Abstract
Foamy virus (FV) vector systems have recently demonstrated their power as efficient gene transfer tools for different target tissues. Unfortunately, FVs cannot be naturally pseudotyped by heterologous viral glycoproteins due to an unusual particle morphogenesis involving a FV Env-dependent particle release process. Therefore, current FV vector systems are constrained to the broad host cell range provided by the cognate viral glycoprotein. We evaluated different approaches for pseudotyping of FV vectors, in which the specific FV Gag–Env interaction, essential for particle egress, is substituted by a small-molecule controlled heterodimerization (HD) system. In one system developed, one HD-domain (HDD) is fused to a membrane-targeting domain (MTD), such as the human immunodeficiency virus (HIV) Gag matrix (MA) subunit, with a second fused to the FV capsid protein. Coexpression of both components with different heterologous viral glycoproteins allowed an efficient, dimerizer-dependent pseudotyping of FV capsids. With this system FV vesicular stomatitis virus glycoprotein (VSV-G) pseudotype titers greater than 1 × 106 IU/ml were obtained, at levels comparable to authentic FV vector particles. As a proof-of-principle we demonstrate that Pac2 cells, naturally resistant to FV vectors, become permissive to FV VSV-G pseudotypes. Similar to other retroviral vectors, this FV pseudotyping system now enables adaptation of cell-specific targeting approaches for FVs.
Introduction
The natural exchange of compatible viral structural proteins in host cells infected with two different viruses is frequently observed. As a result, progeny viruses with altered features in comparison to the parental viruses are generated. This component exchange includes the viral glycoprotein, which, via its binding to specific viral entry receptors is one of the predominant determinants for target cell tropism of membrane-enveloped viruses including retroviruses. Viral vector systems exploit this phenomenon by incorporating heterologous viral glycoproteins into recombinant vector particles, a process called pseudotyping. Pseudotyping is used to specifically alter the host range, increase particle stability, and/or enhance the gene transfer efficiency into specific target tissues.1 In the human immunodeficiency virus (HIV) vector systems for example, the authentic HIV Env protein is not commonly used, because it would restrict transduction to CD4+ target cells. Instead, different types of heterologous glycoproteins are employed. Heterologous Env proteins, such as vesicular stomatitis virus glycoprotein (VSV-G), the feline endogenous retrovirus RD114 envelope protein, amphotropic murine leukemia virus Env and the gibbon ape leukemia virus (GALV) envelope protein all confer a broad host range and are frequently used for transduction of cells from the human hematopoietic system. A more restricted tropism is achieved by pseudotyping with other viral glycoproteins such as the Mokola Env for astrocytes,2 lymphochoriomenigitis virus (LCMV) Env for glioma cells,3 or rabies virus Env for neuronal cells and enabling axonal transport in vivo.4 Furthermore, retrovirus vector particles pseudotyped with measles virus glycoproteins of wild-type or vaccine strains have recently become popular.5 In particular, variants that have blinded natural receptor binding domains and are retargeted to specific cell surface receptors are promising tools for cell-specific gene transfer.
Spuma or foamy viruses (FV) are a special kind of retrovirus that, while showing homology to hepadnaviruses such as hepatitis B, have adopted a replication strategy deviating in many ways from those of all other viruses.6 Several of these features, including a very broad host range, a particle-associated infectious viral DNA genome as well as efficient transduction of hematopoietic stem cells have made them an interesting candidate as a gene transfer tool for therapeutic treatments of different diseases.7
FV particle morphogenesis is characterized by the requirement of coexpression of FV capsid and glycoprotein for particle egress, which is unique amongst retroviruses. The FV Gag protein lacks a membrane-targeting signal and unlike other retroviruses is therefore unable to promote virus-like particle release when expressed alone.8 A very specific interaction of FV Gag and the Env protein, involving domains in the capsid N-terminus and the glycoprotein cytoplasmic leader peptide domain, is essential for budding of FV virions.9,10 This function of the FV glycoprotein for particle release cannot be complemented by any other heterologous viral glycoproteins examined.11 As a result, current generations of FV vectors lack the capacity to be pseudotyped by glycoproteins of other viruses. Approaches involving transfer of FV Gag–Env interaction domains to heterologous viral proteins have so far proved unsuccessful in generating functional vector pseudotypes (data not shown).
Here, we describe two approaches for the functional replacement of the natural prototype FV (PFV) Gag–Env protein interaction, essential for capsid egress, by small-molecule-controlled, nonviral heterodimerization domains (HDD). In our first approach, one HDD is fused to the PFV capsid protein, and a second is fused to the cytoplasmic domain of the heterologous viral glycoprotein. The second approach involves the same HDD-PFV capsid fusion protein as the first approach. However, it also includes a heterologous membrane-targeting domain (MTD), such as the matrix (MA) subunit of the HIV-1 Gag protein, fused to a second HDD domain and coexpression of authentic heterologous viral glycoproteins. Both approaches allow small-molecule-dependent pseudotyping of PFV vector particles, with the second resulting in production of vector titers at a comparable level to those obtained with authentic FV vector components.
Results
Functional replacement of the natural PFV Gag–Env interaction by heterologous HDD
PFV capsid membrane-targeting and particle release is dependent on a very specific interaction of PFV Gag with the cognate viral glycoprotein involving a highly conserved WxxW motive (W10,13A) in the envelope leader peptide (LP) subunit.9 We examined two different strategies to replace the natural Gag–Env interaction with a small-molecule-regulated protein-protein HD system (HDS, Argent Regulated Heterodimerization Kit; Ariad, Cambridge, MA) to allow production of infectious PFV vector particles pseudotyped by heterologous viral glycoproteins (Figure 1).
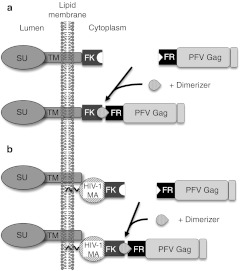
Figure 1.
Strategies for HDD-mediated pseudotyping of FV vectors. Schematic illustration of the strategies employed to pseudotype FV vectors with heterologous viral glycoproteins. (a) Replacement of the natural PFV Gag-Env interactions by fusing small-molecule-controlled, heterologous HDD to the PFV Gag protein and cytoplasmic domain of heterologous glycoproteins. (b) Small-molecule-controlled PFV Gag membrane association by fusing heterologous HDD to PFV Gag and heterologous MTD. FV, foamy virus; HDD, heterodimerization domain; MTD, membrane-targeting domain; PFV, prototype foamy virus.
