Abstract
Ixmyelocel-T is a patient-specific, expanded, multicellular therapy evaluated in patients with lower extremity critical limb ischemia (CLI) with no options for revascularization. This randomized, double-blind, placebo-controlled, phase 2 trial (RESTORE-CLI) compared the efficacy and safety of intramuscular injections of ixmyelocel-T with placebo. Patients received one-time injections over 20 locations in a single leg and were followed for 12 months. Safety assessments included occurrence of adverse events. Efficacy assessments included time to first occurrence of treatment failure (TTF; major amputation of injected leg; all-cause mortality; doubling of total wound surface area from baseline; de novo gangrene) and amputation-free survival (AFS; major amputation of injected leg; all-cause mortality). A total of 77 patients underwent bone marrow or sham aspiration; 72 patients received ixmyelocel-T (48 patients) or placebo (24 patients). Adverse event rates were similar. Ixmyelocel-T treatment led to a significantly prolonged TTF (P = 0.0032, logrank test). AFS had a clinically meaningful 32% reduction in event rate that was not statistically significant (P = 0.3880, logrank test). Treatment effect in post hoc analyses of patients with baseline wounds was more pronounced (TTF: P < 0.0001, AFS: P = 0.0802, logrank test). Ixmyelocel-T treatment was well tolerated and may offer a potential new treatment option.
Introduction
Peripheral arterial disease (PAD) occurs in ~7% of people over 40 and sharply increases with age to affect ~12–20% of Americans ≥70 years of age.1,2,3 Critical limb ischemia (CLI) represents the most serious form of PAD and is characterized by ischemic rest pain with or without tissue loss (ulcers and/or gangrene).4 It is estimated 5–10% of PAD patients over 50 years of age will develop CLI within 5 years.4 CLI is associated with significant morbidity and mortality: up to 30% of patients will require an amputation within 1 year and another 25% will die of cardiovascular events.1,4 The 10-year survival rate has been estimated to be as low as 10%.4
Treatment of CLI includes risk factor modification (quitting smoking and managing diet, exercise, diabetes, hyperlipidemia, hypertension, and use of antiplatelet medications), ischemic pain control, management of ischemic ulcers, and revascularization either by open bypass surgery or endovascular approaches.4 Although revascularization is a mainstay of treatment, it often is not feasible due to medical comorbidities or no anatomic options, and is associated with significant rates of complications.5,6,7 In addition, in CLI patients with tissue loss, survival and limb-related outcomes are poor as compared with CLI patients with rest pain only.8 Patients with severe ischemia who are unable to undergo revascularization or with a failed revascularization have limited options and amputation is often necessary.9 As it is estimated that there are 150,000–300,000 new cases of CLI every year,4 there remains a significant need for less invasive therapeutic options in patients with CLI.
Several early clinical studies of bone marrow–derived mononuclear cells have demonstrated promising safety and improved clinical outcomes.10,11 The goal for new therapies will be to increase perfusion enough to allow wound healing, resolution of rest pain, and limb salvage.8
Ixmyelocel-T is a patient-specific, expanded, multicellular therapy. In preclinical studies, it has been shown to have multifunctional properties including: tissue remodeling, immune modulation, and the promotion of angiogenesis. These processes could potentially address the multiple underlying causes of severe, chronic ischemic cardiovascular diseases such as CLI. Ixmyelocel-T is produced from a small amount of a subject's own bone marrow in an automated closed-culture system developed by the Sponsor. The process enhances bone marrow–derived mononuclear cells by expanding the mesenchymal stromal cells and alternately activated macrophages that have been reported to augment the return of blood flow in animal models of ischemia (internal Sponsor documents). The ixmyelocel-T manufacturing process significantly expands cells that express the surface marker CD90 (mesenchymal cells), and also expands a CD14+ monocyte/macrophage subset of CD45+ hematopoietic cells.
RESTORE-CLI was a phase 2, double-blind, placebo-controlled, randomized trial conducted with the objective of assessing both the safety and efficacy of ixmyelocel-T in patients with CLI and no options for revascularization. Results from an interim analysis have been previously reported;12 results from the completed trial are presented here.
Results
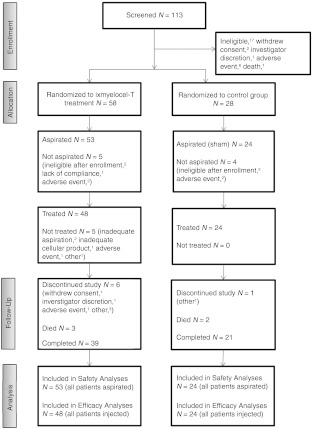
A total of 86 patients at 18 active centers in the United States were randomly assigned to treatment groups between June 2007 and March 2010; patient follow-up was completed in March 2011. Figure 1 summarizes the flow of patients throughout the trial, including reasons for discontinuation. During the 30 days that were allowed between randomization and aspiration, nine patients withdrew from the study due to adverse events, lack of compliance, or no longer meeting eligibility criteria. A total of 77 patients proceeded with the study and underwent aspirations (or sham aspirations for patients in the placebo group), and 72 patients received injections of either ixmyelocel-T or the placebo solution. Of these 72 patients, 13 (18%) received injections in the thigh and calf and 59 (82%) received injections in the thigh, calf, and foot (after a protocol amendment). All available data were included in the specified safety and efficacy summaries and analyses, regardless of whether the patient had completed the trial. Three of the six patients treated with ixmyelocel-T who did not complete the 12-month follow-up period had experienced one or more treatment failure events before they withdrew from the trial; the one control patient who did not complete the 12-month follow-up period did not experience a treatment failure event before study withdrawal.
