Abstract
Loss of microRNA-29 (miR-29) is known to be a mechanism of transforming growth factor-β (TGF-β)-mediated pulmonary fibrosis, but the therapeutic implication of miR-29 for pulmonary fibrosis remains unexplored. The present study investigated whether miR-29 had therapeutic potential for lung disease induced by bleomycin in mice. In addition, the signaling mechanisms that regulated miR-29 expression were investigated in vivo and in vitro. We found that miR-29 was a downstream target gene of Smad3 and negatively regulated by TGF-β/Smad signaling in fibrosis. This was evidenced by the findings that mice or pulmonary fibroblasts null for Smad3 were protected against bleomycin or TGF-β1-induced loss of miR-29 along with fibrosis in vivo and in vitro. Interestingly, overexpression of miR-29 could in turn negatively regulated TGF-β and connective tissue growth factor (CTGF) expression and Smad3 signaling. Therefore, Sleeping Beauty (SB)-mediated miR-29 gene transfer into normal and diseased lung tissues was capable of preventing and treating pulmonary fibrosis including inflammatory macrophage infiltration induced by bleomycin in mice. In conclusion, miR-29 is negatively regulated by TGF-β/Smad3 and has a therapeutic potential for pulmonary fibrosis. SB-mediated miR-29 gene therapy is a non-invasive therapeutic strategy for lung disease associated with fibrosis.
Introduction
Idiopathic pulmonary fibrosis is a chronic, progressive, and lethal pulmonary disease with unknown cause or cure.1 Idiopathic pulmonary fibrosis is characterized by the excessive deposition of collagen and other extracellular matrix proteins within the lung interstitium, which is mediated by transforming growth factor-β1 (TGF-β1) through its downstream signaling pathway, called Smad2 and Smad3.2,3,4 Recently, we have demonstrated that Smad3, but not Smad2, is a key signaling pathway of fibrogenesis in vivo and in vitro.5 The critical role for Smad3 in fibrogenesis has also been reported in a number of disease models including bleomycin-induced pulmonary fibrosis,6 ischemic and hypertensive cardiac remodeling,7,8 dimethylnitrosamine-induced hepatic fibrosis,9 and unilateral ureteral obstructive nephropathy.10 However, treatment for fibrosis by directly targeting the TGF-β/Smad3 signaling pathway may be problematic. This may be largely attributed to an essential role for TGF-β/Smad3 in balancing the immune system because deletion of TGF-β1 or Smad3 impairs immunity and results in a deathly inflammation in multiple organs.11,12,13,14 Thus, strategies aiming to prevent or treat fibrosis need to precisely target the downstream specific gene(s) of TGF-β/Smad3 that are directly regulating fibrosis, rather than blocking the general effects of the TGF-β signaling pathway.
Increasing evidence shows that microRNAs (miRNAs) are key biological regulators that control gene expression by translational suppression and destabilization of target mRNAs during the pathophysiological processes.15 Aberrant expression of miRNAs has been regarded as a common feature of fibrotic diseases, among which microRNA-29 (miR-29) is one of the known TGF-β1-associated microRNAs involved in fibrogenesis because many of ECM genes are targets of miR-29, including ELN, FBN1, COL1A1, COL1A2, and COL3A1, etc.16,17 Several studies have demonstrated that miR-29 was reduced or lost in a number of diseases associated with fibrosis in heart, lung, and liver, which was downregulated by TGF-β1.16,17,18 However, a fundamental question whether miR-29 has therapeutic potential for pulmonary fibrosis remains unexplored. In addition, the signaling mechanisms by which TGF-β regulates miR-29 expression remain to be determined. Taken together for critical roles of Smad3 and miR-29 in the pathogenesis of fibrosis, we thus hypothesize that miR-29 may be a downstream target gene of TGF-β/Smad3 related to fibrosis and may function as an antifibrotic agent with therapeutic potential for pulmonary fibrosis. The hypothesis was examined in a mouse model of bleomycin-induced pulmonary fibrosis in Smad3 knockout (KO) mice and lung fibroblasts. Importantly, the therapeutic potential of miR-29 in pulmonary fibrosis was examined in both the induction phase and established lung disease induced by bleomycin using the Sleeping Beauty (SB) transposon-mediated gene transfer system.
Results
Loss of miR-29 in bleomycin-induced pulmonary fibrosis is Smad3-dependent
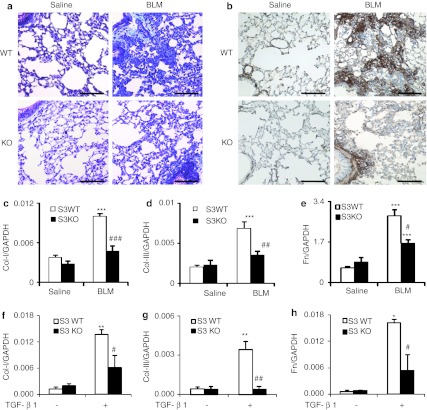
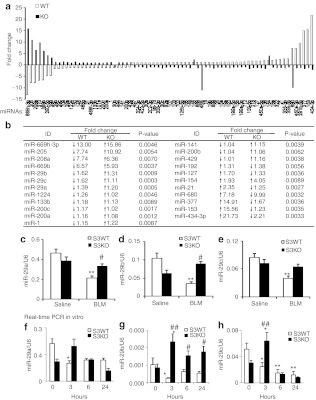
We first examined whether miR-29 expression was Smad3-dependent during lung fibrogenesis in a mouse model of bleomycin-induced pulmonary fibrosis in Smad3 KO and wild-type (WT) mice. Consistent with a previous report,6 mice lacking Smad3 were protected against severe pulmonary fibrosis as determined by Masson's trichrome staining (Figure 1a), immunohistochemistry (Figure 1b), and quantitative PCR analysis for expression of collagen I, collagen III and fibronectin (Figure 1c–e). miRNA microarray revealed that microRNAs were differentially expressed in the diseased lung of Smad3 KO and WT mice at day 14 after disease induction (Figure 2). Of them, the miR-29 family including miR-29a, miR-29b, and miR-29c was significantly downregulated in Smad3 WT mice along with pulmonary fibrosis, but upregulated in Smad3 KO mice with minor fibrotic changes (Figures 1 and 2b). Quantitative real-time PCR confirmed these results that Smad3 KO mice were protected from the loss of the miR-29 family (Figure 2c–e).
