Abstract
Antisense technologies for the targeted inhibition of gene expression could provide an effective strategy for the suppression of inflammation. However, the effective use of antisense oligonucleotides (ODN) has been limited because of several problems. Therefore, a delivery system for antisense ODNs that enhances antisense stability, while maintaining the specificity of antisense for its target RNA or DNA is needed. We have developed a delivery system for antisense ODN using schizophyllan (SPG), a polysaccharide that belongs to the β-(1-3) glucan family. This system has several advantages enabling the effective suppression of targeted RNA or DNA: the SPG complex is stable in vivo and does not dissolve in the presence of deoxyribonuclease, and the SPG complex is effectively taken up into macrophages by phagocytosis through Dectin-1. Macrophage-migration inhibitory factor (MIF), which is mainly produced by macrophages has been shown to have a pathogenetic role in inflammatory bowel disease (IBD). We developed a technique to create an SPG complex that highly conformed to the antisense MIF. The administration of antisense MIF/SPG complex effectively suppressed MIF production and significantly ameliorated intestinal inflammation. Our result demonstrated a possible new therapeutic approach, i.e., the administration of antisense MIF/SPG complex, for the treatment of IBD.
Introduction
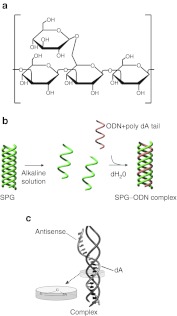
Schizophyllan (SPG) is a member of the β-(1-3) glucan family (Figure 1a) and adopts a triple helix formation in neutral water and a single-chain formation in alkaline solution (>0.25 N NaOHaq) (Figure 1b). When an alkaline solution of SPG is neutralized to pH 7–8, the single chain reverts to its original triple helix as a result of hydrophobic and hydrogen bonding interactions. When a particular polynucleotide such as poly(C) or poly(dA) is present during this process, two main-chain glucoses of β-1,3-glucans and one oligonucleotide (ODN) base form a stoichiometric complex rather than reverting to the original triple helix (Figure 1b,c).1 Contrary to these long homo sequences, a short hetero-sequence such as antisense or CpG DNA does not form a complex with SPG. An SPG-based drug delivery system was formed using antisense ODN and poly (dA) to deliver functional ODNs as antisense DNA to targeted cells. The advantages of using SPG are that medical-grade SPG is available2 and that the complex is presumably recognized by Dectin-1 on antigen-presenting cells such as macrophages and dendritic cells.3
Figure 1.
Schematic illustration showing a complex structure composed of an oligonucleotide (ODN) with a dA tail and schizophyllan (SPG) as well as antisense migration inhibitory factor (MIF)/SPG complex. (a) Chemical structure of SPG. (b) Schematic illustration of SPG–ODN complex. (c) A triple-stranded complex is formed from one DNA and two SPG strands with interactions between the two SPG main-chain glucoses and one dA.
Macrophage-migration inhibitory factor (MIF) was originally discovered as a lymphokine derived from activated T cells that inhibit the random migration of macrophages in vitro.4,5 Several studies have revealed that MIF is expressed in several cell types, including monocytes/macrophages, vascular smooth muscle and cardiomyocytes, and is released upon stimulation from pre-formed storage pools.6 MIF enhances tumor necrosis factor (TNF) and nitric oxide production by lipopolysaccharide (LPS)-stimulated macrophages. Moreover, conversely, TNF-α and interferon-γ upregulate MIF production in macrophages forming a proinflammatory loop within the cytokine network.7 As a product of cells of the innate immune system, MIF enhances phagocytosis, intracellular killing, and the production of nitric oxide, H2O2 and TNF-α in macrophages, thus representing an important factor in the protection of the host against various infectious agents.8 With this important role in innate immunity, MIF is also essential for the adaptive immune response. MIF has been shown to induce interleukin-12 (IL-12) production from macrophages thereby activating the T-helper 1 response.9 Meanwhile, the neutralization of MIF inhibited T cell proliferation as well as IL-2 and interferon-γ production.10
Crohn's disease and ulcerative colitis constitute two major categories of human inflammatory bowel disease (IBD). Although the precise mechanisms responsible for the inflammation and immune responses in IBD have not been fully elucidated, several reports have suggested the involvement of proinflammatory cytokines such as IL-1β, TNF-α, and IL-6, in the pathogenesis of this disease.11,12 These cytokines have also been implicated in the pathogenesis of several autoimmune diseases and their inhibition has been beneficial in both animal and human studies.13 Several in vitro and in vivo data support the concept that MIF is located upstream of events leading to the possible dysregulation of immunoinflammatory responses inducing autoimmune reactions. In fact, MIF has been implicated in the pathogenesis of IBD.9 MIF also reportedly contributed to the development of colitis in several studies using neutralizing antibodies and MIF-gene knockout mice.9,14,15 Furthermore, recent reports have shown that MIF is involved in the pathogens of other inflammatory diseases, such as arthritis, experimental autoimmune encephalomyelitis, type1 diabetes mellitus, and glomerulonephritis.16 These reports have revealed a central role of MIF in the inflammatory process. Therefore, we targeted MIF using a new drug delivery system utilizing SPG for the treatment of IBD.