Figure 1.
Patient recruitment and disposition throughout the trial. N, number of patients.
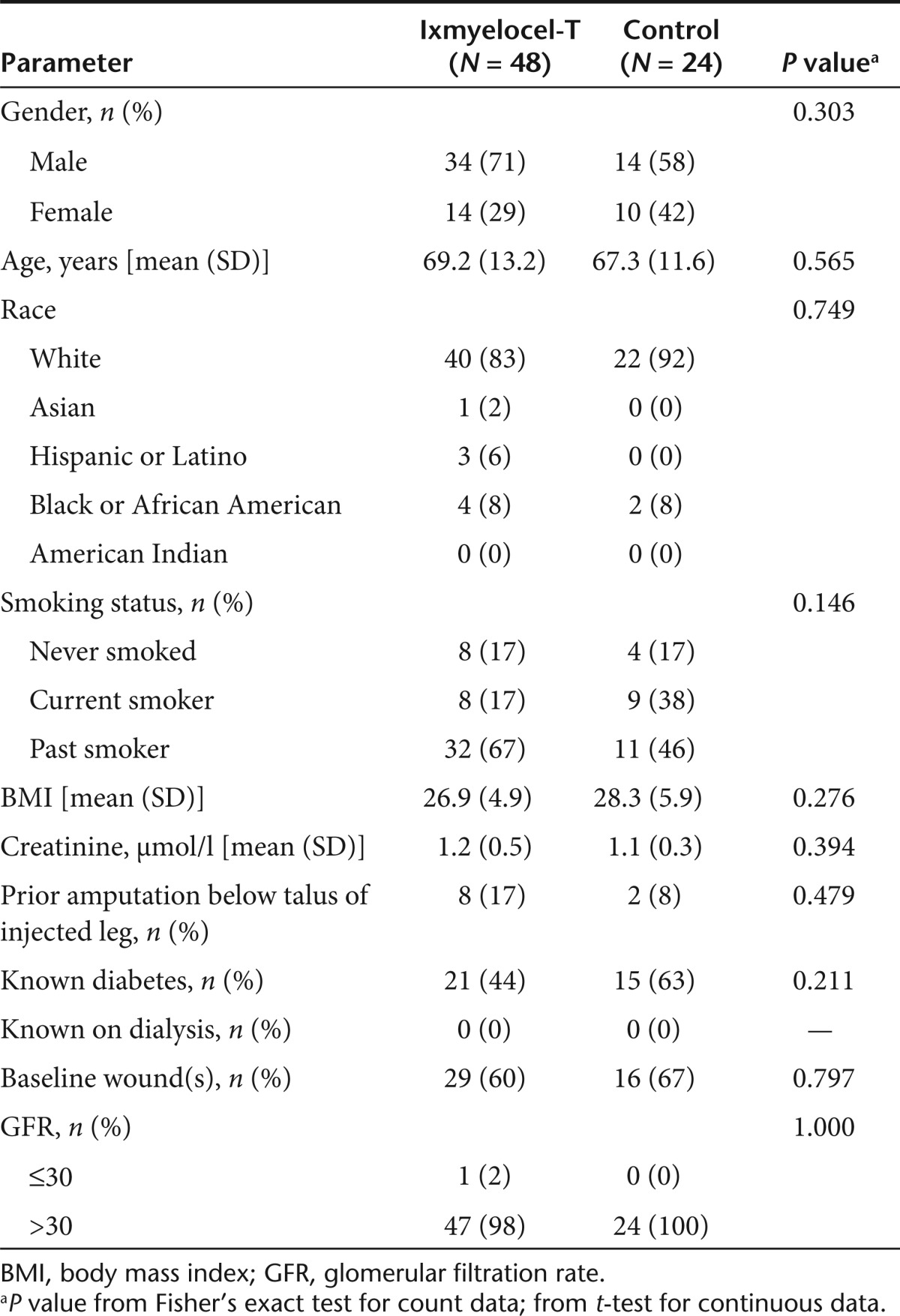
The characteristics of treated patients were similar between the two treatment groups at baseline (Table 1). In the ixmyelocel-T and control groups, 29 (60%) and 16 (67%) patients, respectively, had wounds present at baseline (P = 0.797). One ixmyelocel-T-treated patient (2%) had a glomerular filtration rate ≤30 ml/ min/1.73 m2, all others were >30 ml/min/1.73 m2.
Table 1. Baseline characteristics of injected patients.