Figure 1.
Smad3 knockout (KO) mice are protected against pulmonary fibrosis in vivo and transforming growth factor-β1 (TGF-β1) induced fibrotic response in vitro in lung fibroblasts. (a) Masson's trichrome staining. (b) Immunohistochemistry staining of collagen I. (c–e) Real-time PCR analysis for mRNA expression of (c) collagen I, (d) collagen III, and (e) fibronectin in Smad3 wild-type (WT) and KO mice at day 14 after bleomycin treatment. (f–h) Real-time PCR for (f) collagen I, (g) collagen III, and (h) fibronectin (Fn) mRNA expression at 3 hours after TGF-β1 (2 ng/ml) stimulation. Each bar represents mean + SEM for five mice or four independent experiments in vitro. *P < 0.05, **P < 0.01, ***P < 0.001 versus saline group or no treatment cells; #P < 0.05, ##P < 0.01, ###P < 0.001 versus bleomycin-treated Smad3 WT mice or TGF-β1-treated cells. BLM, bleomycin. Bar = 100 µm.
Figure 2.
MicroRNA (miRNA) microarray and microRNA-29 (miR-29) expression in bleomycin-treated lungs at day 14 in Smad3 wild-type (WT) and knockout (KO) mice and transforming growth factor-β1 (TGF-β1)-treated cells. (a) miRNA profile in the fibrotic lungs of Smad3 WT and KO mice. (b) A list of fold changes of miRNAs in the fibrotic lungs of Smad3 WT and KO mice. Data are normalized to saline-treated Smad3 WT or KO mice. (c–e) Real-time PCR analysis of miR-29a,b,c in vivo. Note that miR-29 family members are significantly decreased in Smad3 WT mice, but increased in Smad3 KO mice. (f–h) Real-time PCR analysis of miR-29a,b,c in vitro in response to TGF-β1. Each bar represents the mean + SEM for five mice or four independent experiments. *P < 0.05, ** P < 0.01 versus saline group or no treatment cells; #P < 0.05, ##P < 0.01 versus bleomycin-treated Smad3 WT mice or TGF-β1-treated cells. BLM, bleomycin.
Expression of miR-29 is negatively regulated by TGF-β/Smad3 in lung fibroblasts in vitro
To investigate the regulating role of Smad3 in miR-29 expression during pulmonary fibrosis, primary lung fibroblasts isolated from Smad3 WT or KO mice were stimulated with TGF-β1. Consistent with the findings in vivo (Figures 1a–e and 2a–e), real-time PCR detected that addition of TGF-β1 was able to downregulate expression of miR-29a-c along with a marked upregulation of collagen I, III, and fibronectin in Smad3 WT lung fibroblasts (Figure 1f–h and 2f–h), whereas deletion of Smad3 prevented TGF-β1-induced downregulation of the miR-29 family and fibrosis response (Figure 1f–h and 2f–h). Because miR-29b was the one with the most significant changes among the miR-29 family member during fibrosis in vivo and in vitro, it was used for in vivo study to explore the functional role and therapeutic potential for pulmonary fibrosis.
Effect of SB-transposon system on delivering miR-29b into the lung
To determine the efficiency of SB-mediated miR-29b therapy in lung fibrosis, the exogenous pre-miR-29b (pT2/BH-pre-miR-29b) or empty control plasmid was transfected into the lung tissue with the SB-transposase plasmid (SB11). In situ hybridization detected that the use of SB-transposase system had resulted in a much higher expression rate of the exogenous miR-29b by all cell type within the lung tissue (Supplementary Figure S2a), which was consistent with the previous study using the luciferase as a reporter plasmid.19 In contrast, no positive signals were detected in those treated with empty control plasmids (Supplementary Figure S2a).
To prove evidence for a critical role of SB-transposase-sustained miR-29 expression in the lung tissue, we transfected the transposon-containing pre-miR-29 (pT2/BH-pre-miR-29b) with or without SB-transposase plasmid (SB11) into the lung tissue. As shown in Supplementary Figure S2b, mouse lungs cotransfected with both pT2/BH-pre-miR-29b and SB11 had much higher levels of miR-29b expression at day 14 when compared with pT2/BH-pre-miR-29b-treated alone, demonstrating that in the presence of the SB-transposase, miR-29 could integrate into the genome to sustain its expression.
To confirm SB-tranposase-mediated excision of the exogenous miR-29b from the transposons within the transfected lung tissues, we performed excision assay by amplifying a PCR product of ~161 bp from mouse lung tissues cotransfected with SB11 and pT2/BH-pre-miR-29b or treated with pT2/BH-pre-miR-29b plasmid without SB11, empty control plasmids, or saline. As shown in Supplementary Figure S2c, PCR clearly demonstrated that the exogenous miR-29b was specifically cut down from the lung tissues which received the cotransfection with SB11 and pT2/BH-pre-miR-29b only.