For the first approach (Figure 1a) we explored the possibility of functionally replacing the authentic Gag–Env interaction with a small-molecule-controlled, nonviral protein-protein HDS. For this purpose various PFV Gag and Env packaging constructs were generated with their FK or FR HDD genetically fused to the N- or C-terminus of either PFV Gag or PFV Env (wt and W10,13A mutant) (Figure 2a). Subsequently, corresponding PFV Gag- and PFV Env-HDD packaging constructs in different combinations and in context of a four-component PFV vector system were cotransfected into 293T packaging cells in the presence or absence of dimerizer. Harvested cell culture supernatants were analyzed for their ability to support generation of infectious PFV vector particles in the presence of dimerizer using a flow cytometric enhanced green fluorescent protein (EGFP) marker gene transfer assay (Figure 2b, Supplementary Figure S1, Supplementary Results).
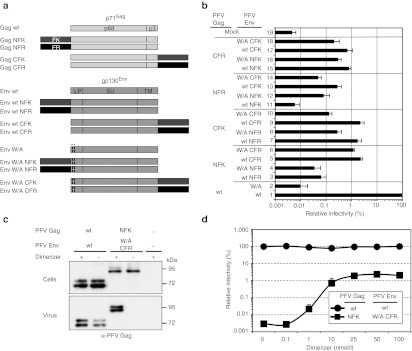
Figure 2.
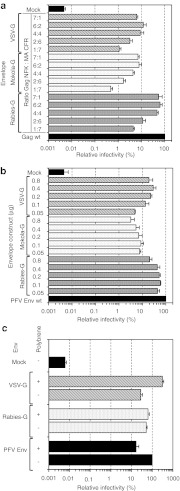
Functional replacement of the essential natural PFV Gag–Env interaction. (a) Schematic outline of the PFV Gag and PFV Env packaging constructs used. (b–d) 293T cells were cotransfected with different Gag and Env packaging constructs in context of a four-component replication-deficient PFV vector system as indicated. Subsequently infectivity of supernatants was determined on HT1080 target cells using a flow cytometric EGFP transfer assay or protein composition of pelleted particles was analyzed by western blot. (b) Relative infectivity (n = 3) of recombinant vector particles generated with different PFV Gag and PFV Env packaging constructs using 10 nmol/l dimerizer. Absolute titers of plain wt supernatants set to 100% were 1.8 ± 0.75 × 106 EGFP ffu/ml. (c) Western blot analysis of small-molecule-dependent physical particle release by HDD-tagged, interaction-deficient PFV Env W10,13A (W/A) using a polyclonal antiserum specific for PFV Gag (α-PFV Gag). (+) 10 nmol/l dimerizer; (−) 0 nmol/l dimerizer. (d) Dimerizer dose-dependency of infectious PFV vector particle production. Absolute titers of plain wt supernatants set to 100% (n = 3) were 4.4 ± 2.2 × 106 EGFP ffu/ml. EGFP, enhanced green fluorescent protein; HDD, heterodimerization domain; ORF, open reading frame; PFV, prototype foamy virus **, W/A mutation in the Env ORF.
The results revealed that most combinations yielded infectious particles, although viral titers varied strongly. For combinations with HDD-tagged interaction-deficient PFV Env W/A packaging constructs, production of infectious particles was strictly dimerizer-dependent (data not shown). The highest titers were obtained when the interaction-deficient PFV Env W/A protein was C-terminally tagged with an FR (CFR) domain (Env W/A CFR) and combined with an N-terminal FK (NFK)-domain-tagged Gag (Gag NFK) packaging construct (Figure 2b, bar 6). Vector titers were about 50-fold lower in comparison to wild-type particles but only twofold lower in comparison to particles composed of Gag NFK and CFR-tagged natural interaction proficient wild-type Env (Figure 2b, compare bar 6 to bar 1, 5). The recovery of physical particle release by using HDD-tagged PFV Gag and PFV Env W/A packaging constructs was strictly dimerizer-dependent (Figure 2c). Western blot analysis of viral particles purified from cell culture supernatants demonstrated that in the presence of the dimerizer, virions containing Env W/A were released at levels comparable to the wild-type. Titration of the dimerizer concentration during vector production showed that viral titers plateau at a final concentration of 25 nmol/l (Figure 2d).
Taken together the data demonstrate that the natural PFV Gag–Env interaction can be functionally replaced by small-molecule-controlled nonviral HDDs.
Pseudotyping of PFV particles by VSV-G using HDD-tagged glycoprotein and capsid packaging constructs
Next, the successful replacement strategy for the essential natural PFV Gag–Env interaction by heterologous HDDs was utilized for VSV-G incorporation into PFV vector particles. HDD-tagged VSV-G packaging constructs, analogous to the PFV Env packaging constructs described above, were generated (Figure 3a). Different combinations of HDD-tagged VSV-G and PFV Gag packaging constructs were then examined with respect to their capacity to support production of infectious pseudotyped PFV vector particles in the presence of dimerizer (Figure 3b). VSV-G proteins with N-terminal HDD fusion (VSV-G NFK and VSV-G NFR) were unable to support production of infectious pseudotyped PFV vector particles, most probably due to cotranslational cleavage of the VSV-G signal peptide, removing the HDD from the viral glycoprotein (Figure 3b, data not shown). In contrast, all VSV-G proteins with C-terminal fusion (VSV-G CFK and VSV-G CFR) yielded infectious pseudotyped PFV particles. Coexpression of PFV Gag CFK together with VSV-G CFR gave rise to the highest titers, although only minor differences to the other combinations were observed (Figure 3b). Again, the physical release of HDD-tagged PFV capsids supported by the C-terminal HDD-tagged VSV-G glycoproteins was dimerizer-dependent (Figure 3c). Similar to the results with HDD-tagged PFV Env proteins, the dimerizer concentration yielding the highest vector titers was around 25 nmol/l (Figure 3d). Although infectious VSV-G pseudotyped PFV particles could be generated by this approach, the pseudotype titers were still about 1,000-fold below those obtained with standard PFV vector particles using the authentic PFV Env protein (Figure 3b,d). Attempts to improve vector titers by optimizing the ratios of the transfected packaging components were unsuccessful (data not shown). This might point to functional restrictions of the HDD-tagged VSV-G proteins.
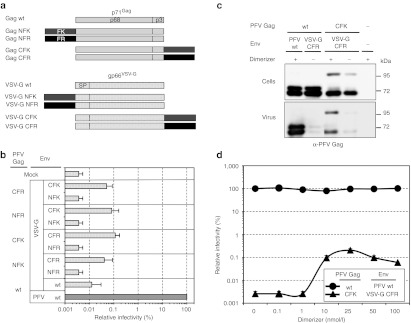
Figure 3.