Safety
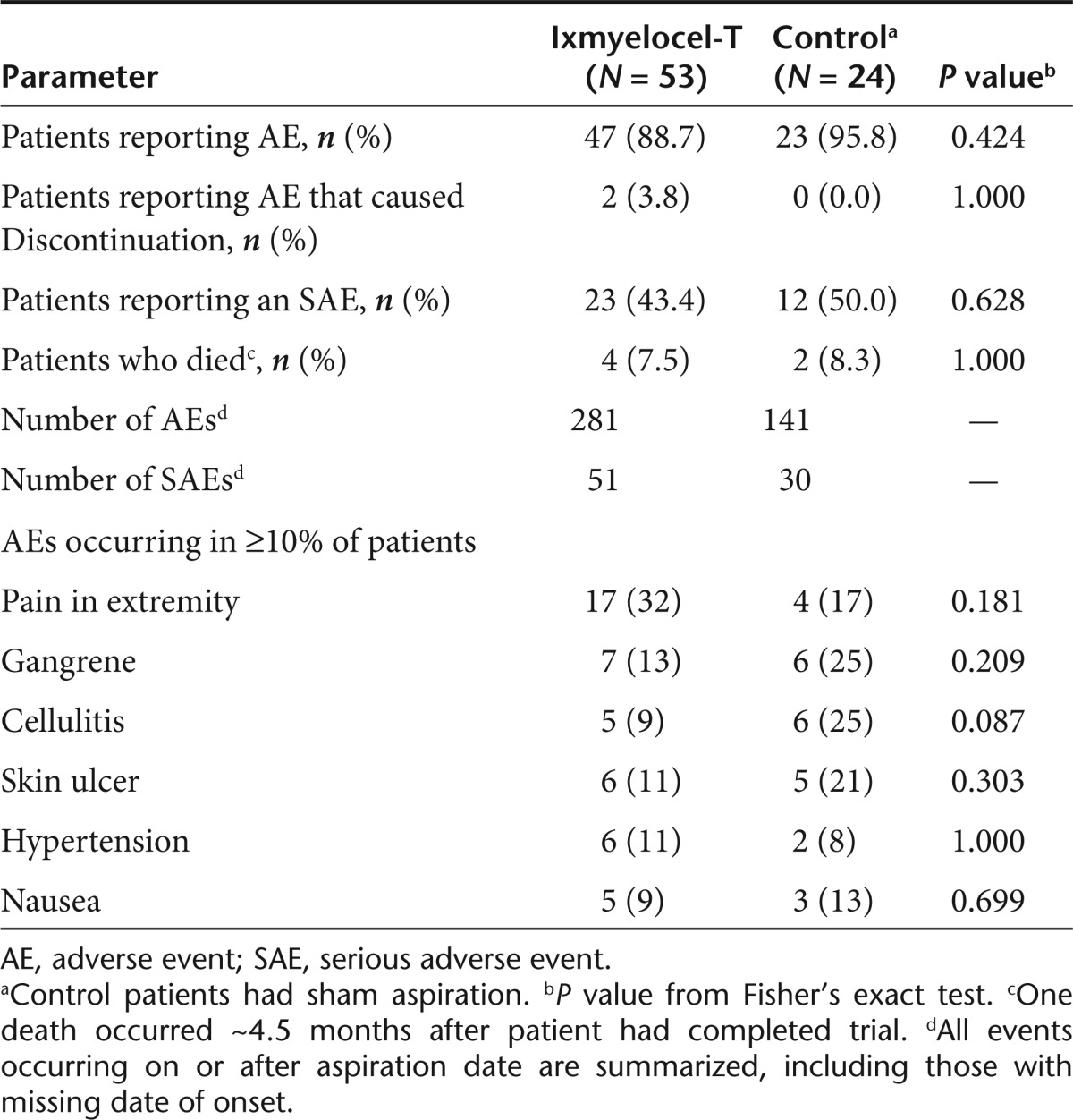
The occurrence of adverse events and serious adverse events was similar between the two treatment groups (Table 2). Almost all patients reported adverse events during the trial. The most commonly reported adverse events tended to be those characteristic of the disease process: pain in extremity, gangrene, cellulitis, and skin ulcer occurring in 17 (32%), 7 (13%), 5 (9%), and 6 (11%) ixmyelocel-T-treated patients and 4 (17%), 6 (25%), 6 (25%), and 5 (21%) control patients, respectively.
Table 2. Summary of adverse events for all patients aspirated.
Of the 77 patients who underwent aspiration (or sham aspiration), two patients withdrew from the trial due to adverse events: one patient randomized to the ixmyelocel-T group withdrew due to a urinary tract infection, a serious adverse event, which hospitalized the patient and prevented him from receiving treatment injections within the product's expiration window. The second patient, also in the ixmyelocel-T group, withdrew due to pain in the extremity on day 63. Neither event was felt by the investigator to be due to the aspiration procedure, the injection procedure, nor the (blinded) treatment.
There were four deaths (8%) in the ixmyelocel-T treatment group and two (8%) in the control group and none was considered related to treatment. In the ixmyelocel-T treatment group, three of the deaths occurred while patients were still participating in the trial (one patient died on day 148 from complications resulting from a hip fracture; one patient died on day 132 due to congestive cardiac failure and one patient died on day 333 due to renal impairment). The fourth ixmyelocel-T patient who died had completed the trial on day 361 but died from a glioblastoma on day 498. One of the patients who died in the control group died on day 37 of hypovolemic shock and the other control patient died on day 258 from a cardiac disorder. Each of the five patients who died while still ongoing in the trial contributed a death to the treatment failure end point and to the amputation-free survival (AFS) end point; the one patient who completed the study and then died did not contribute a death to either the treatment failure or the AFS end point.