Gene transfer of miR-29b prevents bleomycin-induced pulmonary fibrosis in mice
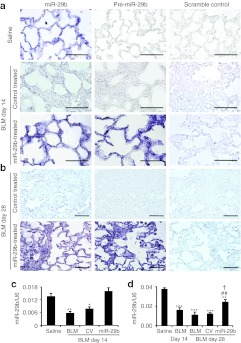
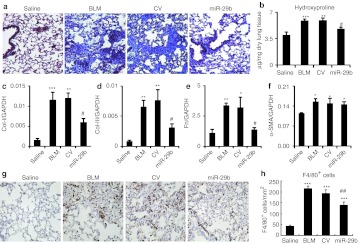
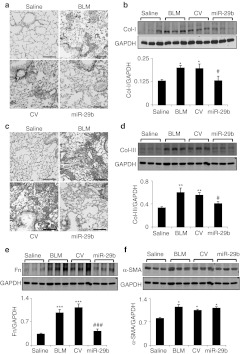
After demonstrating the efficiency and specificity of SB-mediated miR-29 gene transfer into the lung tissue, the functional role of miR-29 in pulmonary fibrosis was tested in a mouse model of bleomycin-induced pulmonary fibrosis by SB-mediated gene transfer of miR-29b. As shown in Figure 3a, in situ hybridization detected that a moderate endogenous miR-29b was expressed in saline-treated mouse lungs, but was virtually lost in bleomycin-induced, control vector-treated lung tissues with severe fibrosis. In contrast, SB -mediated miR-29b gene transfer resulted in higher levels of miR-29b transgene (exogenous miR-29b) expression, presumably by endothelial cells, alveolar epithelial cells as previously described using the SB-mediated luciferase reporter assay.19 Quantitative real-time PCR also confirmed that SB-based miR-29b gene transfer restored miR-29 expression to the normal levels in the lung tissues at day 14 after bleomycin injection (Figure 3c), thereby preventing bleomycin-induced lung fibrosis as demonstrated by Masson's trichrome staining, hydroxyproline content, and collagen I, III, and fibronectin mRNA expression (Figure 4). The functional role for miR-29 in blockade of bleomycin-induced pulmonary fibrosis including collagen I, III, and fibronectin expression was further demonstrated at the protein levels by immunohistochemistry and western blot analysis (Figure 5). Interestingly, overexpression of miR-29 was also able to block macrophage infiltration (Figure 4g and h), but failed to suppress α-smooth muscle actin (α-SMA) expression at both mRNA and protein levels within the diseased lung tissue (Figures 4f and 5f).
Figure 3.
Sleeping Beauty (SB) transposon-mediated microRNA-29b (miR-29b) gene therapy prevents a loss of miR-29b from the fibrotic lung tissues at day 14 (preventional experiment) and day 28 (interventional experiment) after bleomycin treatment. (a,b) In situ hybridization of miR-29b and transgene miR-29b2 (pre-miR-29b2) in saline or bleomycin-treated lung tissues at (a) day 14 and (b) day 28. (c,d) Real-time PCR results of miR-29b expression. Results show that miR-29b is highly expressed in normal lung tissues treated with saline, but lost in the bleomycin-treated tissues with severe fibrosis. In contrast, SB-mediated pre-miR-29b gene therapy results in higher levels of transgene miR-29 (pre-miR-29b) expression, thereby preventing a loss of miR-29b from the diseased lung tissues at both day 14 and day 28. Note that the exogenous miR-29 gene transfection rate and transgene expression in the lung tissues are indicated by pre-miR-29b by in situ hybridization and the specificity of miR-29b transgene expression is verified by a negative detection in control-treated lung tissues. Each bar represents mean + SEM for at least five mice. *P < 0.05, **P < 0.01, ***P < 0.001 versus saline-treated mice; #P < 0.05, ##P < 0.01 versus control vector treatment. †P < 0.05 versus bleomycin-treated mice at day 14. BLM, bleomycin; CV, control vector. Bar = 50 µm.
Figure 4.
Sleeping Beauty (SB) tansposon system-based microRNA-29b (miR-29b) gene transfer prevents bleomycin-induced pulmonary fibrosis at day 14. (a) Masson's trichrome staining. (b) Hydroxyproline content. (c–f) Real-time PCR analysis of (c) collagen I, (d) collagen III, (e) fibronectin (Fn), and (f) α-smooth muscle actin (α-SMA) mRNA expression. Note that SB-mediated miR-29b gene transfer results in inhibition of pulmonary fibrosis as demonstrated by histology, hydroxyproline content, and collagen I, III, fibronectin, but not α-SMA mRNA expression.(g,h) Immunohistochemistry and semiquantification of F4/80+ macrophages infiltration in the lung. Each bar represents mean + SEM for at least five mice. *P < 0.05, **P < 0.01, ***P < 0.001 versus saline-treated mice; #P < 0.05 versus control vector-treated mice. BLM, bleomycin; CV, control vector. Bar = 100 µm.
Figure 5.
Immunohistochemistry and western blot analysis detect that Sleeping Beauty (SB) tansposon system-based microRNA-29b (miR-29b) gene transfer prevents bleomycin-induced pulmonary fibrosis at day 14. (a,b) Collagen I expression by (a) immunohistochemistry and (b) western blotting. (c,d) Collagen III expression by (c) immunohistochemistry and (d) western blotting. (e) Fibronectin (Fn) expression by western blotting. (f) α-Smooth muscle actin (α-SMA) expression by western blotting. Each bar represents mean + SEM for at least five mice. *P < 0.05, **P < 0.01, ***P < 0.001 versus saline-treated mice; #P < 0.05, ##P < 0.001 versus control vector-treated mice. BLM, bleomycin; CV, control vector. Bar = 100 µm.
