee.
Pleural effusion xenograft model. Athymic nude mice were engrafted with 1–2 × 106 exponentially grown MBA231-lu-P4 cells in 10 µl PBS. Cells were implanted by t.t. injection in the left pleural cavity of 4–5-week-old female nude mice as described previously.9 Intrapleural tumor engraftment was confirmed by live body imaging using the Xenogen Ivis 200 System (Caliper Life Sciences, Hopkinton, MA) on day 3 after the tumor cell injection.9 Animal groups (9–11 mice per group) were arranged according to the bioluminescent signal intensity of the pleural tumors to ensure comparable tumor burden. Mice received three therapeutic injections (once per week, starting on day 4–6 of the study) of MV strains in 50 µl volume via t.t. injection under brief Isoflurane inhalation anesthesia. Survival was monitored and compared to the control group injected with heat-inactivated (60 °C/1 hour) virus. Animals were euthanized if they developed weight loss >15–20% and dyspnea. Pleural fluid samples from the euthanized mice were collected for MV isolation and histological examination.
Cytokine expression analysis following MV treatment of advanced pleural effusion. Mice with advanced intrapleural tumors (3–4 weeks postengraftment) were injected with a single t.t. injection of 5 × 105 TCID50 of MV-s-NAP or MV-lambda control virus. Two to six mice per group were sacrificed at different time points (day 1, 2, 4, and 9) and pleural fluid and serum samples were collected. Pleural fluid specimens were examined for the presence of tumor cells and virus-induced syncytia by light microscopy using Giemsa's staining and immunohistochemistry as described above. MV infection in the tumor cells was also confirmed by overlay on Vero cell monolayers.9 For measurement of cytokine levels and transgene expression analysis, pleural fluid samples were centrifuged to remove cells and the supernatant was collected and frozen at −80 °C. The concentration of proinflammatory cytokines released in the pleural fluid and serum was measured using mouse IL-12/23 p40, IL-6, and TNF-α ELISA kits according to the manufacturer's instructions (eBioscience). Expression of the NAP transgene in the pleural fluid of treated animals was confirmed by immunoblotting. Briefly, 10 µl of 1:10 diluted in PBS pleural fluid were mixed with 10 µl sample buffer and were resolved on 15% Criterion gel (Bio-Rad, Hercules, CA). Proteins were transferred to polyvinylidene fluoride membrane (Bio-Rad) and probed with the NAP-specific monoclonal antibody 16F4 or antibody against human lambda light chain as previously described.17
Disseminated breast cancer animal model. Female nude mice were injected i.v. with 1–2 × 106 exponentially growing MDA231-lu-P3 or MDA231-lu-P4 cells resuspended in 100 µl PBS. Forty-eight to seventy-two hours postinjection, engraftment of the tumor metastases in the lungs was verified by the Xenogen system imaging (Caliper Life Sciences, Hopkinton, MA).9 Animals were assigned to groups (8–9 animals per group) based on the bioluminescence intensity of the lung metastases to ensure comparable tumor burden. Mice were treated with 7–10 repeated i.v. injections of oncolytic MV strains (1–2 × 106 TCID50 in 100 µl) starting on day 5–6 postengraftment. Survival of the treated groups was compared to that of mice injected with heat-inactivated virus.
Toxicology studies. To assess a possible toxicity of the NAP-expressing MV strains, 11 female 3–4 week-old Ifnarko-CD46Ge mice were injected i.p. with 106 TCID50 of MV-s-NAP. Mice were monitored for clinical toxicity, development of neurologic symptoms, and weight loss up to 3 months after virus inoculation. The animals were bled on days 1, 2, 3, 7, and 14 postinjection (3–4 animals per time point) and blood samples were analyzed on the HMT2 Hematology Counter (Abaxis, Union City, CA) for complete blood count. Serum levels of inflammatory cytokines (TNF-α and IL-6) were measured by ELISA. Non-injected mice served as control group. The animals were monitored for >3 months post-MV injection for development of adverse reactions.
In a similar experiment female 4–5 week-old Ifnarko-CD46Ge mice (n = 9) were injected i.v. with 2 × 106 TCID50 of MV-s-NAP. Mice were monitored daily and compared to control mice for development of toxicity. Blood and serum samples were collected on days 1, 2, 3, 7, and 14 and analyzed as described above.
Statistical analysis. The GraphPad Prism 5.0 computer software (GraphPad Software, San Diego, CA) was used in statistical analysis of the data. Kaplan–Meyer curves were generated and median survival was compared using the log-rank test. Statistical significance was based on P values < 0.05.