In this study, we established a technique to create a complex form consisting of antisense MIF and two single SPG chains as a new delivery system. Furthermore, we demonstrated that the administration of this antisense MIF/SPG complex suppressed MIF safely and effectively and significantly improved intestinal inflammation in a dextran sodium sulfate (DSS)-induced animal model of colitis. Our results suggest that MIF is involved in the development of colitis and that antisense MIF treatment can be effectively performed using our new SPG-based delivery system.
Results
Preparation of antisense MIF/SPG complex
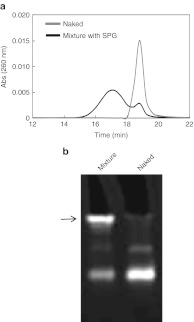
We used the antisense ODN sequence to suppress MIF, as the antisense ODN has a (dA)40 tail that induce the formation of a complex with SPG. The antisense MIF/SPG complex was prepared as described in the Materials and Methods section. Figure 2a compares the gel-permeation chromatography profiles monitored by the UV absorbance at 260 nm before and after the formation of the antisense MIF with the (dA)40 tail. After the formation of the complex, the UV absorbance reading for the antisense MIF shifted to a shorter elution time, indicating an increase in the hydrodynamic volume. By comparing the complexed peak and the free antisense MIF (around 18–20 min), we estimated the complex yield to be 80%. A high yield of complex formation was also confirmed using polyacrylamide gel electrophoresis (Figure 2b).
Figure 2.
Synthesis of schizophyllan (SPG)-antisense migration inhibitory factor (MIF) complex was confirmed using GPC and PAGE. (a) Gel-permeation chromatograms of antisense MIF with a (dA)40 tail (red) and its mixture with SPG (blue). The eluant was measured at a UV absorbance of 260 nm. (b) PAGE patterns for the antisense MIF (naked) and its mixture with SPG (mixture).
MIF was increased in DSS-induced colitis
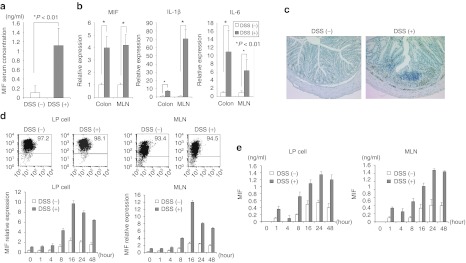
To evaluate MIF production in DSS-induced colitis, we measured the serum MIF production using an enzyme-linked immunosorbent assay. MIF production increased significantly in the DSS-treated mice (Figure 3a). Next, we performed real-time PCR using mesenteric lymph node (MLN) and colon samples to examine IL-1β and IL-6 expression as proinflammatory cytokines. The levels of these proinflammatory cytokines were significantly increased in the DSS-treated mice, as previous reports have shown (Figure 3b).9 MIF mRNA was also increased in the DSS-treated mice. Immunohistochemistry revealed that CD11b+ macrophages had infiltrated the intestines of the DSS-treated mice (Figure 3c).
Figure 3.
Migration inhibitory factor (MIF) expression is upregulated in mesenteric lymph node (MLN) and lamina propria (LP) in dextran sodium sulfate (DSS)-induced colitis. (a) Serum MIF concentration, as measured using an enzyme-linked immunosorbent assay (ELISA) (n = 5 per group). (b) Interleukin (IL)-1β, IL-6, and MIF mRNA expression was determined in MLN and colon specimens using real-time PCR (n = 5 per group). Data were normalized to the expression of β-actin mRNA. (c) Immunohistological staining for MIF in colonic tissue from DSS-treated mice and untreated mice. (d) CD11b+ cells were isolated from LP and MLN in the DSS-treated mice and untreated mice. CD11b+ cells were cultured with 1 µg/ml of LPS. MIF expression was measured at the indicated time point (0, 1, 4, 8, 16, 24, and 48 hours) using real-time PCR and ELISA. The cytokine mRNA expressions were determined in colon and MLN specimens using real-time PCR. Data were normalized to the expression of β-actin mRNA. (e) The supernatants were collected, and the cytokine productions were measured using an ELISA.