Therapeutic effect of miR-29 on the established pulmonary fibrosis induced by bleomycin in mice
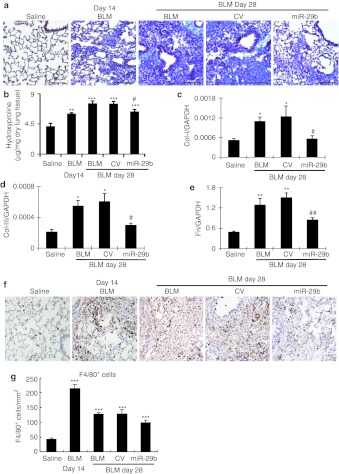
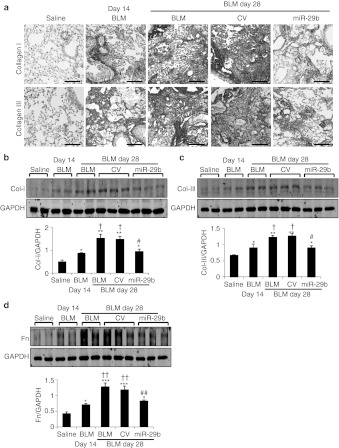
We then examined whether miR-29 had therapeutic potential for lung fibrosis in an established mouse model of bleomycin-induced pulmonary fibrosis. On day 14 after bleomycin, moderate to severe lung fibrosis was established (Figure 1) and the miR-29b precursor or the empty control vector system was delivered into the diseased lung using the SB-based gene transfer system as described above. In situ hybridization and quantitative real-time PCR clearly demonstrated that the SB-gene transfer system was capable of delivering the miR-29b into the established lung disease with fibrosis at day 14, therefore, restoring higher levels of miR-29b at day 28 (Figure 3b and d). This resulted in blockade of progressive lung fibrosis as demonstrated by Masson's trichrome staining, hydroxyproline content, and collagen I, III, and fibronectin mRNA expression (Figure 6). Furthermore, immunohistochemistry and western blot analysis also revealed that restored miR-29 from day 14 onwards inhibited progressive lung fibrosis such as collagen I, III, and fibronectin expression at day 28 (Figure 7). Again, this was associated with inhibition of F4/80+ macrophage infiltration (Figure 6f and g). However, in comparisons with the diseased parameters at day 14 before miR-29 treatment, restoring the miR-29 level from day 14 could not reverse the established lung fibrosis at day 28 (Figures 6 and 7)
Figure 6.
Sleeping Beauty (SB) transposon system-based microRNA-29b (miR-29b) gene transfer attenuates progressive pulmonary fibrosis in an established mouse model of bleomycin-induced pulmonary fibrosis at day 28. (a) Masson's trichrome staining. (b) Hydroxyproline content assay. (c–e) Real-time PCR analysis for mRNA expression of (c) collagen I, (d) collagen III, and (e) fibronectin (Fn). (f,g) Immunohistochemistry and semiquantification of F4/80+ cells infiltration in the lung. Results show that SB-mediated miR-29b gene therapy at day 14 of diseased lung attenuates progressive pulmonary fibrosis at day 28. Each bar represents mean + SEM for at least five mice. *P < 0.05, **P < 0.01, ***P < 0.001 versus saline-treated mice; #P < 0.05 versus control vector treated mice. CV, control vector; BLM, bleomycin. Bar = 100 µm.
Figure 7.
Immunohistochenistry and western blot analysis detect that Sleeping Beauty (SB) transposon system-based microRNA-29b (miR-29b) gene transfer attenuates progressive pulmonary fibrosis in an established mouse model of bleomycin-induced pulmonary fibrosis at day 28. (a) Immunohistochemistry staining of collagen I and collagen III. (b–d) Western blot analysis of (b) collagen I, (c) collagen III, and (d) fibronectin (Fn). Each bar represents mean + SEM for at least five mice. *P < 0.05, **P < 0.01, ***P < 0.001 versus saline-treated mice; #P < 0.05, ##P < 0.01 versus control vector treated mice. †P < 0.05, ††P < 0.01 versus bleomycin-treated mice at day 14. BLM, bleomycin; CV, control vector. Bar = 100 µm.
Gene transfer of miR-29 inhibits bleomycin-induced pulmonary fibrosis by suppressing TGF-β/Smad3 signaling
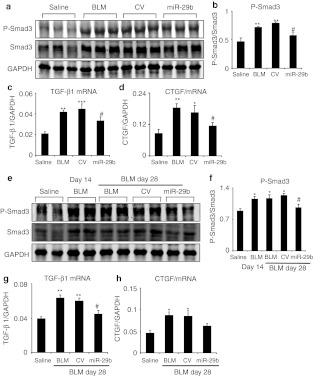
We next investigated whether overexpression of miR-29 could in turn suppress TGF-β/Smad3 signaling during pulmonary fibrosis. Interestingly, SB-mediated gene transfer of miR-29b was able to suppress expression of two major fibrogenic factors, TGF-β1, and connective tissue growth factor (CTGF), in addition to inhibiting Smad3 phosphorylation in both induction and established phases of bleomycin-induced lung fibrosis (Figure 8).
Figure 8.