SUPPLEMENTARY MATERIAL Table S1. Hematological analysis of blood samples from Ifnarko-CD46Ge mice injected with MV-s-NAP via the i.p. or i.v. route of administration compared to controls.
Acknowledgments
We would like to thank Dr Amy G. Andrews from the Mayo Clinic Department of Comparative Medicine for her review of the laboratory data of the Toxicology study, and Ms. Raquel Ostby for her help with manuscript preparation. We wish to thank the Mayo Clinic Viral Vector Production Laboratory for providing MV-NIS. This work is supported by grants P50 CA 116201, P50 CA 136393 and R01 CA 136547, Paul Leibson Memorial Fund, and Atwater grant.
Supplementary Material
Hematological analysis of blood samples from Ifnarko-CD46Ge mice injected with MV-s-NAP via the i.p. or i.v. route of administration compared to controls.
REFERENCES
- Jemal A, Siegel R, Xu J., and, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Light RW. Clinical practice. Pleural effusion. N Engl J Med. 2002;346:1971–1977. doi: 10.1056/NEJMcp010731. [DOI] [PubMed] [Google Scholar]
- Neragi-Miandoab S. Malignant pleural effusion, current and evolving approaches for its diagnosis and management. Lung Cancer. 2006;54:1–9. doi: 10.1016/j.lungcan.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Heffner JE. Diagnosis and management of malignant pleural effusions. Respirology. 2008;13:5–20. doi: 10.1111/j.1440-1843.2007.01154.x. [DOI] [PubMed] [Google Scholar]
- Heffner JE., and, Klein JS. Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc. 2008;83:235–250. doi: 10.4065/83.2.235. [DOI] [PubMed] [Google Scholar]
- Musani AI. Treatment options for malignant pleural effusion. Curr Opin Pulm Med. 2009;15:380–387. doi: 10.1097/MCP.0b013e32832c6a8a. [DOI] [PubMed] [Google Scholar]
- Galanis E. Therapeutic potential of oncolytic measles virus: promises and challenges. Clin Pharmacol Ther. 2010;88:620–625. doi: 10.1038/clpt.2010.211. [DOI] [PubMed] [Google Scholar]
- Griffin D.Measles virus Knipe, DM, Howley, PM.edsFields Virology4th edn. Philadelphia PA: Lippincott, Williams & Wilkins; 20011401–1441. [Google Scholar]
- Iankov ID, Msaouel P, Allen C, Federspiel MJ, Bulur PA, Dietz AB.et al. (2010Demonstration of anti-tumor activity of oncolytic measles virus strains in a malignant pleural effusion breast cancer model Breast Cancer Res Treat 122745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisgerault N, Tangy F., and, Gregoire M. New perspectives in cancer virotherapy: bringing the immune system into play. Immunotherapy. 2010;2:185–199. doi: 10.2217/imt.10.6. [DOI] [PubMed] [Google Scholar]
- Grote D, Cattaneo R., and, Fielding AK. Neutrophils contribute to the measles virus-induced antitumor effect: enhancement by granulocyte macrophage colony-stimulating factor expression. Cancer Res. 2003;63:6463–6468. [PubMed] [Google Scholar]
- Tonello F, Dundon WG, Satin B, Molinari M, Tognon G, Grandi G.et al. (1999The Helicobacter pylori neutrophil-activating protein is an iron-binding protein with dodecameric structure Mol Microbiol 34238–246. [DOI] [PubMed] [Google Scholar]
- Satin B, Del Giudice G, Della Bianca V, Dusi S, Laudanna C, Tonello F.et al. (2000The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor J Exp Med 1911467–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montemurro P, Barbuti G, Dundon WG, Del Giudice G, Rappuoli R, Colucci M.et al. (2001Helicobacter pylori neutrophil-activating protein stimulates tissue factor and plasminogen activator inhibitor-2 production by human blood mononuclear cells J Infect Dis 1831055–1062. [DOI] [PubMed] [Google Scholar]
- Amedei A, Cappon A, Codolo G, Cabrelle A, Polenghi A, Benagiano M.et al. (2006The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses J Clin Invest 1161092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Elios MM, Amedei A, Cappon A, Del Prete G., and, de Bernard M. The neutrophil-activating protein of Helicobacter pylori (HP-NAP) as an immune modulating agent. FEMS Immunol Med Microbiol. 2007;50:157–164. doi: 10.1111/j.1574-695X.2007.00258.x. [DOI] [PubMed] [Google Scholar]