To investigate MIF expression in lamina propria (LP) cells and MLN cells over the course of time, CD11b+ cells were isolated from LP and MLN and were cultured with 1 µg/ml of LPS. MIF expression was then quantified using real-time PCR. MIF mRNA expression was expressed as a percentage of the expression of β-actin. Figure 3d showed the precision of CD11b+ cell isolation. The LPS induced MIF mRNA expression of CD11b+ cells peaked at 16 hours. Furthermore, MIF production was measured in the supernatants following stimulation with LPS. MIF production was detected in the supernatants beginning at 1 hour after stimulation (Figure 3e). However, MIF production was decreased at 4 hours. This transient increase in MIF production at 1hour was caused by secretion from cell storage. After 8 hours, MIF production increased and peaked at 24 hours. Since CD11b+ cells produced MIF in animals with DSS-induced colitis, we targeted MIF from CD11b+ cells for the treatment of intestinal inflammation.
Dectin-1 was significantly increased during intestinal inflammation
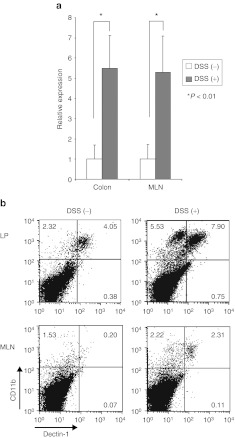
Dectin-1 is a recently discovered pathogen pattern-recognition receptor that binds β-glucans such as SPG.17 Several reports have shown that Dectin-1 is the major β-glucan receptor on macrophages. We investigated the expression of Dectin-1 as an SPG receptor in the normal and DSS-treated mice. Dectin-1 expression was significantly increased in MLN and colon specimens from the DSS-treated mice compared with the expression in samples from normal mice (Figure 4a). Furthermore, we investigated which cells express Dectin-1 in MLN and colon. Mononuclear cells from MLN and colon were analyzed using fluorescence-activated cell sorting (FACS). CD11b+ cells were detected among both the MLN and LP cells. Especially, large numbers of CD11b+ cells were detected in LP cells from the DSS-treated mice (Figure 4b). This result suggested that CD11b+ cells were involved in the development of DSS-induced colitis as previously described. Indeed, the number of Dectin-1 expressing CD11b+ cells and the expression in the DSS-treated mice were much higher than in normal mice (Figure 4b). Therefore, we hypothesized that our delivery system using the SPG complex was effectively taken up by CD11b+ cells.
Figure 4.
Dectin-1 for the receptor of schizophyllan (SPG) was increased in dextran sodium sulfate (DSS) induced acute colitis. (a) Dectin-1 mRNA expression was determined in the mesenteric lymph node (MLN) and colon using real-time PCR. The data were normalized to the expression of β-actin mRNA. (b) Dectin-1 and CD11b expression in the lamina propria (LP) and MLN were analyzed using fluorescence-activated cell sorting (FACS). The data were presented as the mean of three independent experiments.
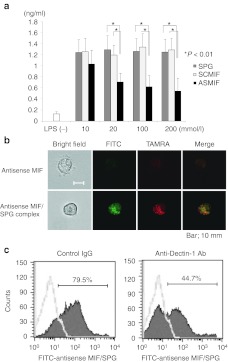
Antisense MIF/SPG complex suppressed MIF production from CD11b+ macrophages
The inhibitory effect of the antisense MIF/SPG complex was assessed in vitro using CD11b+ cells from LP cells and coculturing the cells with 1 µg/ml of LPS with increasing concentrations of antisense MIF/SPG complex. The antisense MIF/SPG complex suppressed MIF production and this effect was dependent on the concentration of antisense MIF (Figure 5a). Both SPG alone and a scramble DNA/SPG complex (as a control for the antisense MIF) did not suppress MIF production from LP cells (Figure 5a). Immunofluorescence was performed to investigate whether the antisense MIF/SPG complex was taken up by CD11b+ cells (Figure 5b). Antisense MIF was labeled with TAMRA, and SPG was labeled with fluorescein isothiocyanate (FITC). CD11b+ cells took up the antisense MIF/SPG complex effectively compared with antisense MIF alone. This data demonstrated that the antisense MIF/SPG complex was taken into CD11b+ cells effectively compared with the antisense MIF alone. Furthermore, to investigate whether the uptake of antisense MIF/SPG complex was dependent on the function of Dectin-1, neutralizing anti-Dectin-1 antibodies were incubated with CD11b+ cells before the addition of the FITC-labeled-antisense MIF/SPG complex. The anti-Dectin-1 antibodies significantly inhibited the uptake of the antisense MIF/SPG complex into CD11b+ cells (Figure 5c). Therefore, our results suggested that Dectin-1 has an important role in the intracellular delivery of the antisense ODN to macrophages.
Figure 5.