Real-time PCR and western blot analysis detect that Sleeping Beauty (SB) transposon system-based microRNA-29b (miR-29b) gene transfer decreases transforming growth factor-β1 (TGF-β1)/Smad3 signaling to prevent or treat the bleomycin-induced pulmonary fibrosis. (a–d) Gene transfer of miR-29b suppresses the TGF-β1 (c), connective tissue growth factor (CTGF) (d) mRNA level and decreases the Smad3 phosphorylation (a,b) to prevent the bleomycin-induced pulmonary fibrosis at day 14. (e–h) Gene transfer of miR-29b downregulates the mRNA level of (g) TGF-β1, (h) CTGF, and (e,f) decreases the Smad3 phosphorylation to treat the established bleomycin-induced pulmonary fibrosis at day 28. Each bar represents mean + SEM for at least five mice. *P < 0.05, **P < 0.01, ***P < 0.001 versus saline-treated mice; #P < 0.05, versus control vector-treated mice. BLM, bleomycin; CV, control vector.
Discussion
Although TGF-β/Smad signaling has been considered as a key pathway leading to pulmonary fibrosis, the specific antifibrotic therapy by targeting this pathway is lacking. The present study identified that miR-29 was a downstream target gene of Smad3 and negatively regulated by TGF-β/Smad signaling during pulmonary fibrosis in vivo and in vitro. More importantly, we also identified that miR-29 was an antifibrotic agent and SB-mediated miR-29 gene therapy had great therapeutic potential for pulmonary fibrosis.
The finding of miR-29 as a downstream target gene of Smad3 that was negatively regulated by TGF-β/Smad3 signaling during pulmonary fibrosis was novel. Indeed, mice and lung fibroblasts lacking Smad3 gene were protected from the loss of miR-29 and fibrosis in vivo and in vitro. These findings are consistent with a recent report in renal fibrosis in which Smad3 mediates TGF-β1-induced downregulation of miR-29 by binding to miR-29 promoter.20 Thus, Smad3-downregulated miR-29 expression might be a mechanism by which mice null for Smad3 were protected against lung fibrosis as seen in this and other study.6 In addition, we have found that overexpression of miR-29b was able to inhibit TGF-β1, CTGF, and Smad3 signaling in bleomycin-induced lung fibrosis. These results indicate that miR-29 is not only a downstream target gene of TGF-β1/Smad3 in the pathogenesis of pulmonary fibrosis, but also acts as an counter-regulator of the TGF-β/Smad3/CTGF axis in lung fibrosis via its negative feedback mechanism. Thus, there are two possible mechanisms by which miR-29 exerts its antifibrotic function. First, miR-29 may directly bind to the 3′UTR of targeted genes (such as collagens etc.) to suppress the fibrotic gene expression as previously reported.17 Second, miR-29 may inhibit lung fibrosis by suppressing TGF-β/Smad3-mediated lung fibrosis as defined in the present study.
In addition, inhibition of macrophage infiltration may also be a mechanism by which overexpression of miR-29 blocks lung fibrosis. Indeed, miR-29 therapy was capable of reducing macrophage infiltration in both preventional and interventional studies of bleomycin-induced lung fibrosis. This finding is consistent with the previous report that macrophages, in particular M2 alternatively activated macrophages, promote bleomycin-induced lung fibrosis.21 Although the mechanisms by which overexpression of miR-29 inhibits macrophage accumulation remain largely unclear, it may be associated with the ability of overexpression of miR-29 in blocking the TGF-β1/Smad3 pathway because deletion of Smad3 has been shown to protect against macrophage infiltration and fibrosis in a variety of diseases including bleomycin-induced pulmonary fibrosis,6 cardiac remodeling,7,8 liver fibrosis,9 and obstructive nephropathy.10
The most important finding in the present study is the identification of miR-29 as an antifibrotic agent and the successful development of SB-transposon system-based, noninvasive miR-29 gene therapy for pulmonary fibrosis. It has been reported that by using antisense inhibitor oligonucleotides (AMO), miRNA-based therapy may become a favorable therapy in cardiovascular and pulmonary diseases.22,23 However, the lower level of tissue uptake of AMO is a key obstacle for developing the AMO strategy into the use clinically. Besides, most experimental designs for gene therapy are viral-based system, which may be hampered by the inherent defects of viral vectors such as pathogenicity and the possibility of triggering host inflammatory and immune responses.24,25 Compared to viral vectors, nonviral vectors-mediated gene therapy has several advantages.26 As a nonviral gene delivery system, the SB-transposon system has been used to effectively and safely deliver gene into the lungs with higher levels of transfection rate and long-term transgene expression.27,28,29 Thus, the gene transfer strategy combining SB-transposon system and polyethylenimine has been used to treat many diseases including pulmonary hypertension.26,30 In the present study, by using polyethyleneimine as the transfection reagent, an enhanced SB-transposon system was used to effectively transfer the miR-29b into the mouse lung tissues via tail vein injection. We found that SB-mediated gene transfer had resulted in higher levels of exogenous pre-miR-29b expression in the lung tissues detected by in situ hybridization, even so in the scarring lung tissues in the established model of lung fibrosis. Thus, by using SB-based miRNA therapy, we were able to overexpress miR-29 in the normal lung to prevent pulmonary fibrosis following bleomycin administration, and also able to restore miR-29 to a significantly higher level within the fibrotic lungs to block progressive fibrosis in the established model of pulmonary fibrosis. One intriguing aspect of this study is that the numbers of α-SMA+ myofibroblasts were not affected by miR-29 treatment, whereas collagen mRNA levels and protein deposition within the diseased lung were substantially reduced. This may be associated with the ability of miR-29 to specifically target collagen I, collagen III, fibrilin, and elastin,16,17,18,31 but not α-SMA gene because no miR-29-binding sites are detected on α-SMA 3′UTR by the programs with Targetscan (http://targetscan.org), miRanda (http://www.microrna.org), and PicTar (http://pictar.mdc-berlin.de). Thus, the finding from the present study suggests that miR-29 may have limited function in the mechanisms regulating myofibroblast differentiation and/or recruitment in the lung under pathophysiological conditions.