As the study progressed, patients may have developed occlusions in their previously placed stents, or the site principal investigator may have felt that the patient had developed an option to revascularize the extremity using endovascular techniques that were not possible at the time of initial evaluation. Three patients (two in the ixmyelocel-T group and one in the control group) at separate study sites had a stent placed in the injected leg during the trial. One ixmyelocel-T patient had a nonserious adverse event of “in-stent stenosis,” which resulted in a “stent placement of right common iliac artery” on day 63; this patient did not experience any treatment failure events. The second ixmyelocel-T patient had a serious adverse event of “bilateral lower extremity pain,” which resulted in “left iliac artery stent placement” on day 293; this patient experienced de novo gangrene, a treatment failure event, on day 318. The control patient had a nonserious adverse event of “right great toe amp site not healing,” which resulted in “angioplasty and stenting of new right superficial femoral artery occlusion” on day 154; this patient experienced wound size doubling, a treatment failure event, on day 91.
Serious adverse events occurred in 23 (43%) ixmyelocel-T-treated patients and in 12 (50%) control patients. Only one serious adverse event was felt by the investigator to be possibly related to blinded treatment. One patient in the ixmyelocel-T treatment group developed wound sepsis on the first toe of the injected leg on day 34 that resulted in progressive amputations of the toe culminating with a below-knee amputation on day 63.
There were five adverse event reports in the ixmyelocel-T treatment group and one in the control group of injection site pain or pain in extremity/foot that the investigator felt was at least possibly related the blinded treatment. There was one additional report of a possibly related injection site reaction described as redness and a raised area at the injection sites, which started on day 3 in the control group. Other adverse events that were felt by the investigator to be at least possibly related to blinded treatment were a burning sensation (occurring in one control patient beginning on day 0), cellulitis (one ixmyelocel-T-treated patient beginning on day 14 and a separate occurrence on day 69), eczema on the injected leg (ixmyelocel-T-treated patient beginning on day 1), and thrombophlebitis (ixmyelocel-T-treated patient beginning on day 86).
Efficacy
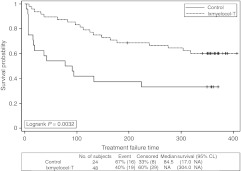
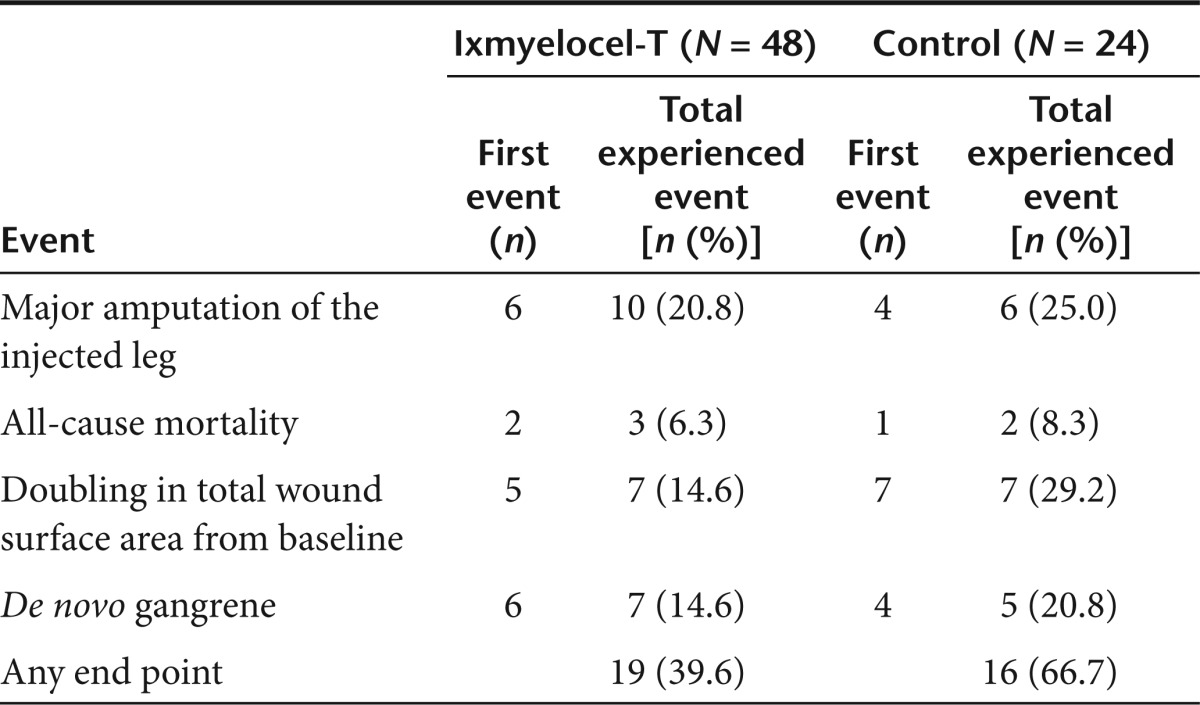
Time to first occurrence of treatment failure (TTF) was significantly longer for patients treated with ixmyelocel-T as compared with control patients (P = 0.0032, logrank test; Figure 2). The survival curves diverged early and the difference between groups was maintained throughout the 12-month follow-up period. The Cox proportional hazards (PH) analysis gave a treatment hazards ratio (HR) = 0.381, 95% confidence interval (CI) = (0.195, 0.744), conveying a significant reduction in the risk of treatment failure in the ixmyelocel-T treatment group of ~62% (P = 0.0047). Table 3 details the contribution of individual events to the composite treatment failure end point and the total number of patients who experienced each end point. The percentage of patients experiencing each of the individual events favored the ixmyelocel-T group with the largest differences between treatment groups seen for doubling of total wound surface area from baseline and de novo gangrene. A total of 19 (39.6%) ixmyelocel-T-treated patients and 16 (66.7%) control patients experienced one or more of the treatment failure events (P = 0.0451, Fisher's exact test).