Pseudotyping of PFV capsids using HDD-tagged PFV Gag and VSV-G. (a) Schematic outline of the PFV Gag and VSV-G packaging constructs used. (b–d) 293T cells were cotransfected with different Gag and Env packaging constructs in context of a four-component replication-deficient PFV vector system as indicated. Subsequently, infectivity of plain supernatants was determined on HT1080 target cells using a flow cytometric EGFP transfer assay, or the protein composition of pelleted particles was analyzed by western blot. (b) Relative infectivity (n = 3) of recombinant vector particles generated with different PFV Gag and glycoprotein packaging constructs using 10 nmol/l dimerizer. Absolute titers of plain wt supernatants set to 100% were 3.1 ± 1.3 × 106 EGFP ffu/ml. (c) Western blot analysis of small-molecule-dependent physical particle release of HDD-tagged VSV-G using a polyclonal antiserum specific for PFV Gag (α-PFV Gag). (+) 10 nmol/l dimerizer; (−) 0 nmol/l dimerizer. (d) Dimerizer dose-dependency of infectious VSV-G pseudotyped PFV vector particle production. Absolute titers of plain wt supernatants set to 100% (n = 3) were 4.4 ± 2.2 × 106 EGFP ffu/ml. EGFP, enhanced green fluorescent protein; HDD, heterodimerization domain; PFV, prototype foamy virus; VSV-G, vesicular stomatitis virus glycoprotein.
These data demonstrate that functional pseudotyping of PFV particles is possible using HDD-tagged capsid and glycoprotein packaging constructs. However, infectivity of such vector particles is greatly diminished in comparison to authentic PFV vector particles.
Pseudotyping of PFV vector particles with native heterologous viral glycoproteins by inducible PFV capsid membrane-targeting
HDD-tagging of heterologous glycoproteins as demonstrated above for VSV-G allows pseudotyping of PFV vectors but appears to interfere with full biological glycoprotein function. We therefore sought to develop an alternative approach for PFV vector pseudotyping that provides a glycoprotein-independent, regulable membrane-targeting of PFV capsids using the Argent HDS. In this approach (Figure 1b), one HDD was genetically fused to a MTD (e.g., HIV-1 Gag MA (H1G-MA) subunit, c-src myristoylation signal (Src-M]) with the other HDD located on the PFV Gag protein (as described above) (Figure 4a). Coexpression of NFK-tagged PFV Gag (Gag NFK) and CFR-tagged HIV-1 Gag MA (H1G-MA CFR) in the absence of any viral glycoprotein coexpression resulted in a physical PFV capsid release into the cell culture supernatant that was strictly dimerizer-dependent (Figure 4b–e, lane 1, 2). This indicated that HDD-tagged PFV Gag and a HDD-tagged MTD (e.g., H1G-MA) can interact in a dimerizer-dependent manner enabling a PFV Env-independent release of PFV virus-like particles (VLPs). As expected, these “bald” PFV VLPs were non-infectious (Figure 4e). In contrast, upon cotransfection of the untagged wild-type PFV Env protein (wt) expression construct with the HDD-tagged PFV Gag and MTD packaging vectors, a dimerizer-independent particle release of infectious particles was observed. This demonstrated that the natural PFV Gag–Env interaction is not disturbed by coexpression of an HDD-MTD and its dimerizer-dependent interaction with the HDD-tagged PFV capsid protein (Figure 4b–e, lane 3, 4). The reconstitution of infectious PFV particles upon coexpression of the untagged, naturally interaction-deficient PFV Env W/A mutant was most interesting (Figure 4b–e, lane 5, 6). Physical release of this type of infectious particles was strictly dimerizer-dependent suggesting that PFV Env located in the plasma membrane, which is devoid of any natural capsid interaction capacity, can be functionally incorporated into PFV particles that bud in a PFV Env-independent but MTD-dependent manner. The infectivity of these PFV Env W/A mutant particles was similar to those containing wild-type PFV Env (Figure 4e). PFV Env W/A protein that was detected in viral particle preparations in the absence of dimerizer (Figure 4c, lane 5) might be derived from capsidless, subviral PFV particles containing only the glycoprotein. Alternatively MTD expression alone might be sufficient to induce a release of particulate structures that can incorporate glycoprotein, even in the absence of a MTD—PFV Gag interaction.
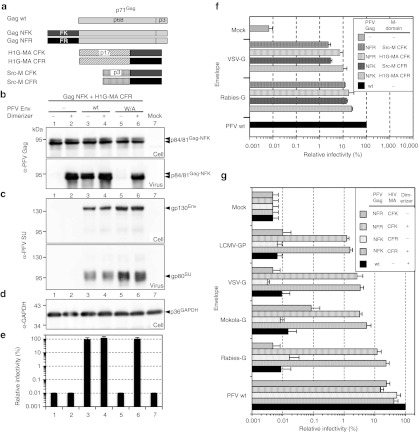
Figure 4.
Small-molecule regulated PFV pseudotyping using separate HDD-tagged, PFV Gag and MTD packaging constructs. (a) Schematic outline of the PFV Gag as well as the HIV-1 Gag MA (H1G-MA) and Src myristoylation signal (Src-M) MTD packaging constructs used. (b–g) 293T cells were cotransfected with different PFV Gag, H1G-MA or Src-M and Env packaging constructs in context of a five-component replication-deficient PFV vector system as indicated. (b-d) Western blot analysis of 293T cell lysates (cell) and viral particles (virus) purified by ultracentrifugation through 20% sucrose of NFK-tagged PFV Gag and CFR-tagged HIV-1 Gag MA in the absence (−) or presence (+) of 25 nmol/l dimerizer. Proteins were detected using (b) an anti-PFV Gag (α-PFV Gag) antiserum or (c) an anti-PFV Env SU (α-PFV SU) specific monoclonal antibody, or (d) an anti-GAPDH monoclonal antibody (α-GAPDH). (e) Relative infectivity (n = 3) of the corresponding plain supernatants determined on HT1080 target cells using a flow cytometric EGFP transfer assay. Absolute titers of plain wt supernatants (lane 3) set to 100% were 0.68 ± 0.18 × 106 EGFP ffu/ml. (f) Comparison of the pseudotyping efficiency (n = 3) using HDD-tagged H1G-MA or Src-M MTD. MTD and PFV Gag packaging constructs were cotransfected at a 1:1 ratio (wt/wt) in combination with the other components of the PFV vector system as indicated using 25 nmol/l dimerizer. Absolute titers of plain wt supernatants set to 100% were 1.3 ± 0.68 × 106 EGFP ffu/ml. (g) Relative infectivity (n = 4) of recombinant vector particles generated with different HDD-tagged PFV Gag and H1G-MA packaging constructs (1:1 ratio) and various Env expression vectors in the absence (−) or presence (+) of 25 nmol/l dimerizer, as indicated. Absolute titers of plain wt supernatants set to 100% were 1.9 ± 0.57 × 106 EGFP ffu/ml. EGFP, enhanced green fluorescent protein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HDD, heterodimerization domain; LCMV, lymphochoriomenigitis virus; MTD, membrane-targeting domain; PFV, prototype foamy virus; VSV-G, vesicular stomatitis virus glycoprotein.