In conclusion, the present study detects that miR-29 is a downstream target gene of TGF-β/Smad3 related to pulmonary fibrosis and is a potential therapeutic agent as an effective antifibrotic agent for pulmonary fibrosis. The ability of SB-transposon-mediated gene transfer of miR-29 to prevent and treat bleomycin-induced pulmonary fibrosis in mice may provide the evidence for the use of miR-29 as a novel and effective therapy for lung disease associated with fibrosis.
Materials and Methods
Mouse model of bleomycin-induced pulmonary fibrosis. Eight to ten weeks old littermate female Smad3 KO and Smad3 WT mice (C57BL/6J background) and C57BL/6J mice were used in this study. Smad3 WT and Smad3 KO mice were identified by genotyping as described previously.13 To induce pulmonary fibrosis, bleomycin (Sigma-Aldrich, St Louis, MO) was dissolved in sterile saline at 1 U/ml. After anesthesia with intraperitoneal ketamine (80 mg/kg) and xylazine (15 mg/kg), bleomycin was injected intratracheally at a dose of 1.5 U/kg body weight. Control groups received the same volume of sterile saline only. Groups of 5–8 mice were sacrificed at day 14 or 28 after bleomycin treatment and lungs were collected for histology, immunohistochemistry, in situ hybridization, miRNA microarray, real-time PCR, and western blot analysis. The experimental procedure was approved by the Animal Experimentation Ethics Committee at The Chinese University of Hong Kong.
SB-miR-29 construction. The SB-transposon system (a gift from Dr Perry Hackett from University of Minnesota)32 was used to deliver miR-29b into the mouse lung. Briefly, a murine pre-miR-29b2 sequence was amplified by PCR with forward primer: 5′- gttaGGATCCaCACAGGAAA-CAAGCTTTGCAT -3′ and reverse primer: 5′- cttaGTCGACaCACTGCCTCTAAACCAA- ACAGA -3′ (underlined: BamHI and SalI sites respectively). The PCR amplified product was subsequently cloned into pcDNA3.0 to obtain pcDNA3-miR-29b2. Then the CMV-miR-29b2 fragment was amplified by PCR from linearized pcDNA3-miR-29b2 (SmaI digested) by forward primer: 5′- gtttaGAATTCAGATCTCCCGATCCCCTATG – 3′ and reverse primer: 5′- gtttaGAATTCGTGGGGATACCCCCTAGAGC -3′ (underlined: SmaI site), and this fragment was subsequently cloned within the EcoRI site of SB-transposon vector, pT2/BH, to obtain pT2/BH-miR-29b2 (Supplementary Figure S1).The empty pT2/BH was used as a control. All plasmids were prepared using the EndoFree Plasmid kit (Qiagen, Valencia, CA) following the manufacturer's protocol.
SB transposon-mediated gene transfer of miR-29. A total of 75 µg plasmid DNA consisting of 50 µg SB transposon-miR-29b (pT2/BH-miR-29b2) or empty pT2/BH and 25 µg SB transposase (pCMV-SB11) was complexed with 16 µl of linear cationic polymer polyethylenimine (Exogen 500 in vivo; Fermentas, Glen Burnie, MD) using a charge ratio of 1:7.27 The DNA/polyethyleneimine complex was prepared and delivered to mouse in vivo via lateral tail vein injection as described previously.27 To test whether overexpression of miR-29 could prevent bleomycin-induced pulmonary fibrosis, groups of 5–8 mice were intravenously injected with 75 µg SB-miR-29b plasmids (pCMV-SB11/pT2/BH-miR-29b2) or control empty plasmids (pCMV-SB11/pT2/BH) in total of 500 µl of volume, followed by intratracheal injection with bleomycin or saline control at 24 hours as described. Mice were sacrificed on day 14 after disease induction. To examine the therapeutic effect of miR-29 on the established mouse model of pulmonary fibrosis, groups of 5–8 mice were firstly received bleomycin treatment, then at day 14 these mice were intravenously injected with SB-miR-29b or control plasmids as described above and sacrificed at day 28 after miR-29 gene therapy.
Excision assay. To prove that SB-mediated excision of the transposons in mouse lung, plasmid-based excision product assay was performed as previously described.33 Briefly, DNA was isolated from ~30 mg mouse right lower lung specimens using the Qiagen DNA Extraction Kit (Qiagen). PCR was performed in two rounds of amplification. PCR conditions were: 95 °C for 5 minutes followed by 45 cycles of (95 °C, 40 seconds, 58 °C, 30 seconds, 72 °C for 1 minute) with the final extension of 5 minutes at 72 °C. A 2 µl of the primary PCR product was used for secondary amplification in a 30 µl reaction with nested primers and the same cycling conditions except that the number of cycles was 35. The expected size of the amplified excision product was ~161 bp. The primers used for the excision assay were: FP1: 5′-TGACGTTGGAGTCCACGTTC-3′and RP1: 5′-GGCTCGTATGTTGTGTGG-3′, and FP2: 5′-GTAATA CGACTCACTATAGG-3′and RP2: 5′-GGAAACAGCTATGACCATG-3′. Then the PCR products were separated on 1.5% agarose gel containing 1:30,000 dilution of Gel Red.
microRNA assays. Total RNA from fibrotic lungs of Smad3 WT and KO mice were extracted using the miRNeasy miRNA Isolation kit (Qiagen). The quality of RNA samples was first accessed by Bioanalyzer (Agilent Technologies, Palo Alto, CA), and then labeled for microarray. A rodent miRNA array (Agilent Technologies) was used to identify the miRNAs and data were analyzed using GeneSpring GX 11 (Agilent Technologies).