- Iankov ID, Haralambieva IH., and, Galanis E. Immunogenicity of attenuated measles virus engineered to express Helicobacter pylori neutrophil-activating protein. Vaccine. 2011;29:1710–1720. doi: 10.1016/j.vaccine.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iankov ID, Hillestad ML, Dietz AB, Russell SJ., and, Galanis E. Converting tumor-specific markers into reporters of oncolytic virus infection. Mol Ther. 2009;17:1395–1403. doi: 10.1038/mt.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrkic B, Pavlovic J, Rülicke T, Volpe P, Buchholz CJ, Hourcade D.et al. (1998Measles virus spread and pathogenesis in genetically modified mice J Virol 727420–7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RM, Greiner SM, Harvey ME, Griesmann G, Kuffel MJ, Buhrow SA.et al. (2007Preclinical pharmacology and toxicology of intravenous MV-NIS, an oncolytic measles virus administered with or without cyclophosphamide Clin Pharmacol Ther 82700–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Msaouel P, Dispenzieri A., and, Galanis E. Clinical testing of engineered oncolytic measles virus strains in the treatment of cancer: an overview. Curr Opin Mol Ther. 2009;11:43–53. [PMC free article] [PubMed] [Google Scholar]
- Biology, Table 1–2. HematologyIn: Laboratory Mouse Handbook, by the American Association for Laboratory Animal Science, 2006. p. 2
- Grote D, Russell SJ, Cornu TI, Cattaneo R, Vile R, Poland GA.et al. (2001Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice Blood 973746–3754. [DOI] [PubMed] [Google Scholar]
- Peng KW, Ahmann GJ, Pham L, Greipp PR, Cattaneo R., and, Russell SJ. Systemic therapy of myeloma xenografts by an attenuated measles virus. Blood. 2001;98:2002–2007. doi: 10.1182/blood.v98.7.2002. [DOI] [PubMed] [Google Scholar]
- Peng KW, TenEyck CJ, Galanis E, Kalli KR, Hartmann LC., and, Russell SJ. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 2002;62:4656–4662. [PubMed] [Google Scholar]
- Phuong LK, Allen C, Peng KW, Giannini C, Greiner S, TenEyck CJ.et al. (2003Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme Cancer Res 632462–2469. [PubMed] [Google Scholar]
- McDonald CJ, Erlichman C, Ingle JN, Rosales GA, Allen C, Greiner SM.et al. (2006A measles virus vaccine strain derivative as a novel oncolytic agent against breast cancer Breast Cancer Res Treat 99177–184. [DOI] [PubMed] [Google Scholar]
- Msaouel P, Iankov ID, Allen C, Morris JC, von Messling V, Cattaneo R.et al. (2009Engineered measles virus as a novel oncolytic therapy against prostate cancer Prostate 6982–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanis E, Hartmann LC, Cliby WA, Long HJ, Peethambaram PP, Barrette BA.et al. (2010Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer Cancer Res 70875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bernard M., and, D'Elios MM. The immune modulating activity of the Helicobacter pylori HP-NAP: Friend or foe. Toxicon. 2010;56:1186–1192. doi: 10.1016/j.toxicon.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Codolo G, Mazzi P, Amedei A, Del Prete G, Berton G, D'Elios MM.et al. (2008The neutrophil-activating protein of Helicobacter pylori down-modulates Th2 inflammation in ovalbumin-induced allergic asthma Cell Microbiol 102355–2363. [DOI] [PubMed] [Google Scholar]
- Del Prete G, Chiumiento L, Amedei A, Piazza M, D'Elios MM, Codolo G.et al. (2008Immunosuppression of TH2 responses in Trichinella spiralis infection by Helicobacter pylori neutrophil-activating protein J Allergy Clin Immunol 122908–913.e5. [DOI] [PubMed] [Google Scholar]
- Lombardi G, Zustovich F, Nicoletto MO, Donach M, Artioli G., and, Pastorelli D. Diagnosis and treatment of malignant pleural effusion: a systematic literature review and new approaches. Am J Clin Oncol. 2010;33:420–423. doi: 10.1097/COC.0b013e3181aacbbf. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MM, Smit HJ, Barbierato SB, Havenith CE, Beelen RH., and, Postmus PE. Talc-induced inflammation in the pleural cavity. Eur Respir J. 1998;12:1419–1423. doi: 10.1183/09031936.98.12061419. [DOI] [PubMed] [Google Scholar]