These results then set the stage for examining the potential of this small-molecule-dependent PFV capsid membrane-targeting and release system to enable the generation of infectious PFV vectors pseudotyped with heterologous viral glycoproteins. For these experiments a set of authentic heterologous viral glycoproteins was used (Figure 4f,g). First, we compared the potential of two different HDD-tagged MTDs, the HIV-1 Gag MA subunit (H1G-MA) and the c-Src myristoylation signal (Src-M), to enable pseudotyping by the glycoproteins of VSV and rabies virus (Figure 4f). The analysis revealed that the combination of NFK-tagged PFV Gag and CFR-tagged MTD was always slightly better than the reciprocally tagged constructs. Furthermore, membrane-targeting by the HDD-tagged HIV-1 Gag MA subunit (H1G-MA) yielded superior titers compared to Src-M-mediated membrane-targeting. Therefore an extended pseudotyping analysis using several other glycoproteins was performed focusing on H1G-MA in combination with HDD-tagged PFV Gag (Figure 4g). Both HDD-tagged packaging constructs, used at a 1:1 weight ratio, were able to support the production of a variety of differently pseudotyped vector particles (Figure 4g). All envelope proteins evaluated (VSV, rabies, mokola, and LCMV) gave rise to infectious pseudotyped PFV particles in a dimerizer-dependent manner with titers that were only five to 100-fold below those of standard PFV vectors (Figure 4g). The combination of NFK-tagged PFV Gag and CFR-tagged H1G-MA resulted in slightly higher titers as compared to the reverse combination and was therefore used for all following evaluations.
VSV-G expression alone is known to result in subviral particle production that can be used to generate infectious vector particles by a “fusion from without” mechanism when combined in vitro with Env-less retroviral VLPs.12 Examining the contribution of this mechanism to PFV-VSV-G pseudotype infectivity we found that it accounts for up to 5‰ of the viral titers obtained (Supplementary Figure S2). Interestingly, although also derived from a rhabdovirus, no “fusion from without” activity could be detected for Rabies-G and efficiently pseudotyping HD PFV vectors (Supplementary Figure S2).
Improvement of viral titers and transduction efficiencies require individual optimization of vector production and infection conditions
The viral glycoproteins of rabies, mokola and VSV-G, displaying the highest pseudotype titers in the initial analysis, were chosen to further optimize the system. First, different ratios of HDD-tagged PFV Gag and H1G-MA packaging constructs were evaluated (Figure 5a). A 3:1 ratio of NFK-tagged PFV Gag to CFR-tagged MTD packaging construct proved to be the best for obtaining the highest pseudotype titers for these heterologous Env proteins. Next, the amount of heterologous glycoprotein expression construct was varied using this optimized 3:1 ratio of HDD-tagged PFV Gag and H1G-MA packaging construct (Figure 5b). Here, differences between the individual glycoprotein expression constructs were observed. Rabies and mokola glycoprotein packaging constructs yielded the highest titers when transfected at 0.1 µg, the amount used in all previous examinations, into 293T cells. Interestingly, VSV-G pseudotype titers were the highest at 0.4 µg glycoprotein packaging construct.
Figure 5.
Optimization of HDD-controlled pseudotyping of PFV vector particles. 293T cells were cotransfected with different PFV Gag, H1G-MA and Env packaging constructs in context of a five-component replication-deficient PFV vector system as indicated. Subsequently, infectivity of supernatants was determined on HT1080 target cells using a flow cytometric EGFP transfer assay. (a) Relative infectivity (n = 3) of recombinant vector particles generated by cotransfection of HDD-tagged PFV Gag-NFK and H1G-MA-CFR packaging constructs at different ratios and various Env expression vectors as indicated using 25 nmol/l dimerizer. Absolute titers of plain Gag wt supernatants set to 100% were 1.6 ± 0.3 × 106 EGFP ffu/ml. (b) Relative infectivity (n = 3) of recombinant vector particles generated with HDD-tagged PFV Gag-NFK and H1G-MA-CFR packaging constructs (3:1 ratio) and different amounts of various Env expression vectors as indicated using 25 nmol/l dimerizer. Absolute titers of plain PFV Env wt supernatants set to 100% were 2.2 ± 1.6 × 106 EGFP ffu/ml. (c) Relative infectivity (n = 3) of PFV vectors generated with HDD-tagged PFV Gag-NFK and H1G-MA-CFR packaging constructs (3:1 ratio) and pseudotyped with various heterologous glycoproteins in the absence (−) and presence (+) of polybrene at 8 µg/ml. Absolute titers of plain PFV Env supernatants in the absence of polybrene set to 100% were 1.2 ± 0.19 × 106 EGFP ffu/ml. EGFP, enhanced green fluorescent protein; HDD, heterodimerization domain; PFV, prototype foamy virus; VSV-G, vesicular stomatitis virus glycoprotein.
It is known that cationic polymers like polybrene can increase retrovirus infectivity.13 Therefore we examined whether we could observe such a stimulating effect on the infectivity of PFV pseudotypes as well (Figure 5c). Although polybrene addition led to a fivefold decrease of viral infectivity of authentic PFV vector particles, a tenfold increase was observed for VSV-G pseudotypes. Compared to wt PFV Env (without polybrene) the VSV-G pseudotyped PFV particles yielded threefold higher infectious titers in the presence of polybrene. No major influence of polycation addition on rabies-G pseudotyped particle infectivity was detectable. This demonstrates that the influence of cationic polymer infectivity enhancement is primarily determined by the glycoprotein incorporated into the vector particle.
Modification of target cell tropism by FV vector pseudotyping
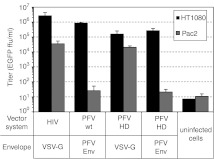
FVs naturally display an extremely broad host range.14 Only recently were cell lines identified which appear to be non-permissive to authentic PFV vectors and lentiviral vectors pseudotyped with PFV Env, such as Pac2 embryonic fibroblasts of the zebrafish.15 As a proof-of-principle demonstrating that pseudotyping of PFV vectors can alter the host range, PFV VSV-G pseudotype infectivity was examined on Pac2 cells. The results summarized in Figure 6 show that Pac2 cells were largely resistant to authentic PFV vectors and HDS-derived PFV particles containing the PFV Env, whereas the same supernatants efficiently transduced HT1080 cells. In contrast, HDS-derived PFV VSV-G as well as HIV-1 VSV-G pseudotype supernatants transduced Pac2 cells at similar high levels.