Quantitative real-time PCR analysis. Total RNAs from both lung tissues and fibroblasts of Smad3 WT and KO mice were extracted by Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Real-time PCR analysis of miR-29a, b, c, collagen matrix, TGF-β1, and CTGF with primers were described previously.5,20
In situ hybridization. Specific 5′ DIG-labeled antisense-locked nucleic acid oligonucleotides for detection mmu-miR-29b (5′ -DigN/AACACTGATTTCAAATGGTGCTA- 3′), an oligo probe (5′–DigN/GAGG+CTTACAT+TGGAT+CCCCGG-3′) for detection of transgene miR-29b2 expression, and a scramble probe (5′ -GTGTAACACGTCTATA CGCCCA- 3′) as negative control were purchased from Exiqon (Vedbaek, Denmark). In situ hybridization of miR-29 or transgene miR-29 was performed on 4% paraformaldehyde/phosphate-buffered saline fixed, paraffin-embedded sections as previously described 20.
Primary lung fibroblasts isolation and culture. Primary lung fibroblasts from Smad3 KO and Smad3 WT mice were isolated and cultured as previously described.34 A recombinant human TGF-β1 (R&D Systems, Minneapolis, MN) at a dose of 2 ng/ml was added into the cell culture for 0, 3, 6, 24 hours for detection of miR-29 and collagen matrix expression by real-time PCR and western blot analysis. At least four independent experiments were performed throughout the study.
Histology and immunohistochemistry. Paraformaldehyde-fixed, paraffin-embedded lung tissues were sectioned (3 µmol/l) for H&E and Masson's trichrome staining, and immunohistochemical staining of collagen I, III (Southern Biotech, Birmingham, AL) and F4/80 (Serotec, Oxford, UK) using a microwave-based antigen retrieval technique 35. Sections were counterstained with hematoxylin. The number of F4/80+ cells in the lung was counted in 10 consecutive fields under power fields (X20) by means of a 0.25-mm2 graticule fitted in the eyepiece of the microscope and expressed as cells/mm2.
Measurement of hydroxyproline. Hydroxyproline assay was performed to determine matrix protein within the lung tissues as described previously with modification.36 Solutions of 0–200 µg/ml hydroxyproline (Sigma-Aldrich) were used to generate the standard curve. Data were expressed as micrograms of hydroxyproline/mg dry weight of lung tissue.
Western blot analysis. Proteins from lung tissues and cultured cells were extracted with RIPA lysis buffer and analyzed by western blotting as described previously.5 The primary antibody against collagen I, III, a-SMA, fibronectin (Fn), phospho-Smad3 (Cell Signaling Technology, Danvers, MA), total Smad3 (Zymed Laboratories, San Francisco, CA), and GAPDH (Chemicon, Temecula, CA) and IRDye 800-conjugated secondary antibody (Rockland Immunochemicals, Gilbertsville, PA) were used in this study. Signals were detected using the LiCor/Odyssey infrared image system (LI-COR Biosciences, Lincoln, NE) and were quantified by using the LiCor/Odyssey followed by analysis with Image J software (NIH).
Statistical analysis. Data were presented as means + SEM. Statistical analyses were performed using one-way ANOVA, followed with the Newman–Keuls post hoc test (Prism 4.0; GraphPad Software, San Diego, CA).
SUPPLEMENTARY MATERIAL Figure S1. Construction of Sleeping Beauty transposon-miR-29b plasmid vectors. Figure S2. Effect of Sleeping Beauty transposon system on delivering miR-29 into the lung.
Acknowledgments
This work was supported by rants from Research Grants Council of Hong Kong (CUHK05/CRF/09) and Chinese University of Hong Kong Focused Investment Scheme B grants (1902059). The authors declared no conflict of interest.
Supplementary Material
Construction of Sleeping Beauty transposon-miR-29b plasmid vectors.
Effect of Sleeping Beauty transposon system on delivering miR-29 into the lung.
REFERENCES
- Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, CAPACITY Study Group et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377:1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- King TE, Jr, Pardo A., and, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- du BRM. Strategies for treating idiopathic pulmonary fibrosis. Nat Rev Drug Discov. 2010;9:129–140. doi: 10.1038/nrd2958. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Russo A, Felici A., and, Flanders KC. Smad3: a key player in pathogenetic mechanisms dependent on TGF-beta. Ann N Y Acad Sci. 2003;995:1–10. doi: 10.1111/j.1749-6632.2003.tb03205.x. [DOI] [PubMed] [Google Scholar]
- Meng XM, Huang XR, Chung AC, Qin W, Shao X, Igarashi P.et al. (2010Smad2 protects against TGF-beta/Smad3-mediated renal fibrosis J Am Soc Nephrol 211477–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Shi W, Wang YL, Chen H, Bringas P, Jr, Datto MB.et al. (2002Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice Am J Physiol Lung Cell Mol Physiol 282L585–L593. [DOI] [PubMed] [Google Scholar]
- Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G.et al. (2007Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling Circulation 1162127–2138. [DOI] [PubMed] [Google Scholar]
- Huang XR, Chung AC, Yang F, Yue W, Deng C, Lau CP.et al. (2010Smad3 mediates cardiac inflammation and fibrosis in angiotensin II-induced hypertensive cardiac remodeling Hypertension 551165–1171. [DOI] [PubMed] [Google Scholar]
- Latella G, Vetuschi A, Sferra R, Catitti V, D'Angelo A, Zanninelli G.et al. (2009Targeted disruption of Smad3 confers resistance to the development of dimethylnitrosamine-induced hepatic fibrosis in mice Liver Int 29997–1009. [DOI] [PubMed] [Google Scholar]