Antisense migration inhibitory factor (MIF)/schizophyllan (SPG) complex inhibited MIF production induced by LPS in vitro. (a) CD11b+ cells from lamina propria (LP) and mesenteric lymph node (MLN) were cultured with several concentrations of antisense MIF/SPG complex (ASMIF), scramble control DNA/SPG complex (SCMIF), and SPG as a control. After 10 hours, 1 µg/ml of LPS was added under each condition and the cells were cultured for 24 hours. MIF expressions were measured using an enzyme-linked immunosorbent assay (ELISA) (n = 5 per group). The data presented are the means of the cytokine concentration ± SD. (b) Immunofluorescence in CD11b+ cells was performed by labeling antisense MIF with TAMRA and the SPG with fluorescein isothiocyanate (FITC). CD11b+ cells took up the antisense MIF/SPG complex effectively compared with the antisense MIF alone. (c) Neutralizing anti-Dectin-1 antibodies inhibited the uptake of FITC-labeled antisense MIF/SPG complex into CD11b+ cells compared with rat IgG2b. The data were presented as the mean of three independent experiments.
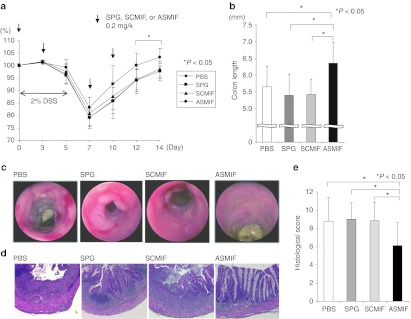
Administration of antisense MIF/SPG complex attenuated DSS-induced colitis
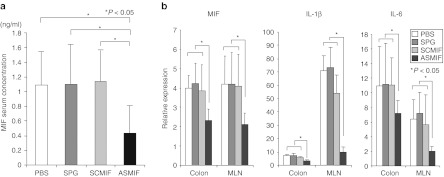
Since our data showed that the antisense MIF/SPG complex suppressed MIF production, this complex was administered twice per week to DSS-treated mice starting on day 0 (Figure 6a). A dose of 0.2 mg/kg of antisense MIF/SPG complex was repeatedly administered by intraperitoneal injection on days 0, 3, 7, and 10. Disease severity and cytokine production were then evaluated on day 14. The administration of the antisense MIF/SPG complex led to significant protection against colitis, as indicated by a significant attenuation in the weight loss and colon shortening (Figure 6a,b). Endoscopic examinations and histological sections of the colon revealed that mucosal injury and inflammation were improved in the antisense treated mice (Figure 6c,d). The histological scores were significantly reduced in the antisense treatment group (Figure 6e). The serum MIF concentration was significantly inhibited by the antisense MIF/SPG complex (Figure 7a). Furthermore, MIF, IL-1β, and IL-6 levels produced in the MLN and colon were significantly decreased by the treatment (Figure 7b). Therefore, the antisense MIF/SPG complex effectively inhibited MIF production from Dectin-1+ cells such as macrophages, thereby attenuating intestinal inflammation.
Figure 6.
Attenuation of dextran sodium sulfate (DSS)-induced colitis by the administration of antisense migration inhibitory factor (MIF)/schizophyllan (SPG) complex. A total of 0.2 mg/kg of antisense MIF/SPG complex (ASMIF), scramble control DNA/SPG complex (SCMIF), SPG, or PBS as a control were injected intraperitoneally (i.p.) twice weekly into mice receiving DSS (n = 8 per group). (a) Body weights as a percentage of the initial weight on day 0 are shown. (b) Colon length from the terminal ileum to the rectum. (c) Endoscopic findings for the colon. (d) Hematoxylin and eosin (H&E) staining of the colon (original magnification: ×100). (e) The histological scores were evaluated.
Figure 7.
Administration of antisense migration inhibitory factor (MIF)/schizophyllan (SPG) complex suppressed cytokines in dextran sodium sulfate (DSS)-induced colitis. (a) The serum MIF concentration was measured using an enzyme-linked immunosorbent assay (ELISA) (n = 5 per group). (b) IL-1β, IL-6 and MIF mRNA expression were determined in the MLN and colon using real-time PCR (n = 5 per group). Data were normalized to the expression of β-actin mRNA.