Figure 2.
Time to first occurrence of treatment failure for all patients injected. Kaplan–Meier survival plot of time to treatment failure (major amputation of injected leg, all-cause mortality, doubling of total wound surface area from baseline, de novo gangrene) for all patients injected. Censored observations are indicated by “+” symbols. CL, confidence limits; NA, not available.
Table 3. Contribution of treatment failure events to composite end point for all patients injected.
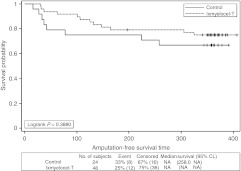
There was a trend for improved AFS in ixmyelocel-T-treated patients as compared with control patients, but the difference did not reach statistical significance (P = 0.3880, logrank test; Figure 3). The Cox PH analysis for AFS gave a treatment HR = 0.676, 95% CI = (0.276, 1.654). Of the 10 ixmyelocel-T-treated patients who experienced a major amputation of the injected leg, 7 (70%) had below-knee amputations and 3 (30%) had above-knee amputations. Three of the seven initial below-knee amputations were followed within 2 months by above-knee amputations. In the control group, six patients experienced a major amputation of the injected leg, four (67%) were below-knee, and 2 (33%) were above-knee. None of the initial below-knee amputations were followed by an above-knee amputation.
Figure 3.
Amputation-free survival for all patients injected. Kaplan–Meier survival plot of amputation-free survival (major amputation of injected leg or all-cause mortality) for all patients injected. Censored observations are indicated by “+” symbols. CL, confidence limits; NA, not available.
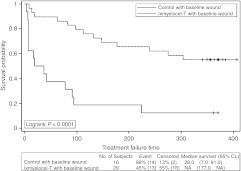
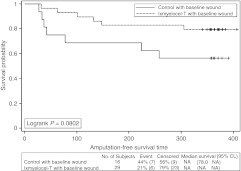
A post hoc analysis of TTF and AFS was performed on the patients who had wounds present at baseline, as this is the patient population that will be studied in phase 3. Thirteen of the 29 (44.8%) ixmyelocel-T-treated patients with wounds at baseline and 14 of the 16 (87.5%) control patients with wounds at baseline experienced a treatment failure event (P = 0.0098, Fisher's exact test). In these patients with wounds at baseline, TTF was longer in the ixmyelocel-T group than the control group (P < 0.0001, logrank test; Figure 4). The Cox PH analysis gave an HR = 0.225, 95% CI = (0.103, 0.490), indicating a risk reduction of treatment failure events of ~78% (P = 0.0002). For AFS, 6 of the 29 (20.7%) ixmyelocel-T-treated patients with baseline wounds and 7 of the 16 (43.8%) control patients with baseline wounds experienced a major amputation of the injected leg or death (P = 0.1687, Fisher's exact test). AFS was longer in the ixmyelocel-T-treated patients with baseline wounds than in control patients with wounds at baseline (P = 0.0802, logrank test; Figure 5). The HR = 0.391, 95% CI = (0.131, 1.164) from the Cox PH analysis, indicating an approximate 61% reduction in risk for ixmyelocel-T-treated patients with baseline wounds (P = 0.0915).
Figure 4.
Time to first occurrence of treatment failure for all patients who had baseline wounds. Kaplan–Meier survival plot of time to treatment failure (major amputation of injected leg, all-cause mortality, doubling of total wound surface area from baseline, de novo gangrene) for post hoc analysis of patients who had baseline wounds. Censored observations are indicated by “+” symbols. CL, confidence limits; NA, not available.
Figure 5.
Amputation-free survival for all patients who had baseline wounds. Kaplan–Meier survival plot of amputation-free survival (major amputation of injected leg or all-cause mortality) for post hoc analysis of patients who had baseline wounds. Censored observations are indicated by “+” symbols. CL, confidence limits; NA, not available.
Discussion
Patients afflicted with CLI have a poor quality of life and high rate of limb loss.1,4 To date, revascularization of the ischemic limb either by endovascular or open surgical approaches is the mainstay of therapy.1,4 Yet revascularization is associated with a significant incidence of failure and complications and is not a viable option in up to 50% of patients.13 In patients with no viable surgical options, avoiding an operative procedure such as an amputation is in the best interest of the patient. After undergoing a major amputation, these patients often suffer from surgical complications either from the amputation procedure itself or from their postoperative clinical state.5,14 Less invasive medical therapies efficacious in the treatment of CLI are, therefore, desirable. Treatment with ixmyelocel-T would require patients to undergo a small volume bone marrow aspiration under conscious sedation followed by intramuscular injections into the affected limb. Both of these procedures provide far fewer complications than those connected to an amputation or surgical revascularization. This trial provides encouraging evidence that treatment with ixmyelocel-T is safe and beneficial in treating lower extremity CLI in a “no-option” population. Because this was primarily a phase 2 safety trial, the sample size was not chosen to try to demonstrate statistical differences between treatment groups. Despite a small number of patients treated, there was improved TTF and AFS in ixmyelocel-T-treated patients as compared with controls. In addition, the treatment effect for both TTF and AFS was even more pronounced in patients who entered the trial with baseline wounds. Treatment with ixmyelocel-T, a patient-specific, multicellular therapy, was safe with a comparable percentage of patients reporting both adverse events and serious adverse events.
