Abstract
A number of novel approaches for repair and regeneration of injured lung have developed over the past several years. These include a better understanding of endogenous stem and progenitor cells in the lung that can function in reparative capacity as well as extensive exploration of the potential efficacy of administering exogenous stem or progenitor cells to function in lung repair. Recent advances in ex vivo lung engineering have also been increasingly applied to the lung. The current status of these approaches as well as initial clinical trials of cell therapies for lung diseases are reviewed below.
Introduction
Lung diseases remain a devastating cause of morbidity and mortality worldwide. Unlike many other major diseases, a number of lung diseases, particularly chronic obstructive pulmonary diseases (COPDs) including both asthma and emphysema, are increasing in prevalence and COPD is expected to become the third leading cause of disease mortality worldwide by 2020.1 Although important advances in symptomatic treatments have occurred, many lung diseases including asthma, emphysema, pulmonary fibrosis, cystic fibrosis, and others have no cure. Lung transplantation is an option; however, there is a critical shortage of donor lungs and transplantation is complicated by acute and chronic rejection requiring lifelong immunosuppression. Further, lung transplantation is not a panacea as 5-year mortality following lung transplantation is ~50%.1 New approaches for lung diseases are thus desperately needed.
Recent advances in lung biology have begun to elucidate the identity and function of resident endogenous progenitor cells in the lung, cells that may provide better understanding of lung disease processes and also which may be potentially manipulated for lung repair and regeneration. In parallel, intense investigation of possible reparative roles of exogenously administered adult and embryonic stem cells has demonstrated potential for structural repair for damaged lungs, notably repair of damaged lung vasculature through paracrine effects of circulating endothelial progenitor cells (EPCs). These studies have also demonstrated potent immunomodulatory effects of adult bone marrow-derived mesenchymal stromal cells (MSCs) in a variety of inflammatory and immune lung diseases. These initial observations have led to a cautious initial but growing exploration of EPCs and MSCs in clinical trials of pulmonary hypertension and COPD, respectively, with other clinical investigations planned.
Ex vivo lung bioengineering has also accelerated at a rapid pace. Tissue engineering has in particular progressed for components of the respiratory system including trachea and diaphragm including recent pioneering clinical use of a bronchus bioengineered from a cadaveric scaffold in which autologous MSCs seeded into the scaffold were utilized to generate cartilage-producing chondrocytes.2 Given the complex anatomical structure and structure-function relationships of the lung itself, ex vivo bioengineering of the lung is a more formidable task. Nonetheless, a variety of three-dimensional (3D) scaffolds, including both biosynthetic constructs as well as decellularized whole lungs have been utilized to explore engineering both airway and vascular systems of the lung. This excitingly has included surgical implantation in rat models of decellularized lungs recellularized with a mix of fetal lung homogenates and other cells with short-term survival and some degree of restitution of gas exchange and vascular perfusion achieved.
Lung is thus a ripe organ for regenerative medicine using a variety of approaches. In the following sections, current state-of-the-art findings for each of the above areas of study will be presented, followed by an assessment of future directions.
Endogenous Lung Progenitor Cells
Endogenous lung stem and progenitor cells are thought to contribute to epithelial maintenance and injury repair. There is a large literature in mouse models and a growing literature describing putative endogenous stem and progenitor cells in human lungs. However, one persistent issue in the literature is the terminology and nomenclature utilized. The terms “stem” and “progenitor” are often used interchangeably and inconsistently. This also applies to the wider range of cells including embryonic and adult-origin stem and progenitor cells obtained from other tissues. For the purposes of this review, endogenous adult tissue-specific stem cells can be best appreciated as cells that have capacity for long-term self-renewal and that can differentiate into progenitors or other more differentiated cells in a particular organ. In general, stem cells have a wide differentiation capacity. In contrast adult endogenous progenitor cells are best appreciated as tissue-specific cells that either do not self-renew or that have short-term renewal potential but that can differentiate into more differentiated cells. The issue of nomenclature as it relates to studies of lung repair and regeneration has been addressed in detail in several recent reviews.3,4
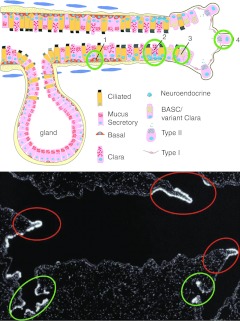
Since the adult lung is a tissue that has a very low turnover rate, murine lung injury models have been used to identify stem/progenitor cells by inducing cellular proliferation and repopulation of the lung epithelium.5 What these studies have detailed is a complex heterogeneity of putative endogenous stem/progenitor cells with different candidates located in different regions of the mouse lung. This is depicted in schematic form in Figure 1. A variety of injury approaches specific to different regions of the lung have been utilized. For example, sulfur dioxide inhalation, which damages the tracheal epithelium, has been utilized for study of proximal airway stem/progenitor cells. Common approaches used to study the distal lung are naphthalene administration, which specifically injures the Clara cells in the bronchiolar epithelium, and bleomycin administration, which injures the alveolar epithelium. Ozone and nitrogen dioxide also damage airway epithelial cells.
Figure 1.
Endogenous lung progenitor cells. (a) A graphic representation of putative lung stem/progenitor cells in the mouse lung. 1) Basal cells in the tracheobronchial region; 2) variant Clara cells associated with pulmonary neuroendocrine cell (PNEC) bodies; 3) variant Clara cells/BASCs present at the bronchiolar–alveolar duct junction; 4) alveolar type II cells present in the alveolar space. See text for further explanation. Modified with permission of the American Thoracic Society. Copyright © American Thoracic Society from Randell, SH (2006). Airway epithelial stem cells and the pathophysiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc 3: 718–725.228 (b) Endogenous regenerative microenvironments in the bronchiolar epithelium of the mouse. In situ hybridization for CCSP mRNA (white autoradiographic grains) was used to identify regions of regenerating epithelium following naphthalene-mediated progenitor cell depletion. Regenerative zones of neuroepithelial bodies were identified located at branch points in the airways (red ovals) and at the bronchoalveolar duct junction (green ovals). Adapted with permission from Stripp, BR, Maxson, K, Mera, R and Singh, G (1995). Plasticity of airway cell proliferation and gene expression after acute naphthalene injury. Am J Physiol 269(6 Pt 1): L791–L799.229 BASC, bronchioalveolar stem cell; CCSP, Clara cell secretory protein; mRNA, messenger RNA.
These studies have demonstrated that basal epithelial cells are a stem/progenitor cell population important in tissue homeostasis and injury repair in the trachea and proximal airways in mice and throughout the airways in humans. These basal cells express high levels of the transcription factor Trp63, cytokeratins 5 and 14, aquaporin 3, and can be isolated by fluorescence-activated cell sorting using expression of the nerve growth factor receptor (Ngfr).6,7,8,9,10 Lineage-tracing studies in the mouse have revealed that basal cells can give rise to Clara and ciliated cells in the proximal airways during homeostasis as well as after sulfur dioxide injury in mice9,11,12,13 Recently, a stem/progenitor population of tracheal submucosal gland duct cells was identified that appears capable of regenerating submucosal gland tubules, ducts, and surface epithelium after hypoxic–ischemic injury.14
In the airways of mouse lungs, the areas surrounding neuroepithelial bodies and the bronchioalveolar duct junction (BADJ) represent two different stem/progenitor cell niches. Variant Clara cells resistant to naphthalene injury were identified near the neuroepithelial bodies in the proximal airways and at the BADJ in distal airways.15,16,17 Bronchioalveolar stem cells (BASCs) are another naphthalene-resistant progenitor cell population located at the BADJ, identified based on their coexpression of the Clara cell marker, Clara cell secretory protein (CCSP, also known as Scgb1a1 or CC10), and the alveolar type II (AT2) cell marker, pro-surfactant protein C (pro-SP-C).18 BASCs can be isolated using fluorescence-activated cell sorting to select for cell expression of the stem cell marker Sca-1 after elimination of hematopoietic cells and endothelial cells (CD45negCD31negSca-1pos). As discussed in ref. 18, CD34 was originally used as a positive selection marker for BASCs, but differences in available antibodies obtained from different companies can result in significant variability in the populations sorted using this marker. Importantly, more recent studies have shown that the CD45negCD31negSca-1pos population is more heterogeneous than previously appreciated.19,20 Based on our own observations that BASCs are contained in the Sca-1low population and express low levels of CD24, and those of other groups, discussed below, a robust marker profile for enriching for BASCs utilizes CD45negCD31negEpCAMposSca-1lowCD24low.21 However, one important point to highlight in studies of endogenous airway stem and progenitor cells is that careful and meticulous use of isolation and characterization techniques, including flow cytometry and immunohistochemistry must be utilized. A better understanding of specific cell surface marker expression profiles is needed to better identify and isolate lung stem and progenitor cells. However, phenotypic characterization is not enough and functional assays including lineage tracing and differentiation capacities are also critical to assess. For example, variant Clara cells localized around neuroepithelial bodies survive severe injury and can re-epithelialize the airways. BASCs can self-renew in culture and express CCSP, pro-SPC, and aquaporin 5 when grown on top of matrigel basement membrane matrix, suggesting they have the ability to differentiate into Clara cells as well as AT2 cells, and type 1 (AT1) alveolar epithelial cells. As such, variant Clara cells at the BADJ and BASCs may be overlapping cell types; however, it is also possible that there are distinct populations of variant Clara cells that lack pro-SPC expression. Robust in vitro assays that recapitulate the in vivo environment are needed to functionally assess these cells in culture. In addition to lineage tracing, other in vivo assays such as cell transplantation are needed to assess the ability of putative lung stem/progenitor cells to reconstitute lung epithelial cell lineages within damaged or diseased tissue.
Recently, another possibly overlapping stem/progenitor cell population was isolated in mouse lungs based on flow cytometric isolation of cells exhibiting the CD45negCD31negEpCAMhi CD49fposCD104posCD24low phenotype.20 These cells form colonies expressing airway and/or alveolar lineage markers in a 3D co-culture matrigel assay with primary lung mesenchymal cells. Also making use of the matrigel approach, Stripp and colleagues demonstrated that the CD45negCD31negCD34negEpCAMpos Sca-1low autofluorescent (AF)low population contains naphthalene-resistant bronchiolar progenitors.22 In contrast, the CD45negCD31negCD34negEpCAMposSca-1lowAFhi population contained the naphthalene-sensitive Clara cells.22 In addition to a role in repair following ozone injury, Clara cells can also give rise to ciliated cells in the terminal airways following nitrogen dioxide exposure, as ciliated cells cannot self-renew.23,24,25,26 As demonstrated by a lineage-tracing study, Clara cells are stem/progenitor cells that have long-term self-renewal ability and can also generate ciliated cells during epithelial homeostasis.27
In the alveolar epithelium, repopulation after bleomycin injury is due to proliferation of AT2 cells, which act as progenitors for AT1 cells.28,29,30,31 BASCs have also been implicated in injury repair after bleomycin-induced injury due to their proliferation after treatment and the in vitro data suggesting their ability to differentiate into pro-SPC positive cells in culture.18 A lineage-tracing study using CCSP-CreER mice showed that the percentage of lineage-labeled cells did not increase in the alveolar space during homeostasis or following hyperoxia injury, suggesting that CCSP-expressing cells did not give rise to alveolar cells.27 However, another lineage-tracing study using the same CCSP-CreER mice found that CCSPpos cells can give rise to both AT1 and AT2 cells following bleomycin injury.31 Recently, Chapman and colleagues identified a mouse airway progenitor population of α6β4pos (also known as CD104) cells located at the BADJ and in the alveolar epithelium capable of contributing to airway and alveolar structures when transplanted with embryonic lung cells under the kidney capsule in vivo.32 It is likely that this population of cells overlaps with that found by Bertoncello and colleagues using the CD45negCD31negEpCAMhiCD49fposCD104posCD24low phenotype.20 In addition, a study by McKeon and colleagues found that p63pos airway cells were capable of forming Krt5pos “pods” in the alveolar space following H1N1 infection-induced injury.33 They also found that p63pos cells from mouse, rat, and human distal airways were capable of forming alveolar-like structures in vitro. These studies suggest that bronchiolar cells may be able to form alveolar structures under certain conditions.
The above studies demonstrate a rich network of endogenous stem and progenitor populations in mouse lungs. Although there may be overlap between cells identified in different investigations, an important concept is that there appears to be regional specificity of the progenitors. The situation is less clear in human lungs. A recent study in human lungs identified a c-kitpos cell that appears to generate both endodermal and mesodermal lineages in tissue culture.34 Further, when injected into mouse lungs following cryoinjury, this cell population appears capable of stimulating or participating in lung repair of airway, pulmonary vasculature, and pulmonary interstitium. If true, this model would overturn the current concept that there is no single multipotent lung stem or progenitor cell capable of generating smooth muscle, vasculature, airways, and alveoli. These findings require further functional characterization and validation using genetic mouse models and/or lineage tracing of c-kitpos cells during homeostasis and injury.35 This is also the first study to show apparent engraftment of human lung cells into the mouse lung. As reviewed in ref. 36, there are several important differences between the structure and cellular composition of mouse and human lungs. For example, submucosal glands and the pseudostratified epithelium containing basal cells, regions that are restricted to the trachea and upper airways in mice, extend from the trachea to the distal airways in humans. Therefore, questions regarding the endogenous niche of these cells in the human or mouse remain to be answered using rigorous techniques including lineage tracing and functional assays. In addition, variant Clara cells or BASCs have not yet been identified in human lung tissue. Therefore, intensive investigation comparing contrasting putative endogenous airway stem and progenitor populations in mouse and human lungs are sorely needed.