- West SD, Davies RJ., and, Lee YC. Pleurodesis for malignant pleural effusions: current controversies and variations in practices. Curr Opin Pulm Med. 2004;10:305–310. doi: 10.1097/01.mcp.0000129756.87090.55. [DOI] [PubMed] [Google Scholar]
- Chung CL, Chen YC., and, Chang SC. Effect of repeated thoracenteses on fluid characteristics, cytokines, and fibrinolytic activity in malignant pleural effusion. Chest. 2003;123:1188–1195. doi: 10.1378/chest.123.4.1188. [DOI] [PubMed] [Google Scholar]
- Yanagawa H, Haku T, Takeuchi E, Suzuki Y, Nokihara H., and, Sone S. Intrapleural therapy with MDP-Lys (L18), a synthetic derivative of muramyl dipeptide, against malignant pleurisy associated with lung cancer. Lung Cancer. 2000;27:67–73. doi: 10.1016/s0169-5002(99)00090-2. [DOI] [PubMed] [Google Scholar]
- Hobohm U. Fever therapy revisited. Br J Cancer. 2005;92:421–425. doi: 10.1038/sj.bjc.6602386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katano M., and, Morisaki T. The past, the present and future of the OK-432 therapy for patients with malignant effusions. Anticancer Res. 1998;18 5D:3917–3925. [PubMed] [Google Scholar]
- Kishi K, Homma S, Sakamoto S, Kawabata M, Tsuboi E, Nakata K.et al. (2004Efficacious pleurodesis with OK-432 and doxorubicin against malignant pleural effusions Eur Respir J 24263–266. [DOI] [PubMed] [Google Scholar]
- Ren S, Terman DS, Bohach G, Silvers A, Hansen C, Colt H.et al. (2004Intrapleural staphylococcal superantigen induces resolution of malignant pleural effusions and a survival benefit in non-small cell lung cancer Chest 1261529–1539. [DOI] [PubMed] [Google Scholar]
- Brisslert M, Enarsson K, Lundin S, Karlsson A, Kusters JG, Svennerholm AM.et al. (2005Helicobacter pylori induce neutrophil transendothelial migration: role of the bacterial HP-NAP FEMS Microbiol Lett 24995–103. [DOI] [PubMed] [Google Scholar]
- Polenghi A, Bossi F, Fischetti F, Durigutto P, Cabrelle A, Tamassia N.et al. (2007The neutrophil-activating protein of Helicobacter pylori crosses endothelia to promote neutrophil adhesion in vivo J Immunol 1781312–1320. [DOI] [PubMed] [Google Scholar]
- Basu A, Mohanty S., and, Sun B. Differential sensitivity of breast cancer cells to tumor necrosis factor-alpha: involvement of protein kinase C. Biochem Biophys Res Commun. 2001;280:883–891. doi: 10.1006/bbrc.2000.4209. [DOI] [PubMed] [Google Scholar]
- Burow ME, Tang Y, Collins-Burow BM, Krajewski S, Reed JC, McLachlan JA.et al. (1999Effects of environmental estrogens on tumor necrosis factor alpha-mediated apoptosis in MCF-7 cells Carcinogenesis 202057–2061. [DOI] [PubMed] [Google Scholar]
- Iankov ID, Blechacz B, Liu C, Schmeckpeper JD, Tarara JE, Federspiel MJ.et al. (2007Infected cell carriers: a new strategy for systemic delivery of oncolytic measles viruses in cancer virotherapy Mol Ther 15114–122. [DOI] [PubMed] [Google Scholar]
- Zuniga A, Wang Z, Liniger M, Hangartner L, Caballero M, Pavlovic J.et al. (2007Attenuated measles virus as a vaccine vector Vaccine 252974–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingli D, Peng KW, Harvey ME, Greipp PR, O'Connor MK, Cattaneo R.et al. (2004Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter Blood 1031641–1646. [DOI] [PubMed] [Google Scholar]
- Duprex WP, McQuaid S, Hangartner L, Billeter MA., and, Rima BK. Observation of measles virus cell-to-cell spread in astrocytoma cells by using a green fluorescent protein-expressing recombinant virus. J Virol. 1999;73:9568–9575. doi: 10.1128/jvi.73.11.9568-9575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfield KK, Walker HJ, Gregory LC., and, Federspiel MJ. Manufacture of measles viruses. Methods Mol Biol. 2011;737:345–366. doi: 10.1007/978-1-61779-095-9_14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hematological analysis of blood samples from Ifnarko-CD46Ge mice injected with MV-s-NAP via the i.p. or i.v. route of administration compared to controls.