Figure 6.
Susceptibility of zebrafish Pac2 cells towards transduction by different retroviral vector pseudotypes. HIV-1 and PFV vector pseudotypes were produced by transient transfection of 293T cells using a three-component or a codon-optimized four- to five-component vector system using different envelope expression constructs as indicated. Subsequently, infectivity of supernatants was determined on HT1080 and Pac2 target cells using 1 ml of 10× concentrated virus stocks and tenfold dilution thereof in a flow cytometric EGFP transfer assay. Absolute titers (n = 2) of the plain vector supernatants on the different target cells are shown. EGFP, enhanced green fluorescent protein; HD, heterodimerization; PFV, prototype foamy virus; VSV-G, vesicular stomatitis virus glycoprotein.
These results demonstrate that PFV vector pseudotyping allows modification of target cell tropism. Furthermore, they strongly suggest that resistance of Pac2 cells towards authentic PFV vectors is not the result of restrictions in post-fusion entry steps.
Discussion
A major difference between FVs and other retroviruses is a very specific Gag–Env protein interaction, which is crucial during the particle release process due to the absence of a MTD within authentic FV Gag proteins.8 As a result, unlike other retroviral vectors, FV Gag does not support VLP release and FV vectors cannot be naturally pseudotyped by heterologous viral glycoproteins.11 This constraint currently limits the target cell host range of FV vectors to that provided by the cognate glycoprotein. Fortunately, the FV Env-mediated tropism is extremely broad, and several therapeutically relevant target tissues, such as hematopoietic or mesenchymal stem cells, can be efficiently transduced by FV vectors.7 However, other target cell types, for example of lymphoid lineage, are only poorly permissive (data not shown). Due to the lack of a pseudotyping feature of current FV vector systems, enhancement of naturally poorly permissive target cell populations, by using more favorable heterologous glycoproteins, or even a more selective targeting of specific target cell types, is not easily possible. Enabling such features to FV vectors requires either a genetic modification of the FV glycoprotein or the development of a FV pseudotyping system.
Here we describe a highly efficient FV pseudotyping system. We demonstrate the functional replacement of the natural Gag–Env interaction, responsible and essential for FV particle egress, by nonviral protein interaction modules. First, we show that fusing small-molecule-regulable HDDs to both PFV Gag and a naturally interaction-deficient PFV Env mutant restores physical particle egress to nearly wild-type levels in a dimerizer-dependent manner. Furthermore, released vector particles generated by this system that contain wild-type or interaction-deficient Env proteins show similar infectivities, although their infectivity was about 100-fold lower in comparison to authentic PFV vectors lacking HDD-tags, indicating some negative effects on their functionality. Adapting this approach to VSV-G, a naturally non-interacting heterologous viral glycoprotein, shows that HDDs can be generally applied to pseudotype PFV capsids. However, vector titers obtained were several orders of magnitude lower in comparison to authentic PFV vectors or lentiviral VSV-G pseudotypes. Different approaches, such as variation of the relative amounts of vector system components used, were unable to further improve the yield of infectious PFV pseudotypes. Potential reasons for reduced vector infectivity of the FV VSV-G pseudotypes include inhibitory effects of the fused HDD on heterologous glycoprotein function. This could either be the result of induced structural changes that interfere with the glycoprotein fusion activity or an altered intracellular trafficking leading to decreased particle incorporation. In line with this, titers of lentiviral vectors pseudotyped with the HDD-VSV-G fusion proteins were significantly lower in comparison to particles harboring the wild-type VSV-G protein (data not shown). Alternatively, intracellular trafficking and localization of PFV capsids might not be fully compatible thereby preventing an efficient dimerizer-dependent interaction between both components. A limitation of this approach is also the requirement to generate separate HDD fusion constructs for every glycoprotein that will be used for FV vector pseudotyping. Every new HDD-Env fusion protein might behave differently with respect to its alterations in cellular expression level, intracellular distribution, and function of the glycoprotein in comparison to the respective parental Env protein. Thus a general application of this approach to a broad variety of different heterologous envelope proteins might not be easily accomplished.
As a consequence we devised a different strategy that potentially allows the use of unmodified heterologous glycoproteins, which have been previously demonstrated to pseudotype other retroviral vectors efficiently. We made use of the observation that FV Env-independent capsid release can be induced by genetic fusion of heterologous MTDs to FV Gag.16,17,18 However, all previous MTD-Gag fusion proteins yielded only non-infectious particles, which was at least in part a result of an aberrant capsid assembly.17 Therefore, our approach was based on a different association of heterologous MTDs to FV Gag using the Argent HDS and thereby minimizing the potential interference with FV capsid morphogenesis. Indeed, this strategy, utilizing an HDD-tagged HIV-1 MA or Src myristoylation signal as MTD for an HDD-tagged PFV Gag proved to be extremely successful. Our results imply that permanent membrane anchorage of FV Gag by genetic fusion of MTDs to the proteins' N-terminus interferes with biological function.16,17,18 This can be circumvented by a transient MTD association, as accomplished by the HD approach applied, and still be compatible with biological activity. This strongly suggests that a free FV Gag N-terminus is essential for biological function. Since the FV Gag protein, unlike other retroviral capsid proteins, displays only a very limited proteolytic processing, stable membrane anchorage interferes with disassembly processes required for productive infection.
The FV HD vector system developed allows for the first time the production of high titer PFV pseudotype supernatants with different unmodified heterologous viral glycoproteins. This possibility will now foster a side by side comparison of the gene transfer potential of FV vectors with lenti- or oncoretroviral vectors into different target tissues using the same mode of host cell entry. Furthermore, it will allow improved transduction efficiencies of target cell tissues that are poorly permissive to FV Env-mediated gene transfer. Finally, the FV HD vector system should pave the way for selective modification of the host range of FV vectors in the near future allowing, for example, the development of specific gene therapeutic approaches by FV vectors. Preliminary experiments suggest that the FV HD vector system can also be used to pseudotype FV particles with measles virus glycoproteins (data not shown). The recently developed powerful cell-specific targeting technology for other retroviral vectors based on measles virus glycoproteins should therefore become available for FV vectors as well by using the FV HD vector system. Another attractive feature of the presented system is its modular structure, in particular the possibility to control pseudotyping by a single dimerizer reagent and to utilize different MTDs for FV Gag membrane association. Both MTDs examined in this study demonstrated a high potential to support PFV vector pseudotyping by different heterologous glycoproteins. However, the use of PFV capsids and alternative MTDs targeting vector assembly to specific membrane subdomains or cellular organelles might give rise to improved viral titers for specific viral glycoproteins or enhanced incorporation of other membrane proteins or targeting molecules into FV particles.