There is also a growing appreciation of endogenous stem or progenitor populations for the other components of lung tissue including airway smooth muscle.37 Several groups have recently identified progenitor cells resident in the lung that share some common characteristics with bone marrow-derived MSCs.38,39,40,41,42,43,44,45,46,47,48,49,50,51 Variably and inconsistently referred to as lung mesenchymal stem cells or lung mesenchymal stromal cells (L-MSCs), the potential physiologic or pathophysiologic role of these cells is not clear. Further, there may be several populations of L-MSCs in both mouse and in human lungs including those isolated from nasal mucosa as well from both proximal and distal lung compartments. In both mouse and human lungs, L-MSCs fulfill criteria for definition of bone marrow-derived MSCs including plastic adherence, expression of CD73, CD90, CD105 while lacking expression of the hematopoietic markers CD14, CD34, and CD45 and the endothelial marker CD31, and trilineage differentiation into adipocytes, chondrocytes, and osteoblasts. Another marker used to identify both human and mouse L-MSCs is the ABCG2 (ATP binding cassette G) transporter and cells designated as L-MSCs have been isolated from Hoechst dye-effluxing side populations of mouse lung homogenates.38,41 Microarray studies demonstrate both similarities and differences of L-MSCs with bone marrow-derived MSCs and further characterization of the L-MSCs is necessary.47Nonetheless, L-MSCs are clonogenic and can be serially passaged while retaining their ability to differentiate. When grown within Matrigel with either basis fibroblast growth factor (bFGF), hepatocyte growth factor (HGF), or epidermal growth factor basis fibroblast growth factor (bFGF), some of these cells express markers suggestive of endothelial or epithelial differentiation into type I and II pneumocytes and Clara Cells.43,44 However, whether these cells actually serve as a source of progenitors utilized during normal epithelial turnover, or are induced to differentiate during injury is a subject of ongoing investigation.
The effects of inflammation and injury on endogenous L-MSC populations in mice have been only minimally investigated to date. In bleomycin-induced pulmonary fibrosis, L-MSCs decrease in overall number as assessed by flow cytometry and in situ staining but little other data is yet available.48 It has also been proposed that L-MSCs could serve to combat lung inflammation through the secretion of immunosuppressive molecules. Similar to bone marrow-derived MSCs, murine L-MSCs suppress T cell proliferation in response to mitogenic or allogeneic stimulation in mixed lymphocyte reaction assays, in part through secretion of prostaglandin E2 (PGE2).42 These properties have been investigated in recent studies assessing administration of L-MSCs in mouse models of lung injury. For example, in an elastase-induced mouse model of emphysema, L-MSC administration after elastase treatment resulted in an increase in survival as well as a decrease in injury, however, not through engraftment and structural repair, but rather through a paracrine, anti-inflammatory effect.50 In a comparison study between L-MSCs and bone marrow-derived MSCs, both were similar in their effectiveness at reducing elastase-induced injury, however, L-MSCs were retained within the injured lung at a higher frequency compared to bone marrow-MSCs.44 This is thought to be possibly due to differences in the expression profile of adhesion molecules and chemokine receptors between bone marrow and lung-derived MSCs but more investigation is needed to clarify this. Most recently, L-MSCs in bronchoalveolar lavage fluid have been suggested as a marker for rejection of lung transplants.51 An important caveat to note when studying L-MSCs is that to date, no in vivo assays such as lineage tracing, have been developed. Most of the data collected pertaining to clonogenicity, differentiation capabilities, and immunomodulatory properties have been derived from isolated and cultured and/or passaged L-MSCs. This requires disaggregation of the lung thereby destroying their anatomical niche and loss of crucial clues as to what their role is in vivo. Thus at present the physiologic or pathophysiologic role of L-MSCs remains unclear.
Lung Cancer Stem Cells
One question with respect to endogenous lung stem or progenitor cells is whether they can act as either lung cancer stem cells or as tumor initiating or propagating cells. Current cancer stem cell hypotheses propose that a subpopulation of cells within a tumor possesses characteristics of stem cells and can give rise to the heterogeneity of cell types often found in many tumor types including lung cancers (reviewed in ref. 52,53). Importantly, cancer stem cells are thought to be resistant to radiation and chemotherapeutics and significantly contribute to resistance of tumors, particularly lung cancers, to available treatment strategies.52,53 Cancer stem cells are typically identified by dissociating tumor cells, using fluorescence-activated cell sorting to select tumor subpopulations, and transplantation in vivo. In some cases, the cancer stem cell population shares marker expression with normal tissue-specific stem cells. Using an orthotopic transplant assay to deliver murine lung adenocarcinoma cells to the mouse lung, Sca-1, one of the cell surface markers used to isolate BASCs from the normal mouse lung, enriched for cells with tumor-propagating ability in primary mouse adenocarcinomas initiated by oncogenic K-ras in combination with p53 deficiency.54 However, in the same study Sca-1 did not enrich for tumor-propagating cells in other mouse tumor models carrying different oncogenic mutations. This study using genetically distinct mouse models suggests that those seeking to identify human lung cancer propagating cells should consider the genotype of their human tumor samples. Side population cells, which efflux Hoechst dye, in six human non-small cell lung cancer cell lines were resistant to chemotherapy, expressed increased levels of drug transporters, showed increased invasiveness in vitro, and were tumorigenic in a subcutaneous transplant assay in mice.55 CD133, originally a cell surface marker of brain and colon cancer stem cells, has been used to identify lung cancer cells with tumorigenic properties. CD133+ cells from human lung tumors formed self-renewing spheres in vitro that could propagate tumors when transplanted subcutaneously in mice, and CD133+ cells from xenografts were enriched for tumorigenicity.56,57,58,59 However, other studies using lung cancer cell lines have shown that CD133 does not always mark a cancer stem cell population.60,61,62,63 Another cell surface marker, CD44, marked tumorigenic cells in lung cancer cell lines transplanted subcutaneously.64 Expression of another marker, aldehyde dehydrogenase (ALDH) has also been used to isolate putative cancer stem cells in leukemia, brain, breast, colon, prostate, liver, bladder, pancreatic, thyroid, and head and neck cancers. ALDH1+ cells from primary human lung tumor cells and from lung cancer cell lines were enriched for tumor-initiating ability in subcutaneous transplant assays.65,66,67 However, a population of human lung cancer stem cells serially transplanted in the lung microenvironment that recapitulates the lung tumor phenotype is yet to be described. Thus, while several lung cancer stem cell candidates have emerged, ongoing studies to further characterize endogenous lung stem cells and their potential malignant transformation will further identify and target important lung cancer stem cells.
Engraftment of Exogenous Cells in the Lung: Structural Repair and Regeneration
The question of whether non-lung stem and/or progenitor cells, whether embryonic or adult in origin, can engraft and acquire phenotype of structural lung cells following either systemic or direct intratracheal administration remains controversial. Several promising and provocative reports appeared in the early 2000's suggesting that bone marrow-derived cells including hematopoietic stem cells, MSCs, multipotent adult progenitor cells, and other populations could structurally engraft as mature differentiated airway and alveolar epithelial cells or as pulmonary vascular or interstitial cells in mouse models as well as following clinical bone marrow or lung transplantation (reviewed in ref. 3,4). Later studies also examined potential engraftment using stem and progenitor cells isolated from other tissues such as adipose, placenta, cord, blood, and others. However, it has become apparent that many of these studies utilized inadequate methodologies and that several technical issues contributed to misinterpretation of results in these initial reports. Notably, inadequate microscopic techniques did not effectively discriminate donor-derived cells superimposed on resident airway or alveolar epithelial cells. Further, a variety of leukocytes, notably airway and alveolar macrophages, reside in the lung. Many of the early reports did not utilize antibodies directed against CD45 or other leukocyte markers to exclude the possibility that cells of donor origin detected in airway or alveolar epithelium were donor-derived leukocytes rather than epithelial cells. Other tools, such as use of green fluorescence protein as a marker of donor-derived marrow cells obtained from transgenic green fluorescence protein mice in recipient mouse lungs can be subject to error in the presence of autofluorescent cells commonly found in the lung.68 Exquisite care and sophisticated microscopic approaches, including confocal and deconvolution techniques, must be utilized to effectively demonstrate potential engraftment.69,70,71,72,73 Better understanding of the mechanisms involved in recruiting exogenously administered cells to lung and inducing them to acquire functional characteristics of airway and alveolar epithelial cells is also necessary. However, at present, structural engraftment of airway or alveolar epithelium by either embryonic or adult-origin stem or progenitor cells is currently felt to be a rare occurrence of uncertain physiologic relevance (Figure 2).
Figure 2.
Systemic administration of different populations of bone marrow-derived cells results in rare epithelial engraftment but can stimulate pulmonary vascular growth. (a) Detection of Cftr expression in female Cftr KO mouse lungs following transplantation with male GFP bone marrow-derived stromal cells. Rare donor derived (Y chromosome, red), Cftr positive (green), and cytokeratin positive (blue) cells are found in airway walls of lungs assessed 1 week after transplantation. Only ~0.01% of the total airway epithelial cells appeared to be of donor marrow origin and expressed Cftr. Original magnification ×1,000. Reprinted with permission of the American Thoracic Society. Copyright © American Thoracic Society from Loi, R, Beckett, T, Goncz, KK, Suratt, BT and Weiss, DJ (2006). Am J Resp Crit Care Med 173: 171–179.70 (b) Representative confocal projection images of lung sections (a) perfused with fluorescent microspheres (green) suspended in agarose (i.e.,fluorescent microangiography) and immunostained for α-smooth muscle actin (red). Normal filling of the microvasculature was observed in control rats (a), whereas rats treated with monocrotaline (MCT) showed a marked loss of microvascular perfusion and widespread precapillary occlusion 21 (b) and 35 (d) days after MCT injection. In the prevention model, animals receiving bone marrow-derived endothelial-like progenitor cells (ELPC) displayed improved microvascular perfusion and preserved continuity of the distal vasculature (c). In the reversal model, eNOS-transduced ELPCs dramatically improved the appearance of the pulmonary microvasculature (f),whereas progenitor cells alone resulted in more modest increases in perfusion and little noticeable reduction in arteriolar muscularization (e, calibration bars = 100 µm). Figure reprinted with permission from Zhao, YD, Courtman, DW, Deng, Y, Kugathasan, L, Zhang, Q and Stewart, DJ (2005). Circ Res 96: 442–450.75 eNOS, endothelial nitric oxide synthase; GFP, green fluorescence protein.
More convincing results have been obtained with regeneration of damaged pulmonary vasculature with exogenously administered EPCs.74,75,76,77,78 This is primarily in the setting of animal models of pulmonary hypertension in which experimentally induced destruction of distal arterioles and capillaries of the pulmonary circulation are restored by systemic EPC administration (Figure 2). Although the EPCs were initially thought to directly contribute to angiogenesis, more recent data suggest that paracrine effects and secretion of growth factors that stimulate native angiogenesis is a more likely mechanism. Nonetheless, regardless of mechanisms, both anatomic and physiologic improvement in pulmonary hypertension is observed. These preclinical studies have led directly to clinical trials of autologous EPCs in both adult and pediatric patients conducted in China and in Canada.79,80 Promising initial results have been obtained in these trials and an expanded number of comparable trials is expected.