Unique features of ixmyelocel-T treatment result from the small volume (~50 ml) bone marrow aspiration and cell manufacturing processes which significantly expand key cell types. Clinical studies on therapeutic angiogenesis using bone marrow–derived progenitor cells have commonly harvested 500 ml or more of bone marrow.10 The large volume of bone marrow harvest required by other stem cell therapy techniques is significantly more uncomfortable for the patients and may entail general anesthesia and/or postprocedure blood transfusion with increased potential for morbidity and mortality.15 Ixmyelocel-T has the potential to overcome these limitations by significantly expanding the number of the cells critical to regeneration and repair through automated, closed ex vivo culture. This allows the bone marrow sample to be collected from a small volume aspiration (~50 ml) under local anesthesia or conscious sedation without general anesthesia in a procedure that lasts ~15 minutes. Ixmyelocel-T also contains large numbers of mesenchymal stromal cells and alternatively activated macrophages and potentially represents a more standardized, and possibly more potent, therapy than fresh bone marrow preparations.
Potential limitations include difficulties with an inadequate aspirate preventing processing into ixmyelocel-T. Two patients from the same site had an inadequate aspirate, meaning that there was an insufficient number of cells to initiate an ixmyelocel-T product. A third sample, while being processed, had a breach in the closed system and was not suitable to be shipped back to the site. There was a fourth instance in which the product was initially thought to be nonsterile but this test result was discovered to be a false positive; the external contract laboratory performing the sterility testing determined that the “positive” test was a result of a sterility breach during their testing procedure. Although events like these can occur, they happen infrequently and can be overcome with additional processing controls or aspiration technique training.
Limitations of this trial include the fact that it was not powered a priori for efficacy evaluations and the small number of patients. A second limitation centers on the collection of hemodynamic data. Though ankle and toe pressures and indexes were collected at screening, follow-up measures of the same parameter were often unavailable or not done due to amputations or noncompressible or nonpulsatile arteries. As a result, there were insufficient data to analyze ankle and toe pressures and indexes. In this CLI patient population, while it would have been optimal to have full data sets on hemodynamic parameters, these measurements are in fact substitutes for the hard end points of patient outcomes. Additionally, it is unclear by what mechanism(s) cellular therapy might have an effect.9,10,11 If it is through a nonarteriogenic pathway, then these hemodynamic indexes may not show improvement and yet the cellular therapy may mechanistically yield clinically meaningful and beneficial patient outcomes.
Every effort was made to maintain a double-blind nature for this trial with separate physicians or designees obtaining the aspirations or sham aspirations, and administering treatment injections, using masked syringes. There was little difference between treatment groups in adverse event reports of injection site pain or reactions investigators felt were at least possibly related to ixmyelocel-T treatment that might unblind a patient or investigator to treatment assignment. However, it is unknown whether the occurrence of one of these adverse events might have led to suspicions of treatment group assignment on a patient-by-patient basis. Despite these limitations, the results were statistically significant for TTF, a more objective end point than those used in other cell therapy studies.11,15,16
In conclusion, there were no major safety issues related to ixmyelocel-T treatment in patients with no-option CLI. Treatment with ixmyelocel-T improved TTF in treated patients as compared with controls. These results suggest that treatment with ixmyelocel-T has the potential to be a promising treatment option in patients with CLI who are unable to undergo revascularization. Based on these data, a larger phase 3 pivotal trial is warranted.
Materials and Methods
Trial design. This randomized, double-blind, placebo-controlled, multicenter trial was conducted in accordance with Declaration of Helsinki principles and approved by the appropriate institutional review boards. All patients gave written informed consent. Patients included were those 18–90 years old with a diagnosis of CLI, defined as persistent, recurring ischemic rest pain ≥2 weeks duration and/or ulceration or gangrene of the toe or foot, with toe systolic pressure ≤50 mm Hg (or absent palpable pedal pulse in patients with diabetes) or ankle systolic pressure ≤70 mm Hg. Other inclusion criteria included infrainguinal occlusive disease deemed by the site principal investigator not amenable to revascularization (confirmed by angiographic imaging results or by color flow duplex ultrasound obtained within the 6 months before randomization), controlled blood pressure with or without antihypertensive therapy, and adequate antiplatelet therapy established before randomization. Statin therapy was required unless contraindicated. Major exclusion criteria were patients who had a previous amputation of the talus or above on the preidentified index (injected) leg; a known, failed ipsilateral revascularization procedure within 2 weeks before randomization; active infection or infection of the target extremity manifested by fever, purulence, and severe cellulitis; wet gangrenous tissue; poorly controlled diabetes (hemoglobin A1c >10%); aortoiliac disease with >50% stenosis; or a wound with exposed tendon or bone. To protect the blinding of the trial, the physician or appropriately trained designee who performed the bone marrow or sham aspirations was different from the physician or designee who later administered the treatment injections and who followed the patients thereafter.
Patients who satisfied selection criteria were randomized 2:1 to receive either ixmyelocel-T injections or placebo injections (electrolyte solution) in a preidentified index leg. Injections were administered one time, intramuscularly, over 20 locations in the lower thigh, calf, and foot. Patients were then followed for 12 months for safety and efficacy outcomes.