The FV HD vector system packaging components might also be used to develop stable FV packaging cell lines. However, the successful generation of stable packaging cell lines probably depends strongly on the potential cytotoxicity of individual packaging components, such as the viral glycoprotein used (i.e., VSV-G), when expressed constitutively. In case of authentic FV vector systems not the constitutive expression of the glycoprotein but of the viral capsid protein appears to be toxic to the packaging cells (data not shown).
Taken together with the described FV HD vector system, a major limitation of FV-mediated gene transfer in comparison with other viral vector systems is compensated and effective pseudotyping with various heterologous glycoproteins offers an important extension to the applications of FV vectors in the future.
Materials and Methods
Cell lines and culture conditions. The human kidney cell line 293T19 and the human fibrosarcoma cell line HT108020 were cultivated at 37 °C and 5% CO2 in Dulbecco's modified Eagle medium supplemented with 10% heat-inactivated fetal calf serum and antibiotics. The zebrafish embryonic fibroblast cell line Pac2 was cultivated at 28 °C in CO2 independent in Leibovitz's L-15 medium with 20% heat-inactivated fetal calf serum and antibiotics.21
Recombinant DNAs and plasmid expression constructs. The expression-optimized PFV four-component vector system consisting of the packaging plasmids pcoPG4 (PFV Gag wt), pcoPP (PFV Pol wt), pcoPE (PFV Env wt), and the EGFP expressing transfer vector puc2MD9 used for production of PFV wt particles was described previously.15,22 The packaging constructs encoding the glycoproteins of rabies virus,23 mokola virus,23 LCMV (Arm53b),23 and VSV11 described previously, were used for pseudotyping of retroviral vectors. For some experiments, lentiviral pseudotypes were generated using the HIV-1 Gag-Pol expressing packaging vector pCD/NL-BH,24 different glycoprotein expression constructs and the HIV-1–based transfer vector p6NST90. This lentiviral transfer vector is a derivative of p6NST50, containing a human ubiquitin C promoter-driven EGFP expression cassette instead of the spleen focus forming virus (SFFV) U3 promoter-driven encephalomyocarditis virus (EMCV) internal ribosomal entry site EGFP-Zeocin expression cassette.25
The HD system components including open reading frames (ORFs) encoding a FK506 binding protein (FKBP, termed FK) or a mutant FRAP fragment (FRB*, termed FR), and the dimerizer (rapamycin analog called rapalog or AP21967) were part of the ARGENTT Regulated Heterodimerization Kit provided by ARIAD.26 In this study, the FK and FR HD domains were genetically fused to different genes, which are shown schematically in Figures 2a, 3a and 4a. Based on the parental PFV Gag packaging construct pcoPG4 variants were generated having the FK or FR HD domains, separated by flexible GS-linker sequences, fused to the N- (Gag NFK: pcoPG4 NFK; Gag NFR: pcoPG4 NFR) or C-terminus (Gag CFK: pcoPG4 CFK; Gag CFR: pcoPG4 CFR). The mutant PFV Env packaging construct pcoPE04 contains the W10,13A (W/A) mutation in the PFV Env LP domain abolishing the natural PFV Env interaction domain with PFV Gag that is essential for PFV particle release.9 For the wild-type (Env wt: pcoPE) and the interaction-deficient PFV Env packaging vector, (Env W/A: pcoPE04) variants were generated having the FK or FR HD domains, separated by flexible GS-linker sequences, fused to the N- (Env wt NFK: pcoPE21; Env W/A NFK: pcoPE22; Env wt NFR: pcoPE23; Env W/A NFR: pcoPE24) or C-terminus (Env wt CFK: pcoPE25; Env W/A CFK: pcoPE26; Env wt CFR: pcoPE27; Env W/A CFR: pcoPE28). Similarly, based on the parental VSV-G encoding packaging vector pcziVSV-G variants with N- (VSV-G NFK: pcziVSV-G NFK; VSV-G NFR: pcziVSV-G NFR) or C-terminal (VSV-G CFK: pcziVSV-G CFK; VSV-G CFR: pcziVSV-G CFR) FK or FR HD domain fusion, were generated. Membrane-targeted variants of the FK and FR HD domain were generated by N-terminal addition of the HIV-1 Gag MA subunit (H1G-MA CFK: pcziH1G-MA CFK; H1G-MA CFR: pcziH1G-MA CFR; aa1-136 of expression optimized HTLVIIIB Gag ORF) or a c-src myristoylation signal sequence (Src-M CFK: pcziSrcM CFK; Src-M CFR: pcziSrcM CFR; aa1-10 of c-src). Further details on the cloning procedures and the individual plasmids are available on request.
Viral supernatant production and viral particle condensation. FV vector particles were produced as previously described.27 Briefly, the individual plasmids of the PFV four-component PFV vector system were transiently cotransfected into 293T cells using polyethyleneimine in 10 cm dishes or PolyFect transfection reagents (QIAGEN, Hilden, Germany) in 12-well plates. 293T cells were cotransfected with different combinations of PFV Gag (wt, wt FK or FR) and glycoprotein (PFV wt, W/A, wt FK or FR, W/A FK or FR, VSV-G FK or FR) packaging constructs as indicated together with PFV Pol packaging constructs (pcoPP) and a transfer vector (puc2MD9) at the ratio of 4:2:1:15–20 in the absence or presence of different amounts of dimerizer (AP21967) as indicated. Alternatively, a five-component PFV vector system was employed consisting of the PFV four-component vector system as described above and a separate packaging plasmid encoding a membrane-targeted HD domain (H1G-MA CFK or CFR, Src-M CFK or CFR). If not indicated packaging plasmids encoding for different HD-tagged PFV Gag proteins, HD-tagged MTD, PFV Pol protein, glycoproteins, and a PFV transfer vector were cotransfected at a ratio of 4:4:2:1:12–20 (total DNA amount: 3 µg/well for 12-well plates; 15 µg/dish for 10 cm dishes) in the absence or presence of a different concentration of dimerizer as indicated. Lentiviral vector supernatant was produced by cotransfecting the transfer vector p6NST90, the Gag/Pol packaging construct pCD/NL-BH and glycoprotein packaging constructs at a ratio of 1:1:1 into 293T cells. If necessary, empty pUC19 plasmid was used in all experiments as stuffer to keep the total DNA amount of transfected DNA constant. At 26–30 hours post-transfection the medium was exchanged for fresh culture medium with or without dimerizer. Cell-free viral vector supernatant was harvested 48 hours post-transfection by centrifugation at 600g for 5 minutes (12-well-plate) or by using 0.45 µm sterile filters (10 cm dish).