MSCs and Immunomodulation of Lung Disease
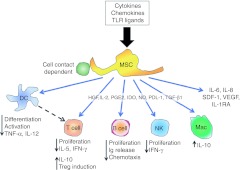
Through in vitro studies, animal models, and clinical trials in non-lung diseases, it has been demonstrated that MSCs can suppress activation, proliferation, and effector functions of cells in both the innate and adaptive immune system, as well as promoting the development of immune cells typically associated with immune suppression such as T-regulatory cells (reviewed in ref. 81,82,83,84,85, schematic representation in Figure 3). MSCs are also capable of influencing CD4+ T cell lymphocyte differentiation (Th1 versus Th2 versus Th17), depending upon the inflammatory microenvironment in which they are placed. The mechanisms by which MSCs exert their effects are thought to be orchestrated mainly through soluble mediators and/or direct cell–cell contact as significant engraftment within the target tissue is not necessary. The general consensus is that no single molecule or mechanism is responsible, thereby demonstrating the ability of the MSCs to sense the specific ongoing inflammatory microenvironment and respond accordingly. Due to low constitutive major histocompatibility complex I expression and no constitutive expression of costimulatory molecules in vitro, MSCs are also thought to be immunoprivileged in that they do not elicit an obvious immune response in allogeneic and xenogeneic transplant models.81,82,83,84,85 However, definition and investigation of MSCs continues to be confounded by several issues. Many of the published studies have utilized different definitions and characterizations of MSCs. This has complicated comparative assessments of published studies. MSCs isolated from different sources generally express comparable cell surface markers and differentiate along recognized lineage pathways. However, differences in gene expression, lineage tendencies, and other properties have been described among MSCs isolated from different sources including both different tissues and different species, particularly in mouse versus human MSCs (reviewed in ref. 3,4). Further, culture variables including culture surface composition and stiffness, oxygen environment, mechanical forces, temperature, and other factors including culture density can profoundly influence phenotype and behavior of MSCs (reviewed in ref. 3,4). It is also becoming increasingly apparent that different inflammatory environments, for example those found in different types of lung injuries, can profoundly influence MSC behavior. Further there is growing evidence that MSCs are heterogeneous and that different MSC subtypes exist. The Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT) has defined minimal criteria for defining (human) MSCs.86 However these criteria are only guidelines and better criteria need to be developed as the MSC field continues to advance. Nonetheless, despite these issues, MSCs have already been utilized in a wide range of clinical trials for immune and inflammatory-based diseases.84,85,86,87,88,89,90 Although efficacy has varied for functional endpoints in the different trials, there have been no significant safety issues with either MSC infusion or with short-term follow-up. All of these factors together make MSCs an attractive cell therapy candidate for immune and inflammatory-based disorders of the lung and there are now more than 50 published investigations in animal models of lung diseases. 91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126, 127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151 Representative studies by disease category are listed below.
Figure 3.
Schematic representation illustrating the range of in vitro immune-modulating effects described for mesenchymal stem cells (MSCs). DC, dendritic cell; HGF, hepatocyte growth factor; IDO, indoleamine 2,3-dioxygenase; IFN-γ, interferon-γ Ig, immunoglobulin; IL, interleukin; IL-1RA, interleukin-1 receptor antagonist; Mac, macrophage; NK, natural killer; NO, nitric oxide; PDL-1, programmed cell death ligand-1; PGE2, prostaglandin E-2; SDF-1, stem-cell derived factor 1; TNF-α, tumor necrosis factor-α TGF-β1, transforming growth factor-β1; TLR, toll-like receptor; VEGF, vascular endothelial growth factor. Reprinted with permission from Sueblinvong, V and Weiss, DJ (2010). Stem cells and cell therapy approaches in lung biology and diseases. Transl Res 156: 188–205.230
Pulmonary fibrosis
Idiopathic pulmonary fibrosis is a heterogenous condition of as yet poorly understood origin marked by excessive deposition of extracellular matrix within the pulmonary interstitium with resulting obliteration of normal airway and alveolar architecture. Current hypotheses suggest that pathogenesis results from a chronic indolent uncontrolled cycle of epithelial damage and fibroblast activation. As idiopathic pulmonary fibrosis is progressive, unresponsive to pharmacologic therapy, and ultimately results in death from respiratory failure with median survival upon diagnosis of 3 years, there is an urgent need for an effective treatment. In the first study to demonstrate ameliorating effects of MSCs in lung injuries, mice were exposed to a lung fibrosis-inducing agent, bleomycin, followed by systemic MSC administration.91 Although only a small number of MSCs appeared to have engrafted in the lung, significant reductions in lung inflammation and damage, collagen deposition, and other markers of injury occurred. A subsequent study suggested interleukin-1α receptor antagonist (IL-1αRA) secreted by the MSCs as responsible for the anti-inflammatory effects.92 A number of other reports have since confirmed the beneficial effects of MSC administration in the bleomycin model of pulmonary fibrosis although the mechanisms by which the MSCs are acting have not yet been fully elucidated.49,93,94,95,96,97,98,99 Further, whether clinical investigations of MSCs in idiopathic pulmonary fibrosis will show potential benefit is currently unclear. One logistic problem is that idiopathic pulmonary fibrosis is most often diagnosed in late stages when there is already significant fibrosis and less inflammation that might be amenable to potential immunomodulation by the MSCs. Nonetheless, other forms of pulmonary fibrosis with more active inflammatory components may be more amenable.
Acute lung injury/sepsis
Acute lung injury (ALI) is a result of local or systemic inflammation that leads to disruption of the alveolar-capillary interface, leakage of protein rich fluid and inflammatory cells into the interstitium and alveolar space, and extensive release of inflammatory cytokines. Despite improvements in supportive care, pharmacologic interventions have not proven effective, and a more severe form of ALI, the adult respiratory distress syndrome (ARDS), still has approximate mortality of 30% and remains a significant cause of death in the intensive care unit. Given the acute inflammatory nature of ALI/ARDS, this is perhaps a more appropriate target for clinical use of MSCs. As such, a number of studies in animal models of ALI and also in a model of ALI in explanted human lungs have shown beneficial effects of MSC administration.100,101,102,103,104,105 Regardless of the specific model of ALI utilized, consistent findings include increased survival, significantly less lung damage including edema, protein leak, and hemorrhage and also decreased levels of proinflammatory cytokines in bronchoalveolar lavage fluid with concurrent increases in anti-inflammatory cytokines such as IL-10, IL-13, and IL-1αRA. Several mechanisms for the beneficial actions of MSCs have been suggested including release of growth factors including keratinocyte growth factor and angiopoietin 1 (Ang-1), which can stimulate repair of damaged epithelia. Further, transducing the MSCs to overexpress Ang-1 results in further repair from injury. ALI is often a result of systemic inflammation, principally sepsis due to systemic bacterial infection. Several recent reports have demonstrated efficacy of systemic MSC administration in mouse models of sepsis and systemic inflammation.106,107,108,109,110 MSC administration generally improved survival and function of sepsis-damaged organs such as the kidney. In parallel, both lung inflammation and levels of circulating proinflammatory cytokines and chemokines were improved by MSC treatment. MSC treatment also resulted in significantly decreased bacterial burden and enhanced bacterial phagocytosis and clearance. Several mechanisms have been proposed to explain these findings including release of soluble mediators by MSCs that may work in part to enhance antibacterial and anti-inflammatory effects of inflammatory cells such as macrophages and neutrophils. In addition human MSCs themselves are capable of secreting at least one antimicrobial peptide, the human cathelicidin antimicrobial peptide hCAP-18/LL-37.111 MSCs may also be useful in preventing ischemia-reperfusion injury.112,113 These preclinical studies demonstrate that MSCs may be useful in clinical sepsis and septic shock, with or without associated ALI, diseases that continue to carry high morbidity and mortality.
Pulmonary hypertension
Although EPCs have been more heavily investigated for use in pulmonary hypertension, several reports have demonstrated efficacy of MSC administration in both mice and rats.114,115,116 As with EPCs, it is not clear whether the MSCs themselves participate in angiogenesis or whether secretion of anti-inflammatory substances ameliorate the experimentally induced injury that results in vascular obliteration. Further, MSCs genetically engineered to overexpress molecules known to have beneficial effects in pulmonary hypertension such as endothelial nitric oxide synthase, prostacyclin, or heme oxygenase 1 were even better able to offer protective effects and in some cases, completely reverse the pulmonary hypertensive phenotype and increase survival.116 MSCs may thus be a valuable alternate or adjunct to use of EPCs for treatment of pulmonary hypertension.
Bronchopulmonary dysplasia
In diseases of prematurity, pulmonary hypoplasia and bronchopulmonary dysplasia account for 70% of neonatal mortality. These processes are characterized by decreased numbers of distal airway and alveolar epithelial cells. Unfortunately currently available treatment regimens can be poorly effective and babies who survive bronchopulmonary dysplasia have significantly impaired pulmonary function and quality of life may be impacted. Several recent studies demonstrate efficacy or MSC administration in rodent models of hyperoxic lung injury, utilized to understand mechanisms of bronchopulmonary dysplasia pathogenesis.117,118,119 PGE2 and tumor necrosis factor-inducible gene 6 (TSG-6) secreted by the MSCs have been suggested to mediate the MSC effects in these models. However, the overall mechanisms of MSC effects to ameliorate hyperoxia-induced lung injury remain unclear. However potential clinical use of MSCs in infants remains a controversial subject.
Chronic obstructive airways diseases: asthma, emphysema, bronchiolitis obliterans
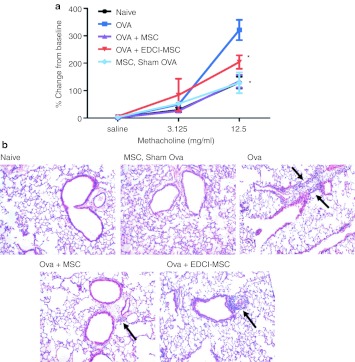
Allergic asthma, defined as airways hyperresponsiveness and inflammation in response to an inhaled allergen, is a disease driven by dendritic cells, CD4 Th2 lymphocytes and B lymphocytes. As proliferation and specific immune effector functions of each of these cell types can be inhibited by MSCs in vitro, allergic asthma is a logical choice for potential intervention with MSCs. As such, there are now a number of studies demonstrating amelioration of airways hyperresponsiveness and lung inflammation in mouse models of allergic airways inflammation.120,121,122,123,124,125 Notably, MSCs can inhibit both the initial allergen sensitization as well as the subsequent response to an allergen challenge in a previously sensitized animal (Figure 4). Some specific mechanisms of MSC actions have been defined in these models including shifting of antigen-specific CD4 T cell phenotype from Th2 to Th1 as well as upregulation of T-regulatory T cells by release of transforming growth factor β (TGFβ) by the MSCs.121,122,124 Each of these actions can ameliorate allergic airways inflammation. Likely there are other mechanisms of MSC actions involved that are still to be elucidated. Given the positive results in these studies, it is likely that clinical trials of MSCs in severe asthma will occur in the near future.
Figure 4.
Systemic administration of syngeneic and allogeneic BMSCs during sensitization to ovalbumin-alum inhibits airways hyperresponsiveness and allergic airways inflammation. (a) Airways hyperresponsiveness following allogeneic administration of bone marrow-derived MSCs, (Ova + MSC), MSCs treated with the cross-linking agent EDCI to inhibit secretion of soluble mediators (Ova + EDCI-MSC), PBS-treated ova immunized mice (Ova), or MSCs administration to sham-treated mice (MSC, sham ova) as determined by peak airways resistance responses at each methacholine dose as a function of % change from resting baseline. (b) Tissue inflammation demonstrating a decrease in ova-stimulated perobronchial infilatrates in mice receiving MSCs but not EDCI-treated MSCs. Reprinted with permission from Goodwin, M, Sueblinvong, V, Eisenhauer, P, Ziats, NP, LeClair, L, Poynter, ME et al. (2011). Bone marrow derived mesenchymal stromal cells inhibit Th2-mediated allergic airways inflammation in mice. Stem Cells 29: 1137–1148.124 *P < 0.05 compared to naive. EDCI, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide; MSC, mesenchymal stromal cell; OVA, ovalbumin; PBS, phosphate-buffered saline.
Emphysema and chronic bronchitis, commonly classified together as COPD are devastating incurable diseases in which chronic inflammation, usually resulting from cigarette smoking, results in progressive destruction of alveoli and progressive respiratory dysfunction leading to death. COPD is one of the only major diseases increasing in prevalence and is predicted to be the third leading cause of death by 2020. Although symptomatic treatments can alleviate some symptoms, there is no cure at present except lung transplantation. An increasing number of studies in rodent models of emphysema, including those induced by either cigarette smoke exposure or by intratracheal administration of destructive enzymes such as elastase, demonstrate protective effects of MSC administration.126,127,128,129,130,131,132,133,134 The mechanisms of the MSC effects in these models are unclear but likely again involve release of anti-inflammatory soluble mediators. Release of soluble mediators such as hepatic growth factor, which can stimulate new alveolar growth, has also been suggested as a potential mechanism of MSC action in these models.
These data suggest potential efficacy of MSCs in clinical COPD. A recent groundbreaking multicenter, double-blind, placebo-controlled phase II trial of MSCs in patients with moderate-severe COPD was initiated in the United States in 2008. The primary goal of the trial is to determine safety of MSC infusions in patients with lung disease. The secondary goal is initial estimation of the potential efficacy of MSCs for decreasing the chronic inflammation associated with COPD thus improving both pulmonary function and quality of life. In the 6-month interim data analysis, no infusional toxicities or significant adverse events were reported.135 Notably, a significant decrease in the circulating inflammatory marker C-reactive protein, commonly elevated in COPD patients, was noted in treated patients as was a trend towards improvement in quality of life indicators. The trial results are expected to be published in the Spring of 2012. Other clinical trials of MSC administration to patients with COPD are currently underway in Europe.