Patients randomized to receive injections of ixmyelocel-T had a small sample of bone marrow (~50 ml) obtained by a percutaneous aspiration from the posterior iliac crest. Patients in the control group underwent a sham aspiration whereby the aspiration needle was inserted through the skin but the periosteum of the iliac crest was not perforated.
Safety analyses were conducted on the Safety Population, defined as all patients who were randomized and underwent aspiration, regardless of whether or not they received their randomized treatment. The primary safety end point was adverse events; aspiration- and treatment-emergent adverse events are summarized. Efficacy analyses were conducted on the Efficacy Population, defined as all randomized patients who received treatment injections. The primary efficacy end point was TTF, with AFS as a secondary efficacy end point. TTF was defined as the earliest trial day on which any of the following treatment failure events occurred: major amputation of the injected leg, all-cause mortality (death), doubling of total wound surface area from baseline, and de novo gangrene. Major amputation was defined as an amputation at or above the talus on the injected leg. For wound size doubling, the patient must have come into the study with a wound (i.e., had a baseline wound) to be eligible to contribute to the event. AFS was defined as the number of days from injection to the first trial day on which a major amputation of the injected leg or death occurred.
Preparation of investigational product. The mononuclear cells from the bone marrow of patients in the ixmyelocel-T treatment group were cultured to expand the mesenchymal stromal cells (CD90) and alternately activated macrophages (CD14+). The process retains cells of other hematopoietic cell lineages from the bone marrow. Ixmyelocel-T product specifications include the total number of viable cells and the proportion of CD90+ cells and CD45+ cells, which together define >98% of the nucleated cells in the product. The ixmyelocel-T product was generated in an automated closed-culture system over 12 ± 1 days at manufacturing facility of Aastrom Biosciences in Ann Arbor, Michigan, and then transported to the clinical site under “cold temperature” storage conditions (between 0 and 12 °C), which was maintained in a qualified shipping container. The ixmyelocel-T product is produced and administered as a “unit dose,” defined as the total number of cells produced from bone marrow–derived mononuclear cells via the Sponsor's manufacturing process. The expansion potential of bone marrow–derived mononuclear cells varies modestly from patient to patient. Product lot release specifications required that the total number of viable cells in the product be in the range of 35–295 × 106 cells, and be primarily composed of two cell types: mesenchymal stromal cells (defined by the CD90+ cell surface marker) and hematopoietic stem, progenitor, and mature cells (defined by the CD45+ hematopoietic cell surface marker). These two markers are mutually exclusive and define two distinct populations of cells. The overall cell viability was measured by membrane integrity by dye exclusion and was 70% or greater. Some excipients, which were added as stabilizing agents, changed over the life of the study. The final volume of the investigational product delivered to the site was 10 ml. The composition of the electrolyte solution (placebo) was composed of the same excipients used in the formulation of the ixmyelocel-T product.
A health-care provider, trained in aseptic technique and unblinded to treatment, prepared 20 syringes from the patient-labeled bags (either ixmyelocel-T product or placebo) that were prepared at Aastrom's manufacturing site. Each syringe contained ~0.5 ml of investigational product. The fluid in the syringes was obscured with frosted tape to ensure the physician or designee administering the injections would not notice a visual difference between syringes. Twenty injection sites in the index leg were mapped by marking four circumferential linear bands around the lower third of the thigh, the greatest diameter of the patient's calf, and at one location proximal and one distal to the greatest calf diameter. Originally, five intramuscular injections of 0.5 ml were given along each band, at least 2.0 cm apart and 0.5 inches into the muscle, to include all major muscle groups. Subsequent to a protocol amendment, the number of circumferential bands was increased to five, to include injections between the interosseous muscles in the foot, and four injections were administered along each band. Patients were observed for injection-related reactions for 2 hours postinjections (considered day 0) and were followed up with a phone call at day 3 and clinic visits on day 7 and at months 3, 6, 9, and 12 for safety and efficacy assessments.
Data safety monitoring board. An independent Data Safety Monitoring Board consisting of three physicians (an interventional cardiologist, a vascular surgeon, and a stem cell therapy physician) and one statistician reviewed deaths and all adverse events in an unblinded fashion on a quarterly basis.
Statistical analyses. Because this was primarily a phase 2 trial that assessed safety and efficacy, the sample size was not chosen to try to demonstrate statistical differences between treatment groups. A total of 86 patients were randomized. The randomization schedule was computer-generated and balanced to adjust the number of patients in each group at each center.
For a patient who did not experience an event, his/her last day of the trial was used to calculate the event-free interval. TTF and AFS were each assessed using Kaplan–Meier curves, with the P value from the logrank test also provided. In addition, Cox PH analyses were performed to obtain an estimate of the treatment effect. For each of the efficacy end points, the HR and its 95% CI from the Cox PH analysis are provided in order to describe the size of the treatment effect of ixmyelocel-T. SAS, version 9.1.3, was used for all data summarization and analysis (SAS, Cary, NC).
There were two formal amendments to the original protocol. Important changes to the eligibility criteria were a modification to the upper age limit (i.e., increased from 82 years to 90 years) and patients with early-end-stage renal disease undergoing dialysis for <6 months were allowed. The randomization schema was updated from 1:1 to 2:1 (ixmyelocel-T to placebo). Furthermore, as noted above, the injection pattern was modified from four circumferential bands of five injections in each band to five circumferential bands (including the foot) with four injections in each band.