- Sato M, Muragaki Y, Saika S, Roberts AB., and, Ooshima A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest. 2003;112:1486–1494. doi: 10.1172/JCI19270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M.et al. (1992Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease Nature 359693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC.et al. (1993Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death Proc Natl Acad Sci USA 90770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Letterio JJ, Lechleider RJ, Chen L, Hayman R, Gu H.et al. (1999Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-beta EMBO J 181280–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, Mizel DE.et al. (1999Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response Nat Cell Biol 1260–266. [DOI] [PubMed] [Google Scholar]
- Inui M, Martello G., and, Piccolo S. MicroRNA control of signal transduction. Nat Rev Mol Cell Biol. 2010;11:252–263. doi: 10.1038/nrm2868. [DOI] [PubMed] [Google Scholar]
- Cushing L, Kuang PP, Qian J, Shao F, Wu J, Little F.et al. (2011miR-29 is a major regulator of genes associated with pulmonary fibrosis Am J Respir Cell Mol Biol 45287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS.et al. (2008Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis Proc Natl Acad Sci USA 10513027–13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roderburg C, Urban GW, Bettermann K, Vucur M, Zimmermann H, Schmidt S.et al. (2011Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis Hepatology 53209–218. [DOI] [PubMed] [Google Scholar]
- Liu H, Liu L, Fletcher BS., and, Visner GA. Sleeping Beauty-based gene therapy with indoleamine 2,3-dioxygenase inhibits lung allograft fibrosis. FASEB J. 2006;20:2384–2386. doi: 10.1096/fj.06-6228fje. [DOI] [PubMed] [Google Scholar]
- Qin W, Chung AC, Huang XR, Meng XM, Hui DS, Yu CM.et al. (2011TGF-ß/Smad3 signaling promotes renal fibrosis by inhibiting miR-29 J Am Soc Nephrol 221462–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons MA, MacKinnon AC, Ramachandran P, Dhaliwal K, Duffin R, Phythian-Adams AT.et al. (2011Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis Am J Respir Crit Care Med 184569–581. [DOI] [PubMed] [Google Scholar]
- Carè A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P.et al. (2007MicroRNA-133 controls cardiac hypertrophy Nat Med 13613–618. [DOI] [PubMed] [Google Scholar]
- Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ.et al. (2010miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis J Exp Med 2071589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji F, Yonemitsu Y, Okano S, Yoshino I, Nakagawa K, Nakashima Y.et al. (2003Airway-directed gene transfer of interleukin-10 using recombinant Sendai virus effectively prevents post-transplant fibrous airway obliteration in mice Gene Ther 10213–218. [DOI] [PubMed] [Google Scholar]
- Aronovich EL, McIvor RS., and, Hackett PB. The Sleeping Beauty transposon system: a non-viral vector for gene therapy. Hum Mol Genet. 2011;20 R1:R14–R20. doi: 10.1093/hmg/ddr140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belur LR, Podetz-Pedersen K, Frandsen J., and, McIvor RS. Lung-directed gene therapy in mice using the nonviral Sleeping Beauty transposon system. Nat Protoc. 2007;2:3146–3152. doi: 10.1038/nprot.2007.460. [DOI] [PubMed] [Google Scholar]
- Belur LR, Frandsen JL, Dupuy AJ, Ingbar DH, Largaespada DA, Hackett PB.et al. (2003Gene insertion and long-term expression in lung mediated by the Sleeping Beauty transposon system Mol Ther 8501–507. [DOI] [PubMed] [Google Scholar]
- Liu L, Sanz S, Heggestad AD, Antharam V, Notterpek L., and, Fletcher BS. Endothelial targeting of the Sleeping Beauty transposon within lung. Mol Ther. 2004;10:97–105. doi: 10.1016/j.ymthe.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Liu L, Liu H, Visner G., and, Fletcher BS. Sleeping Beauty-mediated eNOS gene therapy attenuates monocrotaline-induced pulmonary hypertension in rats. FASEB J. 2006;20:2594–2596. doi: 10.1096/fj.06-6254fje. [DOI] [PubMed] [Google Scholar]
- Maurer B, Stanczyk J, Jüngel A, Akhmetshina A, Trenkmann M, Brock M.et al. (2010MicroRNA-29, a key regulator of collagen expression in systemic sclerosis Arthritis Rheum 621733–1743. [DOI] [PubMed] [Google Scholar]
- Geurts AM, Yang Y, Clark KJ, Liu G, Cui Z, Dupuy AJ.et al. (2003Gene transfer into genomes of human cells by the sleeping beauty transposon system Mol Ther 8108–117. [DOI] [PubMed] [Google Scholar]
- Liu G, Aronovich EL, Cui Z, Whitley CB., and, Hackett PB. Excision of Sleeping Beauty transposons: parameters and applications to gene therapy. J Gene Med. 2004;6:574–583. doi: 10.1002/jgm.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson WE, Polosukhin VV, Zoia O, Stathopoulos GT, Han W, Plieth D.et al. (2005Characterization of fibroblast-specific protein 1 in pulmonary fibrosis Am J Respir Crit Care Med 171899–907. [DOI] [PubMed] [Google Scholar]
- Lan HY, Hutchinson P, Tesch GH, Mu W., and, Atkins RC. A novel method of microwave treatment for detection of cytoplasmic and nuclear antigens by flow cytometry. J Immunol Methods. 1996;190:1–10. doi: 10.1016/0022-1759(95)00233-2. [DOI] [PubMed] [Google Scholar]
- Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, Cunningham A.et al. (2010Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis Am J Respir Crit Care Med 181254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Construction of Sleeping Beauty transposon-miR-29b plasmid vectors.
Effect of Sleeping Beauty transposon system on delivering miR-29 into the lung.