Discussion
An experimental approach to antagonizing MIF actions was attempted using neutralizing anti-MIF antibodies. This strategy has proven to be effective therapeutically in several models of autoimmune disease, such as IBD,9 experimental autoimmune encephalomyelitis,18 and arthritis.19 Antibody-based anti-MIF drugs have significant associated risks and limitations, including their potential immunogenicity, their short half life in vivo, possible side effects, and the high cost of their application. Recently, the development of nonprotein-based anti-MIF therapies has been intensively sought. Several inhibitors reportedly interact with both the MIF catalytic site and its biological activity.20,21,22 Indeed, an aromatic amino acid Schiff's base, 2-[(4-hydroxybenzlidene) amino]-3- (1H-indol-3-yl) propionic acid methyl ester, and an oxidative metabolite of acetaminophen, NAPQI, prevent the MIF–CD74 interaction.20 However, whether such orally active MIF inhibitors can be developed and find the therapeutic application in human clinical trials remains to be seen. Antisense technologies for the targeted inhibition of gene expressions could provide an effective strategy for the suppression of inflammation or oncogenes. Pathologic disorders, that are currently targeted by antisense therapeutics, include viral infections, inflammatory disorders, cardiovascular disease, cancers, genetic disorders, and autoimmune diseases. Therefore, we have approached such treatment using antisense therapeutics against MIF.
Unfortunately, the effective use of antisense ODNs has been limited because of several problems. First, antisense ODNs are often poorly taken up by cells and therefore may never reach their target site. Second, the hydrolysis mediated by deoxyribonuclease causes antisense instability. Antisense MIF alone did not significantly ameliorate DSS-induced colitis (data not shown). Thus, a need exists to develop a delivery system for antisense molecules that enhances antisense stability, while maintaining the specificity of the antisense for its target RNA or DNA. Sakurai et al. discovered that SPG, which constitutes a triplex helix, dissolved into single chains when treated with DMSO and an alkaline solution and that antisense ODNs and two single chains of SPG reformed a triple helix. This new delivery system has several advantages that enable the effective suppression of MIF, as the SPG complex was stable in vivo and did not dissolve in the presence of deoxyribonuclease, and the SPG complex was effectively taken up by macrophages, which are the main producers of MIF, through phagocytosis. In this study, we developed a technique to create an SPG complex that highly conformed to the antisense MIF. This antisense MIF/SPG complex significantly suppressed MIF production both in vitro and in vivo. The downregulation of proinflammatory cytokines such as IL-1β and IL-6 induced by the suppression of MIF subsequently ameliorated intestinal inflammation.
Dectin-1 is the primary receptor for the phagocytosis of β-glucan by macrophages and DC.23,24,25 Recently, immunohistochemical staining showed that Dectin-1 was mainly present on macrophages in the human intestine. Several cytokines such as granulocyte-macrophage colony-stimulating factor, IL-4, IL-13, TNF-α, and interferon-γ upregulated Dectin-1 expression in macrophages.26 Interestingly, Dectin-1 expression appeared to be elevated in inflamed intestinal tissue, but not in a disease specific manner.27 Therefore, the inflammation induced by DSS was thought to arise from the upregulation of Dectin-1 expression on CD11b+ macrophages. The antisense MIF/SPG complex was effectively taken into Dectin-1+ cells such as macrophages at the inflammation site. On the other hand, the function of Dectin-1 in the intestine remains unclear, although Dectin-1 is known to play an important role in anti-fungal immunity.24,25 Although several reports have shown that the Dectin-1 pathway directly activates NF-κB and drives the induction of cytokines, such as TNF-α and IL-6,28 SPG alone and the antisense MIF/SPG complex did not exacerbate DSS-induced colitis compared with the control. Furthermore, SPG did not drive TNF-α production from CD11b+ macrophages in our study (data not shown). Our studies both in vivo and in vitro suggested that the interaction of SPG and Dectin-1 did not produce proinflammatory cytokines and did not induce inflammation.
In summary, the administration of the antisense MIF/SPG complex significantly ameliorated colitis by suppressing MIF production without causing any adverse effects in a mouse model. Our study suggested that this delivery system targeting MIF might be a candidate treatment for IBD. Further experiments are needed to enable human trial.
Materials and Methods
Materials. Schizophyllum commune-derived SPG (Mw = 1.5 × 105, based on gel-permeation chromatography coupled with a multiangle light scattering analysis) was kindly provided by Mitsui Sugar (Tokyo, Japan). All ODN samples, including antisense ODN for MIF (TGGTGTTCACGATGAACATAGGCATG-(dA)40) were synthesized at FASMAC (Kanagawa, Japan) and purified using high-performance liquid chromatography.
Preparation of antisense MIF/SPG complex. SPG was dissolved in 0.25 N NaOHaq for 2–5 days to dissociate the triple helix to a single chain. The SPG solution was then mixed with antisense MIF in water and phosphate buffer solution (330 mmol/l NaH2PO4, pH 7.4). After mixing, the mixture was stored overnight at 4 °C. The molar ratio [(SPG)/(antisense MIF)] was controlled at 0.27. The final concentration of DNA was determined by measuring the ultraviolet absorbance.