Acknowledgments
We thank William Hiatt (University of Colorado Denver School of Medicine) for his comments and review and Lisa McCormick (LSM Pharmaceutical Consulting) for her assistance in manuscript preparation. This trial was funded by Aastrom Biosciences. R.J.P. has been paid consulting fees by AnGes and Aastrom Biosciences. W.A.M. has been paid consulting fees by Aastrom. A.T.L., T.P.S., S.W., and R.L.B. are paid employees of Aastrom.
Participating investigators: R.J.P., Dartmouth-Hitchcock Medical Center, Lebanon, NH; W.A.M., University of North Carolina Medical School, Chapel Hill, NC; Anthony J Comerota, Jobst Vascular Center, Toledo, OH; S.A.B., Malcolm Randall VAMC and University of Florida, Gainesville, FL; R.G., Vanderbilt University Medical Center, Nashville, TN; T.D.H., Minneapolis Heart Institute at Abbott Northwestern, Minneapolis, MN; Edith Tzeng, University of Pittsburgh, Pittsburgh, PA; Omaida Velaquez, Miller School of Medicine at the University of Miami, Miami, FL; Carlo Dall'Olmo, Michigan Vascular Research Center, Flint, MI; Colleen Johnson Moore, Southern Illinois University School of Medicine, Springfield, IL; Jeff Martinez, Peripheral Vascular Associates, San Antonio, TX; Brian Halloran, St Joseph Mercy Hospital, Ann Arbor, MI; Peter Henke, VA Ann Arbor Healthcare System, Ann Arbor, MI; Patrick Stiff, Loyola University, Maywood, IL; Farrell Mendelsohn, Cardiology PC, Birmingham, AL; W Todd Bohannon, Scott and White Memorial Hospital, Temple, TX; Jorge Saucedo, University of Oklahoma Health Sciences Center, Oklahoma City, OK.
REFERENCES
- Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. (2006) et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR.et al. (2007Ethnic-specific prevalence of peripheral arterial disease in the United States Am J Prev Med 32328–333. [DOI] [PubMed] [Google Scholar]
- Ostchega Y, Paulose-Ram R, Dillon CF, Gu Q., and, Hughes JP. Prevalence of peripheral arterial disease and risk factors in persons aged 60 and older: data from the National Health and Nutrition Examination Survey 1999–2004. J Am Geriatr Soc. 2007;55:583–589. doi: 10.1111/j.1532-5415.2007.01123.x. [DOI] [PubMed] [Google Scholar]
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, TASC II Working Group et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33 Suppl 1:S1–S75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Falluji N., and, Mukherjee D. Contemporary management of infrapopliteal peripheral arterial disease. Angiology. 2011;62:490–499. doi: 10.1177/0003319710398011. [DOI] [PubMed] [Google Scholar]
- Powell RJ, Simons M, Mendelsohn FO, Daniel G, Henry TD, Koga M.et al. (2008Results of a double-blind, placebo-controlled study to assess the safety of intramuscular injection of hepatocyte growth factor plasmid to improve limb perfusion in patients with critical limb ischemia Circulation 11858–65. [DOI] [PubMed] [Google Scholar]
- Varu VN, Hogg ME., and, Kibbe MR. Critical limb ischemia. J Vasc Surg. 2010;51:230–241. doi: 10.1016/j.jvs.2009.08.073. [DOI] [PubMed] [Google Scholar]
- The Critical Limb Ischemia (CLI) Performance Goals Work Group of the Society for Vascular Surgery (SVS) . < http://Criticallimb.org >. Accessed 01 June 2011.
- Lawall H, Bramlage P., and, Amann B. Stem cell and progenitor cell therapy in peripheral artery disease. Thromb Haemost. 2010;103:696–709. doi: 10.1160/TH09-10-0688. [DOI] [PubMed] [Google Scholar]
- Sprengers RW, Lips DJ, Moll FL., and, Verhaar MC. Progenitor cell therapy in patients with critical limb ischemia without surgical options. Ann Surg. 2008;247:411–420. doi: 10.1097/SLA.0b013e318153fdcb. [DOI] [PubMed] [Google Scholar]
- Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, for the Therapeutic Angiogenesis using Cell Transplantation (TACT) Study Investigators et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- Powell RJ, Comerota AJ, Berceli SA, Guzman R, Henry TD, Tzeng E.et al. (2011Interim analysis results from the RESTORE-CLI, a randomized, double-blind multicenter phase II trial comparing expanded autologous bone marrow-derived tissue repair cells and placebo in patients with critical limb ischemia J Vasc Surg 541032–1041. [DOI] [PubMed] [Google Scholar]
- Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, BASIL trial participants et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–1934. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, PREVENT III Investigators et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43:742–751. doi: 10.1016/j.jvs.2005.12.058. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Lawson JH, Rapp BM, Dalsing MC, Klein J, Wilson MG.et al. (2011Autologous bone marrow mononuclear cell therapy is safe and promotes amputation-free survival in patients with critical limb ischemia J Vasc Surg 531565–74.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin EC, Guilherme S, Gahremanpour A, Canales J, Zheng Y, Cabreira-Hansen MG.et al (2011A randomized, controlled study of autologous therapy with bone marrow–derived aldehyde dehydrogenase bright cells in patients with critical limb ischemia Catheter Cardiovasc Interv 781060–1067. [DOI] [PubMed] [Google Scholar]