Bronchiolitis obliterans (OB) is a rare chronic progressive fibrous obliteration of the airways of unclear etiology. It can be a significant complication of allogeneic bone marrow transplantation and is also a leading cause of chronic rejection and mortality in lung transplant recipients. As discussed above, presence of lung-derived MSCs in bronchoalveolar lavage fluid may be a marker of chronic rejection in lung transplant recipients.51 However, MSC administration may also alleviate OB in mouse transplantation models.136 One potential area for study of MSC effects on OB is in the context of clinical trials of MSCs in prevention or treatment of graft versus host disease in bone marrow transplant recipients. As OB may be a manifestation of graft versus host disease, addition of lung function testing to outcome measures in these studies may provide invaluable information on potential clinical efficacy of MSC administration for OB.
Lung cancers
Bone marrow-derived MSCs and EPCs may contribute to development of primary and metastatic lung carcinoma and other malignancies, notably breast and ovarian cancers, in mouse models. These cells function, in part, by providing a supportive stroma for the cancers and/or by participating in tumor vascularization.137,138,139,140 In contrast, MSCs and EPCs have been demonstrated to home to areas of tumor development and EPCs and MSCs engineered to secrete antitumor agents have been utilized to suppress tumor growth in mouse tumor models of primary lung cancers, metastatic lung cancers, and of other cancers metastatic to the lung.141,142,143,144,145,146,147,148,149,150,151,152,153 Cell-based therapies may thus be useful in lung cancer therapeutics.
However, whether MSCs may undergo malignant transformation remains a topic of concern.154,155,156,157 Murine MSCs that were extensively expanded in culture through many passages developed chromosomal instability and produced lung sarcomas in mice.158,159 The results emphasize the greater propensity of mouse as compared to human MSCs and other cells to acquire chromosomal abnormalities with serial passages in culture.158,159,160,161,162,163 Concerns about the potential tumorigenicity of human MSCs were raised by three reports that described escape from senescence and generation of malignant cells as the MSCs were expanded in culture.164,165 The reports were unexpected since emergence from senescence had not been observed in laboratories that had studied the cells for over a decade. The discrepancies were largely explained by reports from two of the laboratories indicating that their cultures of human MSCs had been cross-contaminated with human fibrosarcoma or osteosarcoma cell lines.166 This further highlights the need to develop better markers for defining MSCs and the important need to verify the identity of cells even if received from established sources. It is anticipated that additional strategies to maximize therapeutic utility of MSCs while decreasing chance of any adverse effects will develop over the next several years.
Embryonic Stem Cells and Induced Pluripotent Stem Cells
Progress utilizing pluripotent embryonic stem cells (ESCs) or induced pluripotent stem cells (iPS) for lung regeneration or repair has been slower to develop.167 Both mouse and human ESCs can be induced in culture to acquire some phenotypic markers consistent with type 2 alveolar epithelial cells, notably expression of surfactant proteins such as surfactant protein C and also electron microscopic identification of the cellular organelle involved in production and secretion of surfactant, the lamellar bodies.168,169,170,171,172,173,174,175,176 Derivation of airway epithelial cells from ESCs has proven even more elusive although development of cells with phenotypic markers of airway epithelial cells has been demonstrated following culture of the ESCs under air–liquid interface conditions.177,178,179 However, SPC expression can be found in early lung endoderm and not necessarily denote specific development of type 2 alveolar epithelial cells. Further, SPC can be found in non-lung tissues such as placenta and amnion in both mice and humans.180 Organelles that resemble lamellar bodies have been described in other cell types including skin and the lining of the gastrointestinal tract.181 Similarly, phenotypic markers commonly utilized to identify nonciliated airway (Clara) cells, for example CCSP, are also nonspecific and can be found in other tissues such as the uterus where CCSP is known as uteroglobin. As such, multiple phenotypic markers and better still, functional characterization as either airway or alveolar cells are required to clearly identify lung epithelial cells derived from ESCs. Importantly, lessons learned from developmental biology are critical in developing strategies to sequentially differentiate ESCs into definitive endoderm, foregut endoderm, early lung endoderm as opposed to other foregut derivatives such as esophagus, stomach, or thyroid, and finally specific lung epithelial cells. This approach has been successfully utilized in generating pancreatic β-cell precursors and more recently in generating intestinal cells.182
Moreover, there are still only limited available studies of effects of ESC administration to lung in vivo. A recent study demonstrated that endotracheal administration of human ESC (hESC)-derived type 2 alveolar epithelial (hESC-ATII) cells 1 or 2 days following induction of acute lung injury resulting from intratracheal bleomycin administration to immunocompromised severe combined immunodeficiency (SCID) mice resulted in a substantial number of hES-ATII cells appearing to have engrafted in lung.183 Up to 20% of the total surfactant-expressing cells appeared to be of hES-ATII origin. Notably, bleomycin-induced acute lung injury was significantly reduced in mice receiving hES-ATII cells but not cell or vehicle controls. This first published investigation demonstrating amelioration of lung injury by ESC administration suggests that both structural engraftment and possibly a previously unsuspected paracrine effect of the hES-derived cells may have played a role. These results have a number of ramifications, including the study and potential use of ESCs in genetic lung diseases as embryonic stem cell lines obtained from fetuses with cystic fibrosis have been established.184,185,186
Comparably, iPS cells can be induced in tissue culture to develop into definitive endoderm, the embryologic precursor of lung.187 However, it has proven difficult to date to induce further differentiation to cells with any phenotypic markers suggestive of either airway or alveolar epithelium. Similar strategies utilizing information gained from study of embryonic lung development are also critical for differentiating airway and alveolar epithelial cells from iPS cells. Lung disease-specific iPS cell lines have recently been produced from patients with cystic fibrosis and α-1 antitrypsin disease.188 These cells exhibit normal morphology and protein expression compared to other iPS cell lines but have not yet been studied in detail to determine, for example, if they can also be differentiated into definitive endoderm comparable to nondiseased iPS cells. The disease-specific iPS cells, like ESCs obtained from fetuses with cystic fibrosis, are valuable tools for studying potential means of gene correction and the accompanying changes in cellular physiology such as replacing defective CFTR. However, as discussed above in the section on engraftment, it is not clear if these cells will be useful for in vivo applications.
Lung Tissue Bioengineering
Approximately 1,000–1,500 lung transplants per year are performed in the United States, but a significant shortage of suitable donor lungs and the drawbacks of lung transplantation, including lifelong immunosuppression and an approximate 50% 5-year mortality in large part due to chronic rejection, demonstrates a critical need for new approaches. One rapidly growing area of investigation is that utilizing 3D matrices or other artificial scaffolding for ex vivo growth of functional lung tissue from stem cells ex vivo and in vivo. These approaches have had recent initial success for the trachea and major bronchi. MSCs isolated from amniotic fluid, umbilical cord blood, or bone marrow can be seeded on biosynthetic scaffolds or onto cadaveric scaffolds in which the resident tracheal cells have been removed by treatment with detergents, enzymes, and other agents (decellularized tracheal scaffolds). The MSCs differentiate into cartilage-producing chondrocytes and the resulting tissue used in repair of congenital tracheal or bronchial defects.189,190,191,192,193,194,195,196,197 This approach has recently resulted in successful clinical transplantation of a bioengineered bronchus and more recently bioengineered tracheas bioengineered from either cadaveric or synthetic graft scaffolds.2,198 Although only limited long-term follow-up is available for the outcome of tracheal or bronchial replacements, more extensive use of trachea and bronchi bioengineered from both synthetic and cadaveric scaffolds is expected. Similar approaches have also been utilized to generate tendon tissue for use in congenital diaphragmatic defects.198,199,200
Given the complex 3D architecture and structure–function relationships of the lung, ex vivo lung bioengineering is a more challenging task. Nonetheless there has been significant progress in several areas. Synthetic 3D scaffolds have also been utilized as matrices for ex vivo lung parenchymal development and for study of growth factors and mechanical forces on lung remodeling.201,202,203,204,205,206,207,208,209,210 Comparable scaffold approaches have been utilized to study creation of pulmonary vascular networks and to investigate effects of vascular endothelial cells on development of airway and alveolar epithelial tissues.211,212 Several recent studies have demonstrated the power of rotating bioreactor systems to assess functional interactions of pathogenic bacteria with lung epithelial cells.213,214 Growth of lung epithelial cells, dendritic cells, and macrophages on porous filters has been shown to mimic some of the innate immune pathways of lung airways.215 Coating of human lung cells and human blood capillaries onto porous polydimethylsiloxane chips, a “lung-on-a-chip” device mimicking the alveoli of the lungs, has been recently utilized to evaluate how nanoparticles and bacteria enter the lungs and may also be useful for high throughput screening of drugs.216
However, many of these studies were not designed to demonstrate functionality of the engineered lung tissue, such as the potential for ventilation and gas exchange. Further, artificial scaffolds neither fully replicate the complexity of the lung architecture, nor do they contain all the matrix components essential for normal lung development and function. As such, recent work has focused on use of more natural models including nasal septa and, in particular, decellularized whole lung as a more physiologic scaffolds.217 Decellularized whole lungs are produced by sequential treatment of lungs with detergents, hypotonic and hypertonic salt solutions, enzymes such as DNAse, with or without physical methods such as freeze thaw in order to remove all cellular materials but leave intact relevant extracellular matrix components, including collagens, elastin, and laminin, and thus retain the native structure of the lung.218,219,220,221,222,223,224 However, there is no agreement as yet on an optimal lung de-cellularization protocol and the several different published de-cellularization approaches result in lung scaffolds with differing content of extracellular matrix and residual intracellular proteins.225,226 Further, all of these approaches result in substantial removal of glycosaminoglycans and may have different affects on lung mechanics. These may all critically affect growth and differentiation of cells inoculated into the decellularized scaffolds.225 Further, strategies for appropriately recellularizing the decellularized scaffolds are not yet well-developed. It is not clear which type of cells, either ESCs, iPS cells, mature lung endothelial or epithelial cells obtained from the eventual transplant recipient or alternatively stem or progenitor cells obtained from the eventual transplant recipient, will be utilized. Further, it is not clear how the inoculated cells will be directed to appropriately recellularize specific regions of the lungs, for example airways versus alveoli. As such, while use of decellularized whole lung scaffolds is a promising field, there are many fundamental questions to be addressed before potential translational or clinical application. As discussed above, critical lessons from lung developmental biology will be applicable for directing recellularization of the decellularized scaffolds.
Two recent proof-of-concept studies demonstrated that decellularized rat lung lobes, inoculated with mixtures of homogenized fetal lungs, endothelial cells, and A549 carcinoma cells were able to be surgically implanted in rats that had previously undergone unilateral pneumonectomy with short-term survival of the rats and some degree of gas exchange and vascular perfusion achieved222,223 (Figure 5). A more recent follow-up study demonstrates survival with some degree of gas exchange up to 2 weeks.227 However, the resulting cellular architecture did not fully resemble that of native lung, particularly in the longer surviving animals in which substantial fibrosis occurred. These studies highlight the challenges in recapitulating the normal dynamic integrated functional 3D network of epithelial cells, endothelial cells, fibroblasts, neuronal, and inflammatory cells in the appropriate environment and architecture.
Figure 5.
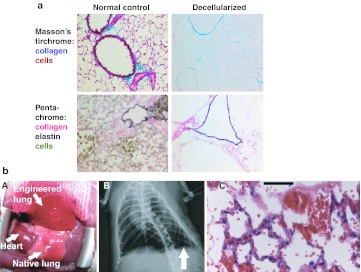
Use of decellularized lung scaffolds for ex vivo lung bioengineering. (a) Massome's trichrome and pentachrome staining of extracellular matrix proteins collagen and elastin in native and decellularized mouse lung. Reprinted with permission from Price, AP, England, KA, Matson, AM, Blazar, BR and Panoskaltsis-Mortari, A (2010). Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng Part A 16: 2581–2591.221 (b) (A) Tissue-engineered left lung was implanted into adult Fischer 344 rat recipient and photographed ~30 minutes later. (B) X-ray image of rat showing the implanted engineered left lung (white arrow) and the right native lung. (C) H and E stain of explanted lung. Red blood cells perfusing septa are evident, and some red blood cells are present in airspaces. Bar 50 µm. Reprinted with permission from Petersen, TH, Calle, EA, Zhao, L, Lee, EJ, Gui, L, Raredon, MB et al. (2010). Tissue-engineered lungs for in vivo implantation. Science 329: 538–541.223 H&E, hematoxylin and eosin.
Summary
Novel approaches for repair and regeneration of injured lungs have progressed rapidly over the past approximate 10 years. Initial excitement about structural engraftment of exogenously administered cells has been tempered with better appreciation that this is a rare phenomenon of unclear physiologic significance. As such, as exciting progress is made in other areas including better understanding of endogenous lung stem and progenitor cells, manipulation of embryonic stem cells and iPS cells to derive functional lung cells, immunomodulatory cell therapy approaches, and in ex vivo lung bioengineering, meticulous techniques and rigorous scientific approaches must be utilized.