Mice. C57BL/6 mice were purchased from the Kyudo Laboratory (Saga, Japan). Mice were maintained under specific pathogen-free conditions at Kurume University (Kurume, Japan). The mice used in all the experiments were handled according to the guidelines and approved protocols of the Kurume University Animal Care and Use Committees.
Induction and assessment of colitis. DSS-induced colitis was induced by the addition of 2 % DSS to the drinking water. Female mice at 8 weeks of age were given 2 % (wt/vol) DSS drinking water (40,000–50,000 MW) (MP Biomedicals, Irvine, CA) for 5 days. The mice were checked for the development of colitis by monitoring their body weights. The mice were sacrificed on day 14. The MLN and colon were then removed and the tissues were fixed in 4% formaldehyde. Cross sections were prepared and stained with hematoxylin and eosin. The histology was scored as follows: for inflammation (I), 0 = none, 1 = mild, 2 = moderate, 3 = severe; for extent (E), 0 = none, 1= mucosa, 2 = mucosa, and submucosa, 3 = transmural; for regeneration (R), 0 = complete regeneration, 1= almost complete regeneration, 2 = regeneration with crypt depletion, 3 = surface epithelium not intact, 4= no tissue repair; for crypt damage (C), 0 = none, 1= 1/3 of basal damaged, 2 = 2/3 of basal damaged, 3 = only surface epithelium intact, 4= entire crypt and epithelium lost; and for percent involvement (P), 1 = 1–25%, 2 = 26–50%, 3 = 51–75%, and 4 = 76–100%. The total histological score was calculated as I + E + R + C + P.
Cell culture and cytokine assay. Mononuclear cells from the LP and MLN were isolated as described previously. Briefly, mononuclear cells from the MLN were isolated after gentle cell dispersion using 25-gauge needles and then passed through a 60-µm nylon membrane. For LP cell isolation, the epithelial cells were removed by washing in 1 mmol/l of EDTA. The colons were cut into small pieces and digested using 100 U/ml collagenase type 2 (Worthington Biochemical, Lakewood, NJ). Next, LP cells were passed through a 100-µm nylon membrane and purified using a 45%/72% Percoll gradient (GE Healthcare, Piscataway, NJ). CD11b+ cells from MLN and LP were isolated using anti-CD11b MicroBeads on a MACS column (Miltenyi Biotec, Auburn, CA).
CD11b+ cells were cultured in 96-well plates at a density of 1 × 106 cells/ml of medium (RPMI 1640 containing 2 mmol/l glutamine and 25 mmol/l HEPES buffer, supplemented with 10% fetal bovine serum) with 1 µg/ml of LPS (Sigma-Aldrich, St Louis, MO). The culture supernatants were then removed and assayed to determine the cytokine concentrations. The cytokine concentration was assayed using an enzyme-linked immunosorbent assay with enzyme-linked immunosorbent assay kits for MIF. The absorbance was measured using an enzyme-linked immunosorbent assay reader at a wavelength of 450 nm.
Real-time PCR. Total RNA was isolated from the colon, MLN, and CD11b+ cells using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol and reverse transcribed into complementary DNA with Thunderbird (Toyobo, Tokyo, Japan). Complementary DNA was quantified using real-time PCR using an ABI PRISM 7000 (Applied Biosystems, Foster City, CA). IL-1β, IL-6, MIF, and β-actin transcripts were amplified using quantitative real-time PCR with SYBR-green. The following primers were used: for IL-1β, IL-1β-Forward (F) 5′-CCC AAG CAA TAC CCA AAG AA-3′, IL-1β-Reverse (R) 5′- CAT CAG AGG CAA GGA GGA AA -3′ for IL-6, IL-6 F 5′- TTC CAT CCA GTT GCC TTC TT -3′, IL-6 R 5′- ATT TCC ACG ATT TCC CAG AG -3′ for MIF, MIF F 5′- TGA CTT TTA GCG GCA CGA AC -3′, MIF R 5′- GAC TCA AGC GAA GGT GGA AC -3′ and for β-actin, β-actin F 5′- TGT CCC TGT ATG CCT CTG GT -3′, β-actin R 5′- GAT GTC ACG CAC GAT TTC C -3′.
Flow cytometric analysis and immunofluorescence. Mononuclear cells from LP and MLN were transferred to a 5 ml tube in phosphate-buffered saline buffer supplemented with 1% bovine serum albumin and 0.5 % sodium azide at a concentration of 1 × 105 cells/25 µl. Fc-blocked cells were incubated with FITC-conjugated rat anti-mouse CD11b mAb (clone M1/70) and phycoerythrin-conjugated rat anti-mouse Dectin-1 mAb (clone 2A11) for 20 minutes on ice. After incubation, the cells were washed with phosphate-buffered saline and resuspended in 400 µl of 2% paraformaldehyde. The cell surface expression of CD11b and Dectin-1 was determined using a flow cytometry analysis.