Viral particles were concentrated by ultracentrifugation of cell-free viral supernatant at 25,000 rpm (82,705g), 4 °C for 1.5 hours in SW28 rotors (Beckman, Krefeld, Germany). Subsequently, pelleted viral particles were resuspended in phosphate-buffered saline with 10% fetal calf serum to obtain 200× volume concentrated stocks that were snap-frozen in aliquots and stored at −80 °C or used immediately.
Optimization of the five-component PFV pseudotyping system. For optimization of the five-component PFV pseudotyping system using HD-tagged MTDs, viral supernatants were generated by PolyFect transfection in 12-well plates as described above. To determine the optimal ratio of HD-tagged Gag to HD-tagged MTDs packaging construct the amount of both plasmids was kept constant at 0.8 µg total while the relative ratio of both components was varied between 7:1, 6:2, 4:4, 2:6, and 1:7. The optimal amounts of the viral envelopes necessary were determined by varying the total amount of glycoprotein packaging plasmids between 0.05 and 0.8 µg. Empty pUC19 plasmid was used as stuffer to keep the total DNA of the glycoprotein packaging component at 0.8 µg. The HD-tagged Gag to HD-tagged MTD packaging plasmid ratio was kept at 3:1 in these experiments. In some experiments polybrene was added to the viral supernatants at 8 µg/ml final concentration as indicated.
Analysis of viral infectivity. Infectivity of viral supernatants produced was determined by a flow cytometric-based EGFP marker gene transfer assay as described previously.15 HT1080 or Pac2 cells were seeded at a density of 2 × 104 cells/well in 12-well plates 16–24 hours before transduction. Target cells were incubated with 1 ml of cell-free viral supernatant generated by transient transfection as described above and tenfold serial dilutions thereof for 4–6 hours before replacement with normal growth medium. For “fusion from without” experiments, 0.75 ml of the different cell-free supernatants were mixed at a 1:1 ratio as indicated. Subsequently, target cells were infected with 1 ml virus supernatant samples and tenfold serial dilutions thereof as indicated above. The percentage of EGFP marker gene expressing cell was determined 72 hours postinfection by flow cytometry using a FACS Calibur (Becton Dickinson, Heidelberg, Germany). Mean viral titers were calculated for all dilutions of a specific type of vector supernatant giving rise to values of 0.09–80% GFP positive cells using the following formula: Titerabsolute (EGFP ffu/ml) = −2 × ln (1 − ((%GFP positive cells − mock)/100)) × dilution factor × number of infected cells. All infectivity experiments were performed at least three times. In each independent experiment, the titers obtained with PFV wild-type constructs were arbitrarily set to 100% and those of the other samples expressed as values relative to the wt control.
Western blot analysis and antisera. Cellular lysates for protein expression analysis were prepared by washing the transfected 293T cells in 10 cm dishes with phosphate-buffered saline and then incubating for 20 minutes with 600 µl cell-lysis buffer (10 mmol/l Tris/HCl pH 8.0, 140 mmol/l NaCl, 0.025% NaN3, 1% Triton-100) on ice. Subsequently, cell lysates were scraped off the cell culture dishes using a rubber policeman and centrifuged through a QIAshredder (QIAGEN) to shear genomic DNA. After rinsing the QIAshredder with 600 µl 2× PPPC (Protein-Proben-Puffer-Comassie) (100 mmol/l Tris-HCl; pH 6.8, 24% Glycerol, 8% SDS, 0.02% Coomassie Brilliant Blue G-250, 2% β-Mercaptoethanol) the combined flow through was boiled at 95 °C for 10 minutes and used directly for SDS-polyacrylamide gel electrophoresis or stored at −20 °C until further use.
For analysis of viral particle-associated protein composition cell-free supernatant of transfected 293T cells (10 cm dish) was harvested using a syringe and a 0.45 µm pore size sterile filter. Viral particles were concentrated by ultracentrifugation at 25,000 rpm, 4 °C in SW32 (76,755 g) or SW40 (78,925 g) rotors through a 20% sucrose cushion. Viral pellets were resuspended in 50 µl phosphate-buffered saline and 50 µl 2× PPPC was added. After boiling for 10 minutes at 95 °C the samples were used directly for SDS-polyacrylamide gel electrophoresis (SDS-PAGE) or stored at −20 °C.
Cell or viral particle protein samples were separated by SDS-PAGE using a 7.5% polyacrylamide gel and analyzed by immunoblotting as described previously.9 A polyclonal rabbit antisera specific for PFV Gag,28 a hybridoma supernatant specific for the PFV Env surface subunit (SU) (clone P3E10),29,30 and a monoclonal mouse anti-rabbit GAPDH antibodies (G8795; Sigma-Aldrich, Hamburg, Germany) were used. After incubation with a suitable horseradish peroxidase (HRP)-conjugated secondary antibody, the blots were developed with Immobilon Western HRP substrate. The chemiluminescence signal was digitally recorded using a LAS-3000 imager (Fuji, Düsseldorf, Germany).
SUPPLEMENTARY MATERIAL Figure S1. Influence of HDD fusion on biological activity of individual viral proteins. Figure S2. Contribution of “fusion from without” glycoprotein activity to FV pseudotype infectivity. Results.
Acknowledgments
We thank Michael Thompson for critically reading the manuscript. Y.P.H. was supported by a DIGS-BB fellowship. This work was supported by grants from the DFG (LI621/3-3 and SPP1230 LI621/6-1), CRTD (seed grant 2011), and BMBF (01ZZ0102) to D.L. The authors declared no conflict of interest.
Supplementary Material
Influence of HDD fusion on biological activity of individual viral proteins.
Contribution of “fusion from without” glycoprotein activity to FV pseudotype infectivity.