REFERENCES
- Orens JB., and, Garrity ER., Jr General overview of lung transplantation and review of organ allocation. Proc Am Thorac Soc. 2009;6:13–19. doi: 10.1513/pats.200807-072GO. [DOI] [PubMed] [Google Scholar]
- Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA.et al. (2008Clinical transplantation of a tissue-engineered airway Lancet 3722023–2030. [DOI] [PubMed] [Google Scholar]
- Weiss DJ, Kolls JK, Ortiz LA, Panoskaltis-Mortari A., and, Prockop DJ.2008Stem cells and cell therapy approaches for lung diseasesConference Report. Proc Am Thorac Soc 5637–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss DJ, Bertoncello I, Borok Z, Kim C, Panoskaltsis-Mortari A, Reynolds S.et al. (2011Stem cells and cell therapies in lung biology and lung diseases Proc Am Thorac Soc 8223–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL., and, Hogan BL. Epithelial stem cells of the lung: privileged few or opportunities for many. Development. 2006;133:2455–2465. doi: 10.1242/dev.02407. [DOI] [PubMed] [Google Scholar]
- Borthwick DW, Shahbazian M, Krantz QT, Dorin JR., and, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol. 2001;24:662–670. doi: 10.1165/ajrcmb.24.6.4217. [DOI] [PubMed] [Google Scholar]
- Daniely Y, Liao G, Dixon D, Linnoila RI, Lori A, Randell SH.et al. (2004Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium Am J Physiol, Cell Physiol 287C171–C181. [DOI] [PubMed] [Google Scholar]
- Schoch KG, Lori A, Burns KA, Eldred T, Olsen JC., and, Randell SH. A subset of mouse tracheal epithelial basal cells generates large colonies in vitro. Am J Physiol Lung Cell Mol Physiol. 2004;286:L631–L642. doi: 10.1152/ajplung.00112.2003. [DOI] [PubMed] [Google Scholar]
- Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y.et al. (2009Basal cells as stem cells of the mouse trachea and human airway epithelium Proc Natl Acad Sci USA 10612771–12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avril-Delplanque A, Casal I, Castillon N, Hinnrasky J, Puchelle E., and, Péault B. Aquaporin-3 expression in human fetal airway epithelial progenitor cells. Stem Cells. 2005;23:992–1001. doi: 10.1634/stemcells.2004-0197. [DOI] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Watkins S, Fuchs E., and, Stripp BR. In vivo differentiation potential of tracheal basal cells: evidence for multipotent and unipotent subpopulations. Am J Physiol Lung Cell Mol Physiol. 2004;286:L643–L649. doi: 10.1152/ajplung.00155.2003. [DOI] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Watkins S, Fuchs E., and, Stripp BR. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol. 2004;164:577–588. doi: 10.1016/S0002-9440(10)63147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Brechbuhl HM, Smith RW, Li B, Hicks DA, Titchner T.et al. (2011Context-dependent differentiation of multipotential keratin 14-expressing tracheal basal cells Am J Respir Cell Mol Biol 45403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegab AE, Ha VL, Gilbert JL, Zhang KX, Malkoski SP, Chon AT.et al. (2011Novel stem/progenitor cell population from murine tracheal submucosal gland ducts with multipotent regenerative potential Stem Cells 291283–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SD, Giangreco A, Power JH., and, Stripp BR. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol. 2000;156:269–278. doi: 10.1016/S0002-9440(10)64727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Giangreco A, Hurley CM., and, Stripp BR. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol. 2001;24:671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- Giangreco A, Reynolds SD., and, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol. 2002;161:173–182. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S.et al. (2005Identification of bronchioalveolar stem cells in normal lung and lung cancer Cell 121823–835. [DOI] [PubMed] [Google Scholar]
- Raiser DM., and, Kim CF. Commentary: Sca-1 and Cells of the Lung: A matter of Different Sorts. Stem Cells. 2009;27:606–611. doi: 10.1002/stem.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQualter JL, Yuen K, Williams B., and, Bertoncello I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci USA. 2010;107:1414–1419. doi: 10.1073/pnas.0909207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharek SJ, Fillmore CM, Lau AN, Gludish DW, Chou A, Ho JW.et al. (2011Lung stem cell self-renewal relies on BMI1-dependent control of expression at imprinted loci Cell Stem Cell 9272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teisanu RM, Chen H, Matsumoto K, McQualter JL, Potts E, Foster WM.et al. (2011Functional analysis of two distinct bronchiolar progenitors during lung injury and repair Am J Respir Cell Mol Biol 44794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Johnson LV, Stephens RJ., and, Freeman G. Renewal of the terminal bronchiolar epithelium in the rat following exposure to NO2 or O3. Lab Invest. 1976;35:246–257. [PubMed] [Google Scholar]
- Evans MJ, Shami SG, Cabral-Anderson LJ., and, Dekker NP. Role of nonciliated cells in renewal of the bronchial epithelium of rats exposed to NO2. Am J Pathol. 1986;123:126–133. [PMC free article] [PubMed] [Google Scholar]
- Barth PJ., and, Müller B. Effects of nitrogen dioxide exposure on Clara cell proliferation and morphology. Pathol Res Pract. 1999;195:487–493. doi: 10.1016/S0344-0338(99)80052-1. [DOI] [PubMed] [Google Scholar]
- Rawlins EL., and, Hogan BL. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am J Physiol Lung Cell Mol Physiol. 2008;295:L231–L234. doi: 10.1152/ajplung.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H.et al. (2009The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium Cell Stem Cell 4525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Yoneda K., and, Kikkawa Y. Morphologic and biochemical study of pulmonary changes induced by bleomycin in mice. Lab Invest. 1976;35:558–568. [PubMed] [Google Scholar]
- Kawamoto M., and, Fukuda Y. Cell proliferation during the process of bleomycin-induced pulmonary fibrosis in rats. Acta Pathol Jpn. 1990;40:227–238. doi: 10.1111/j.1440-1827.1990.tb01556.x. [DOI] [PubMed] [Google Scholar]
- Isakson BE, Lubman RL, Seedorf GJ., and, Boitano S. Modulation of pulmonary alveolar type II cell phenotype and communication by extra-cellular matrix and KGF. Am J Physiol Cell Physiol. 2001;281:C1291–C1299. doi: 10.1152/ajpcell.2001.281.4.C1291. [DOI] [PubMed] [Google Scholar]
- Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J.et al. (2011Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition Proc Natl Acad Sci USA 108E1475–E1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K.et al. (2011Integrin a6ß4 identifies an adult distal lung epithelial population with regenerative potential in mice J Clin Invest 1212855–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D.et al. (2011Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection Cell 147525–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajstura J, Rota M, Hall SR, Hosoda T, D'Amario D, Sanada F.et al. (2011Evidence for human lung stem cells N Engl J Med 3641795–1806. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lung stem cells: looking beyond the hype. Nat Med. 2011;17:788–789. doi: 10.1038/nm0711-788. [DOI] [PubMed] [Google Scholar]
- Rock JR, Randell SH., and, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J, Summer R., and, Fine A. Stem cells in airway smooth muscle: state of the art. Proc Am Thorac Soc. 2008;5:11–14. doi: 10.1513/pats.200704-052VS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summer R, Fitzsimmons K, Dwyer D, Murphy J., and, Fine A. Isolation of an adult mouse lung mesenchymal progenitor cell population. Am J Respir Cell Mol Biol. 2007;37:152–159. doi: 10.1165/rcmb.2006-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennrick KT, Keeton AG, Nanua S, Kijek TG, Goldsmith AM, Sajjan US.et al. (2007Lung cells from neonates show a mesenchymal stem cell phenotype Am J Respir Crit Care Med 1751158–1164. [DOI] [PubMed] [Google Scholar]
- Lama VN, Smith L, Badri L, Flint A, Andrei AC, Murray S.et al. (2007Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts J Clin Invest 117989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Helm K, Ruegg P, Varella-Garcia M, Burnham E., and, Majka S. Adult lung side population cells have mesenchymal stem cell potential. Cytotherapy. 2008;10:140–151. doi: 10.1080/14653240801895296. [DOI] [PubMed] [Google Scholar]
- Jarvinen L, Badri L, Wettlaufer S, Ohtsuka T, Standiford TJ, Toews GB.et al. (2008Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator J Immunol 1814389–4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoubi G, Cortes-Dericks L, Breyer I, Schmid RA., and, Dutly AE. Identification of mesenchymal stromal cells in human lung parenchyma capable of differentiating into aquaporin 5-expressing cells. Lab Invest. 2009;89:1100–1114. doi: 10.1038/labinvest.2009.73. [DOI] [PubMed] [Google Scholar]
- Hegab AE, Kubo H, Fujino N, Suzuki T, He M, Kato H.et al. (2010Isolation and characterization of murine multipotent lung stem cells Stem Cells Dev 19523–536. [DOI] [PubMed] [Google Scholar]
- Bentley JK, Popova AP, Bozyk PD, Linn MJ, Baek AE, Lei J.et al. (2010Ovalbumin sensitization and challenge increases the number of lung cells possessing a mesenchymal stromal cell phenotype Respir Res 11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob M, Hemeda H, Janeschik S, Bootz F, Rotter N, Lang S.et al. (2010Human nasal mucosa contains tissue-resident immunologically responsive mesenchymal stromal cells Stem Cells Dev 19635–644. [DOI] [PubMed] [Google Scholar]
- Ingenito E, Tsai L, Murthy S, Tyagi S, Mazan M., and, Hoffman A.2011Autologous lung-derived mesenchymal stem cell transplantation in experimental emphysema Stem Cells Dev 201779–1792.21585237 [Google Scholar]
- Bozyk PD, Popova AP, Bentley JK, Goldsmith AM, Linn MJ, Weiss DJ.et al. (2011Mesenchymal stromal cells from neonatal tracheal aspirates demonstrate a pattern of lung-specific gene expression Stem Cells Dev 201995–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun D, Garat C, West J, Thorn N, Chow K, Cleaver T.et al. (2011The pathology of bleomycin-induced fibrosis is associated with loss of resident lung mesenchymal stem cells that regulate effector T-cell proliferation Stem Cells 29725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AM, Paxson JA, Mazan MR, Davis AM, Tyagi S, Murthy S.et al. (2011Lung-derived mesenchymal stromal cell post-transplantation survival, persistence, paracrine expression, and repair of elastase-injured lung Stem Cells Dev 201779–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badri L, Murray S, Liu LX, Walker NM, Flint A, Wadhwa A.et al. (2011Mesenchymal stromal cells in bronchoalveolar lavage as predictors of bronchiolitis obliterans syndrome Am J Respir Crit Care Med 1831062–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ. Cancer stem cells. Clin Adv Hematol Oncol. 2005;3:171–172. [PubMed] [Google Scholar]
- Giangreco A, Groot KR., and, Janes SM. Lung cancer and lung stem cells: strange bedfellows. Am J Respir Crit Care Med. 2007;175:547–553. doi: 10.1164/rccm.200607-984PP. [DOI] [PubMed] [Google Scholar]
- Curtis SJ, Sinkevicius KW, Li D, Lau AN, Roach RR, Zamponi R.et al. (2010Primary tumor genotype is an important determinant in identification of lung cancer propagating cells Cell Stem Cell 7127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MM, Ng AV, Lam S., and, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–4833. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A.et al. (2008Identification and expansion of the tumorigenic lung cancer stem cell population Cell Death Differ 15504–514. [DOI] [PubMed] [Google Scholar]
- Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L.et al. (2009Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment Proc Natl Acad Sci USA 10616281–16286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirino V, Camerlingo R, Franco R, Malanga D, La Rocca A, Viglietto G.et al. (2009The role of CD133 in the identification and characterisation of tumour-initiating cells in non-small-cell lung cancer Eur J Cardiothorac Surg 36446–453. [DOI] [PubMed] [Google Scholar]
- Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ.et al. (2007CD133(+) and CD133(-) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles Cancer Res 674010–4015. [DOI] [PubMed] [Google Scholar]
- Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T.et al. (2008CD133 expression is not restricted to stem cells, and both CD133+ and CD133- metastatic colon cancer cells initiate tumors J Clin Invest 1182111–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM., and, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Li M, Wang X, Wang Y., and, Ma D. Both CD133+ and CD133- subpopulations of A549 and H446 cells contain cancer-initiating cells. Cancer Sci. 2009;100:1040–1046. doi: 10.1111/j.1349-7006.2009.01144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui F, Wang J, Chen D., and, Chen YJ. CD133 is a temporary marker of cancer stem cells in small cell lung cancer, but not in non-small cell lung cancer. Oncol Rep. 2011;25:701–708. doi: 10.3892/or.2010.1115. [DOI] [PubMed] [Google Scholar]
- Leung EL, Fiscus RR, Tung JW, Tin VP, Cheng LC, Sihoe AD.et al. (2010Non-small cell lung cancer cells expressing CD44 are enriched for stem cell-like properties PLoS ONE 5e14062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Qiu Q, Khanna A, Todd NW, Deepak J, Xing L.et al. (2009Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer Mol Cancer Res 7330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JP, Spinola M, Dodge M, Raso MG, Behrens C, Gao B.et al. (2010Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on notch signaling Cancer Res 709937–9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D., and, Shi Y. Aldehyde dehydrogenase-1 is a specific marker for stem cells in human lung adenocarcinoma. Med Oncol. 2011;28:315–321. doi: 10.1007/s12032-011-9933-9. [DOI] [PubMed] [Google Scholar]
- Swenson ES, Price JG, Brazelton T., and, Krause DS. Limitations of green fluorescent protein as a cell lineage marker. Stem Cells. 2007;25:2593–2600. doi: 10.1634/stemcells.2007-0241. [DOI] [PubMed] [Google Scholar]
- Beckett T, Loi R, Prenovitz R, Poynter M, Goncz KK, Suratt BT.et al. (2005Acute lung injury with endotoxin or NO2 does not enhance development of airway epithelium from bone marrow Mol Ther 12680–686. [DOI] [PubMed] [Google Scholar]
- Loi R, Beckett T, Goncz KK, Suratt BT., and, Weiss DJ. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med. 2006;173:171–179. doi: 10.1164/rccm.200502-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueblinvong V, Loi R, Eisenhauer PL, Bernstein IM, Suratt BT, Spees JL.et al. (2008Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells Am J Respir Crit Care Med 177701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotton DN, Fabian AJ., and, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol. 2005;33:328–334. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JC, Summer R, Sun X, Fitzsimmons K., and, Fine A. Evidence that bone marrow cells do not contribute to the alveolar epithelium. Am J Respir Cell Mol Biol. 2005;33:335–342. doi: 10.1165/rcmb.2005-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Nakamura T, Toba T, Kajiwara N, Kato H., and, Shimizu Y. Transplantation of endothelial progenitor cells into the lung to alleviate pulmonary hypertension in dogs. Tissue Eng. 2004;10:771–779. doi: 10.1089/1076327041348563. [DOI] [PubMed] [Google Scholar]
- Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q., and, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res. 2005;96:442–450. doi: 10.1161/01.RES.0000157672.70560.7b. [DOI] [PubMed] [Google Scholar]
- Raoul W, Wagner-Ballon O, Saber G, Hulin A, Marcos E, Giraudier S.et al. (2007Effects of bone marrow-derived cells on monocrotaline- and hypoxia-induced pulmonary hypertension in mice Respir Res 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam CF, Liu YC, Hsu JK, Yeh PA, Su TY, Huang CC.et al. (2008Autologous transplantation of endothelial progenitor cells attenuates acute lung injury in rabbits Anesthesiology 108392–401. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Kitaichi T, Urata M, Kurobe H, Kanbara T, Motoki T.et al. (2009Syngeneic bone marrow mononuclear cells improve pulmonary arterial hypertension through vascular endothelial growth factor upregulation Ann Thorac Surg 88418–424. [DOI] [PubMed] [Google Scholar]
- Wang XX, Zhang FR, Shang YP, Zhu JH, Xie XD, Tao QM.et al. (2007Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial J Am Coll Cardiol 491566–1571. [DOI] [PubMed] [Google Scholar]
- Zhu JH, Wang XX, Zhang FR, Shang YP, Tao QM, Zhu JH.et al. (2008Safety and efficacy of autologous endothelial progenitor cells transplantation in children with idiopathic pulmonary arterial hypertension: open-label pilot study Pediatr Transplant 12650–655. [DOI] [PubMed] [Google Scholar]
- Phinney DG., and, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair–current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- Nauta AJ., and, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther. 2009;17:939–946. doi: 10.1038/mt.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano A, Galderisi U., and, Marino IR. From the laboratory bench to the patient's bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Moretta L., and, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D.et al. (2006Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement Cytotherapy 8315–317. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M.et al. (2004Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells Lancet 3631439–1441. [DOI] [PubMed] [Google Scholar]
- Ringdén O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lönnies H.et al. (2006Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease Transplantation 811390–1397. [DOI] [PubMed] [Google Scholar]
- Fang B, Song YP, Liao LM, Han Q., and, Zhao RC. Treatment of severe therapy-resistant acute graft-versus-host disease with human adipose tissue-derived mesenchymal stem cells. Bone Marrow Transplant. 2006;38:389–390. doi: 10.1038/sj.bmt.1705457. [DOI] [PubMed] [Google Scholar]
- Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP.et al. (2009A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction J Am Coll Cardiol 542277–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N.et al. (2003Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects Proc Natl Acad Sci USA 1008407–8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K.et al. (2007Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury Proc Natl Acad Sci USA 10411002–11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Zhang YF, Liu YG, Zhou JJ, Li ZK, Wu CG.et al. (2008Therapeutic effects of bone marrow-derived mesenchymal stem cells engraftment on bleomycin-induced lung injury in rats Transplant Proc 401700–1705. [DOI] [PubMed] [Google Scholar]
- Moodley Y, Atienza D, Manuelpillai U, Samuel CS, Tchongue J, Ilancheran S.et al. (2009Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury Am J Pathol 175303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germano D, Blyszczuk P, Valaperti A, Kania G, Dirnhofer S, Landmesser U.et al. (2009Prominin-1/CD133+ lung epithelial progenitors protect from bleomycin-induced pulmonary fibrosis Am J Respir Crit Care Med 179939–949. [DOI] [PubMed] [Google Scholar]
- Aguilar S, Scotton CJ, McNulty K, Nye E, Stamp G, Laurent G.et al. (2009Bone marrow stem cells expressing keratinocyte growth factor via an inducible lentivirus protects against bleomycin-induced pulmonary fibrosis PLoS ONE 4e8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto M, Nishiwaki T, Matsuo N, Kimura H., and, Matsushima K. Minimally cultured bone marrow mesenchymal stem cells ameliorate fibrotic lung injury. Eur Respir J. 2009;34:740–748. doi: 10.1183/09031936.00128508. [DOI] [PubMed] [Google Scholar]
- Cargnoni A, Gibelli L, Tosini A, Signoroni PB, Nassuato C, Arienti D.et al. (2009Transplantation of allogeneic and xenogeneic placenta-derived cells reduces bleomycin-induced lung fibrosis Cell Transplant 18405–422. [DOI] [PubMed] [Google Scholar]
- Lee SH, Jang AS, Kim YE, Cha JY, Kim TH, Jung S.et al. (2010Modulation of cytokine and nitric oxide by mesenchymal stem cell transfer in lung injury/fibrosis Respir Res 1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC., and, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4:e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Qu J, Cao L, Sai Y, Chen C, He L.et al. (2008Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice J Pathol 214472–481. [DOI] [PubMed] [Google Scholar]
- Gupta N, Su X, Popov B, Lee JW, Serikov V., and, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- Xu J, Woods CR, Mora AL, Joodi R, Brigham KL, Iyer S.et al. (2007Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice Am J Physiol Lung Cell Mol Physiol 293L131–L141. [DOI] [PubMed] [Google Scholar]
- Fang X, Neyrinck AP, Matthay MA., and, Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem. 2010;285:26211–26222. doi: 10.1074/jbc.M110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Fang X, Gupta N, Serikov V., and, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA. 2009;106:16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K.et al. (2009Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production Nat Med 1542–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Anderson P, González MA, Rico L, Büscher D., and, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- Mei SH, Haitsma JJ, Dos Santos CC, Deng Y, Lai PF, Slutsky AS.et al. (2010Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis Am J Respir Crit Care Med 1821047–1057. [DOI] [PubMed] [Google Scholar]
- Yagi H, Soto-Gutierrez A, Kitagawa Y, Tilles AW, Tompkins RG., and, Yarmush ML. Bone marrow mesenchymal stromal cells attenuate organ injury induced by LPS and burn. Cell Transplant. 2010;19:823–830. doi: 10.3727/096368910X508942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi H, Soto-Gutierrez A, Navarro-Alvarez N, Nahmias Y, Goldwasser Y, Kitagawa Y.et al. (2010Reactive bone marrow stromal cells attenuate systemic inflammation via sTNFR1 Mol Ther 181857–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW.et al. (2010Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37 Stem Cells 282229–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning E, Pham S, Li S, Vazquez-Padron RI, Mathew J, Ruiz P.et al. (2010Interleukin-10 delivery via mesenchymal stem cells: a novel gene therapy approach to prevent lung ischemia-reperfusion injury Hum Gene Ther 21713–727. [DOI] [PubMed] [Google Scholar]
- Yang Z, Sharma AK, Marshall M, Kron IL., and, Laubach VE. NADPH oxidase in bone marrow-derived cells mediates pulmonary ischemia-reperfusion injury. Am J Respir Cell Mol Biol. 2009;40:375–381. doi: 10.1165/rcmb.2008-0300OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baber SR, Deng W, Master RG, Bunnell BA, Taylor BK, Murthy SN.et al. (2007Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction Am J Physiol Heart Circ Physiol 292H1120–H1128. [DOI] [PubMed] [Google Scholar]
- Umar S, de Visser YP, Steendijk P, Schutte CI, Laghmani el H, Wagenaar GT.et al. (2009Allogenic stem cell therapy improves right ventricular function by improving lung pathology in rats with pulmonary hypertension Am J Physiol Heart Circ Physiol 297H1606–H1616. [DOI] [PubMed] [Google Scholar]
- Kanki-Horimoto S, Horimoto H, Mieno S, Kishida K, Watanabe F, Furuya E.et al. (2006Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension Circulation 1141 SupplI181–I185. [DOI] [PubMed] [Google Scholar]
- van Haaften T, Byrne R, Bonnet S, Rochefort GY, Akabutu J, Bouchentouf M.et al. (2009Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats Am J Respir Crit Care Med 1801131–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA.et al. (2009Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease Am J Respir Crit Care Med 1801122–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YS, Oh W, Choi SJ, Sung DK, Kim SY, Choi EY.et al. (2009Human umbilical cord blood-derived mesenchymal stem cells attenuate hyperoxia-induced lung injury in neonatal rats Cell Transplant 18869–886. [DOI] [PubMed] [Google Scholar]
- Cho KS, Park HK, Park HY, Jung JS, Jeon SG, Kim YK.et al. (2009IFATS collection: Immunomodulatory effects of adipose tissue-derived stem cells in an allergic rhinitis mouse model Stem Cells 27259–265. [DOI] [PubMed] [Google Scholar]
- Park HK, Cho KS, Park HY, Shin DH, Kim YK, Jung JS.et al. (2010Adipose-derived stromal cells inhibit allergic airway inflammation in mice Stem Cells Dev 191811–1818. [DOI] [PubMed] [Google Scholar]
- Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Gorham JD.et al. (2010Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma Proc Natl Acad Sci USA 1075652–5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfield TL, Koloze M, Lennon DP, Zuchowski B, Yang SE., and, Caplan AI. Human mesenchymal stem cells suppress chronic airway inflammation in the murine ovalbumin asthma model. Am J Physiol Lung Cell Mol Physiol. 2010;299:L760–L770. doi: 10.1152/ajplung.00182.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin M, Sueblinvong V, Eisenhauer P, Ziats NP, LeClair L, Poynter ME.et al. (2011Bone marrow-derived mesenchymal stromal cells inhibit Th2-mediated allergic airways inflammation in mice Stem Cells 291137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang HJ, Cho KS, Park HY., and, Roh HJ. Adipose tissue-derived stem cells for cell therapy of airway allergic diseases in mouse. Acta Histochemica. 2011;113:501–507. doi: 10.1016/j.acthis.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Ishizawa K, Kubo H, Yamada M, Kobayashi S, Numasaki M, Ueda S.et al. (2004Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema FEBS Lett 556249–252. [DOI] [PubMed] [Google Scholar]
- Shigemura N, Okumura M, Mizuno S, Imanishi Y, Nakamura T., and, Sawa Y. Autologous transplantation of adipose tissue-derived stromal cells ameliorates pulmonary emphysema. Am J Transplant. 2006;6:2592–2600. doi: 10.1111/j.1600-6143.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- Shigemura N, Okumura M, Mizuno S, Imanishi Y, Matsuyama A, Shiono H.et al. (2006Lung tissue engineering technique with adipose stromal cells improves surgical outcome for pulmonary emphysema Am J Respir Crit Care Med 1741199–1205. [DOI] [PubMed] [Google Scholar]
- Yuhgetsu H, Ohno Y, Funaguchi N, Asai T, Sawada M, Takemura G.et al. (2006Beneficial effects of autologous bone marrow mononuclear cell transplantation against elastase-induced emphysema in rabbits Exp Lung Res 32413–426. [DOI] [PubMed] [Google Scholar]
- Adachi Y, Oyaizu H, Taketani S, Minamino K, Yamaguchi K, Shultz LD.et al. (2006Treatment and transfer of emphysema by a new bone marrow transplantation method from normal mice to Tsk mice and vice versa Stem Cells 242071–2077. [DOI] [PubMed] [Google Scholar]
- Zhen G, Liu H, Gu N, Zhang H, Xu Y., and, Zhang Z. Mesenchymal stem cells transplantation protects against rat pulmonary emphysema. Front Biosci. 2008;13:3415–3422. doi: 10.2741/2936. [DOI] [PubMed] [Google Scholar]
- Schweitzer KS, Johnstone BH, Garrison J, Rush NI, Cooper S, Traktuev DO.et al. (2011Adipose stem cell treatment in mice attenuates lung and systemic injury induced by cigarette smoking Am J Respir Crit Care Med 183215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsha AM, Ohkouchi S, Xin H, Kanehira M, Sun R, Nukiwa T.et al. (2011Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model Mol Ther 19196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen G, Xue Z, Zhao J, Gu N, Tang Z, Xu Y.et al. (2010Mesenchymal stem cell transplantation increases expression of vascular endothelial growth factor in papain-induced emphysematous lungs and inhibits apoptosis of lung cells Cytotherapy 12605–614. [DOI] [PubMed] [Google Scholar]
- < http://investor.osiris.com/releasedetail.cfm?ReleaseID=311598 >
- Grove DA, Xu J, Joodi R, Torres-Gonzales E, Neujahr D, Mora AL.et al. (2011Attenuation of early airway obstruction by mesenchymal stem cells in a murine model of heterotopic tracheal transplantation J Heart Lung Transplant 30341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Direkze NC, Hodivala-Dilke K, Jeffery R, Hunt T, Poulsom R, Oukrif D.et al. (2004Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts Cancer Res 648492–8495. [DOI] [PubMed] [Google Scholar]
- Hilbe W, Dirnhofer S, Oberwasserlechner F, Schmid T, Gunsilius E, Hilbe G.et al. (2004CD133 positive endothelial progenitor cells contribute to the tumour vasculature in non-small cell lung cancer J Clin Pathol 57965–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN.et al. (2004Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents J Natl Cancer Inst 961593–1603. [DOI] [PubMed] [Google Scholar]
- Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K., and, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- Arap W., and, Pasqualini R. Engineered embryonic endothelial progenitor cells as therapeutic Trojan horses. Cancer Cell. 2004;5:406–408. doi: 10.1016/s1535-6108(04)00121-7. [DOI] [PubMed] [Google Scholar]
- Loebinger MR., and, Janes SM. Stem cells as vectors for antitumour therapy. Thorax. 2010;65:362–369. doi: 10.1136/thx.2009.128025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN.et al. (2004Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst 961593–1603. [DOI] [PubMed]
- Wei J, Blum S, Unger M, Jarmy G, Lamparter M, Geishauser A.et al. (2004Embryonic endothelial progenitor cells armed with a suicide gene target hypoxic lung metastases after intravenous delivery Cancer Cell 5477–488. [DOI] [PubMed] [Google Scholar]
- Hall B, Dembinski J, Sasser AK, Studeny M, Andreeff M., and, Marini F. Mesenchymal stem cells in cancer: tumor-associated fibroblasts and cell-based delivery vehicles. Int J Hematol. 2007;86:8–16. doi: 10.1532/IJH97.06230. [DOI] [PubMed] [Google Scholar]
- Kanehira M, Xin H, Hoshino K, Maemondo M, Mizuguchi H, Hayakawa T.et al. (2007Targeted delivery of NK4 to multiple lung tumors by bone marrow-derived mesenchymal stem cells Cancer Gene Ther 14894–903. [DOI] [PubMed] [Google Scholar]
- Rachakatla RS, Marini F, Weiss ML, Tamura M., and, Troyer D. Development of human umbilical cord matrix stem cell-based gene therapy for experimental lung tumors. Cancer Gene Ther. 2007;14:828–835. doi: 10.1038/sj.cgt.7701077. [DOI] [PubMed] [Google Scholar]
- Stoff-Khalili MA, Rivera AA, Mathis JM, Banerjee NS, Moon AS, Hess A.et al. (2007Mesenchymal stem cells as a vehicle for targeted delivery of CRAds to lung metastases of breast carcinoma Breast Cancer Res Treat 105157–167. [DOI] [PubMed] [Google Scholar]
- Xin H, Kanehira M, Mizuguchi H, Hayakawa T, Kikuchi T, Nukiwa T.et al. (2007Targeted delivery of CX3CL1 to multiple lung tumors by mesenchymal stem cells Stem Cells 251618–1626. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhao P, Kennedy C, Chen K, Wiegand J, Washington G.et al. (2008Treatment of pulmonary metastatic tumors in mice using lentiviral vector-engineered stem cells Cancer Gene Ther 1573–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebinger MR, Kyrtatos PG, Turmaine M, Price AN, Pankhurst Q, Lythgoe MF.et al. (2009Magnetic resonance imaging of mesenchymal stem cells homing to pulmonary metastases using biocompatible magnetic nanoparticles Cancer Res 698862–8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebinger MR, Eddaoudi A, Davies D., and, Janes SM. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009;69:4134–4142. doi: 10.1158/0008-5472.CAN-08-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzuka T, Rachakatla RS, Doi C, Maurya DK, Ohta N, Kawabata A.et al. (2010Human umbilical cord matrix-derived stem cells expressing interferon-beta gene significantly attenuate bronchioloalveolar carcinoma xenografts in SCID mice Lung Cancer 7028–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ., and, Olson SD. Clinical trials with adult stem/progenitor cells for tissue repair: let's not overlook some essential precautions. Blood. 2007;109:3147–3151. doi: 10.1182/blood-2006-03-013433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameshwar P. Casting doubt on the safety of “off-the-shelf” mesenchymal stem cells for cell therapy. Mol Ther. 2009;17:216–218. doi: 10.1038/mt.2008.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ, Brenner M, Fibbe WE, Horwitz E, Le Blanc K, Phinney DG.et al. (2010Defining the risks of mesenchymal stromal cell therapy Cytotherapy 12576–578. [DOI] [PubMed] [Google Scholar]
- Mishra PJ, Mishra PJ, Glod JW., and, Banerjee D. Mesenchymal stem cells: flip side of the coin. Cancer Res. 2009;69:1255–1258. doi: 10.1158/0008-5472.CAN-08-3562. [DOI] [PubMed] [Google Scholar]
- Aguilar S, Nye E, Chan J, Loebinger M, Spencer-Dene B, Fisk N.et al. (2007Murine but not human mesenchymal stem cells generate osteosarcoma-like lesions in the lung Stem Cells 251586–1594. [DOI] [PubMed] [Google Scholar]
- Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S.et al. (2007Sarcoma derived from cultured mesenchymal stem cells Stem Cells 25371–379. [DOI] [PubMed] [Google Scholar]
- Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A.et al. (2007Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms Cancer Res 679142–9149. [DOI] [PubMed] [Google Scholar]
- Qin Y, Ji H, Wu Y., and, Liu H. Chromosomal instability of murine adipose tissue-derived mesenchymal stem cells in long-term culture and development of cloned embryos. Cloning Stem Cells. 2009;11:445–452. doi: 10.1089/clo.2009.0006. [DOI] [PubMed] [Google Scholar]
- Tarte K, Gaillard J, Lataillade JJ, Fouillard L, Becker M, Mossafa H, Société Française de Greffe de Moelle et Thérapie Cellulaire et al. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood. 2010;115:1549–1553. doi: 10.1182/blood-2009-05-219907. [DOI] [PubMed] [Google Scholar]
- Rubio D, Garcia S, Paz MF, De la Cueva T, Lopez-Fernandez LA, Lloyd AC.et al. (2008Molecular characterization of spontaneous mesenchymal stem cell transformation PLoS ONE 3e1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio D, Garcia-Castro J, Martín MC, de la Fuente R, Cigudosa JC, Lloyd AC.et al. (2005Spontaneous human adult stem cell transformation Cancer Res 653035–3039. [DOI] [PubMed] [Google Scholar]
- Røsland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H.et al. (2009Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation Cancer Res 695331–5339. [DOI] [PubMed] [Google Scholar]
- Torsvik A, Røsland GV, Svendsen A, Molven A, Immervoll H, McCormack E.et al. (2010Spontaneous malignant transformation of human mesenchymal stem cells reflects cross-contamination: putting the research field on track – letter Cancer Res 706393–6396. [DOI] [PubMed] [Google Scholar]
- Weiss DJ., and, Finck C. Embryonic stem cells and repair of lung injury. Mol Ther. 2010;18:460–461. doi: 10.1038/mt.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vranken BE, Romanska HM, Polak JM, Rippon HJ, Shannon JM., and, Bishop AE. Coculture of embryonic stem cells with pulmonary mesenchyme: a microenvironment that promotes differentiation of pulmonary epithelium. Tissue Eng. 2005;11:1177–1187. doi: 10.1089/ten.2005.11.1177. [DOI] [PubMed] [Google Scholar]
- Samadikuchaksaraei A., and, Bishop AE. Derivation and characterization of alveolar epithelial cells from murine embryonic stem cells in vitro. Methods Mol Biol. 2006;330:233–248. doi: 10.1385/1-59745-036-7:233. [DOI] [PubMed] [Google Scholar]
- Rippon HJ, Polak JM, Qin M., and, Bishop AE. Derivation of distal lung epithelial progenitors from murine embryonic stem cells using a novel three-step differentiation protocol. Stem Cells. 2006;24:1389–1398. doi: 10.1634/stemcells.2005-0465. [DOI] [PubMed] [Google Scholar]
- Denham M, Cole TJ., and, Mollard R. Embryonic stem cells form glandular structures and express surfactant protein C following culture with dissociated fetal respiratory tissue. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1210–L1215. doi: 10.1152/ajplung.00427.2005. [DOI] [PubMed] [Google Scholar]
- Samadikuchaksaraei A, Cohen S, Isaac K, Rippon HJ, Polak JM, Bielby RC.et al. (2006Derivation of distal airway epithelium from human embryonic stem cells Tissue Eng 12867–875. [DOI] [PubMed] [Google Scholar]
- Wang D, Haviland DL, Burns AR, Zsigmond E., and, Wetsel RA. A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:4449–4454. doi: 10.1073/pnas.0700052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vranken BE, Rippon HJ, Samadikuchaksaraei A, Trounson AO., and, Bishop AE. The differentiation of distal lung epithelium from embryonic stem cells. Curr Protoc Stem Cell Biol. 2007;Chapter 1:Unit 1G.1. doi: 10.1002/9780470151808.sc01g01s2. [DOI] [PubMed] [Google Scholar]
- Winkler ME, Mauritz C, Groos S, Kispert A, Menke S, Hoffmann A.et al. (2008Serum-free differentiation of murine embryonic stem cells into alveolar type II epithelial cells Cloning Stem Cells 1049–64. [DOI] [PubMed] [Google Scholar]
- Roszell B, Mondrinos MJ, Seaton A, Simons DM, Koutzaki SH, Fong GH.et al. (2009Efficient derivation of alveolar type II cells from embryonic stem cells for in vivo application Tissue Eng 153351–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coraux C, Nawrocki-Raby B, Hinnrasky J, Kileztky C, Gaillard D, Dani C.et al. (2005Embryonic stem cells generate airway epithelial tissue Am J Respir Cell Mol Biol 3287–92. [DOI] [PubMed] [Google Scholar]