CD11b+ cells were incubated with TAMRA-antisense MIF and TAMRA-antisense MIF/FITC–SPG complex for 4 hours. Fluorescence microscope observations of the antisense MIF or antisense MIF/ SPG complex taken up into CD11b+ cells were performed using a BZ-9000 digital fluorescence microscope (Keyence, Osaka, Japan). For the Dectin-1 blocking study, 5 µg/ml of neutralizing anti-Dectin-1 antibodies (clone 2A11) and rat IgG2b as a control were incubated with CD11b+ cells for 1 hour before the addition of the antisense MIF/SPG complex. FITC-labeled antisense MIF/SPG complex was added and the cells were cultured for 2 hours. Then, a flow cytometry analysis was performed.
Immunohistochemical analysis. Fresh tissue samples from the colon were frozen in OCT (optimal cutting temperature) compound (Ames Company, Elkhart, IN) and stored at −80 °C until use. The frozen tissue sections (4-mm thick) were air dried again for 20 minutes and then stained using the avidin–biotin complex method as described previously.29
Treatment using antisense MIF/SPG complex. For DSS-induced colitis, 0.2 mg/kg of SPG, scramble DNA/SPG complex, or antisense MIF /SPG complex was administered intraperitoneally to mice twice weekly (day 0, 3, 7, and 10). To monitor the colitis activity, a high resolution video endoscopic system (Karl Storz, Tuttlingen, Germany) designed for mice was used.30 Prominent endoscopic signs of inflammation in DSS-treated mice were the abrogation of the normal vascular pattern, the presence of mucosal granularity, and the appearance of ulcers. To determine colitis activity in the DSS-treated mice, mice were monitored using mini-endoscopy performed on day 14. Then, the mice were sacrificed on day 14 and the colitis was evaluated by a trained pathologist who was blinded to the treatment group.
Statistical analysis. The statistical analysis was performed using the Student t-test or the Mann–Whitney U-test for quantitative variables and a χ2 analysis for qualitative variables. Two-sided P values <0.05 were considered statistically significant.
Acknowledgments
The present work was supported in part by a National Institute of Biomedical Innovation Grant (ID07-16). The authors declared no conflict of interest.
REFERENCES
- Sakurai K, Mizu M., and, Shinkai S. Polysaccharide–polynucleotide complexes. 2. Complementary polynucleotide mimic behavior of the natural polysaccharide schizophyllan in the macromolecular complex with single-stranded RNA and DNA. Biomacromolecules. 2001;2:641–650. doi: 10.1021/bm000121r. [DOI] [PubMed] [Google Scholar]
- Noda K, Takeuchi S, Yajima A, Akiya K, Kasamatsu T, Tomoda Y.et al. (1992Clinical effect of sizofiran combined with irradiation in cervical cancer patients: a randomized controlled study. Cooperative Study Group on SPG for Gynecological Cancer Jpn J Clin Oncol 2217–25. [PubMed] [Google Scholar]
- Minari J, Mochizuki S, Matsuzaki T, Adachi Y, Ohno N., and, Sakurai K. Enhanced cytokine secretion from primary macrophages due to Dectin-1 mediated uptake of CpG DNA/ß-1,3-glucan complex. Bioconjug Chem. 2011;22:9–15. doi: 10.1021/bc1001196. [DOI] [PubMed] [Google Scholar]
- Bloom BR., and, Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966;153:80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- David JR. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci USA. 1966;56:72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EJ, Li J, Leng L, McDonald C, Atsumi T, Bucala R.et al. (2008Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart Nature 451578–582. [DOI] [PubMed] [Google Scholar]
- Calandra T, Bernhagen J, Mitchell RA., and, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994;179:1895–1902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera S, Suzuki K, Matsuno T, Kaneda K, Takagi M., and, Nishihira J. Macrophage migration inhibitory factor induces phagocytosis of foreign particles by macrophages in autocrine and paracrine fashion. Immunology. 1997;92:131–137. doi: 10.1046/j.1365-2567.1997.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong YP, Abadia-Molina AC, Satoskar AR, Clarke K, Rietdijk ST, Faubion WA.et al. (2001Development of chronic colitis is dependent on the cytokine MIF Nat Immunol 21061–1066. [DOI] [PubMed] [Google Scholar]