References
- Cronin J, Zhang XY., and, Reiser J. Altering the tropism of lentiviral vectors through pseudotyping. Curr Gene Ther. 2005;5:387–398. doi: 10.2174/1566523054546224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin A, Faideau M, Dufour N, Auregan G, Hassig R, Andrieu T.et al. (2009Engineered lentiviral vector targeting astrocytes in vivo Glia 57667–679. [DOI] [PubMed] [Google Scholar]
- Miletic H, Fischer YH, Neumann H, Hans V, Stenzel W, Giroglou T.et al. (2004Selective transduction of malignant glioma by lentiviral vectors pseudotyped with lymphocytic choriomeningitis virus glycoproteins Hum Gene Ther 151091–1100. [DOI] [PubMed] [Google Scholar]
- Kato S, Inoue K, Kobayashi K, Yasoshima Y, Miyachi S, Inoue S.et al. (2007Efficient gene transfer via retrograde transport in rodent and primate brains using a human immunodeficiency virus type 1-based vector pseudotyped with rabies virus glycoprotein Hum Gene Ther 181141–1151. [DOI] [PubMed] [Google Scholar]
- Buchholz CJ, Mühlebach MD., and, Cichutek K. Lentiviral vectors with measles virus glycoproteins - dream team for gene transfer. Trends Biotechnol. 2009;27:259–265. doi: 10.1016/j.tibtech.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Rethwilm A. Molecular biology of foamy viruses. Med Microbiol Immunol. 2010;199:197–207. doi: 10.1007/s00430-010-0158-x. [DOI] [PubMed] [Google Scholar]
- Lindemann D., and, Rethwilm A. Foamy virus biology and its application for vector development. Viruses. 2011;3:561–585. doi: 10.3390/v3050561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer N, Heinkelein M, Lindemann D, Enssle J, Baum C, Werder E.et al. (1998Foamy virus particle formation J Virol 721610–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann D, Pietschmann T, Picard-Maureau M, Berg A, Heinkelein M, Thurow J.et al. (2001A particle-associated glycoprotein signal peptide essential for virus maturation and infectivity J Virol 755762–5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk T, Geiselhart V, Frech M, Fuller SD, Flügel RM., and, Löchelt M. Specific interaction of a novel foamy virus Env leader protein with the N-terminal Gag domain. J Virol. 2001;75:7995–8007. doi: 10.1128/JVI.75.17.7995-8007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietschmann T, Heinkelein M, Heldmann M, Zentgraf H, Rethwilm A., and, Lindemann D. Foamy virus capsids require the cognate envelope protein for particle export. J Virol. 1999;73:2613–2621. doi: 10.1128/jvi.73.4.2613-2621.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe A, Chen ST, Miyanohara A., and, Friedmann T. In vitro cell-free conversion of noninfectious Moloney retrovirus particles to an infectious form by the addition of the vesicular stomatitis virus surrogate envelope G protein. J Virol. 1998;72:6356–6361. doi: 10.1128/jvi.72.8.6356-6361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang BL, Ikeda Y, Cosset FL, Collins MK., and, Takeuchi Y. Characterization of HIV-1 vectors with gammaretrovirus envelope glycoproteins produced from stable packaging cells. Gene Ther. 2004;11:591–598. doi: 10.1038/sj.gt.3302189. [DOI] [PubMed] [Google Scholar]
- Hill CL, Bieniasz PD., and, McClure MO. Properties of human foamy virus relevant to its development as a vector for gene therapy. J Gen Virol. 1999;80 (Pt 8):2003–2009. doi: 10.1099/0022-1317-80-8-2003. [DOI] [PubMed] [Google Scholar]
- Stirnnagel K, Lüftenegger D, Stange A, Swiersy A, Müllers E, Reh J.et al. (2010Analysis of prototype foamy virus particle-host cell interaction with autofluorescent retroviral particles Retrovirology 745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman SW., and, Linial ML. Identification of a conserved residue of foamy virus Gag required for intracellular capsid assembly. J Virol. 2001;75:6857–6864. doi: 10.1128/JVI.75.15.6857-6864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Life RB, Lee EG, Eastman SW., and, Linial ML. Mutations in the amino terminus of foamy virus Gag disrupt morphology and infectivity but do not target assembly. J Virol. 2008;82:6109–6119. doi: 10.1128/JVI.00503-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhadina M, McClure MO, Johnson MC., and, Bieniasz PD. Ubiquitin-dependent virus particle budding without viral protein ubiquitination. Proc Natl Acad Sci USA. 2007;104:20031–20036. doi: 10.1073/pnas.0708002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBridge RB, Tang P, Hsia HC, Leong PM, Miller JH., and, Calos MP. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol. 1987;7:379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S, Nelson-Rees WA, Toth EM, Arnstein P., and, Gardner MB. Characterization of a newly derived human sarcoma cell line (HT-1080) Cancer. 1974;33:1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Whitmore D, Foulkes NS., and, Sassone-Corsi P. Light acts directly on organs and cells in culture to set the vertebrate circadian clock. Nature. 2000;404:87–91. doi: 10.1038/35003589. [DOI] [PubMed] [Google Scholar]
- Müllers E, Uhlig T, Stirnnagel K, Fiebig U, Zentgraf H., and, Lindemann D. Novel functions of prototype foamy virus Gag glycine- arginine-rich boxes in reverse transcription and particle morphogenesis. J Virol. 2011;85:1452–1463. doi: 10.1128/JVI.01731-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena-Esteves M, Tebbets JC, Steffens S, Crombleholme T., and, Flake AW. Optimized large-scale production of high titer lentivirus vector pseudotypes. J Virol Methods. 2004;122:131–139. doi: 10.1016/j.jviromet.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Schwartz JP, Tanaka K, Brady RO., and, Reiser J. High-titer human immunodeficiency virus type 1-based vector systems for gene delivery into nondividing cells. J Virol. 1998;72:8873–8883. doi: 10.1128/jvi.72.11.8873-8883.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer DA, Kretzschmar G, Müller N, Stanke N, Lindemann D., and, Vollmer G. Activation of transgenic estrogen receptor-beta by selected phytoestrogens in a stably transduced rat serotonergic cell line. J Steroid Biochem Mol Biol. 2010;120:208–217. doi: 10.1016/j.jsbmb.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Belshaw PJ, Ho SN, Crabtree GR., and, Schreiber SL. Controlling protein association and subcellular localization with a synthetic ligand that induces heterodimerization of proteins. Proc Natl Acad Sci USA. 1996;93:4604–4607. doi: 10.1073/pnas.93.10.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann D, Bock M, Schweizer M., and, Rethwilm A. Efficient pseudotyping of murine leukemia virus particles with chimeric human foamy virus envelope proteins. J Virol. 1997;71:4815–4820. doi: 10.1128/jvi.71.6.4815-4820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannigel I, Stange A, Zentgraf H., and, Lindemann D. Correct capsid assembly mediated by a conserved YXXLGL motif in prototype foamy virus Gag is essential for infectivity and reverse transcription of the viral genome. J Virol. 2007;81:3317–3326. doi: 10.1128/JVI.01866-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda A, Stange A, Lüftenegger D, Stanke N, Westphal D, Pietschmann T.et al. (2004Prototype foamy virus envelope glycoprotein leader peptide processing is mediated by a furin-like cellular protease, but cleavage is not essential for viral infectivity J Virol 7813865–13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SF, Eastman SW., and, Linial ML. Foamy virus capsid assembly occurs at a pericentriolar region through a cytoplasmic targeting/retention signal in Gag. Traffic. 2006;7:966–977. doi: 10.1111/j.1600-0854.2006.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Influence of HDD fusion on biological activity of individual viral proteins.
Contribution of “fusion from without” glycoprotein activity to FV pseudotype infectivity.