- Van Haute L, De Block G, Liebaers I, Sermon K., and, De Rycke M. Generation of lung epithelial-like tissue from human embryonic stem cells. Respir Res. 2009;10:105. doi: 10.1186/1465-9921-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wong LB., and, Mao H. Induction of ciliated cells from avian embryonic stem cells using three-dimensional matrix. Tissue Eng Part C Methods. 2010;16:929–936. doi: 10.1089/ten.tec.2009.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Paananen R, Karjalainen MK, Tuohimaa A, Luukkonen A, Ojaniemi M.et al. (2009Genetic association of SP-C with duration of preterm premature rupture of fetal membranes and expression in gestational tissues Ann Med 41629–642. [DOI] [PubMed] [Google Scholar]
- Mason RJ., and, Williams MC. Phospholipid composition and ultrastructure of A549 cells and other cultured pulmonary epithelial cells of presumed type II cell origin. Biochim Biophys Acta. 1980;617:36–50. doi: 10.1016/0005-2760(80)90222-2. [DOI] [PubMed] [Google Scholar]
- Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K.et al. (2011Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro Nature 470105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Morales JE, Calame DG, Alcorn JL., and, Wetsel RA. Transplantation of human embryonic stem cell–derived alveolar epithelial type II cells abrogates acute lung injury in mice. Mol Ther. 2010;18:625–634. doi: 10.1038/mt.2009.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering SJ, Minger SL, Patel M, Taylor H, Black C, Burns CJ.et al. (2005Generation of a human embryonic stem cell line encoding the cystic fibrosis mutation deltaF508, using preimplantation genetic diagnosis Reprod Biomed Online 10390–397. [DOI] [PubMed] [Google Scholar]
- Mateizel I, De Temmerman N, Ullmann U, Cauffman G, Sermon K, Van de Velde H.et al. (2006Derivation of human embryonic stem cell lines from embryos obtained after IVF and after PGD for monogenic disorders Hum Reprod 21503–511. [DOI] [PubMed] [Google Scholar]
- Deleu S, Gonzalez-Merino E, Gaspard N, Nguyen TM, Vanderhaeghen P, Lagneaux L.et al. (2009Human cystic fibrosis embryonic stem cell lines derived on placental mesenchymal stromal cells Reprod Biomed Online 18704–716. [DOI] [PubMed] [Google Scholar]
- Sommer CA, Stadtfeld M, Murphy GJ, Hochedlinger K, Kotton DN., and, Mostoslavsky G. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells. 2009;27:543–549. doi: 10.1634/stemcells.2008-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers A, Jean JC, Sommer CA, Omari A, Ford CC, Mills JA.et al. (2010Generation of transgene-free lung disease-specific human induced pluripotent stem cells using a single excisable lentiviral stem cell cassette Stem Cells 281728–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisaki SM, Fuchs JR, Steigman SA., and, Fauza DO. A comparative analysis of cartilage engineered from different perinatal mesenchymal progenitor cells. Tissue Eng. 2007;13:2633–2644. doi: 10.1089/ten.2006.0407. [DOI] [PubMed] [Google Scholar]
- Kunisaki SM, Armant M, Kao GS, Stevenson K, Kim H., and, Fauza DO. Tissue engineering from human mesenchymal amniocytes: a prelude to clinical trials. J Pediatr Surg. 2007;42:974–979; discussion 979–980. doi: 10.1016/j.jpedsurg.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Kunisaki SM, Jennings RW., and, Fauza DO. Fetal cartilage engineering from amniotic mesenchymal progenitor cells. Stem Cells Dev. 2006;15:245–253. doi: 10.1089/scd.2006.15.245. [DOI] [PubMed] [Google Scholar]
- Kunisaki SM, Freedman DA., and, Fauza DO. Fetal tracheal reconstruction with cartilaginous grafts engineered from mesenchymal amniocytes. J Pediatr Surg. 2006;41:675–682; discussion 675–682. doi: 10.1016/j.jpedsurg.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Fuchs JR, Hannouche D, Terada S, Zand S, Vacanti JP., and, Fauza DO. Cartilage engineering from ovine umbilical cord blood mesenchymal progenitor cells. Stem Cells. 2005;23:958–964. doi: 10.1634/stemcells.2004-0310. [DOI] [PubMed] [Google Scholar]
- Kojima K, Ignotz RA, Kushibiki T, Tinsley KW, Tabata Y., and, Vacanti CA. Tissue-engineered trachea from sheep marrow stromal cells with transforming growth factor beta2 released from biodegradable microspheres in a nude rat recipient. J Thorac Cardiovasc Surg. 2004;128:147–153. doi: 10.1016/j.jtcvs.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Omori K, Nakamura T, Kanemaru S, Magrufov A, Yamashita M., and, Shimizu Y. In situ tissue engineering of the cricoid and trachea in a canine model. Ann Otol Rhinol Laryngol. 2008;117:609–613. doi: 10.1177/000348940811700811. [DOI] [PubMed] [Google Scholar]
- Omori K, Tada Y, Suzuki T, Nomoto Y, Matsuzuka T, Kobayashi K.et al. (2008Clinical application of in situ tissue engineering using a scaffolding technique for reconstruction of the larynx and trachea Ann Otol Rhinol Laryngol 117673–678. [DOI] [PubMed] [Google Scholar]
- Gilbert TW, Gilbert S, Madden M, Reynolds SD., and, Badylak SF. Morphologic assessment of extracellular matrix scaffolds for patch tracheoplasty in a canine model. Ann Thorac Surg. 2008;86:967–74; discussion 967. doi: 10.1016/j.athoracsur.2008.04.071. [DOI] [PubMed] [Google Scholar]
- Jungebluth P, Alici E, Baiguera S, Le Blanc K, Blomberg P, Bozóky B.et al. (2011Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: a proof-of-concept study Lancet 3781997–2004. [DOI] [PubMed] [Google Scholar]
- Kunisaki SM, Fuchs JR, Kaviani A, Oh JT, LaVan DA, Vacanti JP.et al. (2006Diaphragmatic repair through fetal tissue engineering: a comparison between mesenchymal amniocyte- and myoblast-based constructs J Pediatr Surg 4134–9; discussion 34. [DOI] [PubMed] [Google Scholar]
- Fuchs JR, Kaviani A, Oh JT, LaVan D, Udagawa T, Jennings RW.et al. (2004Diaphragmatic reconstruction with autologous tendon engineered from mesenchymal amniocytes J Pediatr Surg 39834–838; discussion 834–838. [DOI] [PubMed] [Google Scholar]
- Urita Y, Komuro H, Chen G, Shinya M, Saihara R., and, Kaneko M. Evaluation of diaphragmatic hernia repair using PLGA mesh-collagen sponge hybrid scaffold: an experimental study in a rat model. Pediatr Surg Int. 2008;24:1041–1045. doi: 10.1007/s00383-008-2212-y. [DOI] [PubMed] [Google Scholar]
- Cortiella J, Nichols JE, Kojima K, Bonassar LJ, Dargon P, Roy AK.et al. (2006Tissue-engineered lung: an in vivo and in vitro comparison of polyglycolic acid and pluronic F-127 hydrogel/somatic lung progenitor cell constructs to support tissue growth Tissue Eng 121213–1225. [DOI] [PubMed] [Google Scholar]
- Ingenito EP, Sen E, Tsai LW, Murthy S., and, Hoffman A. Design and testing of biological scaffolds for delivering reparative cells to target sites in the lung. J Tissue Eng Regen Med. 2010;4:259–272. doi: 10.1002/term.237. [DOI] [PubMed] [Google Scholar]
- Mondrinos MJ, Koutzaki S, Lelkes PI., and, Finck CM. A tissue-engineered model of fetal distal lung tissue. Am J Physiol Lung Cell Mol Physiol. 2007;293:L639–L650. doi: 10.1152/ajplung.00403.2006. [DOI] [PubMed] [Google Scholar]
- Mondrinos MJ, Koutzaki S, Jiwanmall E, Li M, Dechadarevian JP, Lelkes PI.et al. (2006Engineering three-dimensional pulmonary tissue constructs Tissue Eng 12717–728. [DOI] [PubMed] [Google Scholar]
- Lin YM, Boccaccini AR, Polak JM, Bishop AE., and, Maquet V. Biocompatibility of poly-DL-lactic acid (PDLLA) for lung tissue engineering. J Biomater Appl. 2006;21:109–118. doi: 10.1177/0885328206057952. [DOI] [PubMed] [Google Scholar]
- Choe MM, Sporn PH., and, Swartz MA. Extracellular matrix remodeling by dynamic strain in a three-dimensional tissue-engineered human airway wall model. Am J Respir Cell Mol Biol. 2006;35:306–313. doi: 10.1165/rcmb.2005-0443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Xu J, Souza P, Tanswell B, Tanswell AK., and, Post M. The effect of mechanical strain on fetal rat lung cell proliferation: comparison of two- and three-dimensional culture systems. In Vitro Cell Dev Biol Anim. 1995;31:858–866. doi: 10.1007/BF02634570. [DOI] [PubMed] [Google Scholar]
- Liu M, Tanswell AK., and, Post M. Mechanical force-induced signal transduction in lung cells. Am J Physiol. 1999;277 4 Pt 1:L667–L683. doi: 10.1152/ajplung.1999.277.4.L667. [DOI] [PubMed] [Google Scholar]
- Liu M., and, Post M. Invited review: mechanochemical signal transduction in the fetal lung. J Appl Physiol. 2000;89:2078–2084. doi: 10.1152/jappl.2000.89.5.2078. [DOI] [PubMed] [Google Scholar]
- Andrade CF, Wong AP, Waddell TK, Keshavjee S., and, Liu M. Cell-based tissue engineering for lung regeneration. Am J Physiol Lung Cell Mol Physiol. 2007;292:L510–L518. doi: 10.1152/ajplung.00175.2006. [DOI] [PubMed] [Google Scholar]
- Hoganson DM, Pryor HI 2nd., and, Vacanti JP. Tissue engineering and organ structure: a vascularized approach to liver and lung. Pediatr Res. 2008;63:520–526. doi: 10.1203/01.pdr.0000305879.38476.0c. [DOI] [PubMed] [Google Scholar]
- Mondrinos MJ, Koutzaki SH, Poblete HM, Crisanti MC, Lelkes PI., and, Finck CM. In vivo pulmonary tissue engineering: contribution of donor-derived endothelial cells to construct vascularization. Tissue Eng Part A. 2008;14:361–368. doi: 10.1089/tea.2007.0041. [DOI] [PubMed] [Google Scholar]
- Carterson AJ, Höner zu Bentrup K, Ott CM, Clarke MS, Pierson DL, Vanderburg CR.et al. (2005A549 lung epithelial cells grown as three-dimensional aggregates: alternative tissue culture model for Pseudomonas aeruginosa pathogenesis Infect Immun 731129–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbé A, De Boever P, Van Houdt R, Moors H, Mergeay M., and, Cornelis P. Use of the rotating wall vessel technology to study the effect of shear stress on growth behaviour of Pseudomonas aeruginosa PA01. Environ Microbiol. 2008;10:2098–2110. doi: 10.1111/j.1462-2920.2008.01631.x. [DOI] [PubMed] [Google Scholar]
- Rothen-Rutishauser BM, Kiama SG., and, Gehr P. A three-dimensional cellular model of the human respiratory tract to study the interaction with particles. Am J Respir Cell Mol Biol. 2005;32:281–289. doi: 10.1165/rcmb.2004-0187OC. [DOI] [PubMed] [Google Scholar]
- Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY., and, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panoskaltsis-Mortari A., and, Weiss DJ. Breathing new life into lung transplantation therapy. Mol Ther. 2010;18:1581–1583. doi: 10.1038/mt.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwebuga-Mukasa JS, Ingbar DH., and, Madri JA. Repopulation of a human alveolar matrix by adult rat type II pneumocytes in vitro. A novel system for type II pneumocyte culture. Exp Cell Res. 1986;162:423–435. doi: 10.1016/0014-4827(86)90347-2. [DOI] [PubMed] [Google Scholar]
- Antunes MB, Woodworth BA, Bhargave G, Xiong G, Aguilar JL, Ratner AJ.et al. (2007Murine nasal septa for respiratory epithelial air-liquid interface cultures BioTechniques 43195–196, 198, 200 passim. [DOI] [PubMed] [Google Scholar]
- Price AP, England KA, Matson AM, Blazar BR., and, Panoskaltsis-Mortari A. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng Part A. 2010;16:2581–2591. doi: 10.1089/ten.tea.2009.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortiella J, Niles J, Cantu A, Brettler A, Pham A, Vargas G.et al. (2010Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation Tissue Eng Part A 162565–2580. [DOI] [PubMed] [Google Scholar]
- Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB.et al. (2010Tissue-engineered lungs for in vivo implantation Science 329538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L.et al. (2010Regeneration and orthotopic transplantation of a bioartificial lung Nat Med 16927–933. [DOI] [PubMed] [Google Scholar]
- Daly AB, Wallis JM, Borg ZD, Bonvillain RW, Deng B, Ballif BA.et al. (2012Initial binding and re-cellularization of de-cellularized mouse lung scaffolds with bone marrow-derived mesenchymal stromal cells Tissue Eng Part A 181–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JM, Borg ZD, Daly AB, Deng B, Ballif BA, Allen GB.et al. (2012Comparative Assessment of Detergent-Based Protocols for Mouse Lung De-Cellularization and Re-Cellularization Tissue Eng Part C Methodsepub ahead of print). [DOI] [PMC free article] [PubMed]
- Song JJ, Kim SS, Liu Z, Madsen JC, Mathisen DJ, Vacanti JP.et al. (2011Enhanced in vivo function of bioartificial lungs in rats Ann Thorac Surg 92998–1005; discussion 1005–1006. [DOI] [PubMed] [Google Scholar]
- Randell SH. Airway epithelial stem cells and the pathophysiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:718–725. doi: 10.1513/pats.200605-117SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stripp BR, Maxson K, Mera R., and, Singh G. Plasticity of airway cell proliferation and gene expression after acute naphthalene injury. Am J Physiol. 1995;269 6 Pt 1:L791–L799. doi: 10.1152/ajplung.1995.269.6.L791. [DOI] [PubMed] [Google Scholar]
- Sueblinvong V., and, Weiss DJ. Stem cells and cell therapy approaches in lung biology and diseases. Transl Res. 2010;156:188–205. doi: 10.1016/j.trsl.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]