- Bacher M, Metz CN, Calandra T, Mayer K, Chesney J, Lohoff M.et al. (1996An essential regulatory role for macrophage migration inhibitory factor in T-cell activation Proc Natl Acad Sci USA 937849–7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- Xavier RJ., and, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- Hoffman HM. Therapy of autoinflammatory syndromes. J Allergy Clin Immunol. 2009;124:1129–38; quiz 1139. doi: 10.1016/j.jaci.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawara T, Nishihira J, Takeda H, Hige S, Kato M, Sugiyama T.et al. (2002Amelioration of dextran sulfate sodium-induced colitis by anti-macrophage migration inhibitory factor antibody in mice Gastroenterology 123256–270. [DOI] [PubMed] [Google Scholar]
- Ohkawara T, Mitsuyama K, Takeda H, Asaka M, Fujiyama Y., and, Nishihira J. Lack of macrophage migration inhibitory factor suppresses innate immune response in murine dextran sulfate sodium-induced colitis. Scand J Gastroenterol. 2008;43:1497–1504. doi: 10.1080/00365520802273017. [DOI] [PubMed] [Google Scholar]
- Stosic-Grujicic S, Stojanovic I., and, Nicoletti F. MIF in autoimmunity and novel therapeutic approaches. Autoimmun Rev. 2009;8:244–249. doi: 10.1016/j.autrev.2008.07.037. [DOI] [PubMed] [Google Scholar]
- Ariizumi K, Shen GL, Shikano S, Xu S, Ritter R 3rd, Kumamoto T.et al. (2000Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning J Biol Chem 27520157–20167. [DOI] [PubMed] [Google Scholar]
- Denkinger CM, Denkinger M, Kort JJ, Metz C., and, Forsthuber TG. In vivo blockade of macrophage migration inhibitory factor ameliorates acute experimental autoimmune encephalomyelitis by impairing the homing of encephalitogenic T cells to the central nervous system. J Immunol. 2003;170:1274–1282. doi: 10.4049/jimmunol.170.3.1274. [DOI] [PubMed] [Google Scholar]
- Santos L, Hall P, Metz C, Bucala R., and, Morand EF. Role of macrophage migration inhibitory factor (MIF) in murine antigen-induced arthritis: interaction with glucocorticoids. Clin Exp Immunol. 2001;123:309–314. doi: 10.1046/j.1365-2249.2001.01423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dios A, Mitchell RA, Aljabari B, Lubetsky J, O'Connor K, Liao H.et al. (2002Inhibition of MIF bioactivity by rational design of pharmacological inhibitors of MIF tautomerase activity J Med Chem 452410–2416. [DOI] [PubMed] [Google Scholar]
- Senter PD, Al-Abed Y, Metz CN, Benigni F, Mitchell RA, Chesney J.et al. (2002Inhibition of macrophage migration inhibitory factor (MIF) tautomerase and biological activities by acetaminophen metabolites Proc Natl Acad Sci USA 99144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KF., and, Al-Abed Y. Critical modifications of the ISO-1 scaffold improve its potent inhibition of macrophage migration inhibitory factor (MIF) tautomerase activity. Bioorg Med Chem Lett. 2006;16:3376–3379. doi: 10.1016/j.bmcl.2006.04.038. [DOI] [PubMed] [Google Scholar]
- Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L.et al. (2002Dectin-1 is a major β-glucan receptor on macrophages J Exp Med 196407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H.et al. (2007Dectin-1 is required for β-glucan recognition and control of fungal infection Nat Immunol 831–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki K.et al. (2007Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans Nat Immunol 839–46. [DOI] [PubMed] [Google Scholar]
- Willment JA, Lin HH, Reid DM, Taylor PR, Williams DL, Wong SY.et al. (2003Dectin-1 expression and function are enhanced on alternatively activated and GM-CSF-treated macrophages and are negatively regulated by IL-10, dexamethasone, and lipopolysaccharide J Immunol 1714569–4573. [DOI] [PubMed] [Google Scholar]
- de Vries HS, Plantinga TS, van Krieken JH, Stienstra R, van Bodegraven AA, Festen EA.et al. (2009Genetic association analysis of the functional c.714T>G polymorphism and mucosal expression of dectin-1 in inflammatory bowel disease PLoS ONE 4e7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodridge HS, Wolf AJ., and, Underhill DM. β-glucan recognition by the innate immune system. Immunol Rev. 2009;230:38–50. doi: 10.1111/j.1600-065X.2009.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi E, Mizoguchi A., and, Bhan AK. Role of cytokines in the early stages of chronic colitis in TCR alpha-mutant mice. Lab Invest. 1997;76:385–397. [PubMed] [Google Scholar]
- Becker C, Fantini MC, Wirtz S, Nikolaev A, Kiesslich R, Lehr HA.et al. (2005In vivo imaging of colitis and colon cancer development in mice using high resolution chromoendoscopy Gut 54950–954. [DOI] [PMC free article] [PubMed] [Google Scholar]